Abstract
Rotator cuff tears are common musculoskeletal injuries often requiring surgical intervention with high failure rates. Currently, pulsed electromagnetic fields (PEMFs) are used for treatment of long-bone fracture and lumbar and cervical spine fusion surgery. No studies have investigated PEMF in healing soft tissue. Therefore, we investigated the effect of PEMF on rotator cuff healing using a rat rotator cuff repair model. We hypothesized that PEMF exposure following rotator cuff repair would improve tendon mechanical properties, tissue morphology, and alter in vivo joint function. 70 adult male Sprague-Dawley rats were assigned to three groups: bilateral repair with PEMF (n=30), bilateral repair followed by cage activity (n=30), uninjured control with cage activity (n=10). Rats in the surgical groups were sacrificed at 4, 8, and 16 weeks. Control group was sacrificed at 8 weeks. Passive joint mechanics and gait analysis were assessed over time. Biomechanical analysis and µCT was performed on left shoulders; histological analysis on right shoulders. Results indicate no differences in passive joint mechanics and ambulation. At 4 weeks the PEMF group had decreased cross-sectional area and increased modulus and maximum stress. At 8 weeks the PEMF group had increased modulus and more rounded cells in the midsubstance. At 16 weeks the PEMF group had improved bone quality. Therefore, results indicate that PEMF improves early tendon healing and does not alter joint function in a rat rotator cuff repair model.
Statement of Clinical Significance
PEMF exposure following rotator cuff repair improves early tendon healing.
Keywords: PEMF, supraspinatus repair, animal model
INTRODUCTION
Rotator cuff tears are common musculoskeletal injuries which often require surgical intervention. Unfortunately, post-repair prognosis is poor, with surgical repairs that fail in up to 94% of cases1. Repaired tissue tends to be fibrotic, disorganized, and reattaches poorly to the bony insertion. To improve tendon-to-bone healing, many non-invasive therapeutic devices have been utilized post-operatively including therapeutic ultrasound and shock wave therapy2; 3.
In orthopaedics, the use of these non-invasive therapeutic devices has become increasingly popular during the post-operative recovery period in an effort to enhance tissue healing. These devices tend to be relatively inexpensive and easily obtainable. They are relatively easy to use, and are especially enticing as they can be brought into the patient’s home and do not require frequent visits to the clinic. Additionally, non-invasive therapeutic devices can be used for a variety of applications, including promoting tissue healing prior to surgical intervention or in lieu of surgical intervention all together.
Although there are numerous advantages to using non-invasive therapeutic devices, their efficacy has not yet been maximized. For example, a recent study comparing shock wave therapy and corticosteroid injection found corticosteroids significantly reduced pain in patients with plantar fasciitis4. Although corticosteroids are commonly used as anti-inflammatory pain reducers in orthopaedics, their safety is controversial, and they do not play a role in tissue healing. Therefore, the investigation of alternative non-invasive therapeutic technologies is desirable.
Currently, non-invasive devices emitting pulsed electromagnetic fields (PEMFs) have been approved by the FDA for treatment of long-bone fracture non-unions and as an adjunct to lumbar and cervical spine fusion surgery5–9. Several pre-clinical studies have been conducted examining the effect of PEMF in various biological models including a study examining the effect of PEMF in a rat fibular osteotomy model which found increased bone callus volume, stiffness, and modulus in the PEMF treated group10. Another study examining whether PEMF would improve vertebral bone mass in a rat osteoporosis model found that PEMF exposure improved trabecular bone volume in osteoporotic rat vertebrae11. Because PEMF therapy is commonly used in bone fracture healing, there may be a potential therapeutic application using PEMF therapy to enhance soft tissue healing as PEMF has also been shown to decrease inflammatory markers12. Currently, the effect of PEMF exposure on tendon-to-bone healing has not yet been evaluated.
Therefore, the objective of this study was to investigate the effect of PEMF exposure on rotator cuff healing using an established rat rotator cuff acute detachment and repair model13–18. In this study, we asked whether PEMF exposure for 3 hours a day, 7 days per week would improve tissue mechanical and morphologic properties, as well as joint function. We hypothesized that PEMF exposure following rotator cuff detachment and repair would 1) improve healing tendon mechanical properties, 2) improve tissue morphology including cell shape, cellularity, and collagen fiber organization, and 3) alter in vivo joint function including ambulation and passive joint mechanics.
