Abstract
Background
Tibial slope angle is a nonmodifiable risk factor for anterior cruciate ligament (ACL) injury. However, the mechanical role of varying tibial slopes during athletic tasks has yet to be clinically quantified.
Purpose
To examine the influence of posterior tibial slope on knee joint loading during controlled, in vitro simulation of the knee joint articulations during athletic tasks.
Study Design
Descriptive laboratory study.
Methods
A 6 degree of freedom robotic manipulator positionally maneuvered cadaveric knee joints from 12 unique specimens with varying tibial slopes (range, −7.7° to 7.7°) through drop vertical jump and sidestep cutting tasks that were derived from 3-dimensional in vivo motion recordings. Internal knee joint torques and forces were recorded throughout simulation and were linearly correlated with tibial slope.
Results
The mean (6SD) posterior tibial slope angle was 2.2° ± 4.3° in the lateral compartment and 2.3° ± 3.3° in the medial compartment. For simulated drop vertical jumps, lateral compartment tibial slope angle expressed moderate, direct correlations with peak internally generated knee adduction (r = 0.60–0.65), flexion (r = 0.64–0.66), lateral (r = 0.57–0.69), and external rotation torques (r = 0.47–0.72) as well as inverse correlations with peak abduction (r = −0.42 to −0.61) and internal rotation torques (r = −0.39 to −0.79). Only frontal plane torques were correlated during sidestep cutting simulations. For simulated drop vertical jumps, medial compartment tibial slope angle expressed moderate, direct correlations with peak internally generated knee flexion torque (r = 0.64–0.69) and lateral knee force (r = 0.55–0.74) as well as inverse correlations with peak external torque (r = −0.34 to 20.67) and medial knee force (r = −0.58 to −0.59). These moderate correlations were also present during simulated sidestep cutting.
Conclusion
The investigation supported the theory that increased posterior tibial slope would lead to greater magnitude knee joint moments, specifically, internally generated knee adduction and flexion torques.
Clinical Relevance
The knee torques that positively correlated with increased tibial slope angle in this investigation are associated with heightened risk of ACL injury. Therefore, the present data indicated that a higher posterior tibial slope is correlated to increased knee loads that are associated with heightened risk of ACL injury.
Keywords: tibial slope angle, ACL injury risk, knee kinetics, joint biomechanics
Over the past 30 years, a significant amount of time and funding has been utilized to identify risk factors for and mechanisms of anterior cruciate ligament (ACL) injury.20,21 Approximately 70% of these injuries occur in noncontact situations, likely as a result of numerous risk factors collectively resulting in a knee orientation that places excessive and abnormal mechanical demand on the ligament.1,8 Risk factors for ACL injury are commonly divided into 2 categories: modifiable and nonmodifiable.2 Modifiable risk factors are related to neuromuscular control, muscular deficiencies, and other biomechanical mechanisms that can be mitigated or corrected though noninvasive prophylactic interventions. Conversely, nonmodifiable factors represent those control mechanisms that either require surgical intervention to improve or that realistically are entirely unalterable. One such nonmodifiable risk factor is the posterior angulation of the tibial articular surface, commonly referred to as the tibial plateau slope.45
In a recent systematic review and meta-analysis, Wordeman et al45 demonstrated that the majority of literature supports a moderate to strong association between ACL injury and medial and lateral tibial slope. However, a number of shortcomings in the literature were also described. Specifically, the authors noted that the preponderance of studies available utilized a retrospective case-control design and reported significantly differing values for the magnitude of tibial plateau slope in ACL-injured and uninjured subjects. While a relatively sound theoretical and biomechanical basis is established for tibial plateau slope as a risk factor for ACL injury,11,15,22,29,39,40,45 the clinical implications are unclear. Furthermore, the extent to which neuromuscular control and gross biomechanics are able to compensate for or mitigate the biomechanical risk imposed by tibial slope remains unanswered.40,45 To elucidate the extent of the role(s) of posterior tibial slope in vivo, it is critical that any ex vivo analyses closely mirror the kinematics and kinetics of dynamic, high-risk movements as these tasks have served as greater predictors of injury than more controlled tasks such as gait.21 Further, failure to maintain such physiologic relevance in ex vivo analyses is to exacerbate the gap between model and clinical applicability. Subsequent association of any provocative motions with tibial plateau slope should be carefully scrutinized and independently verified but may ultimately be considered pertinent to injury based on the biomechanical relevance of the task. While a significant body of in vivo and radiographic work demonstrates an association of posterior tibial slope with ACL injury risk,40,45 only a handful of investigations have quantified the mechanical consequences of higher tibial slope during simulations of dynamic tasks. McLean et al29 previously reported significant in vivo associations between lateral tibial plateau slope and anterior knee joint reaction force as well as between medial and lateral tibial plateau slope and peak knee abduction and internal rotation angles. Cadaveric investigations during simulated landing also demonstrate a role for tibial anatomy in anterior tibial acceleration profiles and gross biomechanics.35
The objective of this investigation was to examine the influence of posterior tibial slope on knee joint loading during controlled, in vitro simulation of the knee joint articulations during athletic tasks. Our tested hypothesis was that increased posterior tibial plateau slope would be associated with greater magnitudes of anterior load and internally generated adduction, flexion, and external moments within the knee.
