Abstract
AIM
To assess and compare the diurnal macular choroidal area fluctuation in normal and primary open angle glaucoma (POAG) groups using enhanced depth imaging optical coherence tomography (EDI-OCT).
METHODS
Twenty-eight normal and 27 POAG eyes were enrolled in this study. EDI-OCT was used to measure the macular choroidal area every 3h from 9:00 a.m. to 21:00 p.m.
RESULTS
Significant diurnal fluctuations of macular choroidal area were observed in both normal (P=0.003) and POAG groups (P<0.001). But no significant macular choroidal area difference has been found between the two groups at all the five measurement time-points (512778±166242 vs 455079±207278 µm2, P=0.195 at 9:00 a.m.; 501526±168953 vs 447846±211147 µm2, P=0.245 at 12:00 a.m.; 501982±173158 vs 448024±206653 µm2, P=0.239 at 15:00 p.m.; 508912±174589 vs 457783±207081 µm2, P=0.252 at 18:00 p.m.; 503787±171241 vs 453230±205955 µm2, P=0.274 at 21:00 p.m.; respectively). Furthermore, neither the fluctuation manners nor the change in macular choroidal area between the two adjacent measurement time points showed significant difference between normal and POAG groups (all P>0.05). In the meantime, significant diurnal intraocular pressure fluctuations were also observed in normal and POAG groups (both P<0.001).
CONCLUSION
In diurnal time, the macular choroidal area in both normal and POAG groups fluctuated significantly; moreover, neither the value of macular choroidal area, nor the fluctuation of macular choroidal area in POAG group is significantly different from that in normal group.
Keywords: diurnal fluctuation, macular choroidal area, primary open angle glaucoma, optical coherence tomography
INTRODUCTION
Primary open angle glaucoma (POAG) is a leading cause of irreversible blinding disease characterized by progressive degeneration of retinal ganglion cells, resulting in the glaucomatous change of the optic disc and corresponding defect of visual field[1]. For most POAG patients, we could observe that the progress of visual field defect was from periphery (e.g. paracentral scotoma or arcuate scotoma) to the center. In addition, Rao et al[2] also found the rate of mean deviation (MD) change was less negative in eyes with more severe visual field loss at baseline. Hence, the changing rate of MD would slow down with the progression of POAG. So the central visual acuity of most POAG patients could remain undamaged as the normal individuals for a long time and only in the late severe stage of POAG would the central visual acuity be damaged.
According to the neurovascular unit theory[3]–[4], the function of neuron and vessels were combined together. In addition, the blood supply of macula is only from the choroid due to lack of retinal vessels and the choroid is the main vascular layer of eye[5]. So the macular choroid might be of great importance for the central visual acuity. In 1990s, histological studies observed both thickened and thinned choroidal thickness in POAG patients[6]–[7]. But recently, many studies reported no significant macular choroidal thickness difference between normal and POAG eyes by enhanced depth imaging optical coherence tomography (EDI-OCT)[8]–[9]. Using the same method, Rhew et al[10] and Park et al[11] found the similar result between normal and normal tension glaucoma eyes. Furthermore, the Meta-analysis result of Wang and Zhang[12] suggested that there was no significant difference of the macular choroidal thickness between normal and POAG eyes. So as a neurovascular unit, the function of the macula (central visual acuity) and the macular choroidal thickness in POAG patients remained the same as that of normal individuals.
