Abstract
Sexual selection has been proposed as one mechanism to explain the maintenance of high allelic diversity in MHC genes that control the extent of resistance against pathogens and parasites in natural populations. MHC-based sexual selection is known to involve olfactory mechanisms in fish, mice, and humans. During mate choice, females of the three-spined stickleback (Gasterosteus aculeatus) use an odor-based selection strategy to achieve an optimal level of MHC diversity in their offspring, equipping them with optimal resistance toward pathogens and parasites. The molecular mechanism of odor-based mate-selection strategies is unknown. Because peptide ligands for MHC class I molecules function as individuality signals in mice, we hypothesized that female sticklebacks might assess the degree of MHC diversity of potential partners by means of the structural diversity of the corresponding peptide ligands in perceived odor signals. We show that structurally diverse MHC ligands interact with natural odors of male sticklebacks to predictably modify MHC-related mate choice. For a mating pair with suboptimal numbers of MHC alleles, peptides increase the attractiveness of male water, whereas for a mating pair with superoptimal numbers, attractiveness is decreased. Our results suggest that female sticklebacks use evolutionarily conserved structural features of MHC peptide ligands to evaluate MHC diversity of their prospective mating partners.
Keywords: sexual selection, olfaction, immunogenetics
The maintenance of high allelic diversity in MHC genes that control the extent of resistance against pathogens and parasites in natural populations has been explained by sexual selection (1, 2). MHC-based sexual selection is known to involve olfactory mechanisms in fish (3, 4), mice (5–7), and humans (8, 9). In natural populations of the three-spined stickleback (Gasterosteus aculeatus), individuals with an intermediate number of different MHC alleles are the most frequent genotype (3, 10) that both under field (10) and experimental conditions (11, 12) best resist natural parasites. During mate choice, female sticklebacks use an odor-based selection strategy to achieve this optimal level of MHC diversity in their offspring (3, 4). Therefore, male sticklebacks are postulated to produce and release MHC-related odors that provide information about the individual's composition of MHC alleles. It is also clear that, although the mechanism is as yet unknown, female sticklebacks are capable of assessing the degree of MHC diversity of their prospective mating partners. Because genes of the MHC complex are highly polymorphic and encode structurally related but distinct MHC molecules (13, 14), natural chemosignals that function in MHC-related mate choice must be expected also to be structurally polymorphic, and the extent of their structural diversity should be a function of the number of structurally different MHC molecules expressed by an individual. With regard to the nature of this signal, MHC molecules or their fragments, degradation products of their peptide ligands, and products of the MHC-dependent microflora have been considered as potential odorants (15).
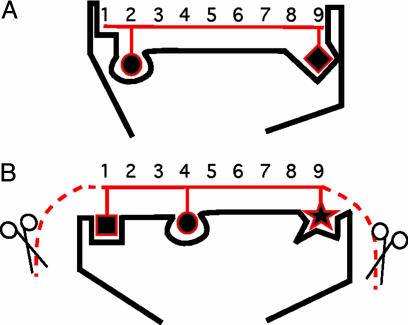
The diversity of ligand-binding pockets of MHC molecules allows them to bind to particular subsets of peptides that are generated as intermediates during intracellular proteolytic degradation and to display them at the cell surface for immune surveillance (14). As a general rule, the greater the number of structurally diverse MHC alleles that are expressed, the greater the number of different peptides that are displayed at the cell surface. Therefore, the sequence space of MHC peptide ligands directly reflects the underlying degree of MHC molecule polymorphism present in an individual. In other words, the range of peptides displayed by an individual's MHC molecules mirrors the structural diversity of its MHC alleles. These peptides may represent the elusive polymorphic signal used during matechoice decisions. The structural diversity of peptide ligands of each MHC molecule as such may be too large to provide an unambiguous signal. However, different peptide ligands of one particular MHC molecule share common residues (so-called anchors) whose side chains fit into the characteristic binding pockets of the MHC molecule (14). Therefore, the anchor residues, rather than the precise sequences, may be the defining feature of peptide signals. MHC class I and class II molecules bind their peptide cargo according to different rules, which are reflected in so-called peptide motifs (14). These motifs mirror the overall structure of the ligand-binding pocket of MHC molecules and describe the lengths and the preferential occurrences (usually less pronounced in MHC class II ligands) of certain amino acid residues at certain positions of peptide