URETHRAL CATHETERIZATION
Acute urinary retention (AUR) is a common urologic condition that often presents to an emergency department (ED) as a sudden inability to pass urine accompanying with lower abdominal pain.1 It increases in incidence with age and most often occurs in men over the age of 60 years.1–3 Generally, the causes of AUR can be classified into 3 categories. The first category relates to any event that increases the resistance to the urine flow, including, for example, benign prostatic hyperplasia (BPH), urethral stricture, or detrusor sphincter dysfunction. Second, AUR may result from an interruption of either the sensory innervation of the bladder wall or the motor supply of the detrusor muscle. It is most commonly seen in spinal cord injuries, progressive neurologic diseases, diabetic neuropathy, and cerebrovascular accidents.1,2,4 The third mechanism relates to any situation that either permits or causes the bladder to over-distend.1 Overdistension of the bladder is most commonly encountered by the pharmacologic use of opiates, anticholinergic administration, and the generalized increase in α-adrenergic activity that exists after surgery.1
The initial management of AUR of urine is prompt relief of retention and pain by catheterization of the bladder.1,5–7 There are no uniform guidelines for bladder decompression but most urologists prefer urethral catheterization for the initial management of AUR.8
Box 1 summarizes the most common indications for urethral catheterization in the ED.
Box 1. Indications for urethral catheterization.
AUR
Hydronephrosis
Continuous bladder irrigation
Neurogenic bladder
Bedridden patients
As in any other procedure performed in the ED, urethral catheterization has several contraindications; among them are exposure to a recent urologic surgery, pelvic or abdominal trauma, and blood in urethral meatus or perineal hematoma. Patients with mentioned conditions should not have urethral catheterization as initial procedure for bladder decompression.8
The equipment is available as a commercial nonreusable kit. Box 2 lists the content of the catheterization tray.
Box 2. Equipment in catheterization tray.
Foley catheter: straight tip, Coudé tip, 3-way irrigation catheter
Sterile gloves
14F Foley catheter
10-mL normal saline prefilled syringe
Collection bag
Cotton balls
Povidone-iodine solution
Lubricant jelly
Water
Sterile drape
Urethral catheterization must be done using sterile technique, always taking into consideration that male and female patients have special anatomic landmarks. After careful exposure, use an antiseptic solution soaked into cotton balls to cleanse the exposed meatus and surrounding tissues. Cleaning should be done in circular motion starting on the urethral meatus and proceeding outward.2,9
In uncircumcised men, total control of the penile foreskin is paramount to ensuring success. Retract the available foreskin to its fullest extent proximal to the glans penis.2,9 The appropriately sized catheter previously lubricated with jelly should be gently passed into the urethra and upward into the bladder. An appropriate initial Foley size is a 14F to 18F Foley catheter. Inject male or female urethra with 5 mL to 7 mL of 2% viscous lidocaine or other similar anesthetic lubricant to help urethral distention and anesthesia. After passing the catheter, slowly inflate the balloon with 10 mL of tap water. Obvious resistance or patient discomfort on balloon inflation should signal potential erroneous urethral positioning and mandates re-evaluation.4 After successful catheter passage and Foley balloon inflation, slowly withdraw the catheter until the approximation of the balloon with the bladder neck precludes further withdrawal.1,2,9 After catheterization, reduce the penile foreskin to its normal anatomic position to prevent the development of iatrogenic paraphimosis. Then connect the catheter to either a sterile leg bag or a closed-system bedside drainage bag. In cases when patient disposition is discharged with an indwelling catheter, it can initially be connected to a leg bag, which is then comfortably fastened to the lower thigh and upper calf. Patients and families must be instructed regarding proper care of the catheter and drainage device.4,5
If obstruction does not allow passage of a flexible catheter, possible causes of obstruction should be taken in to consideration to fix the problem. Patient urethral meatus may be constricted due to trauma or prior transurethral procedure. The scar may prevent admission of a normal-sized catheter. In this case, the obstruction may be bypassed by downsizing the catheter to a 10F to 12F Foley.2,4,10 In the absence of prior instrumentation, the more common cause of obstruction is an enlarged prostate. In this case, a larger catheter (20 or 22 gauge) with a firm Coudé tip may be needed and may require urologic consultation.2 The procedure to place the Coudé uses the same technique as the Foley except that the catheter used is a Coudé. The Coudé catheter is placed into the meatus of the penis with the curved tip pointing up, cephalad, and is advanced with gentle but continuous pressure past the resistance point, typically in the region of an enlarged prostate (Fig. 1).2,6
Fig. 1.
Coudé foley.
A common complication of urinary catheterization is the development of a urinary tract infection (UTI). Patients with catheters for more than 10 days are at higher risk for infection.2 Infection from the urethra and the bladder might spread to cause epididymitis, pyelonephritis, and bacteremia. Other rare complications of long-term indwelling urethral catheterization include bladder stones, recurring bladder spasm, periurethral abscesses, urethral stricture, bladder perforation, and urethral erosion.2,11 Complications that occur during the act of catheterization include false passages in any area of the urethra, urethral catheter retention, and paraphimosis.2,11 Box 3 list the complications associated with urethral catheterization.
Box 3. Complications with urethral catheterization.
Nosocomial UTI
Epididymitis
Pyelonephritis
Bacteremia
Bladder spasm
Periurethral abscess
Bladder perforation
Urethral erosion
Urethral stricture
Urethral catheter retention
Paraphimosis
SUPRAPUBIC CATHETERIZATION
Suprapubic catheterization is indicated in patients who require a urethral catheter, but it cannot be passed or is contraindicated.2,5 Difficulties with urethral instrumentation require a suprapubic catheterization to prevent further urethral injury.6,12 Box 4 summarizes the indications for suprapubic catheterization.
Box 4. Indications for suprapubic catheterization.
Urethral catheterization is contraindicated
Suspected urethral trauma
BPH
Bladder neck mass
Suprapubic catheter placement is contraindicated in an empty bladder.2,5 There must be sufficient urine to allow the needle to penetrate the bladder dome without exiting through the base and also displace the bowel away from the surface of the bladder. Ultrasound should be used to define bladder anatomy.2,5 Blind suprapubic catheterization should be avoided in patients with previous abdominal surgery or previous pelvic irradiation that may have developed adhesions and scaring. Ultrasound guidance is indicated when adhesions are a possibility from prior abdominal surgery.4,5 Major bleeding disorders are a relative contraindication and should be considered on a case-by-case basis (Box 5).2,6
Box 5. Equipment for suprapubic catheterization.
Shaver (to remove hair from suprapubic area)
Lidocaine 1% (5 mL)
Scalpel with no. 11 blade
Skin tape of nylon 3.0 suture (to secure catheter)
Trocar-type cystostomy tube (Cystocath)
Suprapubic catheterization is performed under local anesthesia and using sterile technique. Patients are placed in supine position.2,5 The bladder should be palpated and identified the insertion site, which is midline and 4 cm to 5 cm above the pubic bone.