METHODS
Study design
Seventy adult male Sprague-Dawley rats (400–450g) were used in this University of Pennsylvania Institutional Animal Care and Use Committee approved study. Animals were housed in a conventional facility in 12-hour light/dark cycles and were fed standard rat chow ad libitum. Animals were randomized into one of three treatment groups: 1) acute injury and repair followed by cage activity with PEMF, 2) acute injury and repair followed by cage activity alone, and 3) uninjured control with no surgical intervention. Animals in groups 1 and 2 were sacrificed at 4, 8, and 16 weeks (n=10 per time point) and animals in group 3 (control) were sacrificed at 8 weeks (n=10). Additionally, animals in the 16 week time point (8 week for control group) underwent longitudinal in vivo ambulatory assessment and passive shoulder joint mechanics assessments as described below. At the time of sacrifice, left shoulders (n=10 per group and time point) remained intact for subsequent mechanical testing while right shoulders (n=7 per group and time point) were immediately dissected and processed for histological analysis. The remaining right shoulders were left intact to serve as additional mechanical testing specimens if warranted. The animals were then frozen at −20°C and later thawed for dissection at the time of mechanical testing.
Detachment and Repair Surgery
Animals in groups 1 and 2 were subjected to identical bilateral supraspinatus detachment and repair as described14. For analgesia, buprenorphine (0.05 mg/kg) was administered subcutaneously 30 minutes prior to surgery, 6–8 hours post-operatively, and then every 12 hours for the next 48 hours. Briefly and as described previously14, with the arm held in external rotation and adduction, the deltoid muscle was split in the transverse plane to expose the supraspinatus tendon. The tendon was grasped using double-armed 5-0 polypropylene suture (Surgipro II, Covidien, Mansfield, MA) and was sharply transected from its bony insertion. For repair, a 5 mm diameter high speed bur (Multipro 395, Dremel, Mt. Prospect, IL) was used to remove remaining fibrocartilage from the footprint of the tendon insertion site. A 0.5 mm bone tunnel was drilled from anterior to posterior through the greater tuberosity of the humerus. The suture was passed through the bone tunnel and tied down, affixing the tendon to the greater tuberosity using a modified Mason-Allen technique. The wound was flushed with saline, and the deltoid and skin sutured closed.
PEMF Exposure
Animals in group 1 received daily 3 hour PEMF exposure using a commercial PEMF signal (Physio-Stim®, Orthofix, Inc., Lewisville, TX). Specifically, 24 hours after surgery, animals were placed on a custom built PEMF rack (Orthofix, Inc., Lewisville, TX) with sets of Helmholtz coils for each standard cage (a total of 16 cages). The PEMF rack was equipped with a timer, which automatically turned off the PEMF signal after 3 hours. For quality assurance, the PEMF coil sets were tested twice per week for dB/dt field using a transverse search coil placed at the center of each module between the two coils connected to an amplifier and signal integrator and interfaced with an oscilloscope (Orthofix, Inc., Lewisville, TX).
Quantitative Ambulatory Assessment
Ground reaction forces (medial/lateral, vertical, braking, propulsion) and temporal spatial parameters (step length and width) were measured using an instrumented walkway19. Measurements were taken at baseline and 7, 14, 21, 28, 42, 56, 84, and 112 days (excluding 84 and 112 days in the 8 week control group). Parameters were averaged across walks on a given day and measurements were normalized to body weight.
Passive Joint Mechanics
Passive range of motion and stiffness were measured using a custom device20. Briefly, animals were anesthetized and the forearm was placed in a rotating clamp at 90° elbow flexion and 90° glenohumeral forward flexion. The scapula was stabilized manually to isolate glenohumeral motion and the arm was rotated through the full range of internal and external rotation three times. Range of motion was calculated by the average of the three maximum values for internal and external rotation. Joint stiffness was measured in both the toe and linear regions for both internal and external rotation. Measurements were recorded at baseline and at 4, 8, and 16 weeks (excluding 16 weeks in the 8 week control group). All parameters measured were normalized to baseline values.