ligands (Fig. 1). MHC class I peptides are usually 9 aa long and the C-terminal amino acid residue always serves as an anchor residue; whereas the N-terminal residue never serves as an anchor, an additional anchor is often located at the second position. MHC class II peptides are generally longer than MHC class I peptides, with the binding cleft harboring a central stretch of nine amino acids, with positions 1 and 9 in this core sequence almost always representing anchor residues; a variable number of residues protrudes at the N and C termini. Whereas these ragged ends are prone to exoproteolytic degradation, the core sequence of nine amino acids (9-mer) is protected from degradation by virtue of its interaction with MHC class II molecules (16). Based on the known structure of MHC-binding pockets and their corresponding ligands (14), C-terminal residues of peptides could be a common target of sensory discrimination in the process of evaluation of MHC diversity (Fig. 1). MHC/peptide complexes are shed from the cell surface and their fragments appear in serum, saliva, and urine (6, 17). These truncated MHC molecules are believed to have a reduced affinity to their peptide ligands (18) and are thus likely to release them into the extracellular space. Peptides may then interact with other types of receptors. Indeed, it has recently been shown that sensory neurons of the vomeronasal organ of mice are activated in a sequence-specific manner by peptides with the general characteristics of MHC ligands (19). This finding suggests how MHC genotype can be converted into an olfactorily detectable quality. In behaving mice, MHC peptides function as individuality signals underlying partner recognition in the context of pregnancy block (Bruce effect) (19). We examined therefore whether peptide ligands of MHC molecules function as chemosignals in matechoice decisions by female sticklebacks.
Fig. 1.
Schematic representation of structure of MHC/peptide complexes. (A) The peptide-binding groove of MHC class I molecules accommodates peptides (red) generally of 9 aa in length, with the N and C termini tightly fixed in the edges. The most significant contacts between peptides and the MHC molecules are mediated through the side chains of so-called anchor residues of the peptide (shown as stick symbols) fitting into pockets of the MHC molecule. The specificity and the precise location of these pockets vary among different MHC molecules; in this way, the structural polymorphism of MHC molecules influences peptide-binding specificity. (B) The peptide-binding groove of MHC class II molecules is not closed at the ends, allowing peptides (red) to protrude out of the pocket. As in MHC class I molecules, the central core of the peptide is locked into the groove by means of anchor/pocket interactions. The N- and C-terminal overhangs are thought to be prone to exonucleolytic degradation (symbolized by dashed lines and scissors), whereas the core sequence is protected (16).
Materials and Methods
Animals. Fish were caught in the Grosse Plöner See in autumn and kept in the laboratory under autumn, winter, spring, and summer temperature and light conditions, and were kept under the latter (summer) conditions for a maximum of 6 weeks before use in the experiments. Under summer conditions, fish were fed with frozen chironomid larvae ad libitum once a day and with a rich diet of live food. The experiments were performed in 2002, 2003, and 2004 with fish that had been caught <6 months earlier; the results were thus repeatable with wild-caught fish in different years.
Mate Choice Between a Single Male's Odor With and Without Added Wild-Type Peptides. Female sticklebacks that were ripe for spawning were placed in a flow chamber that was fed by two water columns under conditions of laminar flow (3, 4). Fish were able to freely investigate the composition of water in the two halves of the chamber for two periods of 300 s each, with spatial reversal of water sources after the first period of 300 s. If the two sources were equally attractive, the fish should spend an equal period (i.e., 300 s) with each source of stimulus. Because odor preference as determined in the flow channel setup reliably predicts mate choice (see Figs. 4 and 5 and Supporting Text, which are published as supporting information on the PNAS web site), the effect of peptides was tested in the flow channel. To this end, water was taken from the tank of a single male per trial and used both as stimulus water (after addition of wild-type peptides in solvent) continuously added to one half, and control water [after addition of solvent only (Figs. 2 and 3A), or modified peptides in solvent (Fig. 3B)] added continuously to the other half of the flow channel. The concentrations of solvent and the volume of supplement added to the water columns were identical on both sides in all experiments.