A preliminary bladder ultrasound examination should be performed by an emergency physician to confirm and visualize bladder limits, lower abdominal contents, and midportion of the bladder where catheterization is to occur (Fig. 2).2,5 Local anesthesia should be used at the insertion site, using 5 mL of 1% lidocaine by raising a wheal and then injecting the local tissue toward the bladder with the 22-gauge spinal needle at an angle aiming 20° to 30° caudal from midline (toward patient legs).1,2 While the needle is advanced and lidocaine is injected, intermittent stopping and withdrawing to assess for urine return should be performed. All suprapubic catheter units are placed with a similar approach until this stage. The following steps describe the placement of the Cook Medical Peel-Away Sheath.9,11 As soon as the bladder has been located, remove the syringe from the needle and advance a guide wire through the needle into the bladder. Withdraw the needle, leaving only the guide wire traversing the anterior abdominal wall and positioned inside the bladder. Small vertical skin 4-mm incision should be made with no. 11 or no. 15 blade to facilitate the Peel-Away Sheath and indwelling fascial dilator insertion through abdominal wall in to the bladder.1,2,5 The physician’s nondominant hand should be placed on the lower abdominal wall, and the unit should be stabilized between the thumb and index fingers. The dominant hand should be used to advance the unit. An ultrasound probe should be placed lateral to the incision, at all times, with a diagonal valgus orientation within a sterile glove to visualize and direct the procedure.1,5 Remove the guide wire and fascial dilator, leaving only the Peel-Away Sheath inside the bladder. Then pass a Foley balloon catheter through the indwelling intravesical sheath.2,4,5 Aspirate urine to confirm proper placement. Inflate the Foley balloon with a minimum of 10-mL solution. Withdraw the Peel-Away Sheath leaving only the indwelling suprapubic Foley catheter. Withdraw the catheter slowly until the inflated balloon approximates the cystostomy site. Connect the catheter to a drainage bag, and then dress the wound with 4 × 4 gauze pads to complete the procedure.4 Catheter should be taped or stitched to the skin. All patients who undergo suprapubic tube placement should be referred to a urologist for correction of the underlying disease as well as routine cystostomy tube care.2,5
Fig. 2.
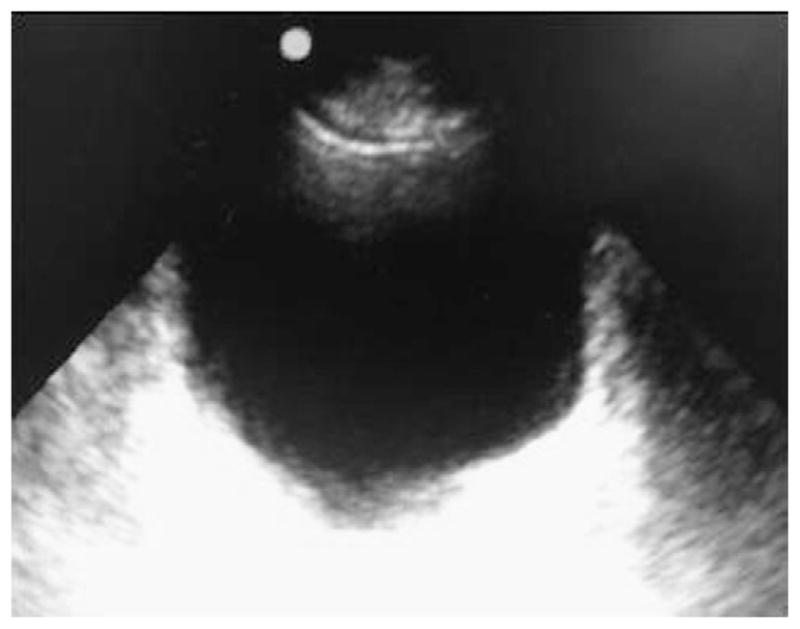
Sonographic view needed for suprapubic catheterization.
Suprapubic catheters carry an increased risk for complications associated with its placement. Serious complications involve perforation of the intraperitoneal contents (bowel perforation, ureteral injury, or large vessel injury) and drains of bladder contents into the peritoneal cavity. Through-and-through bladder penetration with associated rectal, vaginal, or uterine injury has been reported.2,4,5 Infection may occur at the suprapubic insertion site or anywhere along the course of the catheter. Hematuria is rarely more than a transient problem. Other complications include deeper tissue infections that may result from extravasated infected urine or from a superficial infection spreading along the tube. Also common are catheter obstruction by kinking or from blood and inadvertent tube removal.2,5,10
Frequently, patients can be managed as outpatients after bladder decompression. Surgery remains the ultimate management of AUR and is considered the gold standard.1,6,9,12 General recommendation is to wait 30 days or more after an episode of AUR.7,10 Emergency surgery for relief of prostatic obstruction is infrequently directed and carries an increased danger over elective surgery.7,13 Hospital admission is indicated for patients with urosepsis, obstruction related to malignancy, spinal cord compression, or failure to recent urologic procedure.7,13
TESTICULAR DETORSION
Acute scrotal pain with or without swelling and erythema in a male child, adolescent, or adult should always be treated as an emergent condition.14,15 Most of the conditions that cause the signs and symptoms (listed previously) are not emergent but of vital importance are the prompt diagnosis and treatment of torsion of the spermatic cord to avoid permanent ischemic damage to the testicle.14–17 Torsion of the testicle results from twisting of the spermatic cord in its own axis, which compromises testicular blood supply.15,18,19 There is a 4-hour to 8-hour window before significant ischemic damage occurs.15,18,19 The classic clinical presentation of testicular torsion is the sudden onset of severe, unilateral pain, often with nausea and vomiting. It often occurs several hours after vigorous physical activity or minor testicular trauma.16,19,20
A typical finding on physical examination is an asymmetrically high-riding testis on the affected side, with the long axis of the testis oriented transversely, or the epididymis may be located anteriorly.14,15 Cremasteric reflex should be assessed by lightly pinching the skin of the superior thigh while observing the ipsilateral testis.14,15 A normal response, contraction with elevation of the testis, is usually not present in patients with testicular torsion. Other less-specific findings of testicular torsion include the Prehn sign, generalized testicular tenderness, testicular swelling, and erythema. The Prehn sign14,15 is a description of how elevation of affected scrotum relieves pain in patients with epidimytis and aggravates, or has no effect on, patients with testicular torsion. The Prehn sign is not a reliable physical finding to distinguish between torsion and epididymitis.14,15
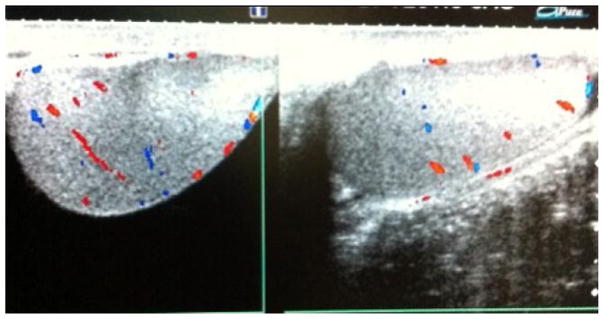
The diagnosis of testicular torsion should be based on clinical suspicion, but there are adjunctive diagnostic studies that may aid in determining the etiology of testicular pain, such as color-flow Doppler ultrasound.17,21 Management for a suspected testicular torsion is immediate surgical repair (Figs. 3 and 4).17
Fig. 3.
