Abstract
The uterine luminal epithelium is the first site of contact between fetal and maternal tissues during therian pregnancy and must undergo specialised changes for implantation of the blastocyst to be successful. These changes, collectively termed the plasma membrane transformation (PMT), allow the blastocyst to attach to the uterine epithelium preceding the formation of a placenta. There are similarities in the morphological and molecular changes occurring in live‐bearing eutherian species during the PMT studied so far. Similar cellular remodelling occurs in a marsupial species, the fat‐tailed dunnart (Sminthopsis crassicaudata), despite the divergence of marsupials from eutherian mammals over 130 mya, which resulted in the evolution of distinct reproductive strategies. Adhesion molecules along the lateral plasma membrane of uterine epithelium provide a barrier to invasion by the embryo. We thus characterised the presence and change in distribution of epithelial cadherin (E‐cadherin) in uterine epithelium from non‐pregnant fat‐tailed dunnarts and compared it to dunnarts in early‐, mid‐ and late‐stage pregnancy. E‐cadherin staining is localised to the lateral plasma membrane in uterine epithelium from non‐pregnant and early‐stage pregnant dunnarts. The E‐cadherin staining is cytoplasmic in epithelium from uteri of mid‐ and late‐stage pregnant dunnarts. This loss of localised staining suggests that the adherens junction dissociates from the lateral plasma membrane, allowing for invasion between the epithelial cells by the blastocyst. As the changes during pregnancy to cadherin were similar in the laboratory rat with highly invasive (haemochorial) placentation, a live‐bearing lizard species with non‐invasive (epitheliochorial) placentation and a marsupial, the fat‐tailed dunnart, which has invasive (endotheliochorial) placentation, we suggest that the molecular mechanisms allowing for successful pregnancy are conserved among mammals during the early stages of pregnancy regardless of placental invasiveness.
Keywords: epithelial cadherin, adherens junction, Sminthopsis crassicaudata, marsupial, uterine epithelium
Introduction
Live birth in mammals requires the development of a placenta for waste removal, nutrient uptake and gas exchange during embryonic growth (Mossman, 1937). Uterine remodelling during early pregnancy is essential for ensuring receptivity of the maternal uterine epithelium to the embryo and ultimately for placental formation (Murphy, 2000). The preparatory remodelling begins with changes to the uterine epithelial cells, which are the first cells to come into direct contact with the early embryo. The uterine epithelial cells undergo rapid, hormonally driven, ultrastructural and protein changes collectively termed the ‘plasma membrane transformation’ (PMT; Murphy, 2000, 2004), leading to successful attachment and implantation of the trophoblast (Murphy, 1993) .
Common mechanisms are responsible for the PMT changes across all amniote species studied so far. The apical surface of the epithelial cells becomes flattened with reduced adhesion between adjacent cells preceding implantation or attachment of the early embryo (Murphy, 2000). In mammalian species with invasive placentation, the loss of lateral adhesion allows the trophoblast to invade between the cells and ‘anchor’ within the maternal uterine stroma (Murphy, 2000; Preston et al. 2004; Dudley et al. 2015). Similar remodelling occurs in squamate species with less invasive placentation, with the reduction in lateral adhesion and change in distribution of adhesion structures to the apical portion of the lateral plasma membrane aiding in the attachment of the early embryo to the apical surface of the epithelium through homophilic binding (Biazik et al. 2010; Wu et al. 2011).
Cadherins are a calcium‐dependent superfamily of polypeptides that undergo post‐translational changes to become cell‐to‐cell adhesion proteins. There are hundreds of cadherin protein isoforms, all of which have been demonstrated to be important for normal cell development (Wheelock & Johnson, 2003). Cadherins are classified into four groups: classical cadherins that form a component of adhesion junctions, desmosomal cadherins, protocadherins and cadherin‐related molecules (Ivanov et al. 2001). Cadherins form the adherens junction in the lateral plasma membrane of cells (Hartsock & Nelson, 2008). They establish cell polarisation and connect the actin cytoskeleton of the cell to the adherens junction of the adjacent cell (Staun‐Ram & Shalev, 2005). Epithelial cadherin (E‐cadherin) is a member of the classical cadherin family most abundant in epithelial tissue. It is an important adhesion molecule that suppresses cell invasion in normal functioning epithelia (van Roy & Berx, 2008). In normal epithelium, E‐cadherin is structurally linked to β‐catenin to create a stable cell–cell adhesion network forming the adherens junction in the lateral plasma membrane of the cell (Harris & Tepass, 2010). E‐cadherin plays a role in tissue formation and migration (Takeichi, 1995; Gumbiner, 1996) and regulates the differentiation of the trophoblast, limiting invasion by the synctiotrophoblast during human embryogenesis (Kokkinos et al. 2010).