METHODS
Experimental Design
An anatomic donations program (Anatomy Gifts Registry Inc) was used to acquire 18 human cadaveric lower extremities from 12 unique donors (mean ± SD: age, 47.6 ± 7.3 years; mass, 829 ± 199 N). These specimens were resected down to the bone and passive structures of the tibiofemoral joint, leaving the articulating surfaces, cruciate ligaments, collateral ligaments, and menisci intact, then mounted to a 6 degree of freedom (DOF) robotic manipulator (KR210; KUKA Robotics Corp). The end effector of the robotic manipulator was mounted with a 6-axis load cell (Theta Model; ATI Industrial Automation) that was capable of recording knee forces and torques along 3 perpendicular axes originating at the tibial joint center point. Six-DOF kinematics recorded from in vivo knee joint motion were input to the robotic manipulator to drive articulation of the cadaveric joint. Barbed 3 mm–microminiature differential variable strain transducers (LORD Microstrain) were implanted on the anteromedial bundle of the ACL to record ligament strain during simulation.4,6,25 Explicit details of this in vivo motion capture and in vitro robotic simulation method have been previously published and are briefly described in the follow-ing.4,5,12 For this investigation, motion simulations were performed with the tibiofemoral articulating surfaces, cruciate ligaments, collateral ligaments, and menisci intact. Before simulation, a digital coordinate measuring machine (CMM; Faro Digitizer F04L2; FARO Technologies Inc) was used to digitize anatomic landmarks on the cadaveric specimen. After the completion of all simulations, the specimen was resected of all loadbearing structures, and each simulation was re-run in a bone-only condition that quantified the effects of inertia and gravity and allowed for compensation of their contributions. The anatomic landmarks (specifically defined later in this text) were used to calculate the posterior tibial slope of the medial and lateral compartments, which were then correlated with the respective 6-DOF joint loading observed during simulation.
Kinematic Model
Bates et al4 previously described the development of a model that performs in vitro simulations of knee articulation during athletic tasks based on in vivo recorded kinematics. Briefly, 3D motion data collected on a matched male (age, 24 years; height, 175 cm; mass, 68.8 kg) and female (age, 25 years; height, 170 cm; mass, 64.4 kg) were processed into joint kinematics using a computational biomechanical model (Visual3D, version 4.0; C-Motion Inc).12 Previously published mathematical factors were systematically applied to these in vivo kinematic data to convert them to physiologically relevant robotic inputs capable of driving in vitro knee articulations.4
Specimen Preparation
To be included in the current investigation, specimens could not have a history of knee trauma, knee surgery, bone cancer, or implants at the shin, knee, or ankle. Specimens were also required to be younger than 55 years, as tissue stiffness has been shown to degrade with age.44 Specimens were frozen at −20°C and thawed at room temperature beginning 24 hours before testing. Thawed specimens were resected of all soft tissue down to the knee joint capsule, leaving the cruciate and collateral ligaments and menisci intact. Anatomic landmarks were identified and used to locate the tibial and femoral axes according to the joint coordinate system.14,19 Custom mechanical fixtures were then aligned with and affixed to the distal end of the tibia such that it could be mounted directly to the robot end effector.9,18,19,34 This rigid mount directly aligned the mechanical axis of the tibia with the compressive axis of the end effector. The femur was secured to a rigid base mounted on the floor, which permitted the robotic manipulator to articulate the tibia about the static femur. The CMM was used to digitize the joint center point and anatomic landmarks across the specimen that were used to define the joint coordinate system relative to the robotic axis for precise articulation of the joint segments.
Robotic Simulation
All simulations were performed at room temperature, and the joint was consistently hydrated with a saline solution. Simulation cycles were performed during the landing phase of each of 4 athletic tasks (male drop vertical jump [DVJ], female DVJ, male sidestep cut, female sidestep cut), beginning at initial contact with the ground and ending at the point where minimum center of gravity was achieved.3 All 4 tasks were simulated on all cadaveric specimens in a randomized order. An initial orientation for each of the 4 motion tasks simulated was selected from in vivo motion analysis. The kinematics of each of these start positions were unique and were matched to within 0.5° of the in vivo kinematics for all 3 rotational DOFs. For the translational DOFs, the limb was incrementally loaded in compression, and simulation cycles were performed until a peak force of 2.0 to 2.5 body weights was achieved for DVJ and 1.5 to 2.0 body weights was achieved for sidestep cutting. The magnitudes represent the peak force generated on a single limb during a double leg DVJ performed in vivo.3 This iterative approach of compression assured that the selection of the initial position promoted a physiologically appropriate loading profile throughout the remainder of the test. Additional details regarding initial positioning for specimens during robotic simulation have been published previously.4,19
Once the starting point was determined, the specimen was run through 10 preconditioning cycles to minimize viscoelastic effect and ensure force drop did not occur. After successful preconditioning, a second set of 10 simulation cycles was run while joint forces and torques were recorded. This process was repeated for each of the four athletic tasks. After all simulations were completed in the intact condition, specimens were resected of load-bearing structures including the cruciate and collateral ligaments, medial and lateral menisci, and femoral condyles. All simulations were re-run in this tibial bone-only scenario to quantify and then compensate for (by subtraction) the effects of gravity and robot inertia. Testing methods were approved by the institutional review board of Cincinnati Children’s Hospital and the University of Cincinnati.
