Abstract
AIM
To analyze the relationship between clinical features and epithelial mesenchymal transition (EMT) in retinoblastoma (RB), further to investigate whether miR-200c regulates the EMT and migration of RB cells.
METHODS
Expression of EMT-related markers and tumor-related factors were detected by immuno-histochemistry analysis in RB tissue from 29 cases. Correlations between their expression and clinical characteristics were analyzed. The regulation effects of miR-200c on EMT-related markers, tumor-related factors were observed in mRNA level and protein level by real-time polymerase chain reaction (PCR) and Western blot, respectively, in Y79 and Weri-rb1 cells. Its effects on migration force of these RB cell lines were also detected with Transwell test.
RESULTS
Lower expression of E-cadherin was present in the cases with malignant prognosis. MiR-200c promoted the expression of E-cadherin and decreased the expression of Vimentin and N-cadherin in Y79 and Weri-rb1 cells. Migration force of RB cells could be inhibited by miR-200c.
CONCLUSION
EMT might be associated with bad prognosis in RB. MiR-200c suppresses the migration of retinoblastomatous cells by reverse EMT.
Keywords: retinoblastoma, epithelial mesenchymal transition, miR-200c, E-cadherin
INTRODUCTION
As a key developmental program, epithelial to mesenchymal transition (EMT) occurs in many important biological processes[1]–[2]. Moreover, it plays important functions in cancer invasion and metastasis that lead to malignant prognosis in many tumors[2]–[4]. The symbol of EMT is the loss of epithelial phenotypes such as expression of specific marker E-cadherin and intercellular tight adhesion. By this procedure, the relative differentiated epithelial carcinoma cells transit to mesenchymal cells with more invasive dedifferentiated characteristics. A direct link between EMT and the generation of cancer stem cells had been verified[5]. Moreover, EMT enables the transmitted cells with capacities of colonization to build distant metastases[4]–[5].
MicroRNAs (miRNAs) are small noncoding single-stranded RNAs (18-25 nucleotides) that widely exist in mammalian cells and have essential regulating effects in many cell processes, such as differentiation, proliferation, and apoptosis of cancer[6]–[7]. They are essential regulators of cancer progressions. Some miRNAs had been demonstrated to act as oncogenes[8]–[9], while the others were cancer suppressors[10]–[12]. MiR-200c had been verified to suppress the EMT program and therefore down-regulate the metastasis by targeting Zeb family, the repressor of E-cadherin, in several malignant tumors[7],[13]–[14].
Retinoblastoma (RB) is the most common malignant intraocular tumor in children[15]. It originates from the inner nuclear layer of retina and is directly caused by the mutations of tumor repressor-RB1 gene[6],[16]. Intracranial infiltration and the secondary metastatic tumors are the major causes of death[17]. In clinical practices, we find that not all the tumors at late stage will definitely lead to bad outcomes, while some patients with early stage tumors even step to death. EMT has been showed correlation with the prognosis in many tumors, including lung[18], colorectal[19], cervical[20], tongue[21] carcinoma and so on. One of the most critical hallmarks of EMT is the loss of E-cadherin[22]. The expression of E-cadherin has been demonstrated to be correlated inversely with prognosis in some epithelial-derived cancers[18],[20],[23]. However, as far as we know, no related research had been reported on RB. In order to detect which EMT factor can predict prognosis of RB, in addition to EMT factors including E-cadherin, Vimentin and N-cadherin, we also selected EMT-related genes such as cancer stem cell factor CD133 and drug resistant factor ABCG2 as candidate factors. In our study, for the first time we investigate the correlation of these target factors and clinical features in the RB specimens.
It has been demonstrated that miR-200c up-regulates the expression of E-cadherin by targeting its transcription repressor-zeb1, a double negative feedback loop between miR-200c and zeb1 regulates EMT in several tumor cell lines[7],[14],[24]. However, there is no such research had been done on RB cell lines. In order to investigate whether the EMT changes the features of tumor cells and whether the negative feedback loop between miR-200c and zeb1 regulates the EMT process in RB cell lines, Weri-RB1 and Y79 were cultured for in vitro studies.
SUBJECTS AND METHODS
Tumor Specimens
Clinical data and tissue specimens from 29 RB cases were collected from the Second Xiangya Hospital of Central South University between 2005 and 2011. All the cases were diagnosed and classified according to the Internatioanl Classification of Retinoblastoma (ICRB)[25]. Diagnosis of RB was confirmed by histopathological examination. The procedure of this study was approved by the Ethics Committee of the Second Xiangya Hospital.
