Abstract
AIM
To investigate the change of anterior chamber angle morphology and intraocular pressure (IOP) reduction after cataract surgery in patients with normal-tension glaucoma (NTG) using swept-source optical coherence tomography (SS-OCT).
METHODS
This prospective, comparative, observational study recruited patients into two groups. Group 1 was the control group including normal subjects except those with cataracts (cataract group, n=67 eyes of 67 patients), and group 2 was NTG group including patients who were diagnosed with NTG and cataracts (n=43 eyes of 43 patients), which were treated with phacoemulsification and intraocular lens implantation. Before surgery, and at postoperative 1 and 6mo, anterior chamber angles were evaluated by SS-OCT under dark conditions using three-dimensional angle analysis scan protocol. Angle opening distance (AOD), angle recess area (ARA), and trabecular-iris surface area (TISA) at four quadrants (temporal, nasal, superior, and inferior) were calculated automatically by SS-OCT, after the observer marked scleral spurs.
RESULTS
A total of 106 patients (54 males and 52 females) were enrolled in the study. Angle parameters, AOD, ARA, and TISA were increased after surgery in both groups. However, changes of angle parameters were only significant in group 2. In group 2, preoperative IOP was 13.2±2.9 mm Hg, and postoperative IOP at 1 and 6mo were 10.5±3.0 and 10.7±2.8 mm Hg, respectively. In group 1, preoperative IOP was 12.4±2.8 mm Hg, and postoperative IOP at 1 and 6mo were 11.6±2.5 and 12.0±2.8 mm Hg, respectively. After cataract surgery, angle parameters changed significantly while IOP significantly reduced and was maintained in group 2 (P<0.001). The changes in angle parameters (ΔAOD500, ΔTISA500 at temporal; ΔAOD500, ΔARA500 at nasal) were linearly correlated with postoperative IOP changes.
CONCLUSION
Cataract surgery may have improved anterior chamber angle parameters and decreased IOP in NTG patients.
Keywords: normal-tension glaucoma, cataract surgery, intraocular pressure reduction, swept-source optical coherence tomography, angle parameters
INTRODUCTION
Cataract and glaucoma are both very common eye diseases in older patients, and their prevalence significantly increases with increasing age. Thus, large numbers of glaucoma patients also have cataracts, which can decrease visual acuity, contrast sensitivity, and examination accuracy. For these reasons, many glaucoma patients undergo cataract surgery. Cataract and glaucoma are major causes of blindness in the world[1]. Cataract is the leading cause of blindness, and it accounts for 50% of blindness worldwide. Glaucoma is the leading cause of irreversible blindness, with an estimated prevalence of about 2.4% in the world[2]–[3]. According to previous reports, in Asia, the prevalence of normal-tension glaucoma (NTG) refers to glaucoma with an intraocular pressure (IOP) below 21 mm Hg, is higher than other regions around the world[4]. It is widely known that glaucoma progression is associated with IOP and hemodynamic status, and controlling IOP is the only existing treatment to prevent NTG progression[3].
The structures and functions of angles are important in aqueous humour outflow. It is well-known that open angle status, rather than closed-angle status, is a more favorable structure in aqueous humour drainage. Lens extraction using cataract surgery creates more space in posterior chamber as well as in the angle, especially in older patients. With aging, lens becomes thicker and anterior chamber narrows. Oxidative stress markers hinder trabecular meshwork functioning, and increasing levels of superoxide dismutase (SOD) and catalase (CAT) activities in anterior chamber have been correlated with the severity of cataracts[5]–[6].
Cataract surgery is a positive factor that benefits the prevention of closed-angle glaucoma, and multiple studies have reported that cataract surgery can lower IOP in glaucoma patients.Lens extraction using cataract surgery is helpful for closed-angle glaucoma with severely narrowed angles. After cataract surgery, configuration of angle was widened, and it lowered IOP[7]–[9]. However, many controversies remain for open angle glaucoma patients, which led us to investigate the effects of cataract surgery in open angle glaucoma.
Recently, anterior segment swept-source optical coherence tomography (AS-SS-OCT) has been used for evaluation of anterior segment (AS) configurations, although previous evaluations only used gonioscopy. Ultrasound biomicroscopy (UBM) assessment of the angle is more objective and reproducible, but its contact may be uncomfortable to the patient[10]. Anterior segment optical coherence tomography (AS-OCT) is a non-contact method that can perform both quantitative and objective evaluations[10]–[11]. When only scleral spur is marked, the built-in software can automatically calculate many AS parameters. Compared to time-domain optical coherence tomography (TD-OCT), the currently used SS-OCT has more horizontal and depth resolution, so that high speed, high resolution, and three-dimensional imaging of the angle, anterior lens surface, and full thickness morphology of the cornea can be obtained. Furthermore, analytical properties of AS-SS-OCT are being continuously refined[11]–[12].