Posterior Tibial Slope
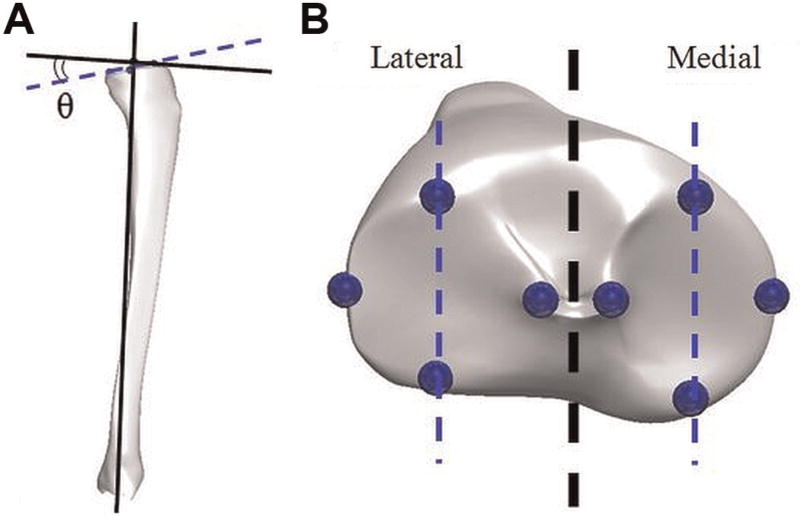
The method used to calculate posterior tibial slope in the present investigation was adapted from previously published literature.15,16 The referenced method was developed for use with sagittal plane radiographs. However, the authors adapted this technique for use with cadaveric specimens by using the CMM to digitize the anatomic landmarks that would normally be identified in radiographic images. Specifically, CMM points were taken at the most anterior and posterior points of the tibial plateau articulating surface along the approximate midline of both the medial and lateral compartments (Figure 1). The slope of the tibial plateau was then calculated as the angle between the line formed by joining these points and a second line drawn perpendicular to the mechanical axis of the tibia in the sagittal plane as described by Hashemi et al. In radiographic studies, the calculation of tibial slope using the prescribed method has been shown to be highly reliable.15 For reference, as the point on the posterior aspect of either compartment moved inferiorly, the posterior tibial slope angle for that compartment would increase.
Figure 1.
(A) Depiction of the method used to calculate tibial slope angle, which was the angular difference θ between a line perpendicular to the mechanical axis of the tibia in the sagittal plane and a line passing through the anterior- and posterior-most points of each tibial compartment. The slope shown is positive. (B) Points collected by the coordinate measuring machine for calculation of tibial slope angle. Notice that the anterior- and posterior-most points of the articulating surface fall on the midline of each tibial compartment.
Data Analysis
All forces and torques presented in the current investigation were based on the knee joint coordinate system and analyzed in the tibial reference frame.14 Forces and torques were filtered through a 12-Hz Fourier transform to reduce noise. Translational forces were normalized to percentage body weight, while rotational torques were normalized to N·m/kg. Data were analyzed from an average of the eighth and ninth cycles of each 10-cycle simulation to eliminate cycle effects. Before data analysis, output measures collected on limbs that originated from the same subject were averaged to eliminate confounding effects on the data. For each motion task, Pearson correlation coefficients were used to determine linear relationships between posterior tibial slope angle and peak force and torque values in all DOFs at the knee. Correlations were interpreted as bad (r < 0.25), poor (0.25 < r < 0.50), moderate (0.50 < r < 0.75), or good (r > 0.75).36 Statistical significance for a linear correlation was determined at the α < 0.05 level. Paired t tests were also run to determine significant differences in tibial slope angle between compartments (α <0.05). All statistical analyses were performed using built-in functions of MATLAB (version 2012b, The MathWorks Inc).
It should be noted that all knee moments in the present study are reported as internally generated torques. This is in contrast to the externally generated knee moments that are typical for in vivo investigations. Externally generated torques provide estimates of the loads that drive limb motion and are extrapolated from external loads recorded on a force platform during athletic tasks. In the present study, the alignment of the force sensor with the mechanical axis of the tibia, and thus the joint center point of the knee, allowed for the calculation of torques inside the knee joint that resist the motion being performed. As such, internally generated knee moments have the opposite orientation of externally generated knee moments. Therefore, an internally generated adduction torque corresponds with an externally generated abduction torque.