Immunohistochemistry
All specimens were fixed in 10% paraformaldehyde and embedded in paraffin, then sectioned in 5 µm, and stained with hematoxylin and eosin. The RB specimens were divided into differentiated and non-differentiated groups according to the presence or absence of rosettes. The specimens were also divided into optic nerve-infiltrated and non-infiltrated groups according to tumor infiltration. The paraffin-embedded sections were dewaxed, dehydrated, and heated in pressure cooker for antigen retrieval (2min after reaching full pressure), then incubated with 3% peroxidase for 10min at room temperature. After incubated in primary antibodies overnight at 4°C the sections were incubated with Polymer Helper reagent (KIT-5020, MaxVisionTM, China) for 20min at 37°C and rinsed with phosphate buffer saline (PBS). Primary antibody information: E-cadherin (#3195 1:400 Cell Signaling Technology); N-cadherin (ab12221, 1:300, Abcam); Vimentin (sc-66002, 1:200, Santa Cruz); ABCG2 (BXP-21, 1:40, Abcam); CD133 (AC133, 1:100, Miltenyi Biotec, Germany). Afterwards, the samples were incubated with poly peroxidase-anti-rabbit IgG for 20min at room temperature. After PBS washing, the sections were stained with 3,3′-diaminobenzidine (DAB) solution (Beijing Zhongshan, China), counterstained with hematoxylin, routinely dehydrated, and then mounted. PBS replaced the antibodies as a negative control, and a known positive section was used as a positive control.
Scoring
In the preliminary observation, we found the clue to association with E-cadherin and outcomes, then H score of the immunochemistry staining of E-cadherin was done[26]–[27]. The stain intensity was scored as: 0, 1, 2, or 3. A score of 0+ for negative, less than 10% discernible membranous staining; 1+ for weak and/or incomplete membranous staining required at least ×200 magnification to confirm positive staining; 2+ for weak to moderate, continuous membranous staining that was not readily apparent at low (×40) magnification; 3+ for strong membranous staining that was readily apparent at low magnification. Only membranous staining was scored. We estimated the fraction of tumor cells in each case that had 0+, 1+, 2+, and 3+ intensity to allow the calculation of an H score. The H score was calculated based on the following formula: H score=(% Tumor 1+)+ 2×(% Tumor 2+)+ 3×(% Tumor 3+) The H score can, therefore, range from 0 (cases with absent staining) to 300 (cases with 100% 3+ staining)[26].
Cell Culture
Human RB cell lines Y79 and Weri-RB1 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, India). The cells were grown under a humidified atmosphere of 5% CO2-95% air at 37°C and the media was changed every other day.
miRNA Transfection
The short interfering mimics and inhibitor of miR-200c and negative control mRNA were chemically synthesized by GenePharma (China). The miR-200c were transiently transfected in Y79 and Weri-RB1 cell lines with Lipofectamine 2000 (Invitrogen) as described in manufacture's protocol respectively. Briefly, Lipofectamine®2000 (Invitrogen) (5 µL) combined with miRNA at a concentration of 30 nmol/L and incubated in OPTI-MEM I reduced serum medium (Invitrogen, CA, USA) for 20min prior to transfection. The combined medium was then added into a 6-well plate with 5×104 cells per well. Cells were incubated at 37°C for 6h before replacement of medium. Then cells were harvested for 48h before further analysis.
Western Blot
For the analysis of protein expression in the Y79 and Weri RB cell lines, 1×106 cells were washed with ice-cold PBS twice and lysed with cell lysis buffer at 4°C for 30min. Cell debris were removed by centrifugation at 15 000× g for 15min at 4°C. Equal amounts of proteins were separated by 12% SDS-PAGE and transferred onto nitrocellulose membrane. The membranes were first stained to confirm the uniform transfer of all samples and then incubated in the blocking solution for 2h at room temperature. The membranes were first incubated with monoclonal antibodies (rabbit Zeb1 antibody, SANTA, SC-25388n,1:500; Goat E-cadherin antibody, SANTA, SC-31020, 1:500; Mouse Vimentin antibody, SANTA, SC-58901, 1:800; Rabbit N-cadherin antibody, Abcam, ab12221, 1:500; Mouse GAPDH antibody, SANTA, SC-365062, 1:800) for 2h, after washed with PBS twice and tris buffered saline tween (TBST) twice. The membranes were incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (SANTA, USA) before washing with TBST. GAPDH was used as the internal control. A BIO-RAD Western blotting detection system was used to detect the immune-reactive proteins.