We investigated angle configuration change and IOP reduction after cataract surgery in NTG patients. Before surgery, not a lot of differences could be found between glaucoma and non-glaucoma patients in terms of angle configuration and function. However, we hypothesized that the changes in angle structures might be different between two groups after cataract surgery. According to the changes in angle configuration, greater IOP reduction might be obtained in NTG. We can expect better IOP control by cataract surgery in NTG patients.
SUBJECTS AND METHODS
Ethics Statement
The study protocol followed tenets of the Declaration of Helsinki, and was approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University College of Medicine. Informed consent was obtained from all subjects.
Study Patients
Between January and December 2015, patients from Gangnam Severance Hospital Eye Center (Seoul, Korea) were recruited. This prospective, comparative, observational study divided patients into two groups. Group 1 was cataract group, including normal patients except for those with cataracts. Group 2 was NTG group, consisting of NTG patients with cataracts; this group was further defined as either having or not having glaucomatous optic nerve changes and visual field (VF) defects. NTG was defined by a glaucoma specialist based on the following factors: 1) glaucomatous VF defect confirmed by two reliable VF tests; 2) typical appearance of glaucomatous optic nerve head (ONH) that includes cup/disc (C/D) ratio >0.7 and C/D ratio asymmetry >0.2, with diffuse or focal neuroretinal rim thinning, disc haemorrhage, or vertical elongation of the optic cup; 3) maximum untreated IOP at <21 mm Hg, as determined by three repeated measurements taken at different times on separate visits during follow-up; 4) normal anterior chamber with open angle status on slit-lamp and gonioscopic examinations.
Inclusion and Exclusion Criteria
All patients were Shaffer grade ≥3, with open angle status. Gonioscopy was performed on the first visit (day 1) using four-mirror goniolens (G-4 High Mag, Volk Optical, Inc., OH, USA) in non-dilated status. Under topical anaesthesia (Proparacaine Hydrochloride, Alcaine®; Alcon, Fort Worth, TX, USA), quadrants were studied with the four-mirror lens. When scleral spur was visible and angle opened wide (about 30°-45°) in all quadrants, which was graded by Shaffer grade ≥3. Patients did not have any other ocular disease affecting the aqueous outflow or angle morphology, except for cataract and glaucoma. Clinical exclusion criteria included closed-angle glaucoma, neovascular glaucoma, age-related macular degeneration, and proliferative diabetic retinopathy. Patients with prior corneal surgery, trabeculoplasty, cycloablation, or any incisional glaucoma procedure (such as trabeculectomy, tube shunt, or deep sclerectomy) were also excluded. If cataract surgery was performed on both eyes, the most affected eyes (worse visual acuity in group 1 and worse glaucoma field in group 2) were enrolled.
Study Examinations
All patients completed an assessment of visual acuity, Goldmann applanation tonometry, gonioscopy, axial length measurement, and indirect ophthalmoscopy. IOP measurement was performed at the clinic in Gangnam Severance Hospital, using Goldmann applanation tonometry (AT900®; Haag-Streit, Koeniz, Switzerland) with topical anaesthesia (proparacaine HCl, Alcaine®; Alcon, Fort Worth, TX, USA). A single ophthalmologist (Choi W) performed IOP measurement three times, and the average value was used for analysis. Patients' data or study enrolment statuses were masked. IOP measurement was performed at the latest procedure (OCT was performed ahead of IOP measurements) during clinical operation time (from 9:00 a.m. to noon), with well-calibrated Goldmann applanation tonometry. Axial length was determined by non-contact type laser biometry (IOL-Master500®; Carl Zeiss Meditec, Dublin, CA, USA). Central corneal thickness was measured by a contact-type ultrasound pachymeter (US-500 Echoscan, Nidek Co., Ltd., Gamagori, Japan). AS-SS-OCT was performed before surgery and on postoperative 1 and 6mo. Preoperative assessments (visual acuity, tonometry, gonioscopy, and AS-SS-OCT) were performed before surgery.
Surgical Procedures
Patients were prescribed pupil dilating medication [5 mg phenylephrine HCl, 5 mg tropicamide (Mydrin-P®; Taejoon Pharmaceutical, Seoul, Korea)] before surgery. One surgeon (Seong GJ) performed all cataract operations under topical anaesthesia (proparacaine HCl, Alcaine®; Alcon, Fort Worth, TX, USA). A 2.75 mm clear corneal incision was made at temporal side of the cornea, and anterior chamber was filled with an ophthalmic viscoelastic device (Healon®; Abbott Laboratories, Chicago, IL, USA). An approximate 5.5-6.0 mm of continuous curvilinear capsulorrhexis was performed. Lens extraction was done by phacoemulsification (INFINITI®; Alcon), and foldable intraocular lens (Hoya iSert®, Hoya, Tokyo, Japan) was inserted into the capsular bag. Corneal wound was sutured with one knot at the temporal incision site, and suture knot was removed at postoperative 2wk. Patients were then treated with gatifloxacin eye drops (Handok, Seoul, Korea) four times per day for 2wk. Prednisolone acetate eye drops (Allergan, Irvine, CA, USA) were used four times per day for 4wk. There were no complications during or after the surgery.