Testicular ultrasound with bilateral color flow. (A) Right testicle. (B) Left testicle.
Fig. 4.
Testicular ultrasound with right testicular torsion and normal left testicle.
All patients with suspected testicular torsion should be immediately consulted to a urologist service. Manual detorsion should be attempted while awaiting patient transport to the operating room.17,22
Manual detorsion objective is to regenerate blood flow to the affected testis but it should never delay operative intervention. Manual detorsion is not recommended if duration of torsion is more than 6 hours.17,22
Patients should be placed in supine position with the hips and knees flexed and the thighs apart (lithotomy position). This position allows the physician easy access and prevents patients from withdrawing during the procedure. Analgesia or sedation use during manual detorsion is controversial.
Most torsions are in medial direction; therefore, clinicians should detorse testes from medial to the lateral side, rotating outward toward the thigh (open book rotation).17 Patients must be as comfortable as possible in lithotomy position.17 Some experts recommend that physicians should be positioned in front of the patient while others endorse that clinicians should be standing at the side of the bed (right side if the clinician is right handed or vice versa).17 Manual detorsion starts as a book would be opened. Detorsion of the patient’s right testis is done in a counterclockwise fashion while the patient’s left testis is detorsed in a clockwise fashion.17 If pain is partially relieved, continue with another rotation; the degree of torsion my range from 180° to 1080°.17 If detorsion is too difficult or makes the pain worse, the emergency physician must attempt to detorse the testis in the opposite direction (Fig. 5).17
Fig. 5.
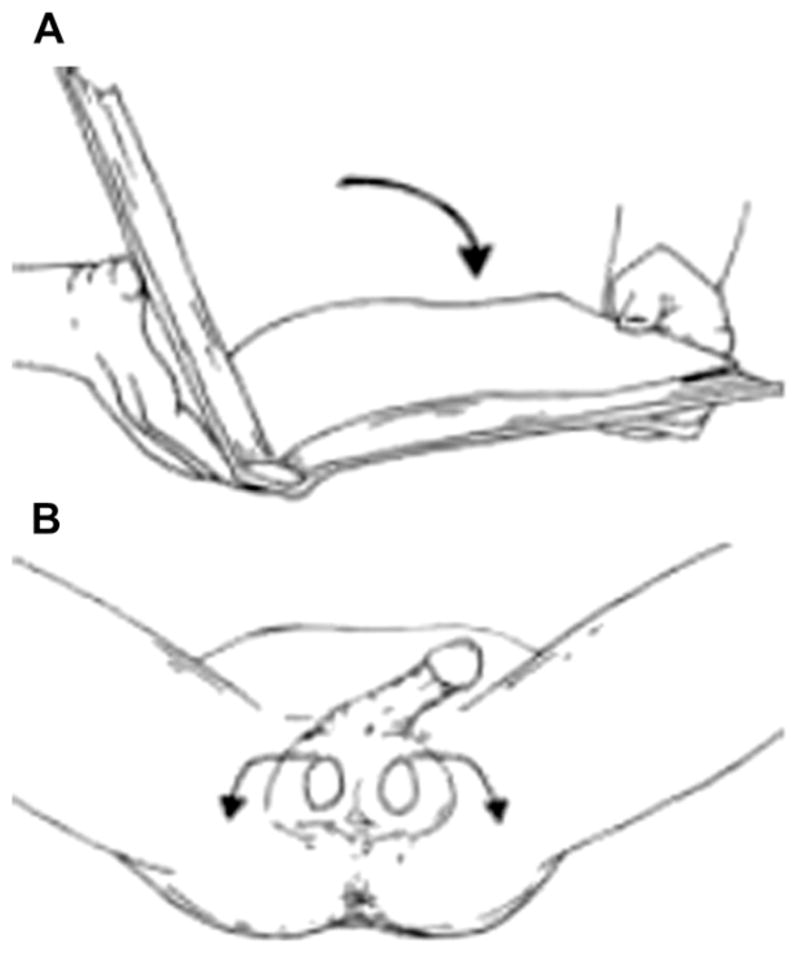
(A) Open Book rotation for manual testicular detorsion (B) Rotate affected testicle away from midline. This is a counterclockwise rotation for right testicle and a clockwise rotation for the left testicle.
Manual detorsion has no complications17,23 but the procedure may be difficult due to acute pain during manipulation.
Successful detorsion is suggested by relief of pain, resolution of the transverse lie of the testis to a longitudinal orientation, lower position of the testis in the scrotum, and return of normal arterial pulsations detected with a Doppler stethoscope22,23 Surgical exploration is necessary even after clinically successful manual detorsion. Manual detorsion is not a substitute for definitive surgical exploration.22,23
DORSAL PENILE NERVE BLOCK
The penis receives its innervation by the pudendal nerve (S2–S4).24,25 This nerve divides into the right and left dorsal nerves of the penis; it travels under the pubis symphysis and enters just below the Buck fascia to provide sensory innervation to the penis.24,25
Many causes of penile pain may be managed with a dorsal nerve block. The procedure is indicated for circumcision, phimosis reduction, paraphimosis reduction, penile laceration repairs, and release of entrapped penile skin from zippers.24–26 Small children may require the use of conscious sedation.24
The procedure is contraindicated if testicular torsion is suspected or there is evidence of skin infection at the site of injection (Box 6).27
Box 6. Equipment for dorsal penile nerve block.
Povidone-iodine solution
4 × 4 Sterile gauze
Local anesthetic without epinephrine
5-mL syringe
16-Gauge and 27-gauge needles
Sterile drapes
Patients should be placed in the supine position with genitals exposed. Gross debris should be cleansed and copious amounts of povidone-iodine solution applied to the penis and scrotum using a soaked 4 × 4 gauze. The glans and shaft of the penis should be cleaned at least twice using a circular motion. The sterile field is created by placing sterile drapes between the scrotum and shaft, above the shaft, and on both sides of the shaft.