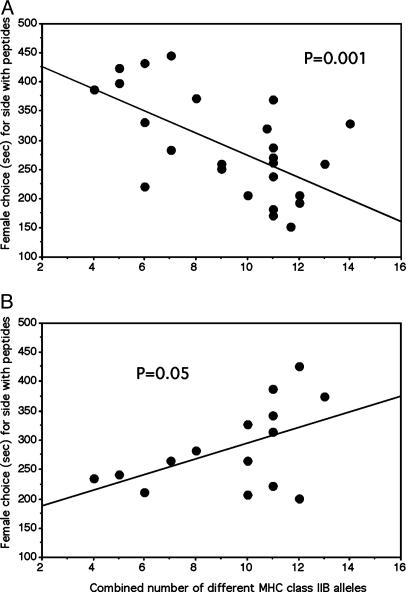
Fig. 2.
Modulation of odor-based preference of females for a potential mate through four different stickleback 9-mer peptides. (A) In a two-choice flow channel, single gravid females chose between the water from the tank of a single male supplemented with either solvent only or four stickleback peptides in solvent. The time females spent in the side of the flow channel to which water from the tank of a single male plus peptides in solvent was added (of a total of 600 s) is shown as a function of the combined number of different MHC class IIB alleles of male and female. (B) Reversal of female response to added peptides on the day after spawning; results are shown otherwise as in A.
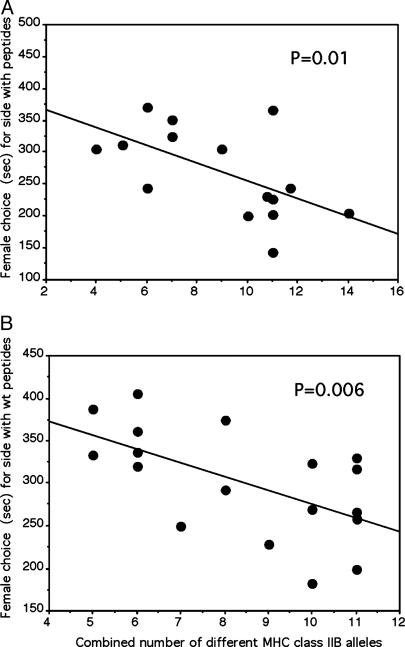
Fig. 3.
Structure/function relationship of peptides in modulation of mate choice. (A) Modulation of odor-based preference of gravid females for a potential mate through five xenogeneic 9-mer peptides, shown otherwise as in Fig. 2. (B) Structural requirements for peptides as odor cues in MHC-related mate choice. Females chose between four different xenogeneic 9-mer peptides and the same number and concentration of the corresponding modified peptides (see text for details), shown otherwise as in A.
Supplements were added to the stimulus water at the start of the experiment, beginning at a concentration of 0.25 mM per peptide and becoming diluted to a final concentration of ≈1.25 nM per peptide in the water column passing through the female's flow chamber. Under such conditions, peptides are not detectably degraded in the water for at least 2 h, as determined by MS (data not shown). Thus, it is unlikely that free amino acids resulting from peptide degradation are responsible for any effect. The molarity of peptides was chosen to correspond to the same order of magnitude as the concentration of MHC molecules observed in the serum and urine of mammals (6, 17). The presence of MHC peptide ligands in conditioned tank water has not yet been directly proven but is suggested by the presence, in bodily fluids, of MHC molecules in other species (6, 17). All experiments were performed in double-blind fashion in the Plön laboratory and the corresponding coding information kept in the Freiburg laboratory.