RESULTS
Posterior Tibial Slope
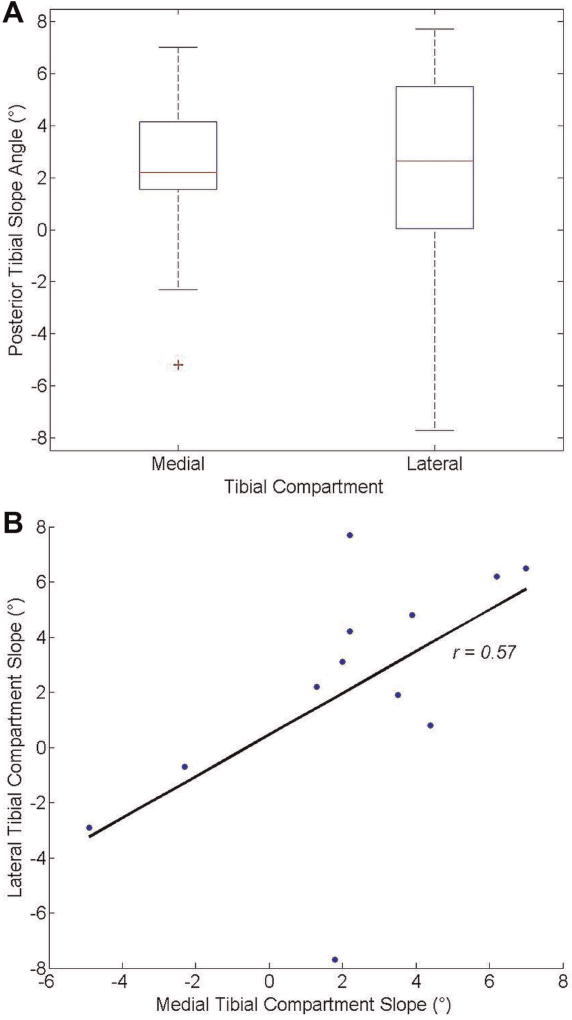
Mean (±SD) posterior tibial slope angle in the lateral compartment (2.2 ° ± 4.3 °) was not significantly different than mean posterior tibial slope angle in the medial compartment (2.3 ° ± 3.3 °; P = .93). However, the range of values for the lateral compartment tibial slope angle (−7.7 ° to 7.7 °) was 3.5° greater than the same range for the medial compartment (−4.9 ° to 7.0 °). Tibial slope angle from the lateral compartment demonstrated a moderate, significant, and direct correlation with tibial slope angle in the medial compartment (r = 0.57, P = .05) (Table 1 and Figure 2).
TABLE 1.
Posterior Tibial Slope Angle for Both Compartments of Each Unique Donor Tested
| Posterior Tibial Slope Angle, deg
|
|||
|---|---|---|---|
| Donor | Medial Compartment |
Lateral Compartment |
Difference |
| Subject 1 | −4.9 | −2.9 | 2.0 |
| Subject 2 | 3.9 | 4.8 | 0.9 |
| Subject 3 | 4.4 | 0.8 | 3.6 |
| Subject 4 | 6.2 | 6.2 | 0.0 |
| Subject 5 | 1.3 | 2.2 | 0.9 |
| Subject 6 | 1.8 | −7.7 | 9.5 |
| Subject 7 | 3.5 | 1.9 | 1.6 |
| Subject 8 | 7.0 | 6.5 | 0.5 |
| Subject 9 | 2.0 | 3.1 | 1.1 |
| Subject 10 | 2.2 | 7.7 | 5.5 |
| Subject 11 | 2.2 | 4.2 | 2.0 |
| Subject 12 | −2.3 | −0.7 | 1.6 |
Figure 2.
(A) Mean posterior tibial slope angles in the medial and lateral compartments for the specimen population. Error bars represent standard deviations. The “+” sign indicates an outlier. (B) Linear correlation between medial and lateral compartment posterior tibial slope angles.
Lateral Compartment
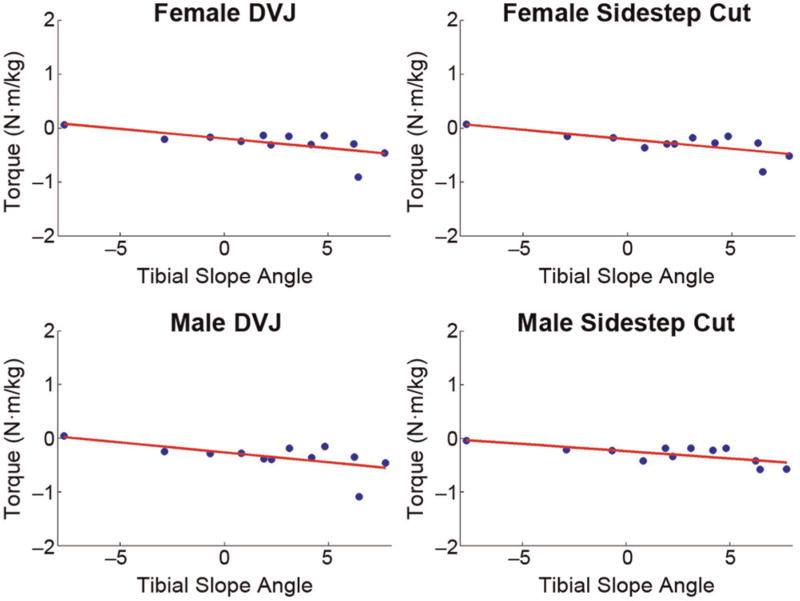
Posterior tibial slope angle in the lateral compartment exhibited moderate to good correlations with peak magnitudes of rotational torque in the frontal plane (Table 2). Increased slope angle had a direct relationship with peak internally generated adduction torque (Figure 3), which corresponds to the external knee abduction torque, and an inverse relationship with peak abduction torque. Lateral compartment tibial slope also exhibited moderate correlations with internally generated knee flexion torques. These sagittal plane correlations were significant during DVJ simulations but not during sidestep cut simulations (P <.1). In the transverse plane, lateral tibial slope had a good, direct correlation with peak internally generated external rotation moments and a moderate, inverse correlation with peak internally generated internal rotation moments during the male DVJ task. However, correlations with peak transverse plane moments were bad to poor in all other simulated tasks. Lateral compartment slope angle had bad to poor correlation with peak anterior knee force in all simulated tasks.