The following steps are involved in the technique and much has remained unchanged since the original description of the procedure by Kirya and colleagues27 in 1978 (Fig. 6). The dorsal penile nerves should be blocked as proximately to the base of the penis as possible. A 27-gauge needle is used to create a small wheal at the 2 o’clock and 10 o’clock positions of the base of the penis.28 Then, the needle is slowly inserted through the center of each wheal, advancing the needle approximately 0.5 cm or until loss of resistance is felt, at which point the needle is within Buck fascia. At this point, the syringe is aspirated to ensure that needle is not within a blood vessel. Anesthetic (2 mL) is injected on each side. When dealing with children and neonates (<10 kg), 0.2 mL to 0.4 mL of 1% lidocaine is injected on each side using a 30-gauge needle. No more than 4.5 mg/kg of lidocaine should be injected.29
Fig. 6.
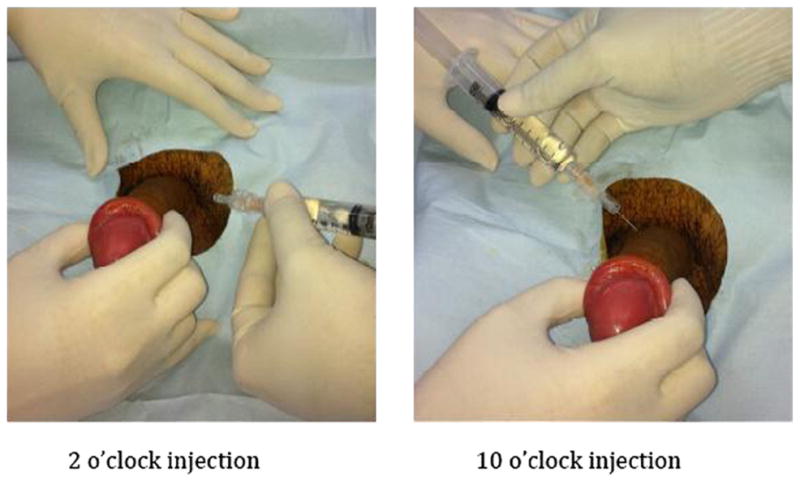
Dorsal penile nerve block.
Complications associated with dorsal penile block include bleeding and hematoma formation, which can be controlled with local pressure.30,31 Inadequate anesthesia should prompt an attempt with a different nerve block.
CAVERNOSAL ASPIRATION
Priapism is manifested by a persisting erection caused by disturbances in the mechanism controlling penile detumescence and the maintenance of penile flaccidity.32 It is usually painful, unrelated to sexual stimulation, and not relieved by ejaculation. It is associated with high incidence of impotence regardless of treatment.32 Most studies identify priapism as an erection lasting at least 4 hours.32
Anatomically, it involves the corpora cavernosa only, sparing the corpus spongiosum and the glans.32–35 Priapism results from a derangement of the penile hemodynamics, affecting the arterial component or the veno-occlusive mechanism.33 This mechanism explains the 2 types of priapism—high-flow and low-flow types. High-flow priapism commonly follows an episode of trauma to the perineum or the genitalia, resulting in increased flow through the arteries.32,34,35 High-flow priapism does not represent an emergency situation; it resolves spontaneously in up to 62% of untreated cases.32,34,35 In low-flow or ischemic priapism, there is an abnormality in the veno-occlusive mechanism, resulting in venous stasis and accumulation of deoxygenated blood within the cavernous tissue.34,35 Oxygenation of the erectile tissues is compromised and risk of future erectile dysfunction secondary to ischemia and subsequent fibrosis is high if not treated expeditiously.35
The diagnosis of priapism is generally clinical.36 Visual inspection reveals an erect penis, and the erection has been present for more than 2 to 4 hours in the absence of sexual excitation.36 Cavernosa blood gas analysis and Doppler ultrasonography may be performed to differentiate between ischemic and nonischemic types of priapism.32,36,37 The Doppler can also detect cavernous arterial fistula, pseudoaneurysm, or other anatomic abnormalities.32,36–38
Prompt intervention is warranted in all cases of low-flow priapism to prevent long-term erectile dysfunction.32 Emergency clinicians should identify reversible causes of priapism and in conjunction with urologist consultation initiate specific corrective therapy.32 Every patient should receive narcotic analgesia regardless of the cause.32 Some studies recommend empiric terbutaline, 0.25 mg to 0.5 mg subcutaneously for every patient presenting to the ED with low-flow priapism.39 Recent studies favor starting with corpora cavernosa aspiration, with a nonheparinized syringe, as first-line treatment, with a success rate of approximately 30%.39 Aspiration can be combined with flushing the cavernosa using normal saline to clear the sludged blood.40 If this fails, instillation of a vasoconstrictive agent, such as phenylephrine, should be used until complete detumescence is achieved.41,42 If urologic consultation is unavailable or delayed, the emergency physician should initiate therapeutic corporal aspiration.39
Despite widespread administration of pharmacologic substances in the treatment of priapism, there is still a call for surgical intervention if all attempts of conservative treatment fail.32,41 Nonischemic priapism is not an urgent condition and may resolve spontaneously.41 If priapism does not respond to pharmaceutical treatment, it can be treated with penile injection and aspiration.32,36 This procedure entails drainage of blood from the erect penis and instillation of vasoactive medication. Alternatively, irrigation with a dilute vasoactive solution is also effective.
Contraindications for cavernosal aspiration are high-flow priapism, overlying cellulitis, and uncontrolled bleeding disorder.32,36,43,44 Box 7 lists the equipment needed for priapism and cavernosal aspiration and irrigation.
Box 7. Equipment for priapism and cavernosal aspiration and irrigation.
Cardiac monitor with blood pressure monitoring capability
Sterile gloves, sterile basin (for collection of drained blood), and sterile drapes.