Anterior Segment Parameters
Before starting the main study, we checked the repeatability and reproducibility of SS-OCT with scleral spur marking. In our anterior OCT instrument (Casia SS-1000; Tomey, Nagoya, Japan), there was built-in software for automatic calculation of AS parameters that was initiated as soon as scleral spur was marked. Therefore, scleral spur marking was considered very important for reliability of AS parameter determinations in this study. A total of 30 randomly selected patients, involving 30 eyes, were checked by AS-SS-OCT. Two investigators marked the scleral spur site at separate spaces at different times, and this procedure was repeated after 1wk. Intrapersonal and interpersonal correlation coefficients were obtained for these determinations.
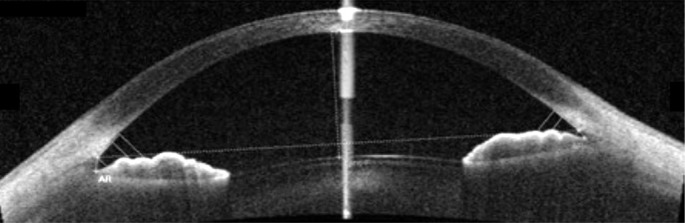
AS parameters were obtained by AS-SS-OCT (Casia SS-1000). One operator obtained all angle images in the undilated state under dark, identical room conditions. To obtain the entire angle images, upper eyelids were gently raised by the examiner using a long cotton tip. If eyelids were tightened and the eye was not exposed in one image, the examiner separately obtained images of the four quadrants. For example, the examiner asked the patient to look forward and raised the upper eyelid for the superior quadrant for imaging, and images of the other quadrants were obtained in a second imaging. Using the angle analysis mode of Casia SS-1000, images were obtained of the nasal, temporal, superior, and inferior angle quadrants by moving the arrow bar. The examiner was blinded to the diagnosis of the patient. Images were also analysed by two other investigators who were blinded to the diagnosis of the patient. The best images were selected after analyses using the automatic calculating software in AS-SS-OCT, in order to obtain several AS parameters. Angle opening distance (AOD) at 500 µm (AOD500), 750 µm (AOD750) from the scleral spur, trabecular-iris surface area at 500 µm (TISA500), 750 µm (TISA750), angle recess area at 500 µm (ARA500), 750 µm (ARA750) were obtained automatically, after marking the scleral spur (Figures 1, 2)[12].
Figure 1. Angle evaluation with SS-OCT and automatically obtained angle parameters.
AOD, ARA, TISA and TIA attemporal side were 0.522, 0.223, 0.189, 38.0 (at 500 µm) and 0.763, 0.392, 0.357, 40.5 (at 750 µm). At nasal side, angle parameters were 0.344, 0.145, 0.127, 26.4 (at 500 µm) and 0.598, 0.273, 0.256, 32.6 (at 750 µm).
Figure 2. Scleral spur (SS), angle recess (AR) and 500/750 µm points from scleral spur were indicated in analyzed program.
Statistical Analysis
The repeated longitudinal data were analysed by SPSS, version 20 software for windows (IBM, Chicago, IL, USA) based on longitudinal, parametric, paired t-test, Chi-square test, and multiple regression test. For intraclass and interclass correlation coefficients, two-way mixed effects model was used. All patients were included and all results were considered significant at P<0.05.
RESULTS
Characteristics of the Study Patients
A total of 110 patients were enrolled. In the end, 4 patients (3 from group 1, 1 from group 2) were excluded due to loss of follow-up. Group 1 included 64 eyes of 64 patients, comprised of 30 males and 34 females. Group 2 included 42 eyes of 42 patients, comprised of 24 males and 18 females. The mean age was 68.87±8.68y in group 1, and 68.00±10.66y in group 2 (Table 1).
Table 1. Patients characteristics.
| Parameters | Group 1 | Group 2 | P |
| Age (a) | 68.87±8.68 | 68.00±10.66 | 0.944 |
| Gender (M:F) | 30:34 | 24:18 | 0.453a |
| Laterality (R:L) | 33:31 | 24:18 | 0.806a |
| Axial length (mm) | 24.01±1.16 | 24.26±1.53 | 0.362 |
| CCT (µm) | 554 ±64 | 556±28 | 0.959 |
| Refractive error (SE) | -0.41±0.85 | -0.50±0.85 | 0.602 |
| MD (dB) | NA | -4.78±5.16 | NA |
| Untreated IOP (mm Hg) | NA | 16.5±3.1 | NA |
| Preop. VA (logMAR) | 0.26±0.15 | 0.21±0.03 | 0.854 |
| Postop. VA (logMAR) | 0.03±0.01 | 0.02±0.01 | 0.886 |
NTG: Normal-tension glaucoma; CCT: Central corneal thickness; SE: Spherical equivalent; VA: Visual acuity; Untreated IOP: Intraocular pressure before anti-glaucoma medications. aP values by Chi-square test.