Tendon mechanical testing
Supraspinatus and humerus tendon-bone units were dissected from the shoulder and cleaned of excess soft tissue under a stereomicroscope. Stain lines were placed along the length of the tendon using Verhoeff’s stain for optical strain measurement. Cross sectional area was measured using a custom laser device as previously described21. The humerus was embedded in polymethylmethacrylate (PMMA) and held in a custom fixture. The tendon was affixed between fine grit sand paper using cyanoacrylate and then fixed in a custom grip. Samples were immersed in a 37°C PBS bath and subjected to a mechanical testing protocol consisting of a preload to 0.08 N, ten cycles of preconditioning (0.1–0.5 N at 1% strain/s), a stress relaxation to 5% strain (5%/s) followed by a 600s hold, and finally a ramp to failure at 0.3%/s. Stress was calculated as force divided by cross sectional area and 2D Lagrangian optical strain was determined from stain line displacements measured from images taken throughout the test using custom tracking software22; 23. Following mechanical testing, humeri were wrapped in PBS soaked gauze and frozen at −20°C for subsequent µCT analysis.
Histological Analysis
Histological analysis was performed to assess cell shape, cellularity, and collagen fiber organization at the injury site and midsubstance of the repaired supraspinatus tendon. At the time of sacrifice, supraspinatus-humerus units were immediately dissected, fixed in formalin, and processed using standard paraffin techniques. 7µm sections were stained with hematoxylin and eosin (H&E) to assess cell shape, cellularity, and collagen fiber orientation. Cell shape and cellularity were evaluated by three blinded graders using a semi-quantitative method. Images were graded between 1 and 3 (1= higher number of cells and more rounded shape. And 3= fewer cells and more spindle shaped). Standards were created within the set of images at each time point, and at least 2 images per region were assessed per specimen. Circular standard deviation of collagen fibers was determined by images taken with a polarizing microscope and analysis with custom software as described previously18; 23; 24.
µ-Computed Tomography
µCT scans were performed on the proximal humerus of a subset of humeri (n=6 per group and time point) (vivaCT 40, Scanco Medical AG, Bruttisellen Switzerland). The proximal growth plate was identified, and the region of interest was defined as the 5 mm proximal from the growth plate, which included the greater tuberosity. Scans were performed at 10.5 µm resolution. Parameters included bone mineral density, bone mineral content, bone volume fraction, total bone volume, connectivity density, trabecular number, trabecular spacing, and trabecular thickness.
Statistical Analysis
Sample sizes were determined using a priori power analyses. All statistical comparisons were made between the PEMF and non-PEMF groups at the same time points. Mechanical testing, µCT, and collagen fiber organization comparisons were made using student’s t-tests with significance set a p≤0.05. Semi-quantitative histological comparisons were made using Mann-Whitney U tests with significance set at p≤0.05. Ambulatory assessment comparisons were made using a 2-way ANOVA with repeated measures on time with follow-up t-tests between groups at each time point. Multiple imputations were calculated for a repeated measures analysis for missing data points (~10% per day). Significance was set at p≤0.05.
RESULTS
Quantitative Ambulatory Assessment
Results indicate no significant difference between the PEMF and non-PEMF groups (Fig. 1).
Figure 1.
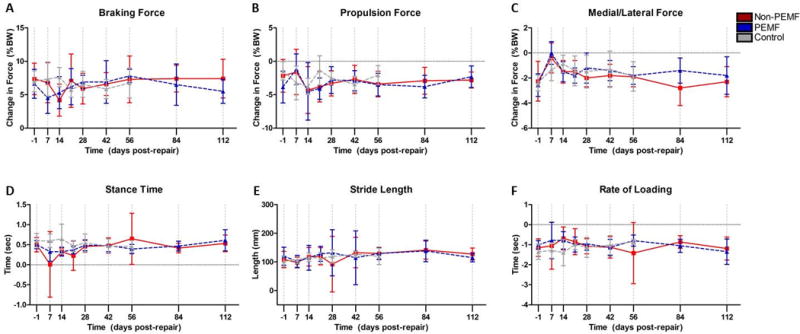
Ambulatory assessment. No significant differences were noted in any parameter at any time point. Data represented as mean ± SD.
Passive Joint Mechanics
Results indicate no significant difference between the PEMF and non-PEMF groups (Fig. 2).
Figure 2.