For normal tension glaucoma patients, intraocular pressure (IOP) could be in normal range but had an abnormal fluctuation with the progress of visual field defect[13], indicating the dynamic parameter is as important as the static parameter. And just as the other ocular parameters, like IOP, axial length (AL) and anterior chamber depth[14]–[17], the macular choroid also did not stay unchanged in the whole day, but had a fluctuation[18]. So although there was no significant choroidal thickness difference between normal and POAG eyes according to the one-off measurement, however whether the fluctuation of choroidal thickness of POAG patients was similar to that of normal individuals was for now still not clear. To answer this question, we aimed to observe and compare the diurnal fluctuation of choroidal thickness between POAG patients and normal controls using EDI-OCT, which was a non-invasive, real-time, high resolution, high speed measurement method. In the meantime, we also hoped to study the correlation between the change in IOP and the change in macular choroidal thickness in both enrolled groups. And considering the higher accuracy of two-dimensional measurement than that of one-dimensional measurement and the reduced measurement bias caused by choroidal thickness variation in different choroidal region, we chose choroidal area instead of thickness measurement.
SUBJECTS AND METHODS
This study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Tongji Hospital, Medical College, Huazhong University of Science and Technology, Wuhan, China. Written informed consents were obtained from all the research participants before enrolling them in this study. Registry number is ChiCTR-RCC-14004831.
In this prospective research, we recruited 28 eyes from 28 normal individuals and 27 eyes from 27 POAG patients (one eye of each subject was randomly selected to eliminate the intereye correlation between two eyes of one subject). All the eyes received the same ophthalmic examinations, including the best-corrected visual acuity (BCVA), refractive error (RE), IOP measurement by non-contact tomometer (NIDEK RT-2100; NIDEK, Co., Ltd., Gamagori, Japan), systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurement (OmronHEM-7201; Omron, Dalian, Liaoning Province, China), slit-lamp microscopy examination, indirect ophthalmoscope, anterior chamber angle examination using gonioscopy, visual field test (30-2, SITA fast) using Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, USA), central corneal thickness (CCT) using pachymetry map (anterior segment OCT, Carl Zeiss Meditec, Dublin, USA), AL using IOL-master (Carl Zeiss Meditec, Dublin, USA), choroid images and retinal nerve fiber layer thickness using spectral-domain optical coherence tomography (SD-OCT; Heidelberg Engineering GmbH, Heidelberg, Germany).
Subjects were included in the POAG group if all of the following were true: 1) at least 18 years of age; 2) cup-to-disc (C/D) ratio ≥0.6 with an interocular C/D ratio difference ≥0.2; 3) retinal nerve fiber layer defect was present; 4) glaucomatous visual field defects corresponding to optic nerve changes were present; 5) normal anterior chamber depth with an open angle; 6) RE between +3.0 and -6.0 diopters (D). Patients who had a history of eye disease (except for POAG disease) or any prior ocular surgeries or poor OCT images quality were excluded from participation. Patients with systemic disease were also excluded. Normal subjects were included if all of the following were true: 1) at least 18 years of age; 2) normal fundus; 3) normal visual field; 4) normal anterior chamber depth with an open angle; 5) RE between +3.0 and -6.0 D. Potential control patients were excluded from participation if they had a family history of glaucoma, a history of ophthalmic disease or surgery, or systemic disease or poor OCT images quality[19].
IOP and the macular choroidal area were measured within 10min every 3h from 9:00 a.m. to 21:00 p.m. in a whole day. And we can calculate the mean arterial pressure (MAP) and mean ocular perfusion pressure (MOPP) according to the following formulas: MAP=DBP+1/3(SBP-DBP) and MOPP=2/3(MAP-IOP). Every examination was performed in sitting position in every tested subject of the whole tested day.
Enhanced Depth Imaging Optical Coherence Tomography
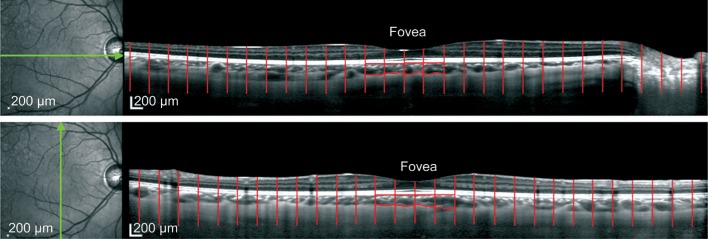
EDI-OCT imaging has been previously reported, and with this technique, we could finally visualize the full thickness of choroid[20]. The image was taken by 100 B-scans with the eye tracing function and the scan angle was 30°[21]. We set the scan angle of the SD-OCT to be 30°, so for every obtained imaging, the whole OCT picture represented 30°. Then from the fovea to both of its sides we divided the image into 30 parts equally, which means every part represented 1°. We chose the continuous 4 parts under the fovea to be the macular choroidal area of that image (Figure 1). For every eye in each measurement time point, a vertical and a horizontal OCT images were obtained. Then the two data were averaged to represent the macular choroidal area of the eye at that measurement time point.