TABLE 2.
Pearson Correlation Coefficients and Corresponding P Values Depicting Linear Relationships Between Posterior Tibial Slope in the Lateral Compartment and Peak Knee Loads in Each DOFa
| Female DVJ
|
Male DVJ
|
Female Sidestep Cut
|
Male Sidestep Cut
|
|||||
|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | |
| Anterior | 0.31 | .32 | 0.32 | .31 | 0.40 | .20 | 0.12 | .70 |
| Posterior | 0.16 | .63 | −0.48 | .11 | −0.13 | .68 | 0.55 | .06 |
| Medial | −0.01 | .98 | 0.03 | .92 | −0.09 | .79 | −0.05 | .89 |
| Lateral | 0.69b | .01b | 0.57b | .05b | 0.35 | .27 | 0.26 | .41 |
| Compression | 0.09 | .78 | 0.26 | .41 | 0.29 | .37 | 0.33 | .29 |
| Distraction | −0.27 | .39 | −0.36 | .25 | −0.09 | .78 | 0.30 | .34 |
| Internal | −0.39 | .20 | −0.79b | .00b | −0.23 | .46 | −0.36 | .26 |
| External | 0.47 | .12 | 0.72b | .01b | 0.48 | .12 | 0.48 | .12 |
| Flexion | 0.64b | .03b | 0.66b | .02b | 0.52 | .08 | 0.55 | .07 |
| Extension | −0.19 | .55 | −0.66b | .02b | −0.34 | .28 | −0.12 | .72 |
| Adduction | 0.65b | .02b | 0.60b | .04b | 0.71b | .01b | 0.71b | .01b |
| Abduction | −0.61b | .03b | −0.42 | .18 | −0.69b | .01b | −0.75b | .00b |
DOF, degree of freedom; DVJ, drop vertical jump.
Statistically significant correlation (P < .05).
Figure 3.
Lateral tibial slope angle directly correlated well with internally generated knee adduction torque in all 4 motion tasks simulated. Note that the directionality of adduction torque in the current setup is negative; therefore, the negative slope is representative of a direct correlation. DVJ, drop vertical jump.
Medial Compartment
Posterior tibial slope angle in the medial compartment exhibited moderate, direct correlation with peak internally generated flexion moment (Table 3). These correlations were significant in 3 of 4 simulated motions. Medial compartment slope also demonstrated a moderate, inverse correlation with peak medial force and moderate, direct correlation with peak lateral force in the knee. Again, these were in 3 of 4 simulated motions. The male cut simulation exhibited fewer significant correlations between tibial slope angle and knee joint loading than did the other simulated tasks. There were no significant relationships between medial tibial slope and frontal plane torques as the Pearson coefficients in this DOF indicated poor or bad correlation.
TABLE 3.
Pearson Correlation Coefficients and Corresponding P Values Depicting Linear Relationships Between Posterior Tibial Slope in the Medial Compartment and Peak Knee Loads in Each DOFa
| Female DVJ
|
Male DVJ
|
Female Sidestep Cut
|
Male Sidestep Cut
|
|||||
|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | |
| Anterior | 0.20 | .53 | 0.41 | .18 | 0.35 | .26 | −0.48 | .11 |
| Posterior | 0.14 | .67 | −0.10 | .75 | 0.01 | .97 | 0.32 | .32 |
| Medial | −0.58b | .05b | −0.59b | .04b | −0.64b | .02b | −0.48 | .11 |
| Lateral | 0.55b | .05b | 0.74b | .01b | 0.64b | .02b | 0.60b | .04b |
| Compression | 0.16 | .61 | 0.51 | .09 | 0.35 | .26 | 0.48 | .12 |
| Distraction | 0.21 | .52 | 0.16 | .61 | 0.20 | .52 | 0.58b | .05b |
| Internal | 0.36 | .25 | −0.15 | .64 | 0.01 | .97 | 0.21 | .52 |
| External | −0.17 | .59 | 0.10 | .75 | −0.06 | .86 | 0.02 | .96 |
| Flexion | 0.64b | .02b | 0.69b | .01b | 0.65b | .02b | 0.52 | .09 |
| Extension | −0.34 | .27 | −0.67b | .02b | −0.60b | .04b | −0.47 | .12 |
| Adduction | 0.43 | .16 | 0.44 | .15 | 0.51 | .09 | 0.45 | .14 |
| Abduction | −0.13 | .68 | −0.35 | .26 | −0.42 | .18 | −0.26 | .41 |
DOF, degree of freedom; DVJ, drop vertical jump.
Statistically significant correlation (P < .05).
ACL Strain
The range of peak strain values in the ACL for each specimen during each simulated task was 2% to 15% for female DVJ, 2% to 19% for male DVJ, 1% to 18% for female sidestep cutting, and 1% to 27% for male sidestep cutting. The mean peak strain for all simulated tasks was under 8% and did not significantly correlate with either medial or lateral compartment posterior tibial slope angle for any of the simulated tasks (P > .05).