RNA Extraction and Quantitative Polymerase Chain Reaction Analysis of MiR-200c
TRIzol reagent (Invitrogen) was used to extract the total RNAs from the fresh cells. The miRNeasy Mini kit (Qiagen, USA) and miRNA Q-polymerase chain reaction (PCR) Detection kit (GeneCopoeia, USA) were used in real-time PCR assays. Quantitative PCR was performed using a ABI prism 7900HT REAL-Time PCR System (Applied Biosystems). The procedure of PCR was performed as follows: denaturation (95°C, 5min), amplification (40 cycles), denaturation (95°C, 10s), annealing (60°C, 20s), elongation (72°C, 10s). Reactions were performed in a total volume of 20 µL in triplicate. The miRNA levels were normalized to U6. The oligonucleotide primers were as follows: has-miR-200c forward (5′-TAATACTGCCGGGTAATGATGGA-3′) and reverse (5′-TGGTGTCGTGGAGTCG-3′); U6RNA forward (5′-GCTTCGGCAGCACATATACTAAAAT-3′) and U6RNA reverse (5′-CGCTTCACGAATTTGCGTGTCAT-3′). The miRNA expression was determined using the 2−ΔCt method.
Real-time Reverse Transcription-polymerase Chain Reaction
Total RNA was prepared and reverse transcribed using RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's protocol. SYBR Green real-time PCR was performed using the following primers (Invitrogen):
GAPDH-F: 5′-AAATTGAGCCCGCAGCCTCCC
GAPDH-R: 5′-GCGCCCAATACGACCAAATCCGT
Zeb1-F: 5′-TGACCTGCCAACAGACCAGACA
Zeb1-R: 5′-CCTTTCCTGTGTCATCCTCCCAGC
E-cadherin-F: 5′-TCCACGCCGAGCCCCAGTAT
E-cadherin-R: 5′-TCAGCCGCTTTAAGGCCCTCAT
Vimentin-F: 5′-TGGCCGCCTGCAGGATGAGAT
Vimentin-R: 5′-AGAGAAATCCTGCTCTCCTCGCCT
N-cadherin-F: 5′-CCCACCACGTACAAGGGTCAGGT
N-cadherin-R: 5′-ACGCTGGGGTATTGGGGGCA
Zeb1, E-cadherin, Vimentin, N-cadherin and GAPDH-specific fragments were amplified by 40 cycles of PCR, respectively, each cycle comprising 20s at 94°C, 20s at 55°C, and 32s at 72°C. The relative mRNA levels were calculated using the 2−ΔCt method.
In Vitro Motility Assay
In vitro motility assay was performed with 6-well transwell chambers with 8-µm porous membrane (Corning Incorporated, Corning, NY, USA)[28]. The upper chamber was filled with serum-free media and the bottom chamber was filled with media containing 10% FBS. Cells were washed three times with PBS and 10 000 cells were added to the top chamber. Cells were incubated in a 5% CO2 humidified incubator for 22h at 37°C. After removed the upper chamber, the migrated cells in the lower chamber were counted.
Statistical Analysis
Linear regression and Pearson correlation were calculated for association between miR-200c and EMT markers (E-cadherin, Vimentin and N-cadherin) at RNA level. The correlation coefficient was reported according to the R2 and P value. P value <0.05 was considered as statistical significance.
RESULTS
Clinical Features of Retinoblastoma Cases
The study included 15 boys (51.7%) and 14 girls (48.3%). The mean age at enucleation was 2.20±1.39y (ranged from 5mo to 8y). Bilateral tumor was detected in 4 children (13.8%) and unilateral in 25 patients (86.2%). According to the ICRB[25],[29], 20 patients were classified in stage D (69.0%), and 9 in stage E (31.0%). The optic nerve was infiltrated in 9 patients (31.0%) and 20 patients were non-infiltrated (69.0%). For differentiation degree, 15 samples (51.7%) were differentiated, and 14 (48.3%) were undifferentiated. Five cases (17.2%) were dead within one year after enucleation, 24 (82.8%) were survival after at least 3y follow-up. Of the 24 survival, 3 got relapse after treatment, and 21 were relapse-free. Details of above information were listed in Table 1.
Table 1. Basic information of the 29 RB patients.