There are changes during early pregnancy to the adherens junction and tight junction proteins in the laboratory rat (Orchard et al. 1999), with pan‐cadherin (a ubiquitous cadherin probe that targets several different cadherin proteins) redistributing apically in the rat during the first few days of pregnancy (Hyland et al. 1998). Pan‐cadherin expression decreases across gestation in live‐bearing lizards with non‐invasive placentae (Wu et al. 2011). Differential expression between barren and pregnant uteri from the same mother suggests that cadherin expression is affected by the presence of the embryo in live‐bearing lizards (Wu et al. 2011). Similar changes to the lateral plasma membrane occur in a range of species, suggesting that common molecular mechanisms regulate changes to the uterine epithelium during pregnancy, regardless of placenta type.
Marsupials diverged from eutherian mammals around 130 mya (Nilsson et al. 2010). Like eutherians, marsupials form a placenta during pregnancy through which the embryo gains nutrients from uterine secretions or the maternal bloodstream, depending on the invasiveness of the placenta (Freyer et al. 2003). A shell membrane, which prevents direct contact between the yolk sac and the uterine epithelium, is present around the embryo during early pregnancy in all marsupials but it allows small molecules to pass through to the developing embryo (Freyer & Renfree, 2009). The shell membrane degenerates during late pregnancy, allowing the yolk sac to be in direct contact with the uterine epithelium (Freyer et al. 2003). Direct uptake of nutrients and gas exchange is then possible through the fetal blood circulation (Freyer et al. 2003). The majority of reproductive biology research has been done in eutherians and there are few studies comparing the outcomes with those from marsupials. It is important to understand the fine details of pregnancy in marsupials as they form the most closely related clade of mammals to eutherians and can thus provide information on the development and commonality of mechanisms for successful reproduction.
The differences in marsupial reproductive modes led us to question whether there are common mechanisms underpinning successful implantation in eutherians and marsupials. We used the fat‐tailed dunnart, Sminthopsis crassicaudata, as a marsupial model because we already understand much about changes to its uterine morphology during pregnancy (Laird et al. 2014; Dudley et al. 2015). Dunnarts are small, carnivorous marsupials from the family Dasyuridae (Morton, 1978). Sminthopsis crassicaudata have one of the shortest gestations of all mammals of 13 days and an endotheliochorial placenta which forms on day 11 of pregnancy when the shell coat is lost (Roberts & Breed, 1994). The dunnart placenta invades on the side of the bilaminar yolk sac (Roberts & Breed, 1994). These reproductive characteristics make the dunnart an ideal model to study the fine molecular changes that underpin successful implantation and pregnancy in marsupials.
Materials and methods
Animal husbandry
All S. crassicaudata used in this study came from an established breeding colony at the University of Sydney. The animals were housed singly or in pairs in cages with clean wood shavings for flooring. Cardboard rolls and nesting boxes with shredded paper were provided along with running wheels and climbing toys for enrichment. Food and water were provided ad libitum. The breeding season in this colony is July to January. The reproductive status of the females was determined through examination of the pouch and the change in vaginal epithelial cells obtained from smears taken from the urogenital sinus and observed and quantified under a light microscope (McAllan et al. 2012). Mated females were monitored for signs of pregnancy, which include an increase in body mass, flushed, moist appearance of the pouch, and reduction of pouch fur.
Tissue harvesting and processing
Reproductive tracts were collected from female dunnarts at early‐ (defined as being close to conception with small, fertilised eggs or early embryos present, n = 8), mid‐(defined as development of the primitive streak and pre‐attachment, n = 5) and late stages (defined as having embryos present that have lost their shell membrane, n = 5) of pregnancy (Dudley et al. 2015). These tissues were compared with the reproductive tracts from eight non‐reproductive females.
Females were euthanised using CO2 inhalation followed by decapitation. Their reproductive tracts were removed. Separate pieces of uterine tissue were dissected, processed and stored for immunohistochemistry and Western blots. All animal experimentation was done with approval from the University of Sydney Animal Ethics Committee K22/5‐2012/1/5764 and the NSW Department of Environment, Conservation and Climate Change (DECC).