DISCUSSION
The objective of this investigation was to examine the influence of posterior tibial slope on knee joint loading during in vitro simulations of the knee joint articulations during athletic tasks. The data partially supported our a priori hypothesis. In concurrence with the hypothesis, increased posterior tibial slope in the lateral compartment consistently exhibited a statistically significant and positive correlation with larger magnitude frontal and sagittal plane knee moments. A similar relationship was present between increased tibial slope in the medial compartment and the magnitude of sagittal plane moments. Frontal plane moments represent the knee loading DOF that is most closely associated with ACL injury risk.21 The peak externally generated knee abduction moment recorded during a DVJ predicts ACL injury risk with 73% specificity and 78% sensitivity,21 which has led to the development of algorithms that can clinically predict this laboratory measure with similarly high sensitivity and specificity.31–33 Thus, the direct relationship between increased tibial slope angle and peak internally generated knee adduction torque during dynamic tasks supports previous literature that shows tibial plateau slope as a risk factor for ACL injury.
Similarly, the portion of the hypothesis that stated internally generated flexion torques would be greater in subjects with more posteriorly oriented tibial slopes was supported. While literature has shown that excessive magnitudes of sagittal plane torque alone are insufficient to rupture the ACL,28 athletes who land with greater knee extension and greater externally generated knee extension torques increase strain on the ACL and are thus likely more susceptible to injury.7,8,17 The present data indicate that increased posterior tibial slope may contribute to this loading in the knee as internally generated flexion torques were moderately correlated with posterior tibial slope angle in both compartments. The few cases of moderate, inverse relationships between posterior slope and internally generated knee extension moment further support this hypothesis by their indication that those specimens with high tibial slope express less knee flexion than their counterparts. Sagittal plane rotation is the primary DOF to absorb torsional impact at the knee.24 A resistance to functional performance in this plane indicates that a greater portion of the impulse forces generated during rapid deceleration tasks will be diverted to frontal and sagittal plane torques more associated with abnormal ACL loading.
The current findings did not support our hypothesis related to anterior tibial force. It was expected that under physiologic compressive loads, an increased anterior tibial slope would result in more compression being carried on the anterior half of the tibial plateau producing a coupled tibial anterior joint force. However, the data indicated no significant correlations between these measures and tibial plateau slope in either compartment. The ACL resists up to 85% of the anterior force generated in the knee.10,26,34 Thus, a loading change in this DOF would have had significant implications on injury risk. The posterior tibial slope range observed in our specimens was −7.7° to 7.7° and averaged 2.3° in medial compartment and 2.2° in the lateral compartment. This range of absolute tibial slope angle is shallower than tibial slope angles calculated from radiographic images but within the standard deviation of some previous reports based on magnetic resonance imaging (MRI).45 The anteriorly oriented slopes observed in some of the current specimens are less common but have been reported in previous literature.39 Overall, the shallow tibial slopes and mismatched anatomic geometry between in vitro and in vivo specimens, as well as the bone bending artifacts that would have worked to alleviate increased anterior loads during simulation, may not have provided sufficient mechanical stimulation to the prescribed kinematic pathway to significantly alter these anterior forces within the joint.
The relative slopes of the medial and lateral tibial plateau are often implicated in the literature as potential contributors to higher risk transverse and frontal plane biomechanics. In the transverse plane, the most common rationale is that a more posteriorly oriented lateral tibial slope compared with the medial tibial slope, in combination with a joint compressive load, will result in greater relative anterior shift of the lateral compartment compared with the medial compartment, creating a net internal rotation of the tibia relative to the femur.30 In the frontal plane, it is frequently argued that a more posteriorly sloped lateral compartment will result in a more distal point of tibio-femoral articulation on the lateral side compared with the medial side, resulting in a valgus orientation.45 Ongoing findings also demonstrate a mechanism by which tibial slope and medial tibial plateau depth of concavity are potentially responsible for unidirectional coupling between valgus knee alignment and internal tibial rotation.23 Specifically, it was found that internal tibial rotation results in coupled valgus, which is the key factor affecting ACL strain in a cadaver simulation. However, valgus induced through direct application of knee abduction moments does not result in any coupled internal tibial rotation. The mechanistic roles of the tibial articular surface in this phenomenon are only theoretical at this point but are as follows: internal rotation of the tibia results primarily in changes in the relative points of tibiofemoral articulation on the medial and lateral compartments. Specifically, it was postulated that a greater concavity of the medial compartment, combined with internal tibial rotation and a lateral slope greater than medial slope, results in a relatively more proximal and anterior point of articulation on the medial plateau and a relatively more distal and posterior point of articulation in the lateral tibial compartment. The distal-proximal aspect of this mechanism results in a net valgus alignment and thus couples valgus to applied internal rotation. During application of pure abduction moments at the knee, the main effect is that of distraction of the medial compartment, which does not result in coupled internal rotation but still significantly affects the strain in the ACL.23 Similar to the present study, these data demonstrated that alterations in the geometry of the tibial plateau lead to changes in the mechanical knee environment that are associated with increased risk of ACL injury.