In tests with the combination of stickleback MHC 9-mer peptides (Fig. 2 A), a total of 31 different females and 31 different males were used. When females were used in two successive gravidities with different males (or males were tested twice with different females), respective data were entered as means per male or female to avoid pseudoreplication. Thus, each individual irrespective of having been tested once or twice contributed to only one data point. All data points in a figure are thus completely independent statistical units. Retesting and averaging the results increases the reliability of data from single fish. Females were retested when they became gravid again, however, only when there were no previously untested gravid females available on a particular day. Males were retested when the number of reproductively active males became limited. Data from 11 retested individuals were entered as means per male or female. In the experiment of Fig. 2B, 15 females and males were used. In one case, the same combination was retested, and the results were averaged and entered as one data point. In tests with the combination of five mouse MHC 9-mer peptides (Fig. 3A), a total of 20 different females and 20 different males were used. Data from 11 retested individuals were entered as means per male or female and given as single data points. The fish from this experiment had also been tested with stickleback MHC 9-mer peptides (Fig. 2 A). In the tests comparing wild-type and modified 9-mer peptides (Fig. 3B), 18 females and 18 males were used. In two cases, the same combinations were retested, the respective results were averaged, and each mean was entered as one data point.
Statistical analysis was performed with statview se (SAS Institute, Cary, NC). We used directed rather than one-tailed statistics to avoid inflation of the α value, when the direction of the predicted effect had been shown by a preceding experiment, otherwise we used two-tailed tests.
Derivation of Stickleback Peptides. To derive stickleback peptide sequences with the properties of MHC ligands, a cDNA library from mRNA isolated from adult stickleback head kidney in the pCMVSport6 vector (Invitrogen) was constructed (A. H.-A. and T.B., unpublished work). Individual cDNA clones were sequenced, and the derived protein sequences were compared with database entries by using the blast suite of search algorithms at the National Center for Biotechnology Information (NCBI) server (www.ncbi.nlm.nih.gov). The following peptides derived from the endogenous stickleback proteins were selected by using algorithms implemented in the SYFPEITHI database suite of programs (www.uni-tuebingen.de/uni/kxi; ref. 20). VDPDNFKLL was derived from Gasterosteus aculeatus β-globin; amino acids 99–107, GenBank accession no. AY184355; NYGVTKTDI was derived from Gasterosteus aculeatus neuronal protein 22, amino acids 47–56, GenBank accession no. AY184357; SYKEKNIFL was derived from Gasterosteus aculeatus signal peptidase complex 25-kDa subunit (Spc25), amino acids 93–101, GenBank accession no. AY184358; and KLYEQGSNK was derived from Gasterosteus aculeatus proteasome subunit α-3, amino acids 57–65, GenBank accession no. AY184356. Xenogeneic MHC ligands were selected from published sources (14): SYFPEITHI, SYIPSAEKI, FAPGNYPAL, ASNENMETM, and AAPDNRETF. In the experiment comparing wild-type and modified peptides, the following peptides were used. Wild-type, SYIPSAEKI, SFVDTRTLL, ASNENMETM, and AAPDNR ETF, and their modified versions, SA IPSAEKA, SAVDTRTLA, ASNEAMETA, and AAPDARETA. Note that SYFPEITHI, SYIPSAEKI, and AAPDNRETF peptides activate vomeronasal sensory neurons in mice, whereas SAFPEITHA and AAPDARETA do not (19). Peptides were chemically synthesized, purified, verified by MALDI-TOF-MS, and dissolved in PBS.
Results and Discussion
In previous experiments, odor-based preference for males by gravid female sticklebacks was determined in a flow channel, where they could be exposed simultaneously to two different water sources, each originating from a tank harboring a single male (3, 4). Under these conditions, female fish appear to compare their own set of MHC alleles with those of the prospective mating partners and show preference for the scent of males providing as optimal a complementation of alleles as possible (4). A female's preference exerted for male odor in the flow channel is a reliable precursor of mate choice, because the flow-channel test reflects a female's ultimate mate choice during spawning (see Figs. 4 and 5).