E‐cadherin immunofluorescence and Western blotting
We used an epithelial cell specific cadherin antibody for immunofluorescence microscopy and Western blotting. Uterine tissue used for immunofluorescence was stored in Tissue‐Tek OCT™ cryoprotectant (Sakura, Tokyo, Japan) in liquid nitrogen (−196 °C). Sections of 8 μm thickness were cut at −25 °C using a Leica CM3050 S cryostat (Leica, Keerbrugg, Switzerland) and mounted on slides coated in gelatin. Four slides with nine sections on each were made for each animal and stored at −20 °C. Two slides were randomly allocated as experimental slides, two as control slides. During initial immunofluorescence work, liver and uterine tissue from rats was used as a positive control. The experimental and control slides were fixed in acetone for 30 min. The slides were then blocked in 1% bovine serum albumin (BSA) in phosphate‐buffered saline (PBS) for 30 min. The experimental slides were covered in the primary antibody [E‐cadherin (4A2) Mouse mAb; Cell Signalling Technology, Danvers, MA, USA] at a 1 : 500 dilution in 1% BSA for 2 h in a humid chamber. The control slides were incubated in a separate humid chamber for 2 h with anti‐mouse primary IgGs (Sigma‐Aldrick, Castle Hill, Sydney, Australia). All slides were rinsed in PBS for 15 min. They were then incubated for 30 min in secondary antibody (sheep anti‐mouse, IgG, GE Healthcare, Little Chalfont, UK) at a dilution of 1 : 500. All slides were rinsed in PBS for 15 min. A drop of Vectorshield with DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) was added to the slide and a coverslip was sealed on with wax. The slides were viewed under a Zeiss Deconvolution microscope (Carl Zeiss Pty. Ltd., Australasia) and images taken using a Zeiss AxioCam HR digital monochrome CCD camera. The tissues were examined for the presence or absence of E‐cadherin and localisation of the staining in the uterine tissue.
Sections of uterine tissue that had been snap‐frozen in liquid nitrogen (−196 ° C) and stored at −80 °C were used for Western blotting. Rat uterine tissue from day 1 of pregnancy was used as a positive control. All rat tissue was shared using University of Sydney Animal Ethics Committee protocol 2014/668. The protein samples were extracted and diluted with lysis buffer and distilled water. Standards were made out of BSA stock solution and 100 μL of each standard and protein dilution were put into a 96‐well plate bicinchoninic acid (BCA) protein assay kit and made up according to the manufacturer's instructions (Micro BSA™ Protein Assay kit; Thermo Scientific, Rockford, IL, USA). The amount of protein in each well was estimated using absorbance readings from a POLARstar Galaxy Microplate Reader (BMG LabTech, Durham, NC, USA). Protein samples of 20 μg were loaded into a 10% SDS‐PAGE gel alongside a normal molecular weight ladder (Page Ruler pre‐stained Protein Ladder) at 200 V for 40 min and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Corporation, Bedford, MA, USA) at 100 V for 1.5 h. The membranes were blocked in 5% skim milk in Tris‐buffered saline and Tween 20 (TBS‐t) solution for 1 h. Membranes were transferred to a primary antibody solution [E‐cadherin (4A2) Mouse mAb; Cell Signalling Technology] in a dilution of 1 : 1000 1% skim milk to be left overnight. Membranes were rinsed three times for 10 min in TBS‐t and transferred to a secondary antibody solution (sheep anti‐mouse, IgG, GE Healthcare) in a dilution of 1 : 2000 in 1% skim milk for 1.5 h. Membranes were rinsed in TBS‐t and immediately imaged using a Chemidoc MP imaging system (Bio‐Rad) using enhanced chemiluminescence (ECL plus Western Blotting Detection System; GE Healthcare). The membrane was stripped and reprobed for β‐actin as a control for equal protein loading.
Results
E‐cadherin staining in uterine epithelium
The E‐cadherin staining is localised to the uterine epithelium and glands in non‐pregnant dunnarts (Fig. 1A). There is uniform staining of E‐cadherin along the lateral plasma membrane of luminal and glandular epithelium (Fig. 2A). In early and mid‐stages of pregnancy, E‐cadherin staining is cytoplasmic throughout the uterine and glandular epithelial cells, with some localised staining along the lateral plasma membrane in early‐stage pregnant (Figs 1B and 2B) and mid‐stage pregnant S. crassicaudata (Figs 1C and 2C). In late stage pregnancy, E‐cadherin staining is cytoplasmic throughout the uterine and glandular epithelial cells, with very little localised staining along the lateral plasma membrane (Figs 1D and 2D). The IgG control images show no localised E‐cadherin staining in the uterine (Fig. 3A) or glandular (Fig. 3B) epithelial cells. The positive control rat tissue shows localised E‐cadherin staining in the uterine epithelium at day 1 of pregnancy and in liver (Figs 3C,D).