Antiseptic solution
Gauze squares
Local anesthetic: 1% lidocaine without epinephrine (penile block)
Syringes: one 1 mL (local anesthetic), two 20 mL or 30 mL
Needles: 19 gauge or 21 gauge (for aspiration, butterfly, or straight for aspiration), 27 gauge (penile block)
-
Irrigation fluid:
Phenylephrine, 10 mg/500 mL of saline
Norepinephrine, 1 mg/500 mL of saline
Epinephrine, 0.5 mg/500 mL of saline
Patients should be placed in the supine position.39 Systemic analgesia should be applied before beginning the procedure. In certain patients, such as children, conscious sedation should be added. An injection of 1% plain lidocaine at the base of the penis for a dorsal penile nerve block or placement of a circumferential penile block is highly recommended.39,41 Patients should be connected to a cardiac monitor with frequent blood pressure measurements. After induction of conscious sedation with intravenous midazolam or other sedatives, the penis, scrotum, and lower abdomen should be cleaned and prepared with the antiseptic solution and allowed to dry. Apply sterile drapes to area.32,39,41
Using left hand thumb and index finger, grasp penis shaft.39 Insert a 19-gauge butterfly needle into the lateral midshaft of the penis at the 3-o’clock or 9-o’clock position, directing the needle straight toward the center of the corpora. Any side of penis could be punctured because there is communication of blood flow (bilateral anastomoses) between both sides.39 The end of the tubing could be placed in a sterile basin, because blood is likely to spontaneously drain from the corpora. Nevertheless, most of the time butterfly or straight needles are attached to a 20-mL or 30-mL syringes for active aspiration.39,41 Too much suction should not be applied because this often stops the aspiration; it should not exceed 20 mL to 30 mL of corporal blood. A common mistake is to use too much suction with a large syringe.39 Aspiration should continue until aspiration of dark blood ceases and bright red arterial blood returns or complete detumescence is obtained and persists.39 Once blood has been drained and the penis has softened, inject 1 mL to 2 mL of the 10-μg/mL phenylephrine solution into the midshaft of each corpora using the same needle that was used for blood aspiration. The injection may be repeated to a maximal dose of 1 mg (1000 μg).32,39
In cases of prolonged priapism or recurrent cases, active irrigation of the old blood might be required. A 21-gauge butterfly needle should be inserted into the proximal penis on the same side of the penis as the aspiration needle.41 Different irrigating solutions have been suggested, but none has proved superiority. Clinicians suggest 20 mL to 30 mL of a phenylephrine/normal saline solution (10 mg of phenylephrine in 500 mL of normal saline) as the exchange for 20 mL to 30 mL of aspirated corporal blood.39 A norepinephrine solution also may be used. Some clinicians add heparin to the solution but value has been unproved.39 Failure to maintain detumescence requires immediate urology evaluation. All other patients require discontinuation of the causal agent and follow-up with a urologist 24 hours after the procedure.43 Vasoactive substance can be absorbed systemically and produce potential side effects, such as arrhythmias, hypertension, and headache.44 The use of vasoactive agents is contraindicated in patients with severe hypertension, dysrhythmias, and monoamine oxidase inhibitor use.44 Patients need to be connected to cardiac monitor and blood pressure monitor at all times, if patients have comorbid conditions. Impotence is a complication after priapism, regardless of the cause or the promptness of therapeutic intervention.43,44 Patients must be advised verbally and in writing of this possible complication.2,6,9 Hematoma and infection can occur, even with correctly performed aspiration (Fig. 7).44
Fig. 7.
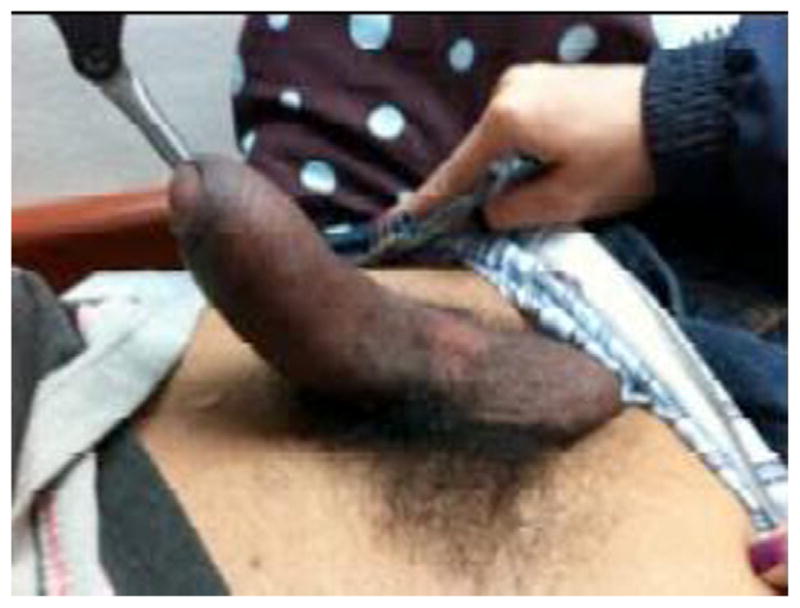
Priapism.
DORSAL SLIT
Phimosis is the inability to retract and expose the glans.45 Up to 10% of boys have physiologic phimosis at 3 years of age, and a larger percentage of children have only partially retractable foreskins. One to 5% have nonretractable foreskins by age 16 years.45 Most healthy adult men should not have phimosis; the presence should raise the suspicion of balanitis, balanoposthitis, diabetes mellitus, or malignancy (Fig. 8).46,47
Fig. 8.
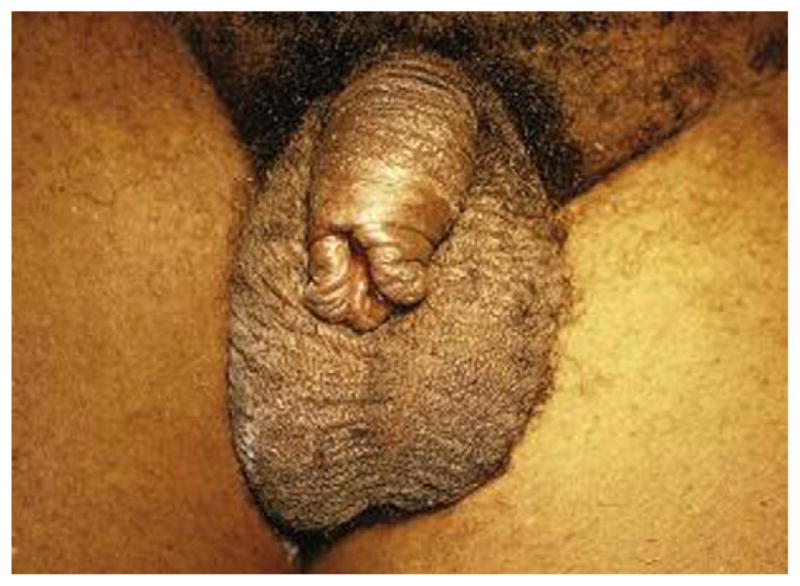
Phimosis.
The indications for performing a dorsal slit include relieving urinary retention in patients with phimosis in whom a urethral catheter cannot be blindly inserted.48 There are no absolute contraindications to the procedure but it should be avoided in patients with bleeding disorders, who are taking anticoagulants, who have infected foreskin, or who are immunocompromised (Box 8).49,50
Box 8. Equipment.