| Case No. | Gender | OD/OS/OU | Age | Differentiation | Optic nerve infiltration | Group of ICRB | Living condition |
| 1 | F | OS | 2a | Differentiated | - | D | Survive |
| 2 | F | OS | 2a | Differentiated | - | D | Survive |
| 3 | F | OD | 1a | Undifferentiated | - | D | Survive |
| 4 | F | OU | 2a | Differentiated | - | D | Survive |
| 5 | F | OU | 4a | Differentiated | - | D | Survive |
| 6 | M | OS | 2a | Differentiated | - | D | Survive |
| 7 | M | OD | 2a | Differentiated | - | D | Survive |
| 8 | F | OD | 3a | Undifferentiated | + | E | Survive |
| 9 | F | OD | 3a | Differentiated | - | D | Survive |
| 10 | M | OS | 3a | Differentiated | - | D | Survive |
| 11 | F | OD | 2a | Differentiated | - | D | Survive |
| 12 | M | OU | 5mo | Differentiated | - | D | Survive |
| 13 | M | OD | 2a | Differentiated | + | E | Survive |
| 14 | F | OS | 6mo | Undifferentiated | - | D | Survive |
| 15 | F | OD | 2a | Undifferentiated | - | D | Survive |
| 16 | M | OS | 2a | Undifferentiated | - | D | Survive |
| 17 | F | OD | 1a | Undifferentiated | + | E | Survive |
| 18 | F | OS | 2a | Undifferentiated | + | E | Survive |
| 19 | M | OS | 3a | Differentiated | - | D | Survive |
| 20 | F | OD | 3a | Differentiated | - | D | Survive |
| 21 | M | OD | 2a | Differentiated | + | E | Survive |
| 22 | M | OS | 2a | Undifferentiated | - | D | Recurrent, survive |
| 23 | M | OU | 2a | Undifferentiated | - | D | Recurrent, survive |
| 24 | M | OD | 1a | Undifferentiated | + | E | Recurrent, survive |
| 25 | M | OD | 1a | Differentiated | - | D | Die |
| 26 | F | OD | 2a | Undifferentiated | + | E | Die |
| 27 | M | OS | 8a | Undifferentiated | + | E | Die |
| 28 | M | OS | 3a | Undifferentiated | + | E | Die |
| 29 | M | OD | 1a | Undifferentiated | - | D | Die |
Decreased Expression of E-cadherin in Retinoblastoma Specimens with Bad Prognosis
To evaluate the association of EMT and clinical features in RB, expression of epithelial marker E-cadherin, mesenchymal marker Vimentin and N-cadherin, stem cell marker CD133 and drug-resistance protein ABCG2 in 29 specimens were analyzed by immunohistochemistry. In the preliminary observation, we found the clue to association with E-cadherin and outcomes, then H score was used to quantitate the expression[26]–[27]. The student t-test analysis showed that the expression of E-cadherin had no significant difference between the two groups of male and female, of differentiated and undifferentiated type, of optic nerve involved and uninvolved. However it is significantly lower expressed in the cases with bad prognosis (Table 1). The expressions of E-cadherin in the specimens of 5 dead patients were significantly lower than those 24 survivals (P<0.05). E-cadherin was significant higher expressed in the 21 relapse-free tumors when compared with 8 recurrent and metastatic tumors (including 5 death) (P<0.01) (Table 2). However when we analyzed the expression of Vimentin, N-cadherin, CD133 and ABCG2 with the above-mentioned clinical features, no significant difference had been detected (data not shown). The immune-histochemical pictures of two patients who had different expression of E-cadherin and prognosis was showed in Figure 1.
Table 2. Association of E-cadherin expression immunohistochemically with clinical features in RB cases.
| Clinical features | Case No. | H-score of E-cadherin |
P | H-score of N-cadherin |
P | H-score of ABCG |
P | H-score of CD133 |
P | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Sex | F | 14 | 106.07 | 35.00 | 0.346 | 149.29 | 24.01 | 0.368 | 139.29 | 21.74 | 0.198 | 150.71 | 23.03 | 0.479 |
| M | 15 | 91.13 | 47.37 | 156.67 | 18.77 | 150.33 | 23.26 | 157.33 | 26.31 | |||||
| Differentiation | Differentiated | 15 | 108.27 | 35.01 | 0.191 | 156.67 | 22.89 | 0.363 | 140.67 | 19.81 | 0.298 | 159.33 | 23.74 | 0.245 |
| Undifferentiated | 14 | 87.71 | 47.02 | 149.29 | 19.79 | 149.64 | 25.61 | 148.57 | 25.07 | |||||
| Optic nerve involvement | Optic nerve not involved | 20 | 99.40 | 38.07 | 0.884 | 145.56 | 16.67 | 0.208 | 144.25 | 21.72 | 0.797 | 155.50 | 22.82 | 0.664 |
| Optic nerve involved | 9 | 96.00 | 51.67 | 156.50 | 22.77 | 146.67 | 26.46 | 151.11 | 29.34 | |||||
| Survival or not | Survival | 24 | 107.00 | 35.24 | 0.012 | 151.30 | 22.01 | 0.385 | 146.04 | 23.45 | 0.600 | 152.50 | 22.31 | 0.442 |
| Death | 5 | 56.80 | 49.92 | 160.00 | 18.97 | 140.00 | 21.21 | 162.00 | 35.64 | |||||
| Recurrence and metastasis | Relapse-free | 21 | 110.48 | 27.62 | 0.009 | 153.33 | 21.76 | 0.369 | 141.19 | 20.61 | 0.149 | 152.38 | 20.71 | 0.542 |
| Relapse | 8 | 66.50 | 56.70 | 161.25 | 18.08 | 155.00 | 26.73 | 158.75 | 33.99 | |||||
P<0.05: Statistical significance in t-test.