Figure 1.
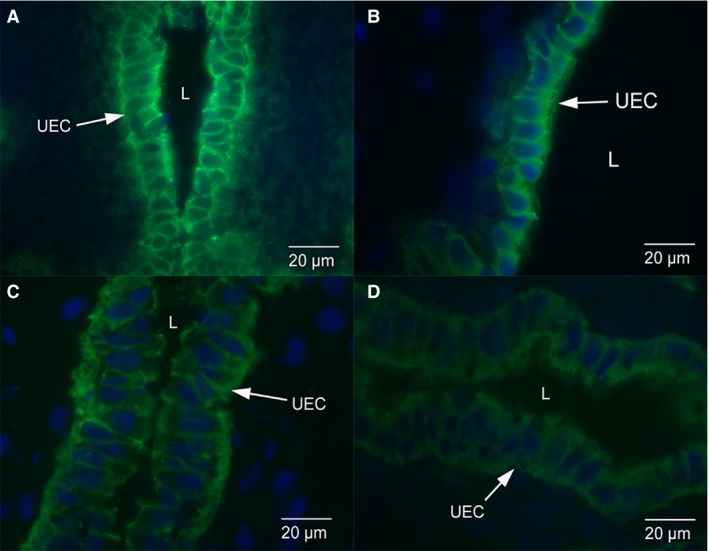
Immunofluorescence micrographs of uterine epithelium from dunnarts across the stages of pregnancy. The E‐cadherin staining is localised to the uterine epithelium and uterine glands. (A) Uterine epithelium from non‐pregnant dunnarts. There is uniform staining of E‐cadherin along the lateral plasma membrane of these cells. (B) Uterine epithelium from dunnarts that are in the early stage of pregnancy. E‐cadherin staining is cytoplasmic throughout the epithelial cells with some localised staining along the lateral plasma membrane. (C) Uterine epithelium from dunnarts that are in the mid‐stage of pregnancy. E‐cadherin staining is cytoplasmic throughout the epithelial cells with some localised staining along the lateral plasma membrane. (D) Uterine epithelium from dunnarts that are in the late stage of pregnancy. E‐cadherin staining is cytoplasmic throughout the epithelial cells with very little localised staining along the lateral plasma membrane. Green staining is E‐cadherin. The nuclei are stained in blue with a DAPI stain. Full arrows point to Uterine Epithelial Cells (UEC). The letter L refers to the lumen of the uterus.
Figure 2.
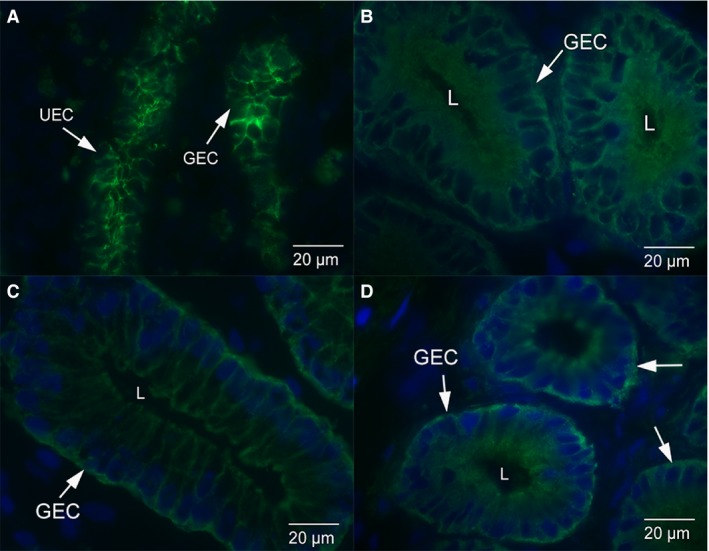
Immunofluorescence micrographs of uterine glandular epithelium from dunnarts across the stages of pregnancy. (A) Glandular epithelium from non‐pregnant dunnarts. There is uniform staining of E‐cadherin along the lateral plasma membrane of these cells. (B) Glandular epithelium from dunnarts that are in the early stage of pregnancy. E‐cadherin staining is cytoplasmic throughout the uterine epithelial cells with some localised staining along the lateral plasma membrane. (C) Glandular epithelium from dunnarts that are in the mid‐stage of pregnancy. E‐cadherin staining is cytoplasmic throughout the epithelial cells with some localised staining along the lateral plasma membrane. (D) Glandular epithelium from dunnarts that are in the late stage of pregnancy. E‐cadherin staining is cytoplasmic throughout the epithelial cells with very little localised staining along the lateral plasma membrane. Green staining is E‐cadherin. The nuclei are stained in blue with a DAPI stain. Full arrows point to glandular epithelial cells (GEC). The letter L refers to the lumen of the gland.