mean±SD
Repeatability and Reproducibility
AS parameters, AOD500, AOD750, ARA500, ARA750, TISA500, and TISA750 had good repeatability and reproducibility. Intraclass correlation coefficients of one investigator at an interval of 1wk were 0.896-0.984, and interclass correlation coefficients for two investigators were 0.906-0.980. Therefore, both repeatability and reproducibility were acceptable for this study.
Intraocular Pressure Reduction After Cataract Surgery
Group 2 (NTG group) had significant changes in IOP. In group 2, preoperative IOP was 13.2±2.9 mm Hg, and postoperative IOP at 1 and 6mo were 10.5±3.0 and 10.7±2.8 mm Hg, respectively. After cataract surgery, significant IOP reduction was observed in group 2 (P<0.001) (Table 2). About 19% IOP reduction was obtained after cataract surgery in NTG patients. Before cataract surgery, the mean IOPs were not significantly different between two groups, recording at 12.4±2.8 mm Hg in group 1 and 13.2±2.9 mm Hg in group 2 (P=0.078). However, significant reduction of IOP was shown only in group 2. In addition, NTG patients used fewer anti-glaucoma eye drops after cataract surgery than before surgery (P=0.005). Before surgery, NTG patients used a mean of 1.53±0.61 species of anti-glaucoma eye drops. Six months after cataract surgery, they used a mean of 0.71±0.83 species of anti-glaucoma eye drops. No patients required IOP-lowering medications after cataract surgery in group 1 (cataract group). Among patients with open angle glaucoma, 33 used prostaglandin analogues, eight used selective beta blockers, 11 used fixed combination of timolol and dorzolamide, and six used alpha agonist (brimonidine). Twenty-one subjects used two species of anti-glaucoma eye drops, and three patients used three species. No significant correlation was found between the use of prostaglandin analogue or alpha agonist and angle parameters.
Table 2. Comparison of IOP.
| Groups | Preop. | Postop. 1mo | Postop. 6mo | P |
| Group 1 | 12.4±2.8 | 11.6±2.5 | 12.0±2.8 | 0.065a; 0.082b |
| Group 2 | 13.2±2.9 | 10.5±3.0 | 10.7±2.8 | <0.001a; <0.001b |
aP values between preop. and postop. 1mo; bP values between preop. and postop. 6mo.
mm Hg; mean±SD
Anterior Chamber Parameters
In both groups, anterior chamber depth (ACD) and anterior chamber volume (ACV), which were measured by three-dimensional reconstruction program of the AS-OCT after cataract surgery, significantly increased compared to before surgery (Table 3). However, there was no significant difference between two groups in regards to ACD and ACV (P=0.576, 0.164, respectively). The decrease of IOP in group 2 might not be due to changes of ACD and ACV, but could be due to increase in angle parameters.
Table 3. Comparison of ACD and ACV.
| Groups | ACD (mm) |
ACV (mm3) |
||||
| Preop. | Postop. | P | Preop. | Postop. | P | |
| Group 1 | 2.92±0.48 | 3.45±0.20 | <0.001a | 159.96±30.66 | 175.22±21.42 | <0.001a |
| Group 2 | 3.07±0.45 | 3.45±0.50 | <0.001a | 165.30±42.59 | 174.05±36.25 | <0.001a |
| P | 0.073b | 0.576b | NA | 0.452b | 0.164b | NA |
ACD: Anterior chamber depth; ACV: Anterior chamber volume. aP value comparison between preop. and postop.; bP value comparison between groups 1 and 2.
Changes of Angle Configurations
Before cataract extraction in group 1, AS parameters at the temporal side were as follows: AOD500, 0.52±0.29 mm; AOD750, 0.70±0.37 mm; ARA500, 0.25±0.13 mm2; ARA750, 0.41±0.21 mm2; TISA500, 0.20±0.11 mm2; and TISA750, 0.35±0.19 mm2. At postoperative 1 and 6mo at the temporal side, AOD500, AOD750, ARA500, ARA750, TISA500, and TISA750 significantly increased. The values at nasal, superior, and inferior sides are also increased. AS-OCT parameters significantly changed when comparing preoperative and postoperative values.