Average peak ACL strain values in this investigation were of comparable magnitude to previous simulations of athletic tasks.5,37,43 The lack of significance between tibial slope angle and ACL strain observed in the current data is somewhat congruent with the literature.45 Many MRI-based investigations have reported limited effect size between ACL-injured and uninjured groups regarding posterior tibial slope angle.45 However, radiograph-based investigations exhibit a clear bias of increased tibial slope angle in ACL-injured populations.45 This would likely be indicative of greater load and strain magnitudes in the ACLs of subjects with larger posterior tibial slope angles. The strain values in this investigation should be taken under consideration with the understanding that this was a position-controlled robotic model. As such, the robotic manipulator regulated the relative position of the bony segments with high precision and no regard for the specimen specific tibial slopes. In vivo the cartilage surfaces of the knee articulate relative to their specific geometry, affording the femoral condyles the potential to slide more posteriorly in subjects with large posterior tibial slope angles.39 This would create a net anterior tibial translation and increase strain on the ACL. Because of the position-controlled nature of the current in vitro model, the only changes in the relative position of the bony structures would have come from bone bending induced by joint contact forces. Such bending would have been unlikely to have a large repositioning effect on the present model due to the relative close proximity of the rigid mounting fixtures to the knee joint. Accordingly, in the present model, force/torque outcomes were a more appropriate variable by which to assess the influence of posterior tibial slope than peak ACL strain.
One limitation of this study was that the reliability of the presented tibial slope measurement technique has not been assessed and thus could not be compared with that published in the literature.16 The present methodology was based on the physical palpation of landmarks, while the previous literature was based on the visual selection of landmarks. In theory, the points collected by CMM in the current investigation match the description of those gathered medical imaging in the literature. However, it is difficult to assess the precision with which this process of tibial slope calculation was recreated without performing both methods on the same cohort of specimens. Unfortunately, neither accurate sagittal plane radiographs nor MRI images were collected of all the specimens used in the current investigation, which prevented such a comparison. It would be worthwhile to assess these issues of reliability in future investigations. A further limitation of this study is that the robotic manipulator had no capacity to account for how differences of tibial slope would affect changes in bony alignment in vivo. The robotic manipulator is a high-precision, position-controlled instrument such that it eliminates, aside from bone bending, the natural kinematic variability observed during in vivo motion.38,41 As such, this was an investigation of how varying specimen geometry influenced mechanical loads produced by identical kinematics. Literature has shown that anatomic differences such as femoral notch width, and the presently examined tibial slope height, that can lead to mechanical loading differences are considered nonmodifiable risk factors to ACL injury.42,45 In vivo, it is likely that variations in tibial slope would influence how the femoral condyles articulate about these geometrical structures and influence kinematics.13,27 However, there exists no physiologically relevant method to incorporate this variation into our model. In the present simulations, this geometry-driven variability would have manifested as kinetic differences between specimens. As mechanical systems, such as diarthrodial joints, tend to gravitate toward the path of least resistance, it is reasonable to infer that increased kinetic magnitudes observed on a given specimen in our in vitro system could have extrapolated to in vivo kinematic variability. In vivo, it would be expected that biological variability would compensate for changes in slope not only with altered mechanical loads but also with altered kinematics.
CONCLUSION
The mechanical knee loading trends observed in the present investigation supported that increased posterior tibial slope would lead to greater knee joint moments. Specifically, greater posterior tibial slope in cadaveric specimens resulted in larger peak magnitudes of internally generated knee adduction, which corresponds to the external knee abduction moments that predict risk of future ACL injury, and knee flexion torques during in vitro simulations of in vivo recorded kinematics. It should be noted that this correlation between posterior tibial slope and peak internally generated knee adduction torque was only significant in the lateral compartment of the tibial plateau. The loading variables specified are associated with increased mechanical demand on the ACL and therefore indicated that athletes with higher posterior tibial slope are likely to be predisposed to additional risk of ACL injury.
Acknowledgments
The authors acknowledge the support of the staff at the Sports Health and Performance Institute at The Ohio State University and the Sports Medicine Biodynamics Laboratory at Cincinnati Children’s Hospital.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by the National Institutes of Health/NIAMS (grant numbers R01-AR049735, R01-AR055563, R01-AR056660, and R01-AR056259).