Figure 3.
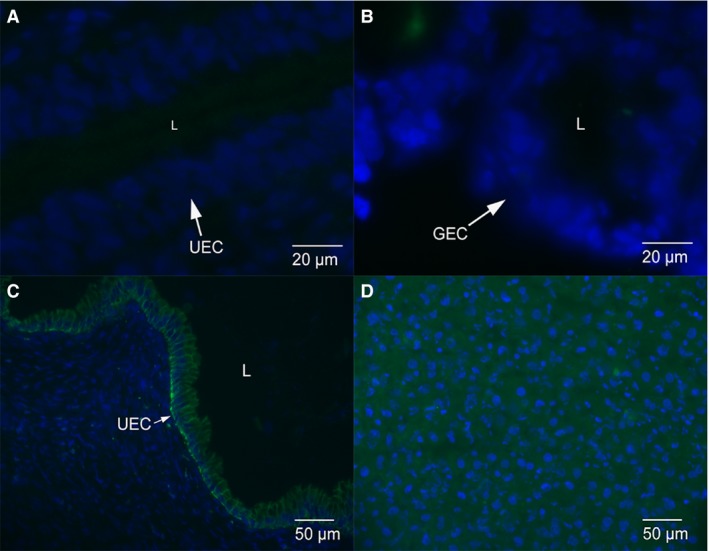
Control images for immunofluorescence micrographs. (A) Negative control anti‐mouse IgG staining in uterine epithelium from early pregnant dunnart. Only blue nuclei staining present. (B) Anti‐mouse IgG staining in glandular epithelium from late pregnant dunnarts. Only blue nuclei staining present. (C) Positive control rat uterine epithelium from day 1 of pregnancy. E‐cadherin staining is localised to the cytoplasm and lateral plasma membrane of the uterine epithelium. (D) Positive control rat liver shows cytoplasmic E‐cadherin staining throughout the cells.
Western blot
A strong band is present for E‐cadherin at 130 kDa in whole uterine tissue in non‐pregnant dunnarts (lane 3), early‐ (lane 4), mid‐ (lane 5) and late‐stage (lane 6) pregnant dunnarts and rat day 1 of pregnancy (lane 2; positive control; Fig. 4). Cadherins have a molecular weight of 120–140 kDa (Takeichi, 1988). There is a smaller band at 34 kDa in the dunnart tissue. β‐Actin (42 kDa) was used as a loading control.
Figure 4.
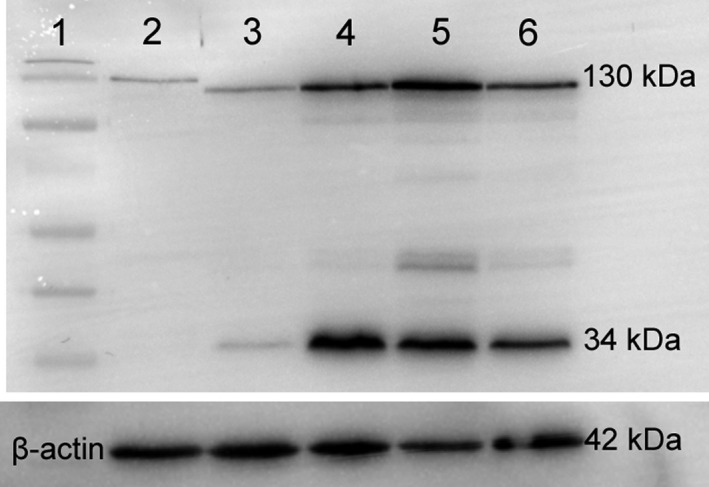
Western blot of whole uterine tissue from the dunnart at early, mid‐ and late pregnancy as well as non‐pregnant dunnarts. Protein 20 μg loaded per well. Rat day 1 of pregnancy was loaded as a positive control. Bands for E‐cadherin are seen around 130 kDa in all loaded tissue and at 34 kDa in the tissue from pregnant dunnarts. Lane 1: normal molecular weight ladder (Page Ruler pre‐stained Protein Ladder). Lane 2: Rat day 1 of pregnancy. Lane 3: Non‐pregnant dunnart. Lane 4: Early pregnant dunnart. Lane 5: Mid‐stage pregnant dunnart. Lane 6: Late pregnant dunnart. β‐Actin (42 kDa) was used as a loading control.