Figure 1. Example of macular choroidal area measurement.
From the fovea to its both sides we divide the imaging into 30 areas, every area represented 1°. For macula, the continuous 4 subfoveal areas (the red encircle zone) represented the macular choroidal area of that image.
The choroidal area was defined as the area between the outer border of the retinal pigment epithelium (RPE) and the inner border of the sclera. For macular imaging, both the sides (left and right border in the imaging) of the measured area should be perpendicular to the RPE and the inner border of the sclera[22]. The observers, who were mask to the subject information, undertook the measurement by using the software Image J (version 1.47, National Institutes of Health, Bethesda, Maryland, USA). And the P-value for the interobserver of the macular choroidal area measurement was greater than 0.05.
To evaluate the correlation between the change in IOP and the change in macular choroidal area, we chose the value of IOP and macular choroidal area at 9:00 a.m., when the diurnal macular choroidal area fluctuation study started, as the baseline. The change in IOP or macular choroidal area was the difference between the value of IOP or macular choroidal area at 12:00 a.m., 15:00 p.m., 18:00 p.m. and 21:00 p.m. and the value of IOP or macular choroidal area at 9:00 a.m.
Statistical Analysis
The statistical analyses were performed using the SPSS software package 19.0. Data are shown as mean±standard deviation. The fluctuation of macular choroidal area was analyzed by using a repeated-measures analysis of variance. Comparison of parameters between POAG group and normal control group are done using Mann-Whitney U test and correlations were evaluated by nonparametric spearman correlation analyses. All tests were two-tailed and statistical significance was defined as P value <0.05.
RESULTS
Demographic Characteristics
This study enrolled 28 normal eyes and 27 POAG eyes. The demographic data are detailed in Table 1. There was no significant difference in the mean age between the normal individuals (36.04±8.04y) and those with POAG group (37.56±7.80y; P=0.367). The percentage of female, RE, BCVA, AL, CCT, MOPP, MAP and DBP were similar in two groups (all P>0.05). Significant difference in SBP can be seen between the normal individuals and POAG patients (111.11±8.17 vs 115.70±5.30 mm Hg; P=0.023). And as expected, the MD of visual field were significantly different between these two groups (-1.96±0.52 vs -9.05±7.02 dB; P<0.001).
Table 1. Demographic characteristics of study subjects.
| Parameters | Normal eyes | POAG eyes | P |
| Age (a) | 36.04±8.04 | 37.56±7.80 | 0.367 |
| Female (%) | 64.3 | 40.7 | 0.080 |
| RE (D) | -2.46±1.57 | -2.41±1.95 | 0.893 |
| BCVA | 0.94±0.18 | 0.93±0.19 | 0.900 |
| AL (mm) | 24.73±0.56 | 24.95±1.46 | 0.674 |
| CCT (µm) | 541.96±31.83 | 544.63±34.12 | 0.926 |
| MOPP (mm Hg) | 46.51±4.77 | 46.61±4.58 | 0.730 |
| MAP (mm Hg) | 86.79±6.01 | 87.90±5.02 | 0.276 |
| SBP (mm Hg) | 111.11±8.17 | 115.7±5.30 | 0.023a |
| DBP (mm Hg) | 74.50±5.88 | 74.00±6.83 | 0.906 |
| MD (dB) | -1.96±0.52 | -9.05±7.02 | <0.001a |
RE: Refractive error; BCVA: Best-corrected visual acuity; AL: Axial length; CCT: Central corneal thickness; MOPP: Mean ocular perfusion pressure; MAP: Mean arterial pressure; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; MD: Mean deviation. aP<0.05.