To determine whether 9-mer peptides representing MHC class I and trimmed MHC class II peptides (Fig. 1) are capable of predictably modifying the outcome of mate choice, we conducted a flow-channel experiment (3, 4) by using a mixture of four different 9-mer peptides, whose sequence was derived from endogenous stickleback proteins. The rationale of this experiment is as follows. If peptides are the natural signal that is linked to a male's MHC alleles and that is evaluated by the choosing female, it should be possible to manipulate the information transmitted by the signal by supplementing further peptides. Because the female prefers the scent of a male that offers the optimal complementation of her own alleles (4), the effect of the peptide supplementation can be predicted for a given pair of female and male. When water conditioned by a male with too few alleles is offered to the female, the addition of peptides should enhance the attractiveness of the spiked water as compared to the unmanipulated sample. This outcome is expected because the structural diversity of peptides (that is responsible for specific binding to particular MHC molecules) in male water is likely increased by the synthetic supplements. Conversely, when water conditioned by an optimal male is supplemented, the spiked version should be less attractive to the female. Spiked water from a male with too many alleles should become even less attractive. Thus, the same mixture of supplemented peptides is predicted to either augment or diminish the endogenous attractiveness of the male's scent. The expected outcome of peptide supplementation depends on the particular combination of alleles of male and female. If the number of unique alleles is below the optimum, attractiveness of the male's water should be increased; when it is at or exceeds the optimum, attractiveness should be decreased. Stickleback test pairs of known MHC class IIB alleles were derived such that the combinations covered a range from 4 to 14 different MHC class IIB alleles per pair, i.e., from sub- to superoptimal to allow for subsequent regression analysis of the results. The number of different alleles was calculated as follows. For example, consider a pair consisting of a female with four different alleles and a male with six different alleles. If they share no alleles, the pair has a combined diversity of 10 different alleles. In this case, on average, five alleles are transmitted to the offspring, because each parent provides a haploid set per gamete (close to the apparent optimum of ≈5.2 alleles in an offspring) (4). Other combinations of male and female may yield allele numbers below or above this optimum. Because we used water from the tank of only one male fed into the two sides of the flow channel, the odor stimuli provided to the female in the choice chamber differed only by supplemented peptides. If peptides were ignored by females, both sides of the flow channel would appear to be equally attractive, and no preference would be expected. Fig. 2 A shows that 9-mer peptides indeed function as attractors (when the combined number of different MHC class IIB alleles of the male and the choosing female are less than optimal) or repellants (when this number is at or exceeds the optimum), respectively. This predicted modulation of female preference for male odor through peptides is highly significant (r = –0.60, n = 26, t = 3.71, P = 0.001, using a two-tailed test) and suggests a strong interaction of the supplemented peptides with natural scent. Note that our analysis does not take into account diversity of MHC class I genes, which cannot be reliably analyzed in sticklebacks at present. As a result, the actual differences among pairs of stimulus males in the diversity of MHC alleles other than class IIB (that is, the overall sequence complexity of peptide ligands) contribute to the overall variance of data, i.e., weaken the relationship. Our results are, therefore, a conservative estimate of the strength of the potential relationship. In contrast to other vertebrates, MHC class I and class II genes are not linked in teleost fish, including sticklebacks (21), making their contribution to MHC-related odor signals independent of each other. Because our results suggest a strong interaction between the diversity of MHC class IIB alleles and peptides, it is possible that endogenous MHC class I peptides play only a minor role in MHC-related scents of sticklebacks.
In flow-channel experiments with a female that compares water sources of two different males, the female prefers the water of the male that best complements her own alleles with respect to the apparent optimum of MHC diversity (3, 4). The experimental approach described above (Fig. 2) shows that synthetic peptides do not obscure the information contained in the male odor. Indeed, the information of natural male odor makes a significant contribution to the outcome of mate choice. The number of alleles of females and males correlated positively within pairs (r = 0.465, n = 26, t = 2.571, P = 0.02, using a two-tailed test), because high numbers can occur only if both mates have high numbers and vice versa. Therefore, the residual number of male MHC class IIB alleles from this relationship was used for a regression analysis with the outcome of female choice. The significant relationship between the residual number of male MHC class IIB alleles and female preference for the side to which peptides were added to male water (r =–0.41, n = 26, t = 2.178, P = 0.04, using a two-tailed test) indicates that male MHC signals contributed significantly to the outcome of mate choice depicted in Fig. 2 A.