Discussion
E‐cadherin is present in the fat‐tailed dunnart (Fig. 4). It is localised to the lateral plasma membrane in non‐pregnant dunnarts, which indicates the presence of a functional adherens junction. However, there is a shift in localisation of E‐cadherin from the lateral plasma membrane of non‐pregnant dunnarts to the cytoplasm of both luminal and glandular epithelium during mid‐ and late‐stage pregnancy (Figs 1 and 2) preceding attachment of the blastocyst which occurs at day 11 of a 13‐day gestation (Roberts & Breed, 1994). These changes were observed uniformly across the uterine epithelium as they are under hormonal control. We interpret the loss of localised E‐cadherin staining from the lateral plasma membrane of the luminal and glandular epithelium as the loss of the adherens junction in dunnarts. The substantial loss of adhesion between uterine epithelial cells likely aids in the invasion of the blastocyst between these cells before the placenta develops and allows the uterine lining to stretch to allow for embryonic growth. The finding suggests that the dissociation of E‐cadherin and loss of the adherens junction in the lateral plasma membrane are likely essential for achieving uterine receptivity and attachment of an embryo in this marsupial species.
Other cadherins localised with a pan‐cadherin antibody also decrease in the plasma membrane of uterine epithelial cells during pregnancy in viviparous lizards with non‐invasive placentae (Wu et al. 2011) and laboratory rats with highly invasive (haemochorial) placentae (Hyland et al. 1998). Staining with this antibody that targets several cadherin proteins shifts from the basal region of the uterine epithelium in laboratory rats on day 1 of pregnancy to the apical region at implantation (Hyland et al. 1998). The localisation of pan‐cadherin to the apical region of the uterine epithelium in the laboratory rat may be to anchor the trophoblast to the maternal tissue at implantation and to contribute to the closure of the uterine lumen via cohesion of opposing epithelial cells (Hyland et al. 1998). Due to the use of antibodies which target several cadherin protein types, it is unknown which specific cadherin proteins are involved in the changes to cadherin during pregnancy in the laboratory rat. However, a shift in E‐cadherin to the apical lateral plasma membrane was identified in human uterine epithelial cells during the implantation window of the menstrual cycle, suggesting that epithelial specific cadherin may be responsible for preparing the endometrium for successful invasive implantation (Buck et al. 2012). Similarly, vascular endothelial (VE) cadherin localises apically in endothelial cells from human placenta to facilitate blood vessel formation signalled by vascular endothelial growth factor (VEGF) (Shimoyama et al. 1989; Abraham et al. 2009).
In dunnarts, E‐cadherin does not shift to the apical region of the uterine epithelium; however, another cadherin protein (desmoglein‐2) does shift to the apical surface during early pregnancy. The shift in desmosomal proteins may aid in the close apposition of the blastocyst to the apical cell membrane preceding invasion between the epithelium at implantation (Dudley et al. 2015). Desmoglein‐2 also occurs on the apical surface of endometrial luminal epithelium in an in vitro model of implantation in Ishikawa cells (Singh & Aplin, 2015). E‐cadherin regulates desmosome assembly in cell culture with the loss of E‐cadherin and the breakdown of the adherens junction reducing the cytoskeletal structure of the cell (Rötzer et al. 2015).
Epithelial cells can be induced to change to mesenchymal cells by an epithelial–mesenchymal transition (EMT) pathway (reviewed by Kokkinos et al. 2010). In mesenchymal cells there is a reduced presence of E‐cadherin in the cellular membrane. These stromal‐type cells lack apico‐basal polarity and have a reduction of other cell‐to‐ cell junction molecules such as Zonula‐occludens‐1 (ZO‐1) and desmoplakin (Hay, 1995). Consequently they are migratory and have invasive potential (Vincent‐Salomon & Thiery, 2003). Uncontrolled EMT can lead to the development of cancers when the transition favours mesenchymal cells or to pregnancy pathologies such as pre‐eclampsia or fetal growth restriction when the invasion of the trophoblast is reduced (reviewed by Kokkinos et al. 2010). E‐cadherin is lost from the cell junction and re‐localised to the cytoplasm or nucleus of the cell in the development of breast cancer (Kokkinos et al. 2007). Uterine epithelial cells undergo a highly controlled process known as the plasma membrane transformation (PMT) during early pregnancy which has some similarities to an EMT but is reversible and allows invasive placentation by the blastocyst at implantation (Png & Murphy, 2002; Murphy, 2004). Our results show that the same changes occur in E‐cadherin in the uterine epithelium, where it is lost from the cell membrane and becomes cytoplasmic during the PMT.