mean±SD
Diurnal Changes of Macular Choroidal Area
Significant diurnal variations of macular choroidal area in normal (P=0.003) and POAG (P<0.001) groups could be detected (Table 2). In both normal and POAG groups, the fluctuation rhythms of macular choroidal area were similar (Figure 2). The macular choroidal area in the five measurement time points all showed no significant difference between the two groups (all P>0.05; Table 2). Furthermore, the change in macular choroidal area between the two adjacent measurement time points (9:00 a.m. vs 12:00 a.m.; 12:00 a.m. vs 15:00 p.m.; 15:00 p.m. vs18:00 p.m.; 18:00 p.m. vs 21:00 p.m., respectively) showed no significant difference between normal and POAG groups (all P>0.05; Table 3).
Table 2. Fluctuations of macular choroid area in normal and POAG groups.
| Choroidal area (µm2) | 9:00 a.m. | 12:00 a.m. | 15:00 p.m. | 18:00 p.m. | 21:00 p.m. | P |
| Normal group | 512778±166242 | 501526±168953 | 501982±173158 | 508912±174589 | 503787±171241 | 0.003a |
| POAG group | 455079±207278 | 447846±211147 | 448024±206653 | 457783±207081 | 453230±205955 | <0.001a |
| P | 0.195 | 0.245 | 0.239 | 0.252 | 0.274 |
aP<0.05.
mean±SD
Figure 2. Fluctuations of macular choroid area of normal and POAG groups.
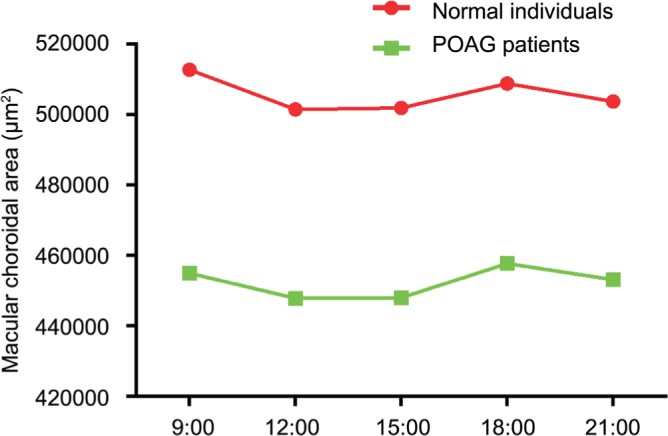
Table 3. The comparison of the macular choroidal area changes between the two adjacent measurement time points in normal and POAG groups.
| The change in choroidal area (µm2) | 9:00 a.m. vs 12:00 a.m. | 12:00 a.m. vs 15:00 p.m. | 15:00 p.m. vs 18:00 p.m. | 18:00 p.m. vs 21:00 p.m. |
| Normal group | -11252±14534 | 455±18882 | 6930±19468 | -5125±14586 |
| POAG group | -7233±12905 | 177±14629 | 9759±13833 | -4553±13540 |
| P | 0.121 | 0.556 | 0.590 | 0.920 |
mean±SD
Diurnal Changes of Intraocular Pressure and the Correlation Between the Change in Macular Choroidal Area and the Change in Intraocular Pressure
We observed a significant fluctuation of IOP in normal and POAG groups (both P<0.001) in diurnal time. In addition, IOP in the five measurement time points all showed significant difference between the two groups (P=0.020, 0.002, 0.004, 0.041, and 0.008; respectively; Table 4).