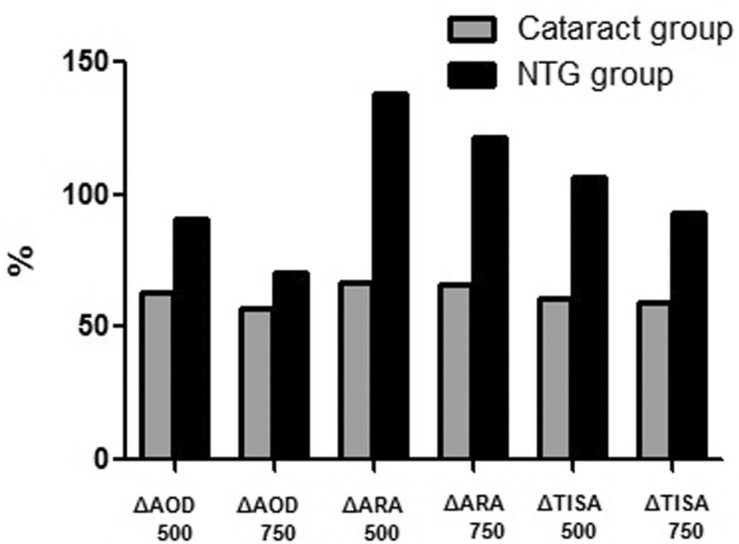
In group 2, the changes of AS parameters were significant. Before cataract extraction, the following parameters were determined at the temporal side: AOD500, 0.52±0.24 mm; AOD750, 0.74±0.38 mm; ARA500, 0.26±0.16 mm2; ARA750, 0.42±0.24 mm2; TISA500, 0.19±0.10 mm2; and TISA750, 0.35±0.18 mm2. Anterior angle parameters were significantly increased at postoperative 1 and 6mo at the temporal side. Data for nasal, superior, and inferior side parameters are also increased. Changes in all parameters before and 1 or 6mo after cataract surgery in NTG patients were all statistically significant in all four quadrants. The variations of angle parameters in group 2 (Figure 3) were greater than those of group 1. In particular, nasal quadrant angle parameters (ARA500, ARA750, and TISA500) were significantly increased compared to those of group 1. There was a 66.66% increase in ARA500 in group 1, and a 138.09% increase in group 2 (P=0.031); a 65.78% increase in ARA750 in group 1, and a 121.32% increase in group 2 (P=0.038); and a 61.11% increase of TISA500 in group 1, and 106.25% increase in group 2 (P=0.045).
Figure 3. Comparisons of angle parameters variations (ΔAOD500/750, ΔARA500/750, ΔTISA500/750) at nasal quadrant in control and NTG group.
Multiple regression analyses of angle parameter changes and IOPs showed that the changes (Δ) of AOD500 at temporal and nasal sides, TISA500 at temporal side, and ARA500 at nasal side were linearly correlated with postoperative IOP (β=-14.686, -11.831, -23.671, and -14.263; P=0.022, 0.050, 0.050, and 0.047, respectively) (Table 4).
Table 4. Multiple regressions of angle parameter changes and postop. IOP changes.
| Angle parameters | β | t | P | R2 |
| Temporal | 0.474 | |||
| ΔAOD 500 | -14.686 | -2.417 | 0.022 | |
| ΔTISA 500 | -23.671 | -1.964 | 0.050 | |
| Nasal | ||||
| ΔAOD 500 | -11.831 | -2.037 | 0.050 | |
| ΔARA 500 | -14.263 | -2.070 | 0.047 |
DISCUSSION
AS-SS-OCT showed good repeatability and reproducibility in our study, as well as in other previous studies[13]–[14]. The present prospective study is the first to evaluate the relationship between angle configuration and IOP in NTG patients before and after cataract surgery. IOP control is the most important factor in glaucoma patients, and lowering IOP will minimize damage to the retinal nerve fiber layer. Taken together, the present study is important in optimizing glaucoma treatment. After cataract surgery, ACD, ACV were significantly increased. However, after surgery, no difference was found between two groups in terms of ACD or ACV. The significant decrease of IOP in group 2 might not be due to changes of ACD and ACV, but could be due to a more significant increase in angle parameters.
However, the effect of IOP reduction in cataract surgery remains controversial. In our study, group 1 (cataract group) also had slightly decreased IOP at 6mo after surgery. According to angle widening, non-glaucoma patients also might have lowered IOP, although the value may not be highly significant (P=0.082). The average IOP of non-glaucomatous eye was 13.4±2.9 mm Hg, according to Namil study (Namil-myon, a rural agricultural area in central Korea). The average IOP in this study was slight lower than that of Namil study; however, preoperative IOP of two groups (groups 1 and 2) were not significantly different. Besides, more IOP reduction was obtained in group 2 at postoperative status. Meanwhile, Prata et al[15] reported that cataract surgery could reduce IOP during the first few days after surgery. However, IOP was not meaningfully reduced for longer time periods (average 5y). Among many studies, the mean decrease of IOP values varied between 1.1 and 5.3 mm Hg. According to Melancia et al[16], 68 patients with open angle glaucoma who were controlled with medication had clinically meaningful IOP (Hayashi group). However, other groups (Shingleton and Mathalone groups) reported that the changes of IOP after cataract surgery were not meaningful in primary open angle glaucoma (POAG).