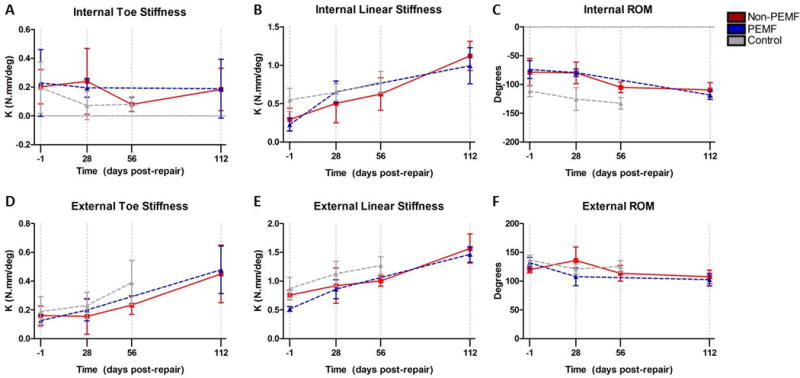
Shoulder joint stiffness and range of motion. No significant differences were noted in any parameter at any time point. Data represented as mean ± SD.
Tendon Mechanical Properties
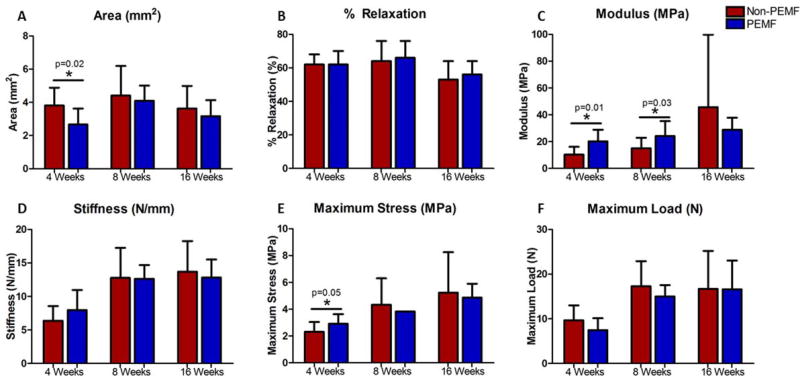
At 4 weeks, the PEMF group had a significantly smaller cross-sectional area compared to the non-PEMF group (Fig. 3A). No differences were detected in cross-sectional area at 8 and 16 weeks. There were no significant differences in percent relaxation at any time point (Fig. 3B). At 4 and 8 weeks, the PEMF group had significantly increased modulus (100% at 4 weeks, 60% at 8 weeks) compared to the non-PEMF group, with no differences detected at 16 weeks (Fig. 3C). No differences were detected in stiffness at any time point (Fig. 3D). At 4 weeks, the PEMF group had significantly increased maximum stress compared to the non-PEMF group, with no differences at 8 and 16 weeks (Fig. 3E). No differences were detected in maximum load at any time point (Fig. 3F).
Figure 3.
Tendon mechanical properties. A) Cross-sectional area was significantly decreased in the PEMF group compared to the non-PEMF group at 4 weeks. No differences were noted at 8 and 16 weeks. (Control area= 1.34 mm2) B) No differences in percent relaxation were noted at any time point. (Control % relaxation= 47%) C) Modulus was significantly increased by 100% at 4 weeks and 60% at 8 weeks in the PEMF group compared to the non-PEMF group. No differences were noted at 16 weeks. (Control modulus= 290.3 MPa) D) No differences were noted in stiffness at any time point. (Control stiffness= 31.15 N/mm) E) Maximum stress was significantly increased at 4 weeks in the PEMF group compared to the non-PEMF group. No differences were noted at 8 and 16 weeks. No error bar is present in the PEMF group at 8 weeks due to small sample size with physiological failure (n=2). F) No differences were noted in maximum load at any time point. Data represented as mean ± SD. Due to non-physiological failure location (typically at 8 and 16 weeks); maximum stress and maximum load data points which did not fail physiologically (i.e., at the injury site) were excluded, resulting in smaller n’s. Control maximum stress and maximum load were not calculated for a similar reason.