Povidone-iodine solution
4 × 4 Sterile gauze
Local anesthetic without epinephrine
5-mL syringe
18-Gauge and 27-gauge needles
Sterile drapes
Straight hemostats or straight Kelly clamps
Straight scissors or no. 15 scalpel
Needle driver
Absorbable sutures 3–0 or 4–0
Petroleum gauze
Topical antibiotic ointment
Using a 5-mL syringe with a 27-gauge needle, a small wheal of local anesthetic is created in the 12-o’clock position of the dorsal midline penis. The needle needs to be advanced through the center of the wheal, injecting subcutaneously. This method does not provide anesthesia to the ventral aspect of the penis and foreskin.
In order to perform the dorsal slit, patients should be placed in the supine position with genitals exposed. The genitalia should be cleaned with copious amounts of povidone-iodine solution using soaked 4 × 4 gauze at least twice. The sterile field is created by placing sterile drapes between the scrotum and shaft, above the shaft, and on both sides of the shaft. Then, the bottom of the jaw of a straight hemostat should be inserted between the foreskin and the glans penis at the 12-o’clock position and the hemostat advanced until its tip reaches the coronal sulcus. The hemostat needs to be swiped to break any adhesions between the foreskin and the glans; the tip of the hemostat should be palpated and cause a tenting of the foreskin at the coronal sulcus. Once placement is confirmed, the hemostat is closed to allow it to crush the foreskin for 2 to 3 minutes. Then, the hemostat should be removed and, with a straight scissors, the crushed foreskin cut. The area should be covered with dry sterile gauze; then, the phimotic foreskin should be removed using a manual technique. Using an absorbable 3–0 or 4–0 suture, the edges of the foreskin are approximated leaving a gap in the 12-o’clock position. Finally, the foreskin is reduced to a natural position covering the glans and topical antibacterial ointment should be applied to the penile skin. The most common complication associated with dorsal slit is bleeding.51–53 Patients should be observed in the ED for at least 30 minutes and urgent follow-up with a urologist within 1 to 2 days should be arranged before discharge (Fig. 9).
Fig. 9.
Phimosis dorsal slit.
PARAPHIMOSIS REDUCTION
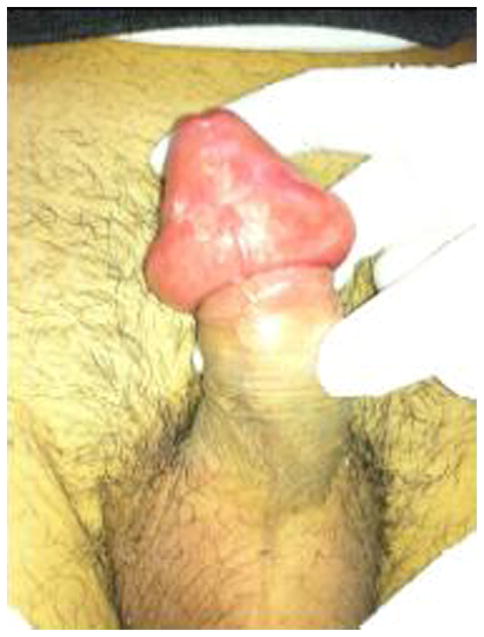
Paraphimosis is the inability to reduce a swollen and proximately positioned foreskin over the glans penis.54,55 Retracted foreskin obstructs lymphatic drainage of the distal penis, causing edema of the retracted foreskin; venous obstruction followed by arterial flow may develop within hours to days. If not corrected in a timely fashion, penile necrosis, infraction of the glans, or gangrene can occur, followed by autoamputation.56,57 The incidence of paraphimosis in the United States and elsewhere is unknown. It can occur at any age but is most common in children and older people (Fig. 10).54
Fig. 10.
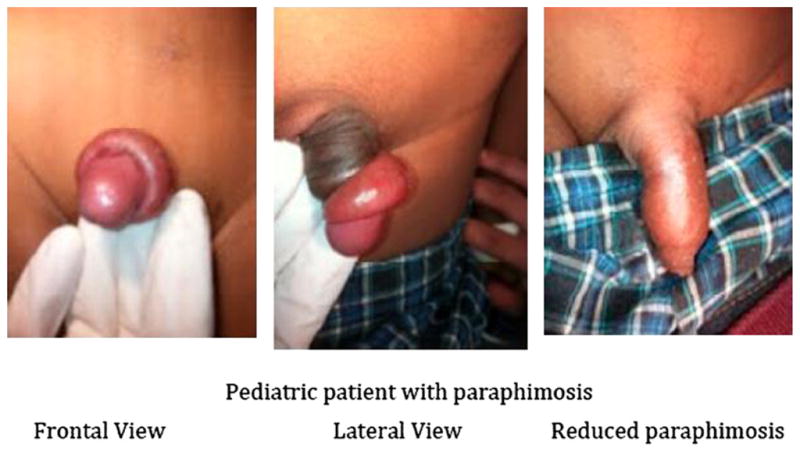
Pediatric paraphymosis.
Patients with phimosis who forcibly retract the foreskin past the glans penis or caretakers who forget to replace the foreskin after retraction can cause paraphimosis. Also reported in the literature, as an associated risk factor for the development of paraphimosis, is penile piercing; there are case reports that described coital paraphimosis leading to penile necrosis.52,53
The clinical presentation most commonly seen is penile pain and swelling. Patients may also present with urinary retention. Although this is a late finding, it requires immediate reduction. Diagnosis of paraphimosis is clinically based on history and physical findings (Fig. 11).
Fig. 11.
Adult paraphimosis.
All patients with paraphimosis require emergent reduction. Contraindications to the procedure include the presence of necrotic tissue or ulcerated foreskin or penis.56,57
Equipment and materials needed for the performance of manual paraphimosis reduction are summarized in Box 9.
Box 9. Equipment for manual paraphimosis reduction.
Topical anesthetic cream (eutectic mixture of local anesthetics [EMLA])
4 × 4 Sterile gauze
Povidone-iodine solution
Sterile drapes
Sterile gloves
Local anesthetic solution without epinephrine
10-mL syringe
Needles, 18-gauge and 27-gauge
Crushed ice
Before the performance of the procedure, patients should be placed in the supine position with genitals exposed. An anesthetic cream should be applied to the glans and foreskin. Then, the area should be cleansed at least twice, with copious amounts of povidone-iodine solution, using a soaked 4 × 4 gauze. After placing sterile drapes between the scrotum and shaft creates a sterile field, reassessment of the anesthetic effect should be evaluated. If adequate anesthetic has not been achieved, a nerve block should be performed. Paraphimosis is a painful condition; consider parenteral analgesia and/or procedural sedation with analgesia.
Steady manual compression over the glans penis and edematous foreskin should be applied, squeezing distally to proximally, and maintained for 5 to 10 minutes. Thumbs should be positioned on both sides of the urethral meatus and the index and middle fingers proximal to the phimotic ring; continuous traction moves the phimotic ring distally over the glans.57
Successful reduction should look like a normal uncircumcised penis. Patients should feel relief of pain; pressure and residual swelling should resolve in a few days. If manual reduction technique fails, a dorsal slit is indicated.58
Patients should be observed for recovery from anesthesia, adequate hemostasis, and ability to urinate. They could be discharge home with instructions to follow-up with a urologist within 1 to 2 days (Fig. 12).