Phacoemulsification results in angle widening and a decrease of IOP in closed-angle glaucoma. After phacoemulsification, ACD and angle parameters change significantly[7]–[10],[15]–[19]. In elderly patients, lens thickness from anterior to posterior surface is increased. The thick lens can influence aqueous humour dynamics, due to shallower anterior chamber and narrower angle. In older open angle glaucoma patients, including NTG, there are frequent senile cataracts. IOP elevation is due to aqueous outflow resistance resulting from trabecular meshwork alterations or collapse of Schlemm's canal, which are major causes of open angle glaucoma[20]–[21]. However, it is well-known that senile cataracts can disrupt aqueous humour outflow. In these cases after cataract extraction, an increase of aqueous outflow has been reported[22]. Cataract extraction alone may at least partially correct the anatomical and physiological problems in aqueous humour dynamics[9]. Senile POAG patients have both conventional pathway dysfunction (trabecular meshwork disruption) and aqueous humour outflow barriers due to increasing lens thickness, which cannot be adequately clinically evaluated by gonioscopy[23]. However, AS-OCT can detect open angles, with narrowed status, more accurately than gonioscopic examination.
Aqueous humour is secreted from ciliary bodies and drained in a balanced pattern at ocular AS, which includes the lens and the trabecular meshwork, to achieve the following: provide nutrients, scavenge metabolic wastes, and maintain the correct ocular pressure. Humans, like other living organisms, are continuously exposed to reactive oxygen species (ROS) as a consequence of biochemical reactions, as well as external pollutants. ROS are causes of many degenerative diseases, including glaucoma, cataract, and macular degeneration. Production of free radicals can result in degeneration of mammalian cells[2]. The cells of trabecular meshwork and Schlemm's canal can therefore be damaged by oxidative stress, which is considered to be the major pathogenic mechanism of POAG. Significant correlations have been found between oxidative damage of human trabecular meshwork DNA, VF defects, and IOP[2]. ROS have also been linked to POAG by increased flow resistance in anterior chamber, due to high levels of hydrogen peroxide[2]–[3],[24]. According to the severity of nuclear cataract, SOD and CAT levels increase in aqueous humour[5]. Increasing ROS in anterior chamber activates scavenging system of ROS in the lens. Imbalance of ROS scavenging system and ROS production are the causes of cataract formation[24]–[25]. Whether ROS increases in anterior chamber precede cataract formation, or cataract formation precedes the formation of ROS is not known. However, many types of ROS can cause cataracts in the lens, so it is possible that cataracts could be a major cause of ROS. Therefore, it is possible that cataract extraction is an effective treatment for decreasing ROS in anterior chamber. As the levels of ROS decrease, trabecular meshwork in anterior chamber could be protected to prevent open angle glaucoma.
During cataract surgery, spontaneous infusion and aspiration were performed with balanced salt solution (BSS). Angles can be enlarged and cleaned by viscoelastic material injection during surgery. One main cause of POAG is alteration of trabecular meshwork due to plaque materials. During cataract surgery, the use of BSS in anterior chamber can remove plaque materials from the angle and trabecular meshwork. In pseudoexfoliation (PEX) syndrome, PEX materials are removed during cataract surgery. Water-jet infusion of BSS removed PEX materials from the angles, and no PEX material recurrence was seen during a mean follow-up of 3y. The mean IOP also decreased significantly after surgery[26]. In a similar manner, anterior chamber irrigation during cataract surgery is helpful in removing plaque materials, which can influence aqueous humour drainage function of trabecular meshwork and Schlemm's canal. In this study, all surgeries were performed through a temporal side clear corneal incision followed by phacoemulsification. The direction of phaco-tip was roughly toward the nasal quadrant; therefore, water-jet effect could be stronger in the nasal quadrant. Water-jet might alter the angle structure; more precisely, the angle geometry in the nasal quadrant could be significantly increased, as shown in this study. Connective tissue structures in glaucoma patients are more elastic to IOP increases than non-glaucoma patients[27]. Prostaglandin analogues, widely used in NTG patients, modulate iris and uvea connective tissues via matrix metalloproteinase (MMPs) in the anterior chamber[28]. Lens extraction using phacoemulsification and IOP implantation create more space in posterior chamber. More loosening of connective tissues in the angle can be directed toward posterior-inferior direction. Angle widenings could then occur during these processes.
According to Koc et al[29], angle width in all quadrants were significantly lower in older group (>41y) than younger groups (<20 or 21-40y) in open angle, healthy subjects. Even with open angle status, older patients have slightly narrowed angle width compared to younger subjects. This might be the reason why ΔAOD 500 at nasal and temporal quadrants was correlated with postoperative IOP. We thought that differences in the composition of connective studies might greatly widen the angle structures in glaucoma group after undergoing the same procedures (cataract surgery), compared to non-glaucoma group (cataract group). Wider angle structure in glaucoma eyes could lead to greater IOP reduction, as compared to non-glaucoma eyes. No significant changes were found in AVD or ACV; however, differences were significant for the angle structures.