Histological Analysis
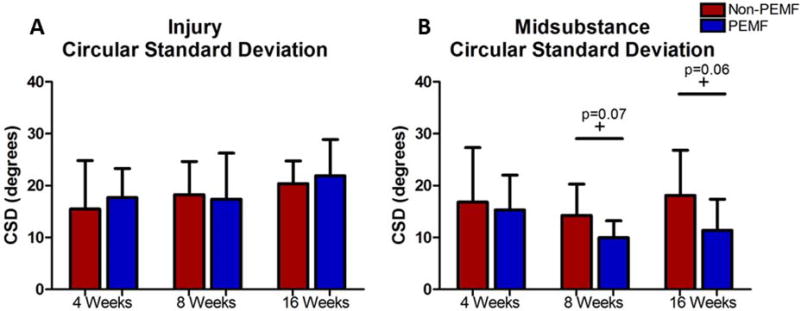
At the injury site, no differences were detected between the PEMF and non-PEMF group in both cell shape and cellularity at any time point (Table 1). Additionally, no differences were observed in collagen fiber organization at the injury site (Fig. 4A). In the midsubstance, at 8 weeks the PEMF group had significantly more rounded cells compared to the non-PEMF group (Table 1). For collagen fiber organization, the PEMF group had trends towards significantly decreased circular standard deviation at both 8 and 16 weeks (Fig. 4B). No other differences were observed in the midsubstance at any time point.
Table 1.
Histological analysis. (NC= no change, ↓ = decreased score in the PEMF group). No differences were noted at the injury site in cell shape and cellularity at any time point. At the midsubstance, cells were more rounded at 8 weeks in the PEMF group compared to the non-PEMF group. No other differences were noted in the midsubstance at any time point.
| Time Point | Injury | Midsubstance | ||
|---|---|---|---|---|
| Cell Shape | Cellularity | Cell Shape | Cellularity | |
| 4 Weeks | NC | NC | NC | NC |
| 8 Weeks | NC | NC | ↓ | NC |
| 16 Weeks | NC | NC | NC | NC |
Figure 4.
Collagen fiber alignment. No differences were noted at the injury sight in collagen fiber alignment. At the midsubstance, circular standard deviation trended toward lower (more organized) at 8 and 16 weeks in the PEMF group compared to the non-PEMF group.
µ-Computed Tomography
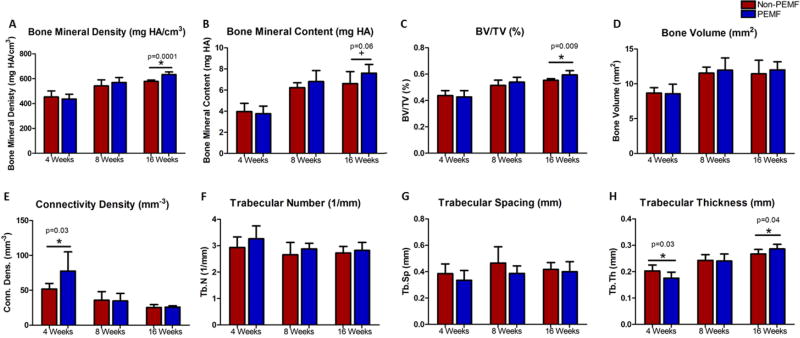
At 4 weeks, trabecular thickness was significantly decreased in the PEMF group compared to the non-PEMF group (Fig. 5H). Additionally, at 4 weeks, connectivity density was significantly increased in the PEMF group compared to the non-PEMF group (Fig. 5E). At 8 weeks, no differences were observed in any parameter. At 16 weeks, the PEMF group had significantly increased bone volume fraction, trabecular thickness, and bone mineral density, and a trend toward increased bone mineral content compared to the non-PEMF group (Fig. 5).
Figure 5.
µCT analysis. A) No differences were noted in bone mineral density at 4 and 8 weeks. At 16 weeks bone mineral density was significantly increased in the PEMF group compared to the non-PEMF group. B) No differences were noted in bone mineral content at 4 and 8 weeks. At 16 weeks bone mineral content trended toward significantly increased in the PEMF group compared to the non-PEMF group. C) No differences were noted in bone volume fraction at 4 and 8 weeks. At 16 weeks bone volume fraction was significantly increased in the PEMF group compared to the non-PEMF group. D) No differences were noted in bone volume at any time point. E) At 4 weeks connectivity density was significantly increased in the PEMF group compared to the non-PEMF group. No differences were noted at 8 and 16 weeks. F) No differences were noted in trabecular number at any time point. G) No differences were noted in trabecular spacing at any time point. H) At 4 weeks trabecular thickness was significantly decreased in the PEMF group compared to the non-PEMF group. No differences were noted at 8 weeks. At 16 weeks trabecular thickness was significantly increased in the PEMF group compared to the non-PEMF group. Data represented as mean ± SD.