Table 4. Fluctuations of IOP of normal and POAG groups.
| IOP (mm Hg) | 9:00 a.m. | 12:00 a.m. | 15:00 p.m. | 18:00 p.m. | 21:00 p.m. | P |
| Normal group | 16.95±2.52 | 16.04±2.65 | 15.57±2.55 | 15.57±2.89 | 14.94±2.54 | <0.001a |
| POAG group | 18.22±2.35 | 18.07±2.31 | 17.50±2.53 | 16.55±2.11 | 16.94±2.25 | <0.001a |
| P | 0.020a | 0.002a | 0.004a | 0.041a | 0.008a | - |
aP<0.05.
mean±SD
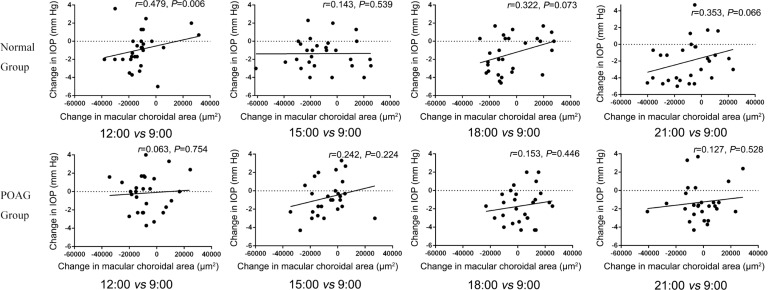
Furthermore, for normal group, the change in IOP was significantly and positively correlated with the change in macular choroidal area only between 12:00 a.m. and the baseline time point (9:00 a.m.) (r=0.479, P=0.006). But no such correlation has been found between 15:00 p.m., 18:00 p.m., 21:00 p.m. and the baseline time-point (9:00 a.m.) (r=0.143, 0.322 and 0.353, respectively; P=0.539, 0.073 and 0.066, respectively). For POAG group, no such correlation has been found between 12:00 a.m., 15:00 p.m., 18:00 p.m., 21:00 p.m. and the baseline time-point (9:00 a.m.) (r=0.063, 0.242, 0.153 and 0.127, respectively; P=0.754, 0.224, 0.446 and 0.528, respectively; Figure 3).
Figure 3. The correlation between the change in macular choroidal area and the change in IOP.
DISCUSSION
In this current study, we set the data at 9:00 a.m. as the baseline, and compared the baseline macular choroid area between the healthy subjects and POAG patients. Based on the results, we confirmed a lack of significant difference of macular choroid area between the two tested groups, just as previous studies have found[8]–[11],[23] and this implied the higher IOP in POAG patients could make the retinal nerve fiber layer thinner but not the macular choroid, the macular choroid remained thick enough to maintain the central visual acuity.
Up to now, the mechanism of the choroid thickness variation is still obscure. Some hypotheses were attempted to explain this physiological phenomenon, including changes in the synthesis of the osmotically active poly proteoglycans, changes of the vasopermeability in the choroid, changes in the amount of the fluid from the anterior chamber, the movement of the fluid from the RPE to the choroid and changes in the contraction of the nonvascular smooth muscle[24]. In addition, the choroidal thickness also has some relationship with the other ocular physiological parameters, like IOP and AL[25] and with the systemic physiological parameters, like the blood pressure[22].
In terms of the fluctuation, Lee et al[26] reported that choroid thickness decreased all the way during the daytime significantly. By the study of Usui et al[22] the choroid thickness has a peak value at 3:00 a.m. and a valley value at 18:00 p.m. The choroid thickness showed a downtrend as well. But conversely, Chakraborty et al[17] found the choroid thickness increased constantly from 12:00 a.m. to 21:00 p.m. Yet those researches were all done in healthy subjects. In our study, we noticed that there exists a significant fluctuation of the macular choroid area not only in healthy subjects but also in POAG patients. But the macular choroid area fluctuation diagram was not a straight line but a curve. It falls down from 9:00 a.m. and then rebounds at 12:00 a.m. all the way up until 18:00 p.m. and then once more falls down to 21:00 p.m. It was not exactly the same with the above mentioned conclusion. This finding might indicate that by POAG patients we should take the measurement time point into consideration when measuring the choroid thickness or area, especially in the forenoon. Besides, we found that the macular choroid area variation curve of normal and POAG groups were similar during the daytime. By the statistical methods, we conclude that, in those two groups, neither the fluctuation manners nor the change value of macular choroidal area between the two adjacent measurement time points differs significantly from each other, indicating that the fluctuation rhythm of macular choroidal area were similar between normal and POAG groups.