This study has some limitations. First, the duration of this study was 6mo. To evaluate long-term effects of cataract extraction for glaucoma patients, longer follow-up periods are needed. Second, this study included a relatively small number of participants (106 eyes of 106 patients). However, data of this prospective study had a normal distribution, and the effects of cataract surgery were statistically significant in NTG patients and normal subjects. Third, we could not evaluate the diurnal variation of IOP (especially at night), and the anti-glaucoma drugs used by all subjects were not identical. However, we made an effort to diminish the bias of IOP variations, and checked IOP at the same time (at 9:00 a.m. to noon). Fourth, subjects enrolled this study were in relatively early stages of glaucoma (-4.78±5.16 dB). We need to study IOP reduction in moderate or severe NTG patients.
During the processes of phacoemulsification and acrylic monofocal intraocular lens insertion, anterior angle can be widened in both elderly glaucoma and normal patients. Especially in NTG patients, nasal quadrant angle parameters can be significantly increased, and IOP can be reduced more than non-glaucoma patients after cataract extraction. Therefore, our results suggest that phacoemulsification and intraocular lens implantation can be simple and convenient adjunctive treatments for glaucoma.
Acknowledgments
My special thanks must go to Dr. Choi W, who had participated in IOP measurement in outpatient clinic with double blind maneuver (patients' data or study enrolment statuses were masked). With his work, we could perform this study.
Conflicts of Interest: Lee W, None; Bae HW, None; Kim CY, None; Seong GJ, None.
REFERENCES
- 1.Chan W, Garcia JA, Newland HS, Muecke J, McGovern S, Selva D, Aung T, Casson RJ. Killing two birds with one stone: the potential effect of cataract surgery on the incidence of primary angle-closure glaucoma in a high-risk population. Clin Exp Ophthalmol. 2012;40(4):e128–134. doi: 10.1111/j.1442-9071.2011.02607.x. [DOI] [PubMed] [Google Scholar]
- 2.Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam Clin Pharmacol. 2012;26(1):86–117. doi: 10.1111/j.1472-8206.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 3.Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD. Oxidative stress in primary open-angle glaucoma. J Glaucoma. 2008;17(4):263–268. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]
- 4.Kim CS, Seong GJ, Lee NH, Song KC, Namil Study Group, Korean Glaucoma Society Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011;118(6):1024–1030. doi: 10.1016/j.ophtha.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Sawada H, Fukuchi T, Abe H. Oxidative stress markers in aqueous humor of patients with senile cataracts. Curr Eye Res. 2009;34(1):36–41. doi: 10.1080/02713680802500960. [DOI] [PubMed] [Google Scholar]
- 6.Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39(8):823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- 7.Roberts TV, Francis IC, Lertusumitkul S, Kappaqoda MB, Coroneo MT. Primary phacoemulsification for uncontrolled angle-closure glaucoma. J Cataract Refract Surg. 2000;26(7):1012–1016. doi: 10.1016/s0886-3350(00)00358-8. [DOI] [PubMed] [Google Scholar]
- 8.Brown RH, Zhong L, Whitman AL, Lynch MG, Kilgo PD, Hovis KL. Reduced intraocular pressure after cataract surgery in patients with narrow angles and chronic angle-closure glaucoma. J Cataract Refract Surg. 2014;40(10):1610–1614. doi: 10.1016/j.jcrs.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and co-existing cataract: a prospective case series. J Glaucoma. 2006;15(1):47–52. doi: 10.1097/01.ijg.0000196619.34368.0a. [DOI] [PubMed] [Google Scholar]
- 10.Zhou AW, Giroux J, Mao AJ, Hutnik CM. Can preoperative anterior chamber angle width predict magnitude of intraocular pressure change after cataract surgery? Can J Ophthalmol. 2010;45(2):149–153. doi: 10.3129/i10-009. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda R, Usui T, Tomidokoro A, Mishima K, Matagi N, Miyai T, Amano S, Araie M. Noninvasive observations of peripheral angle in eyes after penetrating keratoplasty using anterior segment fourier-domain optical coherence tomography. Cornea. 2012;31(3):259–263. doi: 10.1097/ICO.0b013e318226daa9. [DOI] [PubMed] [Google Scholar]