Fig. 12.
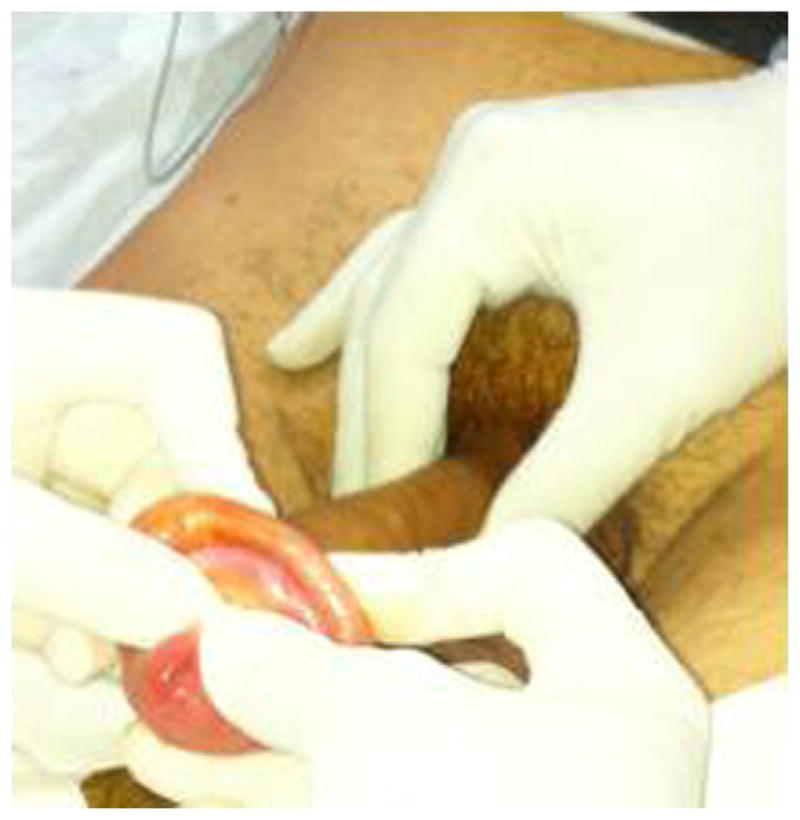
Adult paraphimosis reduction.
MANAGEMENT OF PENILE FRACTURE
A fractured penis occurs when there is a traumatic rupture of the corpus cavernosum. Sudden blunt trauma or abrupt lateral bending of the penis in an erect state can break the markedly thinned and stiff tunica albuginea.59
History and physical examination findings provide the diagnosis. Patients often report penile injury coincident with sexual intercourse, often accompanied by a popping or cracking sound with immediate detumescence. The normal penile appearance is obliterated because of significant deformity, swelling, and ecchymosis, producing the eggplant deformity.60,61 Approximately 30% of men with penile fractures present with blood at the meatus.62 Whenever urethral injury is suspected, a retrograde urethrography is required. The ability to void, however, does not exclude injury.
The treatment of penile fractures is immediate surgical intervention. Fluid resuscitation and stabilization of patients should be the focus in the ED. If surgical therapy must be delayed, initial medical therapy consists of cold compresses, pressure dressings and anti-inflammatory medications, followed by definitive surgical therapy.59–63
All patients must understand that erectile dysfunction, abnormal penile curvature, painful erections, and formation of fibrotic plaques are all possible outcomes that are a result of the nature of the injury. With prompt diagnosis and expedient surgical management, however, outcomes remain excellent and complications are minimal.63
Postsurgical management includes pain medications and oral antibiotics. Patients may be discharged home 1 to 3 days after the surgery (Fig. 13).
Fig. 13.
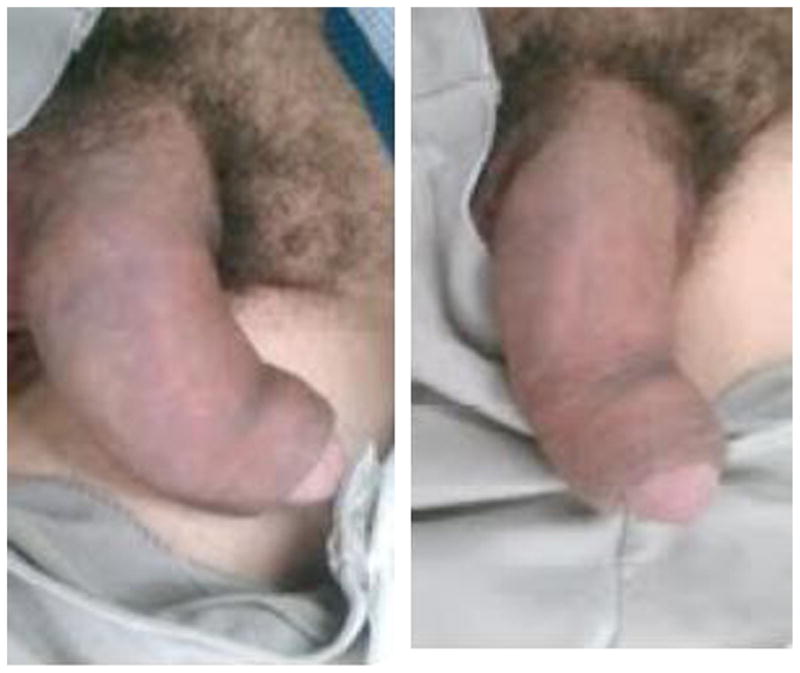
Penile fracture.
MANAGEMENT OF COMMON UROLOGIC PROCEDURES
Emergency medicine providers must be proficient while managing the complications of urologic procedures completed by a urologist. Table 1 lists the most common procedures performed by urologists as well as the associated complications and ED management.
Table 1.
Common complications of urologic procedures
| Procedure | Notes | Complications | ED Management | Disposition |
|---|---|---|---|---|
| ESWL | Preferred treatment modality for renal and ureteric calculi64,65 | Consider U/A, type and screen, renal function test, and CBC Administer IV fluids, antiemetic, narcotic for pain, and prophylaxis antibiotics for all77 |
Urologist ED evaluation vs close outpatient follow-up in 24–48 h | |
| Vasectomy | Most commonly performed urologic surgical procedure78,79 | Scrotal elevation and support. NSAIDs for pain78,84–90 |
Urology follow-up in 2–3 d Long-term follow-up recommended because some patients developed chronic testicular pain78,85–88 |
|
| AUS complications | Device of choice for patients with moderate to severe urinary incontinence91–95 |
|
Urologist ED evaluation for AUS evaluation and removal consideration91,109–111 |
Abbreviations: AUS, artificial urinary sphincter; CBC, complete blood cell count; ESWL, extracorporeal shock wave lithotripsy; NSAID, nonsteroidal anti-inflammatory drug; U/A, urinalysis.