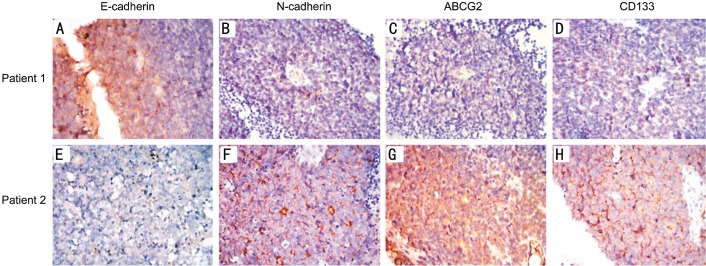
Figure 1. Expression of E-cadherin, N-cadherin, ABCG2 and CD133 in two patients with completely opposite prognosis (100×).
In the immunohistochemical test, positive expression of the target antibody was displayed in brown. Patient 1 (case 13): male, 2 years old of onset, E class in ICRB, survived more than 10y after enucleation. The tumor sample showed strongly positive expression of E-cadherin and nearly negative expression of N-cadherin, ABCG2 and CD133. Patient 2 (case 25): male, 1 year old of onset, D class in ICRB, died one year after enucleation. E-cadherin was low expressed in the tumor, while the expression of N-cadherin, ABCG2 and CD133 was strongly positive.
MiR-200c Represses Zeb1 and Enhances the Expression of E-cadherin in Retinoblastoma Cells
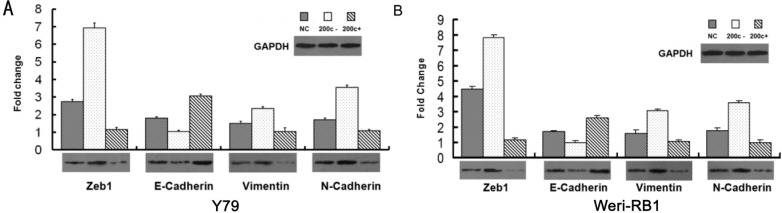
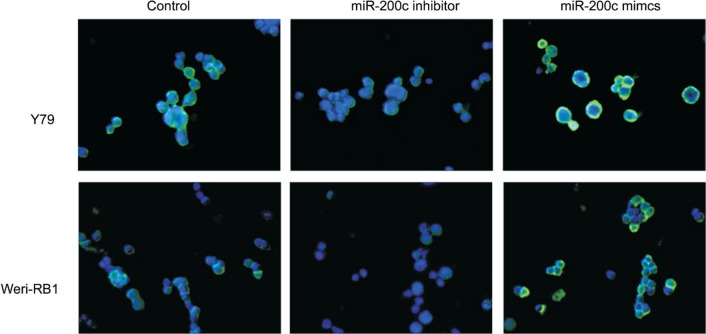
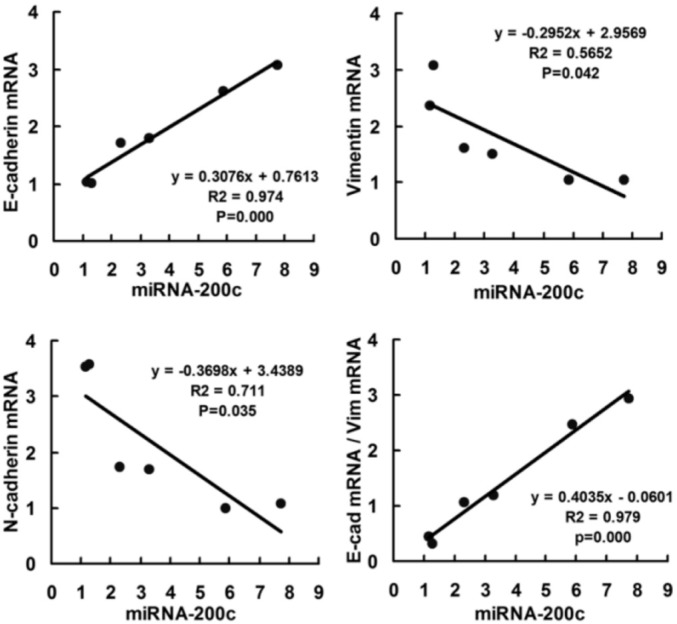
To investigate whether the miR-200c regulates the Zeb1 protein and EMT process in RB cells, the Weri-RB1 and Y79 cells were transfected with miR-200c mimics, inhibitors or control mRNA. The expression of Zeb1 was repressed by the transfection of miR-200c mimics and enhanced by miR-200c inhibitors in both mRNA level and protein level. The suppression of Zeb1 in miR-200c-mimic-transfected cells was accompanied by a restoration of E-cadherin and decreased expression of mesenchymal markers N-cadherin and Vimentin (mRNA and protein) (P<0.05). On the contrary, transfection of miR-200c inhibitor caused a significant reduction of E-cadherin and a concomitant induction of Vimentin and N-cadherin (P<0.05) (Figure 2), which induced EMT. Immunofluorescence expression of E-cadherin was shown in Figure 3. We use Pearson correlation and linear regression to analyze the correlation between miR-200c and E-cadherin, Vimentin, N-cadherin mRNA. It shows that E-cadherin mRNA correlated significantly with miRNA-200c (P<0.01), while Vimentin and N-cadherin mRNA negatively correlated with miRNA-200c (P<0.05). R2 and P values were shown in scatter plot graphs in Figure 4.