The comparative study of proteins that regulate the remodelling of uterine epithelial cells during pregnancy is important to understanding the evolution of placental invasiveness as well as the reproductive similarities between eutherian and marsupial mammals. By identifying the proteins that control cell adhesion and invasion of the placenta, we can understand implantation during normal pregnancy as well as abnormal or unsuccessful pregnancy. Future studies should investigate which hormones control these specific cellular changes in this marsupial species and what role the presence of the blastocyst may play in these changes. The common changes to E‐cadherin and junctional desmosomes across species studied to date suggest that the dissociation of E‐cadherin and loss of the adherens junction in the lateral plasma membrane are likely essential for achieving uterine receptivity and attachment of an embryo across live‐bearing lineages.
Author contributions
J.D. contributed to the planning of the project, acquisition of data, data analysis and interpretation, as well as drafting and revision of the manuscript. C.M., M.T. and B.M. contributed to the design of the project and critical revision of the manuscript as well as providing funding for the project.
Funding
ARC Discovery Grant (C.R.M, M.B.T. and B.M.M.); Macintosh Memorial Fund of the Department of Anatomy and Histology and funds from the Murphy Laboratory.
Acknowledgements
The authors thank M. Laird, J. Herbert and L. Lindsay for technical assistance in the laboratory. The authors acknowledge the facilities and the scientific and technical assistance of the Advanced Microscopy Facility of the Bosch Institute at the University of Sydney. All animal procedures were undertaken with permission from the University of Sydney Animal Ethics Committee (approval number K22/5‐2012/1/5764) and NSW Department of Environment, Conservation and Climate Change (DECC, Licence number SL 100376). This research was supported by an ARC Discovery Grant (M.B.T, C.R.M and B.M.M).
References
- Abraham S, Yeo M, Montero‐Balaguer M, et al. (2009) VE‐Cadherin‐mediated cell‐cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol 19, 668–674. [DOI] [PubMed] [Google Scholar]
- Biazik JM, Thompson MB, Murphy CR (2010) Desmosomes in the uterine epithelium of noninvasive skink placentae. Anat Rec 293, 502–512. [DOI] [PubMed] [Google Scholar]
- Buck VU, Windoffer R, Leube RE, et al. (2012) Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem Cell Biol 137, 777–790. [DOI] [PubMed] [Google Scholar]
- Dudley JS, Murphy CR, Thompson MB, et al. (2015) Desmoglein‐2 during pregnancy and its role in the evolution of viviparity in a marsupial (Sminthopsis crassicaudata; Dasyuridae). J Morphol 276, 261–272. [DOI] [PubMed] [Google Scholar]
- Freyer C, Renfree MB (2009) The mammalian yolk sac placenta. J Exp Zool B Mol Dev Evol 312, 545–554. [DOI] [PubMed] [Google Scholar]
- Freyer C, Zeller U, Renfree MB (2003) The marsupial placenta: a phylogenetic analysis. J Exp Zool Part A 299, 59–77. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357. [DOI] [PubMed] [Google Scholar]
- Harris TJC, Tepass U (2010) Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 11, 502–514. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson JW (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778, 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED (1995) An overview of epithelio‐mesenchymal transformation. Acta Anat (Basel) 154, 8–20. [DOI] [PubMed] [Google Scholar]
- Hyland RA, Shaw TJ, Png FY, et al. (1998) Pan‐cadherin concentrates apically in uterine epithelial cells during uterine closure in the rat. Acta Histochem 100, 75–81. [DOI] [PubMed] [Google Scholar]
- Ivanov DB, Philippova MP, Tkachuk VA (2001) Structure and functions of classical cadherins. Biochemistry Mosc 66, 1174–1186. [DOI] [PubMed] [Google Scholar]
- Kokkinos MI, Wafai R, Wong MK, et al. (2007) Vimentin and epithelial‐mesenchymal transition in human breast cancer – observations in vitro and in vivo. Cells Tissues Organs 185, 191–203. [DOI] [PubMed] [Google Scholar]