DISCUSSION
This study examined the effect of PEMF exposure on tendon-to-bone healing in a rat rotator cuff model. Overall, results suggest that PEMF exposure has a positive effect on early rat rotator cuff healing. Specifically, mechanical properties were drastically improved in the PEMF group at both 4 and 8 weeks. Of note, modulus was increased by 100% at 4 weeks and 60% at 8 weeks, which is a large improvement in tissue properties. Histological analysis showed a more rounded cell shape in the PEMF group at 8 weeks in the midsubstance. This slight but significant finding might suggest inferior tissue, although this difference did not result in inferior mechanical properties and was not noted histologically at 16 weeks. The more rounded cell shape in the PEMF group at 8 weeks might also suggest the cells are metabolically active due to the PEMF exposure, which might support improved mechanical properties at that time point. Additionally, collagen fiber organization in the midsubstance at 8 and 16 weeks showed the PEMF group had trends toward significantly decreased circular standard deviation, suggesting the tissue might be more organized in the PEMF group, which may be related to the improved mechanical properties due to previous findings indicating a strong structure-function relationship in tendon, where fiber alignment was found to correlate significantly with mechanical parameters25. Lastly, in vivo ambulatory assessment and shoulder joint function showed minimal differences between groups. Particularly, when comparing to the control group (although no statistical comparisons were made), it is apparent that the two surgical groups were similar to the control (Figs. 1 and 2). This finding implies that the PEMF and non-PEMF groups recover function concurrently and that PEMF does not negatively affect ambulation and shoulder function. Overall, results suggest that PEMF improves early tendon-to-bone healing specifically through an improvement of tendon mechanical properties.
Results from this study may support previously reported findings using Physio-Stim® PEMF therapy to treat healing bone fractures10; 11. µCT analysis shows improved bone properties in the PEMF group (Fig. 5) after transosseous supraspinatus repair, which further supports findings of PEMF exposure leading to improved bone growth and healing. Therefore, the current study supports the use of PEMF for a rotator cuff tendon-to-bone healing in both tendon and bone.
This study is not without limitations. Although the rat rotator cuff repair model is well established and published in over 140 full-length papers to date, animal models do not exactly mimic the clinical scenario. Additionally, this study utilized an acute supraspinatus detachment and repair whereas clinically rotator cuff tears are typically chronic conditions resultant from repeated overhead motions and overuse. Lastly, animals were exposed to a systemic PEMF signal. Current FDA approved devices deliver PEMF locally to the healing tissue. Although systemic PEMF exposure did not seem to negatively affect the animals, future studies might examine the effects of localized PEMF exposure.
As this is the first known study to examine and support the effect of PEMF exposure on tendon-to-bone healing, many future avenues of study can be investigated. First, the PEMF signal intensity, frequency, and duration are variable, and the PEMF signal might be optimized to even further enhance tendon-to-bone healing. Second, this study only examined supraspinatus tendon-to-bone healing. Investigating the effects on other commonly injured tissues including Achilles tendon, patellar tendon, anterior cruciate ligament, joint replacement, and many other orthopaedic applications may be advantageous. Additionally, PEMF therapy might also be evaluated in tendons and ligaments with diagnosed tendinitis, tendinopathy, or partial tears to avoid or postpone surgical intervention. This application could help to reduce health care costs and potential morbidities associated with anesthesia and surgical procedures.
In conclusion, PEMF exposure improves early tendon-to-bone healing in an acute rat supraspinatus detachment and repair model supporting the use in a clinical scenario of rotator cuff healing.
Acknowledgments
This study was supported by Orthofix, Inc.
Footnotes
Author Contributions Statement: All authors have read and approved final version of manuscript.
Contributions are as follows: JJT- research design, acquisition of data, data analysis and interpretation, drafting and revision of paper, approval of submitted and final versions; JMC- acquisition of data, data analysis and interpretation, approval of submitted and final versions; TRM- acquisition of data, approval of submitted and final versions; CAN- acquisition of data, data analysis, approval of submitted and final versions; EIW- research design, revision of paper, approval of submitted and final versions; NZ- research design, revision of paper, approval of submitted and final versions; JTR- research design, revision of paper, approval of submitted and final versions; LJS- research design, data interpretation, revision of paper, approval of submitted and final versions.