Figure 2. MiRNA-200c inhibits EMT in the Y79 and Weri-RB1 cells in both mRNA level and protein level.
A: Y79 cells; B: Weri-RB1 cells. Cells were transfected with miR-200c inhibitor or miR-200c mimics in vitro. Over-expression of miR-200c improves E-cadherin expression and depresses Zeb1, N-cadherin and Vimentin, statistical significance was detected in both protein and mRNA level (P<0.05). Inhibition of miR-200c up-regulated the expression Zeb1, Vimentin and N-cadherin, while down-regulated E-cadherin (P<0.05). The mRNA and protein expression of Zeb1, E-cadherin, Vimentin and N-cadherin was quantified by real-time RT-PCR (the bar charts) and Western blot (the panels) respectively.
Figure 3. Immunofluorescence analysis of E-cadherin expression (green) in Y79 and Weri-RB1 cells (400×).
Cells were counterstained with DAPI.
Figure 4. Scatter plot showing correlation between miR-200c and EMT markers (E-cadherin, Vimentin and N-cadherin) in mRNA level in two RB cell lines.
Data of 6 groups (control, miR-200c+ and miR-200c- in Y79 and Weri-RB1 cell lines) were plotted and linear regression was applied for correlation analyzed.
MiR-200c Inhibit the Migration of Retinoblastoma Cells
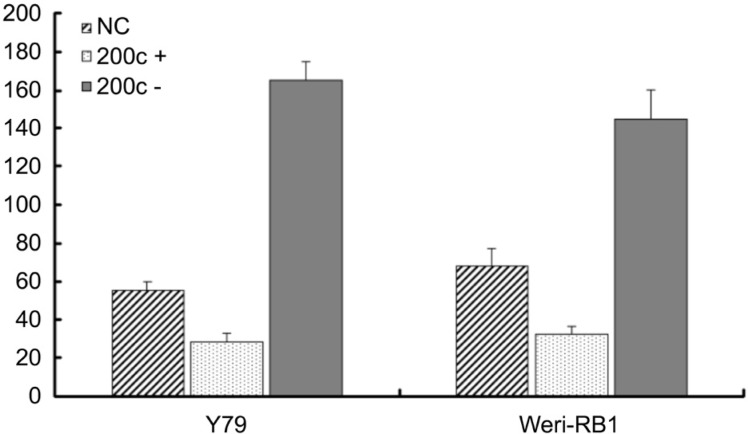
It has been previously demonstrated that the miR-200 family members can decrease the migration and invasion in tumor cells[7],[30]–[31]. We observed that the migration of RB cells can be reversely regulated by the expression of miR-200c. Reintroduction of miR-200c to Y79 and Weri-RB1 cells results in a 67.77% and a 54.74% decrease in migration respectively (Figure 5). On the contrary, when the miR-200c was down-regulated with transfection of miR-200c inhibitors, the migrating force increases 144% in Y79 and 164.25% in Weri-RB1 cells (Figure 5).
Figure 5. MiR-200c inhibited the in vitro migration of retinoblastoma cells in motility assay.
After 24h incubation with miR-200c, the cells migrated into under chambers were quantified. The average cell number of 5 high magnification views was recorded as the cell density. MiR-200c mimics transfected group (miR-200c+) showed reduced cell migration, the difference is statistically significant (P<0.05), however, the transfection of miRNA-200c inhibitors (miR-200c-) significantly enhance the migration of the two RB cell lines (Y79 and Weri-RB1) (P<0.05).