- Kokkinos MI, Murthi P, Wafai R, et al. (2010) Cadherins in the human placenta – epithelial–mesenchymal transition (EMT) and placental development. Placenta 31, 747–755. [DOI] [PubMed] [Google Scholar]
- Laird MK, Thompson MB, Murphy CR, et al. (2014) Uterine epithelial cell changes during pregnancy in a marsupial (Sminthopsis crassicaudata; Dasyuridae). J Morphol 275, 1081–1092. [DOI] [PubMed] [Google Scholar]
- McAllan BM, Feay N, Bradley AJ, et al. (2012) The influence of reproductive hormones on the torpor patterns of the marsupial Sminthopsis macroura: bet‐hedging in an unpredictable environment. Gen Comp Endocrinol 179, 265–276. [DOI] [PubMed] [Google Scholar]
- Morton SR (1978) An ecological study of Sminthopsis crassicauda (Marsupialia: Dasyuridae) III. Reproduction and life history. Aust Wildl Res 5, 183–211. [Google Scholar]
- Mossman H (1937) Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contrib Embryol Carnegie Instn Wash 26, 129–246. [DOI] [PubMed] [Google Scholar]
- Murphy CR (1993) The plasma membrane of uterine epithelial cells: structure and histochemistry. Prog Histochem Cyto 27, 1–16. [DOI] [PubMed] [Google Scholar]
- Murphy CR (2000) The plasma membrane transformation of uterine epithelial cells during pregnancy. J Reprod Fertil Suppl 55, 23–28. [PubMed] [Google Scholar]
- Murphy CR (2004) Uterine receptivity and the plasma membrane transformation. Cell Res 14, 259–267. [DOI] [PubMed] [Google Scholar]
- Nilsson MA, Churakov G, Sommer M, et al. (2010) Tracking marsupial evolution using archaic genomic retroposon insertions. PLoS Biol 8, e1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard MD, Shaw TJ, Murphy CR (1999) Junctional plaque proteins shift to the apical surface of uterine epithelial cells during early pregnancy in the rat. Acta Histochem 101, 147–156. [DOI] [PubMed] [Google Scholar]
- Png FY, Murphy CR (2002) Cytoskeletal proteins in uterine epithelial cells only partially return to the pre‐receptive state after the period of receptivity. Acta Histochem 104, 235–244. [DOI] [PubMed] [Google Scholar]
- Preston AM, Lindsay LA, Murphy CR (2004) Progesterone treatment and the progress of early pregnancy reduce desmoglein 1&2 staining along the lateral plasma membrane in rat uterine epithelial cells. Acta Histochem 106, 345–351. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Breed WG (1994) Embryonic‐maternal cell interactions at implantation in the fat‐tailed dunnart, a dasyurid marsupial. Anat Rec 240, 59–76. [DOI] [PubMed] [Google Scholar]
- Rötzer V, Hartlieb E, Vielmuth F, et al. (2015) E‐cadherin and Src associate with extradesmosomal Dsg3 and modulate desmosome assembly and adhesion. Cell Mol Life Sci 72, 4885–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y, Yoshida T, Terada M, et al. (1989) Molecular cloning of a human Ca2+‐dependent cell‐cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol 109, 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Aplin JD (2015) Endometrial apical glycoproteomic analysis reveals roles for cadherin 6, desmoglein‐2 and plexin b2 in epithelial integrity. Mol Hum Reprod 21, 81–94. [DOI] [PubMed] [Google Scholar]
- Staun‐Ram E, Shalev E (2005) Human trophoblast function during the implantation process. Reprod Biol Endocrinol 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M (1988) The cadherins: cell‐cell adhesion molecules controlling animal morphogenesis. Development 102, 639–655. [DOI] [PubMed] [Google Scholar]
- Takeichi M (1995) Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7(5), 619–627. [DOI] [PubMed] [Google Scholar]
- van Roy F, Berx G (2008) The cell‐cell adhesion molecule E‐cadherin. Cell Mol Life Sci 65, 3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent‐Salomon A, Thiery JP (2003) Host microenvironment in breast cancer development: epithelial‐mesenchymal transition in breast cancer development. Breast Cancer Res 5, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR (2003) Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 19, 207–235. [DOI] [PubMed] [Google Scholar]
- Wu Q, Thompson MB, Murphy CR (2011) Changing distribution of cadherins during gestation in the uterine epithelium of lizards. J Exp Zool B Mol Dev Evol 316, 440–450. [DOI] [PubMed] [Google Scholar]