So based on the results of this study, we could find that not only the macular choroidal area value, but also the fluctuation of the macular choroidal area in the daytime were not different between normal and POAG groups. And as mentioned above, the choroid was the only blood supply to the macula, which was the determinate of the central visual acuity. So we speculated that no matter in terms of statically or dynamically, the macular choroidal blood flow of POAG patients could remain sufficient and change in the normal range within the day, just as the normal individual, and could resist the mechanical compression of IOP and maintain the central visual acuity. So this could be one reason for the long-lasting of central visual acuity of POAG patients, especially in view of the neurovascular unit theory, which combined the blood supply of vessels and the function of nerve.
Furthermore, we also studied the correlation between the change in macular choroidal area and the change in IOP. As the result showed, for normal individuals, the change in IOP was significantly positive correlated with the change in macular choroidal area only between 12:00 a.m. and the baseline time-point (9:00 a.m.), without such correlation between 15:00 p.m., 18:00 p.m., 21:00 p.m. and the baseline time-point (9:00 a.m.). For POAG group, no such correlation has been found between 12:00 a.m., 15:00 p.m., 18:00 p.m., 21:00 p.m. and the baseline time-point (9:00 a.m.). Schuman et al[27] reported that the degree of IOP elevation was associated with uveal thickening in healthy individuals. But by that study, this was achieved by a valsalva maneuver, and the venous pressure was also elevated in the episcleral veins and could contribute to the IOP elevation. In this present study, significant correlation between the change in IOP and the change in macular choroidal area was only observed at 12:00 a.m. in normal group. So in physiological status, there might be no correlation between the change in choroid and IOP in normal and POAG groups because IOP was determined by multi-factors and had autonomic regulation function[28].
The present study had certain limitations. First, the sample size was relatively small and we could not divide the patients with POAG into different subgroups by stages for further analysis. Second, this study was only conducted in diurnal time but not in nocturnal time. Third, the use of anti-glaucoma drugs may affect the choroidal area, we did not take this factor into consideration. Forth, the choroidal area or thickness could not fully represent the actual choroidal microcirculation and metabolic status.
In conclusion, both the macular choroidal area in normal and POAG groups showed significant diurnal fluctuations. When comparing the macular choroidal area between normal and POAG groups, not only the macular choroidal area, but also the fluctuation of macular choroidal area of POAG patients in diurnal time was not significantly different from that of normal individuals.
Acknowledgments
Conflicts of Interest: Li M, None; Guo JM, None; Xu XL, None; Wang JM, None.