DISCUSSION
In the 29 samples of RB patients and the two RB cell-lines (Weri-RB1 and Y79), the co-expression of E-cadherin, Vimentin and N-cadherin was detected in all the samples. It indicated the middle status between the epithelial and mesenchymal phenotypes, like most of cancer cells. Lower expression of E-cadherin protein was detected significantly in the patients with recurrent and metastatic tumor. E-cadherin is a member of Ca2+ dependent transmembrane glycoproteins that regulate cell-cell adhesion[32]–[33]. Loss of E-cadherin reduces the cell-cell adhesion and promotes the cellular mobility and invasion in tumors, so that leads to bad prognosis[18],[20],[23]. Combining with the data in other epithelial-derived tumors including gastrointestinal cancer, non-small cell lung cancer, and cervical carcinoma[18],[20],[23], the prognosis of RB may be somehow forecasted with the expression of E-cadherin. However, only 29 specimens with complete clinical information were involved into our research. The sample size, especially the sample with bad prognosis, is quite small, so the statistical bias is inevitable. A larger sample with longer follow-up information is required to further prove the correlation between E-cadherin and prognosis.
In vitro study of RB cell lines (Y79 and Weri-RB1), we proved that over-expression of miR-200c repressed Zeb1 and improved the expression of E-cadherin. Conversely, transfection of miR-200c inhibitor increased the expression of Zeb1 and repressed E-cadherin. Other researches had showed that miR-200c regulated E-cadherin and EMT by inhibition of Zeb1 in other cancers. Zeb1 binds to the E-box of E-cadherin and inhibits its transcription[7],[24]. The migration of RB cells could also be depressed by over-expression of miR-200c. This might be ascribed to its positive effect to E-cadherin. E-cadherin was involved into the cell-cell adhesion and has been proved depressing migration and invasion[34]. Nevertheless, miR-200c itself could also affect several genes related to migration and invasion like ARHGDIB, NTRK2, EPHB1 and FN1[31]. Park et al[7] transfected colorectal cancer cell HTC116 repeatedly every three days with LNA-200 and detected that cells change from cobblestone to a spindle-like morphology after 15d. However no significant morphological changes of RB cells were observed in our research. The transient transfected RNA could not integrate into the DNA of host cells and only affected the expression of the function protein. Stable transfection of miR-200c and Zeb1 should be done to further investigate its effect on EMT and MET in vitro.
Process of EMT promotes the invasiveness and mobility of tumor cells, so that enables tumor dissemination. However, the disseminated cells need self-renewal capability to form metastatic tumor. Researches showed that EMT cells express higher stem cell markers[5],[35]. MiR-200c had been shown to inhibit tumor-genesis by suppressing stem cell factor Bmi-1 in several cancer cells[14],[36]–[37]. Our research also found that miR-200c can reduce the expression of cancer stemness gene Bmi-1 in Y79 and Weri-RB1 cells (data not shown here). The tumorgenicity regulation of miR-200c would be further investigated in vivo.
In conclusion, E-cadherin is correlated with the prognosis of RB, lower E-cadherin generally indicates bad prognosis. E-cadherin can be up-regulated by over-expression of miR-200c in Y79 and Weri-RB1 cell-lines in vitro, so that MET be activated and migration of tumor cells be inhibited.
Acknowledgments
Authors' Contributions: Gao L was responsible for the study design, statistical analysis and the interpretation of the results. Shao XL and Chen Y were responsible for data acquisition, Shao XL prepared the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.81072221); National Science Foundation of Hunan Province (No.14JJ2005).
Conflicts of Interest: Shao XL, None; Chen Y, None; Gao L, None.