The next experiment was designed to examine the context specificity of the observed effects of peptides. Female sticklebacks avidly forage for food between spawnings; indeed, they often raid nests with stickleback eggs (22). Odor cues used in mate-choice decisions could also be an effective means to restrict this behavior to unrelated eggs. If, after spawning, a stickleback female was repelled rather than attracted by the body odor of her preferred actual or potential mating partners now guarding the nests, it would avoid her cannibalizing her own clutches. Therefore, we tested whether the preference of females for water sources supplemented with peptides changed 1 day after spawning. Indeed, the preference of females for one or the other water source, depending on the combined MHC diversity of the pair was reversed (Fig. 2B). Instead of a negative correlation (as in Fig. 2 A), there is now a significant positive correlation between combined number of different MHC class IIB alleles of the pair and female preference, i.e., the time spent on the side with peptide-spiked male water (r = +0.51, n = 15, t = 2.136, P = 0.05, using a two-tailed test). This behavior is not only adaptive as outlined above but also supports our hypothesis that gravid females use the information provided by water-borne peptides specifically and differentially in the context of reproductive decisions.
We next tested two further predictions of our hypothesis. The first prediction concerns the species origin of peptides. If the sensory mechanism indeed focused on the chemical nature of the C-terminal amino acid residue, rather than on the entire sequence of peptides, 9-mer peptides derived from proteins of other species should also be able to modulate mate choice. Note that MHC molecules not only bind peptides originating from endogenous proteins but also from proteins of parasites that have infected a particular fish, which is the key to their function in the immune system. This result is possible because an MHC molecule binds any peptide that has the appropriate anchor residues irrespective of its origin. As expected, a mixture of such xenogeneic peptides modulated predictably mate choice decisions by female sticklebacks [r = –0.58, n = 15, t = 2.589, P = 0.01, directed (Fig. 3A)]. Clearly, signals about gender, reproductive status, and species identity must be evaluated alongside signals of MHC diversity to ensure a meaningful biological response.
A further prediction of our hypothesis concerns the nature of the chemical diversity of the C-terminal residues of 9-mer peptides. One would expect that mate choice is more effectively modulated by a mixture of peptides differing at their C termini than by a mixture of peptides with the same C-terminal residue. Therefore, a mixture of four 9-mer peptides with four different C-terminal residues was compared with a mixture of related peptides in which the C-terminal residues all comprised of alanine. The rationale of this experiment is as follows. If the C-terminal residues of peptides are indeed decisive determinants for female odor evaluation (as they are for MHC molecule-binding specificity), the choosing female should be able to distinguish between peptide supplements that have high (wild-type) and low (modified) C-terminal diversity. Hence, if given the choice between the two different peptide mixtures added to the water of a single male, females should either prefer or dislike the water with wild-type peptides, depending on the combined number of MHC alleles of the pair in a fashion shown in Figs. 2 and 3A, almost ignoring the supplemented modified peptides. If, however, the C-terminal residues of peptides are not important in this evaluation of odor quality, no preference for the wild-type peptide side, depending on the combined number of MHC alleles of the pair would be expected: the regression line would be parallel to the x axis. This result represents a critical test of our hypothesis, because a preference should only be detected if the C-terminal residues of peptides are important. Fig. 3B shows that when tested against wild-type versions, the effect of modified peptides on mate choice decisions is negligible, because females responded to the wild-type peptides almost exactly as before (Fig. 3A); thus, they are capable of distinguishing between mixtures of peptides that have high and low C-terminal diversity (Fig. 3B; r = –0.59, n = 18, t = 2.95, P = 0.006, directed). The experimental design used here is superior to an experiment that tests the effect of modified peptides in solvent against solvent alone, because, for statistical reasons, the null hypothesis cannot be easily proven correct when no preference is expected. This experiment suggests that structural characteristics of C termini of peptides are an important factor of the female's perception of MHC ligands.