- 12.Nolan WP, See JL, Aung T, Friedman DS, Chan YH, Smith SD, Zheng C, Huang D, Foster PJ, Chew PT. Changes in angle configuration after phacoemulsification measured by anterior segment optical coherence tomography. J Glaucoma. 2008;17(6):455–459. doi: 10.1097/IJG.0b013e3181650f31. [DOI] [PubMed] [Google Scholar]
- 13.Aptel F, Chiquet C, Gimbert A, Romanet JP, Thuret G, Gain P, Campolmi N. Anterior segment biometry using spectral-domain optical coherence tomography. J Refract Surg. 2014;30(5):354–360. doi: 10.3928/1081597X-20140326-01. [DOI] [PubMed] [Google Scholar]
- 14.Szalai E, Berta A, Hassan Z, Modis L., Jr Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. J Cataract Refract Surg. 2012;38(3):485–494. doi: 10.1016/j.jcrs.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Prata TS, Ushida M, Dorairaj S. Cataract surgery alone cannot be considered an IOP-lowering procedure for open-angle glaucoma patients. Arq Bras Oftalmol. 2015;78(5):V–VI. doi: 10.5935/0004-2749.20150072. [DOI] [PubMed] [Google Scholar]
- 16.Melancia D, Abeqao Pinto L, Marques-Neves C. Cataract surgery and intraocular pressure. Ophthalmic Res. 2015;53(3):141–148. doi: 10.1159/000377635. [DOI] [PubMed] [Google Scholar]
- 17.Jamil AZ, Iqbal K, Ur Rahman F, Mirza KA. Effect of phacoemulsification on intraocular pressure. J Coll Physicians Surg Pak. 2011;21(6):347–350. [PubMed] [Google Scholar]
- 18.Eid TM. Primary lens extraction for glaucoma management: a review article. Saudi J Ophthalmol. 2011;25(4):337–345. doi: 10.1016/j.sjopt.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latifi G, Moghimi S, Eslami Y, Fakhraie G, Zarei R, Lin S. Effect of phacoemulsification on drainage angle status in angle closure eyes with or without extensive peripheral anterior synechiae. Eur J Ophthalmol. 2013;23(1):70–79. doi: 10.5301/ejo.5000191. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal P, Daher AM, Agarwal R. Aqueous humor TGF-beta2 levels in patients with open-angle glaucoma: a meta-analysis. Mol Vis. 2015;21:612–620. [PMC free article] [PubMed] [Google Scholar]
- 21.Diestelhorst M, Krieglstein GK. Effect of cataract extraction on aqueous humor dynamics in patients with senile cataract. A prospective fluorophotometric study. Fortschr Ophthalmol. 1991;88(2):128–131. [PubMed] [Google Scholar]
- 22.Mansouri M, Ramezani F, Moghimi S, Tabatabaie A, Abdi F, He M, Lin SC. Anterior segment optical coherence tomography parameters in phacomorphic angle closure and mature cataracts. Invest Ophthalmol Vis Sci. 2014;55(11):7403–7409. doi: 10.1167/iovs.14-14748. [DOI] [PubMed] [Google Scholar]
- 23.Pinazo-Duran MD, Gallego-Pinazo R, Garcia-Medina JJ, Zanon-Moreno V, Nucci C, Dolz-Marco R, Martinez-Castillo S, Galbis-Estrada C, Marco-Ramirez C, Lopez-Galvez MI, Galarreta DJ, Diaz-Llopis M. Oxidative stress and its downstream signaling in aging eyes. Clin Interv Aging. 2014;9:637–652. doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samanta A, Kumar P, Machhua S, Rao GN, Pal A. Incidence of cystoid macular oedema in diabetic patients after phacoemulsification and free radicals link to its pathogenesis. Br J Ophthalmol. 2014;98(9):1266–1272. doi: 10.1136/bjophthalmol-2013-304438. [DOI] [PubMed] [Google Scholar]
- 25.Zoric L, Elek-Vlajic S, Jovanovic M, Kisic B, Djokic O, Canadanovic V, Cosic V, Jaksic V. Oxidative stress intensity in lens and aqueous depending on age-related cataract type and brunescense. Eur J Ophthalmol. 2008;18(5):669–674. doi: 10.1177/112067210801800501. [DOI] [PubMed] [Google Scholar]
- 26.Tran VT. Washout of pseudoexfoliation material combined with cataract surgery: a new surgical approach to lower intraocular pressure in pseudoexfoliationsyndrome. Int Ophthalmol. 2015;35(2):209–214. doi: 10.1007/s10792-014-9934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinhart MR, Cone-Kimball E, Nguyen C, Nguyen TD, Pease ME, Chakravarti S, Oqlesby EN, Quigley HA. Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res. 2014;119:54–60. doi: 10.1016/j.exer.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci. 1997;38(13):2772–2780. [PubMed] [Google Scholar]
- 29.Koc M, Ozulken K, Ayar O, Karakurt A. Measurement of the anterior chamber angle according to quadrants and age groups using Pentacam Scheimpflug camera. J Glaucoma. 2013;22(3):226–229. doi: 10.1097/IJG.0b013e318237c100. [DOI] [PubMed] [Google Scholar]