REFERENCES
- 1.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 2014;40(3):341–348. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sreenivasan S, Thirumalai K, Danda R, Krishnakumar S. Effect of curcumin on miRNA expression in human Y79 retinoblastoma cells. Curr Eye Res. 2012;37(5):421–428. doi: 10.3109/02713683.2011.647224. [DOI] [PubMed] [Google Scholar]
- 7.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotypeof cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22(6):291–301. doi: 10.1038/cgt.2015.19. [DOI] [PubMed] [Google Scholar]
- 9.Pan Y, Meng M, Zhang G, Han H, Zhou Q. Oncogenic microRNAs in the genesis of leukemia and lymphoma. Curr Pharm Des. 2014;20(33):5260–5267. doi: 10.2174/1381612820666140128211724. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Kim EJ, Jeon MJ. MicroRNAs 125a and 125b inhibit ovarian cancer cells through post-transcriptional inactivation of EIF4EBP1. Oncotarget. 2016;7(8):8726–8742. doi: 10.18632/oncotarget.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou CX, Wang CL, Yu AL, Wang QY, Zhan MN, Tang J, Gong XF, Yin QQ, He M, He JR, Chen GQ, Zhao Q. MiR-630 suppresses breast cancer progression by targeting metadherin. Oncotarget. 2016;7(2):1288–1299. doi: 10.18632/oncotarget.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CP, Sun ZL, Lu X, Wu WX, Guo WL, Lu JJ, Han C, Huang JQ, Fang Y. miR-340 suppresses cell migration and invasion by targeting MYO10 in breast cancer. Oncol Rep. 2016;35(2):709–716. doi: 10.3892/or.2015.4411. [DOI] [PubMed] [Google Scholar]
- 13.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126(11):2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 14.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 15.Villegas VM, Hess DJ, Wildner A, Gold AS, Murray TG. Retinoblastoma. Curr Opin Ophthalmol. 2013;24(6):581–588. doi: 10.1097/ICU.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 16.Benavente CA, Dyer MA. Genetics and epigenetics of human retinoblastoma. Annu Rev Pathol. 2015;10:547–562. doi: 10.1146/annurev-pathol-012414-040259. [DOI] [PubMed] [Google Scholar]
- 17.Yu CL, Tucker MA, Abramson DH, Furukawa K, Seddon JM, Stovall M, Fraumeni JF, Jr, Kleinerman RA. Cause-specific mortality in long-term survivors of retinoblastoma. J Nati Cancer Inst. 2009;101(8):581–591. doi: 10.1093/jnci/djp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan B, Zhang W, Jiang LY, Qin WX, Wang X. Reduced E-cadherin expression is a prognostic biomarker of non-small cell lung cancer: a meta-analysis based on 2395 subjects. Int J Clin Exp Med. 2014;7(11):4352–4356. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Liu X, Feng B, Liu N, Wu Q, Han Y, Nie Y, Wu K, Shi Y, Fan D. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34(37):4808–4820. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Qian Y, Wu H, Xie X, Zhou Q, Wang Y, Kuang W, Shen L, Li K, Su J, Shen L, Chen X. Aberrant expression of osteopontin and e-cadherin indicates radiation resistance and poor prognosis for patients with cervical carcinoma. J Histochem Cytochem. 2015;63(2):88–98. doi: 10.1369/0022155414561329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han MW, Lee JC, Kim YM, Cha HJ, Roh JL, Choi SH, Nam SY, Cho KJ, Kim SW, Kim SY. Epithelial-mesenchymal transition: clinical implications for nodal metastasis and prognosis of tongue cancer. Otolaryngol Head Neck Surg. 2015;152(1):80–86. doi: 10.1177/0194599814556061. [DOI] [PubMed] [Google Scholar]
- 22.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26(49):6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bojmar L, Karlsson E, Ellegard S, Olsson H, Björnsson B, Hallböök O, Larsson M, Stål O, Sandström P. The role of microRNA-200 in progression of human colorectal and breast cancer. PLoS One. 2013;8(12):e84815. doi: 10.1371/journal.pone.0084815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22(10):1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, Shields JA. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Radu OM, Foxwell T, Cieply K, Navina S, Dacic S, Nason KS, Davison JM. HER2 amplification in gastroesophageal adenocarcinoma: correlation of two antibodies using gastric cancer scoring criteria, H score, and digital image analysis with fluorescence in situ hybridization. Am J Clin Pathol. 2012;137(4):583–594. doi: 10.1309/AJCPXQVS6YGHPDCY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu K, Shen W, Zhang Y, Zhao Y, Lu Y. MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene. 2015;574(2):330–336. doi: 10.1016/j.gene.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin JP, Shields JA, Eagle RC., Jr High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997–1003. doi: 10.1016/j.ophtha.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8(5):1055–1066. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Scie. 2008;65(23):3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang Z, Sun XY, Xu LC, Fu RZ. Abnormal expression of serum soluble E-Cadherin is correlated with clinicopathological features and prognosis of breast cancer. Med Sci Monit. 2014;20:2776–2782. doi: 10.12659/MSM.892049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina-Ortiz I, Bartolome RA, Hernandez-Varas P, Colo GP, Teixido J. Overexpression of E-cadherin on melanoma cells inhibits chemokine-promoted invasion involving p190RhoGAP/p120ctn-dependent inactivation of RhoA. J Biol Chem. 2009;284(22):15147–15157. doi: 10.1074/jbc.M807834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Tetzlaff MT, Cui R, Xu X. miR-200c inhibits melanoma progression and drug resistance through down-regulation of BMI-1. Am J Pathol. 2012;181(15):1823–1835. doi: 10.1016/j.ajpath.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen L, Chen H, Liu J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J Transl Med. 2014;12:305. doi: 10.1186/s12967-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]