We have previously shown that the sexual selection strategy used by sticklebacks is odor-based and requires the assessment of MHC diversity (3, 4). Our present analysis of the interaction of synthetic peptides with the natural MHC-related odor signal provides evidence for the peptidic nature of natural odorants in MHC-related sexual selection in a teleost fish. It suggests a plausible molecular link between MHC polymorphism and body odor of an individual. It is clear, however, that evolutionary adaptive mate choice requires the integration of additional, presumably species-, gender-, condition-, and maturation-specific signals (reviewed in refs. 22 and 23). The modulation of mate choice by peptides is a specific effect. There are no overall positive or negative responses to peptides; rather, the attractor and repellant functions of peptides solely depend on the MHC status of mating pairs, because both the concentration and composition of peptides in our comparisons is always the same for all pairs in one experiment. This finding rules out a number of trivial explanations, for instance, that peptides are perceived as food or danger signals. In addition, the very same type of information elicits strikingly different behaviors after reproduction, as shown by the reversed response of females to the same mixture of peptides before and after spawning. In the experiment addressing the structural diversity of C-terminal residues of peptides, no difference between wild-type and modified peptides should have been observed, if the response of female sticklebacks to peptides was unrelated to their sequence characteristics. Thus, our results suggest that peptides form part of the MHC-related odor signals that female sticklebacks evaluate in their MHC-related behavioral mate choice. This finding does not rule out the role of other types of molecules that have been implicated in other systems (15, 24, 25). The observed structure/function relationships suggest that the effect of peptides in mate choice of female sticklebacks likely involves sequence-specific activation of olfactory sensory neurons by peptides as described in the mouse (19). The nature of peptide receptors on olfactory neurons is unknown. However, previous studies in the mouse have implicated receptors of the so-called V2R class that are expressed in sensory neurons of the basal zone of the vomeronasal organ (19). Although an anatomically distinct vomeronasal organ does not exist in fish, cells with morphological and physiological characteristics of olfactory neurons of the accessory system are found in the olfactory rosette (26). Furthermore, genes of the V2R class have been cloned from fish (27). This finding suggests that peptide recognition in fish and mouse may occur in a comparable fashion.
Our results suggest that odor-based evaluation of prospective mates is an evolutionarily ancient component of MHC-related sexual selection strategies, designed to achieve an optimal degree of MHC diversity in offspring. We speculate that the evaluation of peptide diversity as a surrogate of genetic relatedness is also used in other contexts, such as kin recognition (28, 29).
Supplementary Material
Acknowledgments
We thank R. Escher for peptide synthesis, M. Held for assistance with sequencing, G. Augustin for fish maintenance, P. Aeschlimann and M. Häberli for assistance with a pilot study, and Dr. D. Penn for helpful discussions. This work was partially supported by Deutsche Forschungs-gemeinschaft Grant Bo 1128/6-1, the Natural Environment Research Council, and the Cardiff University Young Researcher Initiative (to S.G.).
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY184355–AY184358).
References
- 1.Edwards, S. V. & Hedrick, P. W. (1998) Trends Ecol. Evol. 13, 305–311. [DOI] [PubMed] [Google Scholar]
- 2.Apanius, V., Penn, D., Slev, P. R., Ruff, L. R. & Potts, W. K. (1997) Crit. Rev. Immunol. 17, 179–224. [DOI] [PubMed] [Google Scholar]
- 3.Reusch, T. B. H., Häberli, M. A., Aeschlimann, P. B. & Milinski, M. (2001) Nature 414, 300–302. [DOI] [PubMed] [Google Scholar]
- 4.Aeschlimann, P. B., Häberli, M. A., Reusch, T. B. H., Boehm, T. & Milinski, M. (2003) Behav. Ecol. Sociobiol. 54, 119–126. [Google Scholar]
- 5.Yamazaki, K., Boyse, E. A., Mike, V., Thaler, H. T., Mathieson, B. J., Abbott, J., Boyse, J., Zayas, Z. A. & Thomas, L. (1976) J. Exp. Med. 144, 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh, P. B., Brown, R. E. & Roser, B. (1987) Nature 327, 161–164. [DOI] [PubMed] [Google Scholar]
- 7.Penn, D. J. & Potts, W. K. (1999) Am. Nat. 153, 145–164. [DOI] [PubMed] [Google Scholar]
- 8.Wedekind, C., Seebeck, T., Bettens, F. & Paepke, A. J. (1995) Proc. R. Soc. London Ser. B 260, 245–249. [DOI] [PubMed] [Google Scholar]
- 9.Jacob, S., McClintock, M. K., Zelano, B. & Ober, C. (2002) Nat. Genet. 30, 175–179. [DOI] [PubMed] [Google Scholar]
- 10.Wegner, K. M., Reusch, T. B. H. & Kalbe, M. (2003) J. Evol. Biol. 16, 224–232. [DOI] [PubMed] [Google Scholar]
- 11.Wegner, K. M., Kalbe, M., Kurtz, J., Reusch, T. B. H. & Milinski, M. (2003) Science 301, 1343. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz, J., Kalbe, M., Aeschlimann, P. B., Häberli, M., Wegner, K. M., Reusch, T. B. H. & Milinski, M. (2004) Proc. R. Soc. London Ser. B 271, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein, J. (1986) Natural History of the Major Histocompatibility Complex (Wiley, New York).