References
- 1.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lovric V, Ledger M, Goldberg J, et al. The effects of low-intensity pulsed ultrasound on tendon-bone healing in a transosseous-equivalent sheep rotator cuff model. Knee Surg Sports Traumatol Arthrosc. 2013;21:466–475. doi: 10.1007/s00167-012-1972-z. [DOI] [PubMed] [Google Scholar]
- 3.Springer J, Badgett RG. Optimized extracorporeal shock-wave therapy improved pain and functioning in chronic plantar fasciitis. Ann Intern Med. 2015;163:JC8. doi: 10.7326/ACPJC-2015-163-10-008. [DOI] [PubMed] [Google Scholar]
- 4.Mardani-Kivi M, Karimi Mobarakeh M, Hassanzadeh Z, et al. Treatment Outcomes of Corticosteroid Injection and Extracorporeal Shock Wave Therapy as Two Primary Therapeutic Methods for Acute Plantar Fasciitis: A Prospective Randomized Clinical Trial. J Foot Ankle Surg. 2015;54:1047–1052. doi: 10.1053/j.jfas.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8:436–442. doi: 10.1016/j.spinee.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Garland DE, Moses B, Salyer W. Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 1991;22:295–302. [PubMed] [Google Scholar]
- 7.Goodwin CB, Brighton CT, Guyer RD, et al. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine (Phila Pa 1976) 1999;24:1349–1356. doi: 10.1097/00007632-199907010-00013. discussion 1357. [DOI] [PubMed] [Google Scholar]
- 8.Linovitz RJ, Pathria M, Bernhardt M, et al. Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study. Spine (Phila Pa 1976) 2002;27:1383–1389. doi: 10.1097/00007632-200207010-00002. discussion 1389. [DOI] [PubMed] [Google Scholar]
- 9.Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine (Phila Pa 1976) 1990;15:708–712. doi: 10.1097/00007632-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Midura RJ, Ibiwoye MO, Powell KA, et al. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res. 2005;23:1035–1046. doi: 10.1016/j.orthres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Midura RJ. The Effects of Bone Growth Stimulator Treatments on Vertebral Bone Mass in an Osteoporosis Model. North American Spine Society 28th Annual Meeting; New Orleans, Louisiana. 2013. [Google Scholar]
- 12.Willardson RCD, El Naga A, Degmetich S, Sing D, Lotz J. Pulsed electromagnetic fields have an acute anti-inflammatory effect on intervertebral disc cells. Orthopaedic Research Society; San Antonio, Texas, USA: 2013. [Google Scholar]
- 13.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 14.Beason DP, Connizzo BK, Dourte LM, et al. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J Shoulder Elbow Surg. 2012;21:245–250. doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Beason DP, Tucker JJ, Lee CS, et al. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elbow Surg. 2014;23:867–872. doi: 10.1016/j.jse.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caro AC, Tucker JJ, Yannascoli SM, et al. Efficacy of various analgesics on shoulder function and rotator cuff tendon-to-bone healing in a rat (Rattus norvegicus) model. J Am Assoc Lab Anim Sci. 2014;53:185–192. [PMC free article] [PubMed] [Google Scholar]
- 17.Connizzo BK, Yannascoli SM, Tucker JJ, et al. The detrimental effects of systemic Ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014;472:2433–2439. doi: 10.1007/s11999-013-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimbel JA, Van Kleunen JP, Williams GR, et al. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–404. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 19.Sarver JJ, Dishowitz MI, Kim SY, et al. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarver JJ, Peltz CD, Dourte L, et al. After rotator cuff repair, stiffness--but not the loss in range of motion--increased transiently for immobilized shoulders in a rat model. J Shoulder Elbow Surg. 2008;17:108S–113S. doi: 10.1016/j.jse.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favata M. Scarless healing in the fetus: implications and strategies for postnatal tendon repair. University of Pennsylvania; Philadelphia: 2006. [Google Scholar]
- 22.Bey MJ, Song HK, Wehrli FW, et al. A noncontact, nondestructive method for quantifying intratissue deformations and strains. J Biomech Eng. 2002;124:253–258. doi: 10.1115/1.1449917. [DOI] [PubMed] [Google Scholar]
- 23.Thomopoulos S, Williams GR, Gimbel JA, et al. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 24.Gimbel JA, Van Kleunen JP, Mehta S, et al. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Lake SP, Miller KS, Elliott DM, et al. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]