Footnotes
Investigation performed at the University of Cincinnati and the Sports Medicine Biodynamics Center at Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA
References
- 1.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 2.Alentorn-Geli E, Myer GD, Silvers HJ, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: mechanisms of injury and underlying risk factors. Knee Surg Sports Trauma-tol Arthrosc. 2009;17(7):705–729. doi: 10.1007/s00167-009-0813-1. [DOI] [PubMed] [Google Scholar]
- 3.Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46(7):1237–1241. doi: 10.1016/j.jbiomech.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. A novel methodology for the simulation of athletic tasks on cadaveric knee joints with respect to in vivo kinematics. Ann Biomed Eng. 2015;43(10):2456–2466. doi: 10.1007/s10439-015-1285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Relative strain in anterior cruciate ligament and medial collateral ligament during simulated jump landing and sidestep cutting tasks: implications for injury risk. Am J Sports Med. 2015;43(9):2259–2269. doi: 10.1177/0363546515589165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16(1):1–12. doi: 10.1007/BF00182976. [DOI] [PubMed] [Google Scholar]
- 7.Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31(6):519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 8.Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 9.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: is the porcine knee ACL dependent? J Orthop Res. 2011;29(5):641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 10.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 11.Dejour H, Bonnin M. Tibial translation after anterior cruciate ligament rupture. J Bone Joint Surg Br. 1994;76(5):745–749. [PubMed] [Google Scholar]
- 12.Ford KR, Myer GD, Hewett TE. Reliability of landing 3D motion analysis: implications for longitudinal analyses. Med Sci Sports Exerc. 2007;39(11):2021–2028. doi: 10.1249/mss.0b013e318149332d. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto E, Sasashige Y, Tomita T, Iwamoto K, Masuda Y, Hisatome T. Significant effect of the posterior tibial slope on the weight-bearing, midflexion in vivo kinematics after cruciate-retaining total knee arthroplasty. J Arthroplasty. 2014;29(12):2324–2330. doi: 10.1016/j.arth.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi J, Chandrashekar N, Gill B, et al. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90(12):2724–2734. doi: 10.2106/JBJS.G.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashemi J, Chandrashekar N, Mansouri H, et al. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38(1):54–62. doi: 10.1177/0363546509349055. [DOI] [PubMed] [Google Scholar]
- 17.Heijne A, Fleming BC, Renstrom PA, Peura GD, Beynnon BD, Werner S. Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc. 2004;36(6):935–941. doi: 10.1249/01.mss.0000128185.55587.a3. [DOI] [PubMed] [Google Scholar]
- 18.Herfat ST, Boguszewski DV, Nesbitt RJ, Shearn JT. Effect of perturbing a simulated motion on knee and anterior cruciate ligament kinetics. J Biomech Eng. 2012;134(10):104504. doi: 10.1115/1.4007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herfat ST, Boguszewski DV, Shearn JT. Applying simulated in vivo motions to measure human knee and ACL kinetics. Ann Biomed Eng. 2012;40(7):1545–1553. doi: 10.1007/s10439-011-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 21.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 22.Khan MS, Seon JK, Song EK. Risk factors for anterior cruciate ligament injury: assessment of tibial plateau anatomic variables on conventional MRI using a new combined method. Int Orthop. 2011;35(8):1251–1256. doi: 10.1007/s00264-011-1217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiapour AM, Kiapour A, Goel VK, et al. Uni-directional coupling between tibiofemoral frontal and axial plane rotation supports valgus collapse mechanism of ACL injury. J Biomech. 2015;48(10):1745–1751. doi: 10.1016/j.jbiomech.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lephart SM, Abt JP, Ferris CM. Neuromuscular contributions to anterior cruciate ligament injuries in females. Curr Opin Rheumatol. 2002;14(2):168–173. doi: 10.1097/00002281-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41(2):385–395. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72(4):557–567. [PubMed] [Google Scholar]
- 27.Marouane H, Shirazi-Adl A, Adouni M, Hashemi J. Steeper posterior tibial slope markedly increases ACL force in both active gait and passive knee joint under compression. J Biomech. 2014;47(6):1353–1359. doi: 10.1016/j.jbiomech.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 28.McLean SG, Lipfert SW, van den Bogert AJ. Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc. 2004;36(6):1008–1016. doi: 10.1249/01.mss.0000128180.51443.83. [DOI] [PubMed] [Google Scholar]
- 29.McLean SG, Lucey SM, Rohrer S, Brandon C. Knee joint anatomy predicts high-risk in vivo dynamic landing knee biomechanics. Clin Biomech (Bristol, Avon) 2010;25(8):781–788. doi: 10.1016/j.clinbiomech.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 30.McLean SG, Oh YK, Palmer ML, et al. The relationship between anterior tibial acceleration, tibial slope, and ACL strain during a simulated jump landing task. J Bone Joint Surg Am. 2011;93(14):1310–1317. doi: 10.2106/JBJS.J.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br J Sports Med. 2011;45(4):245–252. doi: 10.1136/bjsm.2009.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Clinical correlates to laboratory measures for use in non-contact anterior cruciate ligament injury risk prediction algorithm. Clin Biomech (Bristol, Avon) 2010;25(7):693–699. doi: 10.1016/j.clinbiomech.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Development and validation of a clinic-based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. Am J Sports Med. 2010;38(10):2025–2033. doi: 10.1177/0363546510370933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nesbitt RJ, Herfat ST, Boguszewski DV, Engel AJ, Galloway MT, Shearn JT. Primary and secondary restraints of human and ovine knees for simulated in vivo gait kinematics. J Biomech. 2014;47(9):2022–2027. doi: 10.1016/j.jbiomech.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM. What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med. 2012;40(3):574–583. doi: 10.1177/0363546511432544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practive. Norwalk, CT: McGraw Hill/Appleton & Lange; 1993. [Google Scholar]
- 37.Quatman CE, Kiapour AM, Demetropoulos CK, et al. Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med. 2014;42(1):177–186. doi: 10.1177/0363546513506558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudy TW, Livesay GA, Woo SL, Fu FH. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech. 1996;29(10):1357–1360. doi: 10.1016/0021-9290(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 39.Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43(9):1702–1707. doi: 10.1016/j.jbiomech.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith HC, Vacek P, Johnson RJ, et al. Risk factors for anterior cruciate ligament injury: a review of the literature—part 1: neuromuscular and anatomic risk. Sports Health. 2012;4(1):69–78. doi: 10.1177/1941738111428281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum Mov Sci. 2011;30(5):869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 43.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–227. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 45.Wordeman SC, Quatman CE, Kaeding CC, Hewett TE. In vivo evidence for tibial plateau slope as a risk factor for anterior cruciate ligament injury: a systematic review and meta-analysis. Am J Sports Med. 2012;40(7):1673–1681. doi: 10.1177/0363546512442307. [DOI] [PMC free article] [PubMed] [Google Scholar]