- 14.Rammensee, H.G., Bachmann, J. & Stefanovic, S. (1997) MHC Ligands and Peptide Motifs (Landes Bioscience, Georgetown, TX).
- 15.Penn, D. J. & Potts, W. K. (1998) Adv. Immunol. 69, 411–436. [DOI] [PubMed] [Google Scholar]
- 16.Mouritsen, S., Medal, M., Werdelin, O., Hansen, A. S. & Buus, S. (1992) J. Immunol. 149, 1987–1993. [PubMed] [Google Scholar]
- 17.Stevenson, F. K., George, A. J., Walters, M. T. & Hamblin, T. J. (1986) J. Immunol. Methods 86, 187–190. [DOI] [PubMed] [Google Scholar]
- 18.Singh, P. B. (2001) Reproduction 121, 529–539. [DOI] [PubMed] [Google Scholar]
- 19.Leinders-Zufall, T., Brennan, P., Widmayer, P., Chandramani S. P., Maul-Pavicic, A., Jäger, M., Li, X.-H., Breer, H., Zufall, F. & Boehm, T. (2004) Science 306, 1033–1037. [DOI] [PubMed] [Google Scholar]
- 20.Rammensee, H. G., Bachmann, J., Emmerich, N. P., Bachor, O. A. & Stevanovic, S. (1999) Immunogenetics 50, 213–219. [DOI] [PubMed] [Google Scholar]
- 21.Sato, A., Figueroa, F., Murray, B. W., Malaga-Trillo, E., Zaleska-Rutczynska, Z., Sultmann, H., Toyosawa, S., Wedekind, C., Steck, N. & Klein, J. (2000) Immunogenetics 51, 108–116. [DOI] [PubMed] [Google Scholar]
- 22.Wootton, R.J.A. (1985) Functional Biology of Sticklebacks (Croom Helm, London).
- 23.Bakker, T. C. M. & Milinski, M. (1993) Mar. Behav. Physiol. 23, 287–300. [Google Scholar]
- 24.Beauchamp, G. K. & Yamazaki, K. (2003) Biochem. Soc. Trans. 31, 147–151. [DOI] [PubMed] [Google Scholar]
- 25.Doherty, P. C. (2003) Nat. Immunol. 4, 1043–1045. [DOI] [PubMed] [Google Scholar]
- 26.Hansen, A., Anderson, K. T. & Finger, T. E. (2004) J. Comp. Neurol. 477, 347–359. [DOI] [PubMed] [Google Scholar]
- 27.Cao, Y., Oh, B. C. & Stryer, L. (1998) Proc. Natl. Acad. Sci. USA 95, 11987–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsén, K. H., Grahn, M., Lohm, J. & Langefors, A. (1998) Anim. Behav. 56, 319–327. [DOI] [PubMed] [Google Scholar]
- 29.Neff, B. D. (2003) Nature 422, 716–719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.