REFERENCES
- 1.Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Sur Ophthalmol. 2007;52(Suppl 2):162–173. doi: 10.1016/j.survophthal.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Rao HL, Kumar AU, Babu JG, Senthil S, Garudadri CS. Relationship between severity of visual field loss at presentation and rate of visual field progression in glaucoma. Ophthalmology. 2011;118(2):249–253. doi: 10.1016/j.ophtha.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7(10):3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 5.Delaey C, Van de Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32(6):249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 6.Yin ZQ, Vaegan, Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6(1):23–32. [PubMed] [Google Scholar]
- 7.Cristini G, Cennamo G, Daponte P. Choroidal thickness in primary glaucoma. Ophthalmologica. 1991;202(2):81–85. doi: 10.1159/000310179. [DOI] [PubMed] [Google Scholar]
- 8.Nakakura S, Yamamoto M, Terao E, Nagasawa T, Tabuchi H, Kiuchi Y. The whole macular choroidal thickness in subjects with primary open angle glaucoma. PLoS One. 2014;9(10):e110265. doi: 10.1371/journal.pone.0110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Tatham AJ, Medeiros FA, Zangwill LM, Yang Z, Weinreb RN. Assessment of choroidal thickness in healthy and glaucomatous eyes using swept source optical coherence tomography. PLoS One. 2014;9(10):e109683. doi: 10.1371/journal.pone.0109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhew JY, Kim YT, Choi KR. Measurement of subfoveal choroidal thickness in normal-tension glaucoma in Korean patients. J Glaucoma. 2012;23(1):46–49. doi: 10.1097/IJG.0b013e31825af772. [DOI] [PubMed] [Google Scholar]
- 11.Park HY, Lee NY, Shin HY, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014;23(4):225–231. doi: 10.1097/IJG.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Zhang X. Choroidal thickness and primary open-angle glaucoma: a cross-sectional study and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55(9):6007–6014. doi: 10.1167/iovs.14-14996. [DOI] [PubMed] [Google Scholar]
- 13.Tojo N, Abe S, Ishida M, Yagou T, Hayashi A. The fluctuation of intraocular pressure measured by a contact lens sensor in normal-tension glaucoma patients and nonglaucoma subjects. J Glaucoma. 2017;26(3):195–200. doi: 10.1097/IJG.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 14.Read SA, Collins MJ, Iskander DR. Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci. 2008;49(7):2911–2918. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- 15.Loewen NA, Liu JH, Weinreb RN. Increased 24-hour variation of human intraocular pressure with short axial length. Invest Ophthalmol Vis Sci. 2010;51(2):933–937. doi: 10.1167/iovs.09-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mapstone R, Clark CV. Diurnal variation in the dimensions of the anterior chamber. Arch Ophthalmol. 1985;103(10):1485–1486. doi: 10.1001/archopht.1985.01050100061019. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52(8):5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 18.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, Li M, Chen Z, Zhu Y, Song Y, Zhang H. Schlemm's canal and trabecular meshwork in eyes with primary open angle glaucoma: a comparative study using high-frequency ultrasound biomicroscopy. PLoS One. 2016;11(1):e0145824. doi: 10.1371/journal.pone.0145824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Hirooka K, Fujiwara A, Shiragami C, Baba T, Shiraga F. Relationship between progression of visual field damage and choroidal thickness in eyes with normal-tension glaucoma. Clin Exp Ophthalmol. 2012;40(6):576–582. doi: 10.1111/j.1442-9071.2012.02762.x. [DOI] [PubMed] [Google Scholar]
- 22.Usui S, Ikuno Y, Akiba M, Maruko I, Sekiryu T, Nishida K, Iida T. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Visual Sci. 2012;53(4):2300–2307. doi: 10.1167/iovs.11-8383. [DOI] [PubMed] [Google Scholar]
- 23.Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Visual Sci. 2011;52(6):3430–3435. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hata M, Hirose F, Oishi A, Hirami Y, Kurimoto Y. Changes in choroidal thickness and optical axial length accompanying intraocular pressure increase. Jpn J Ophthalmol. 2012;56(6):564–568. doi: 10.1007/s10384-012-0173-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina. 2014;34(2):385–393. doi: 10.1097/IAE.0b013e3182993f29. [DOI] [PubMed] [Google Scholar]
- 27.Schuman JS, Massicotte EC, Connolly S, Hertzmark E, Mukherji B, Kunen MZ. Increased intraocular pressure and visual field defects in high resistance wind instrument players. Ophthalmology. 2000;107(1):127–133. doi: 10.1016/s0161-6420(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 28.Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog Retin Eye Res. 2016;55:108–148. doi: 10.1016/j.preteyeres.2016.07.002. [DOI] [PubMed] [Google Scholar]