SUMMARY
Emergency physicians must be proficient in the acute management of urologic conditions, especially those that require performing procedures. Most urologic conditions and injuries are initially evaluated in the ED. Several urologic disorders can be evaluated as an outpatient consultation with an urologist; however, a subset of conditions, such as testicular torsion, priapism, and paraphimosis, require immediate identification and expedited intervention and proficiency in the execution of urologic procedures to avoid complications and functional impairment. Early recognition of procedural complications to improve outcomes in these urologic conditions is of utmost importance.
KEY POINTS.
Emergency physicians must be familiar with urologic emergencies; they should be dexterous performing urologic procedures to maintain function while avoiding complications.
Among critical skills and procedures performed by emergency practitioners are urethral and suprapubic catheterization, manual testicular detorsion, dorsal penile nerve block, cavernosal aspiration, dorsal slit, and paraphimosis reduction.
Depending on the urologic condition, emergent consultation and/or close follow-up with a urologist are highly encouraged to assure proper patient care and satisfaction.
Footnotes
Disclosures: None (R.M-C., L.M-C.).
Disclosures: Dr Ramos-Fernandez has funding from Grant Number R25 RR17589 from the National Center for Research Resources (NCRR)/National Institute on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH). The written content and expressions are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Thomas K, Chow K, Kirby RS, et al. Acute urinary retention: a review of the etiology and management. Prostate Cancer Prostatic Dis. 2004;7:32–7. doi: 10.1038/sj.pcan.4500700. [DOI] [PubMed] [Google Scholar]
- 2.Vilke GM, Ufberg JW, Harrigan RA, et al. Evaluation and treatment of acute urinary retention. J Emerg Med. 2008;35(2):193–8. doi: 10.1016/j.jemermed.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen SJ, Jaconsen DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 4.Nyman MA, Schwenk NM, Silverstein MD, et al. Management of urinary retention: rapid versus gradual decompression and risk of complications. Mayo Clin Proc. 1997;72:951. doi: 10.1016/S0025-6196(11)63368-5. [DOI] [PubMed] [Google Scholar]
- 5.Hargreave TB, McNeill SA. Sustained-release alfuzosin and trial without catheter after acute urinary retention: a prospective, placebo-controlled trial. Br J Urol. 1999;84:622–7. doi: 10.1046/j.1464-410x.1999.00277.x. [DOI] [PubMed] [Google Scholar]
- 6.McNeill AS, Rizvi S, Byrne DJ, et al. Long term follow up following presentation with first episode of acute urinary retention. J Urol. 2000;163:559–62. [Google Scholar]
- 7.Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med. 1995;332:75–9. doi: 10.1056/NEJM199501123320202. [DOI] [PubMed] [Google Scholar]
- 8.Desgrandchamps F, De la Taille A, Doublet JD, et al. The management of acute urinary retention in France: a cross-sectional survey in 2618 men with benign prostatic hyperplasia. BJU Int. 2006;97:727. doi: 10.1111/j.1464-410X.2006.06109.x. [DOI] [PubMed] [Google Scholar]
- 9.Horgan AF, Prasad B, Waldron DJ, et al. Acute urinary retention. Comparison of supra-pubic and urethral catheterisation. Br J Urol. 1992;70:149–51. doi: 10.1111/j.1464-410x.1992.tb15693.x. [DOI] [PubMed] [Google Scholar]
- 10.Kessler CS, Bauml J. Non-traumatic urologic emergencies in men: a clinical review. West J Emerg Med. 2009;10(4):281–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilera PA, Choi T, Durham BAl, et al. Ultrasound-guided suprapubic cystostomy catheter placement in the emergency department. J Emerg Med. 2004;26(3):319–21. doi: 10.1016/j.jemermed.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Mc Connell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic enlargement. N Engl J Med. 1998;338:557–63. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 13.Patel MI, et al. The optimal form of urinary drainage after acute retention of urine. BJU Int. 2001;88:26. doi: 10.1046/j.1464-410x.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 14.Beni-Israel T, Goldman M, Bar Chaim S, et al. Clinical predictors for testicular torsion as seen in the pediatric ED. Am J Emerg Med. 2010;28(7):786–9. doi: 10.1016/j.ajem.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Gatti JM, Patrick Murphy J. Current management of the acute scrotum. Semin Pediatr Surg. 2007;16(1):58–63. doi: 10.1053/j.sempedsurg.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Hayn MH, Herz DB, Bellinger MF, et al. Intermittent torsion of the spermatic cord portends an increased risk of acute testicular infarction. J Urol. 2008;180(Suppl 4):1729–32. doi: 10.1016/j.juro.2008.03.101. [DOI] [PubMed] [Google Scholar]
- 17.Sessions AE, Rabinowitz R, Hulbert WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol. 2003;169(2):663. doi: 10.1097/01.ju.0000047381.36380.0e. Department of Urology, University of Rochester School of Medicine, Rochester, New York. [DOI] [PubMed] [Google Scholar]
- 18.Cattolica EV. Preoperative manual detorsion of the torsed spermatic cord. J Urol. 1985;133:803. doi: 10.1016/s0022-5347(17)49233-0. [DOI] [PubMed] [Google Scholar]
- 19.Karmazyn B, et al. Clinical and sonographic criteria of acute scrotum in children: a retrospective study of 172 boys. Pediatr Radiol. 2005;35(3):302–10. doi: 10.1007/s00247-004-1347-9. [DOI] [PubMed] [Google Scholar]
- 20.Cummings JM, Boullier JA, Sekhon D, et al. Adult testicular torsion. J Urol. 2002;167:2109. [PubMed] [Google Scholar]
- 21.Sparano A, Acampora C, Scaglione M, et al. Using color power Doppler ultrasound imaging to diagnose the acute scrotum. A pictorial essay. Emerg Radiol. 2008;15(5):289–94. doi: 10.1007/s10140-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 22.Ransler CW, 3rd, Allen TD. Torsion of the spermatic cord. Urol Clin North Am. 1992;9:245. [PubMed] [Google Scholar]
- 23.Jefferson RH, Perez LM, Joseph DB. Critical analysis of the clinical presentation of acute scrotum: a 9-year experience at a single institution. J Urol. 1997;158:1198. doi: 10.1097/00005392-199709000-00134. [DOI] [PubMed] [Google Scholar]
- 24.Telgarsky B, Karovic D, Wassermann O, et al. Penile block in children, our first experience. Bratisl Lek Listy. 2006;107(8):320–2. [PubMed] [Google Scholar]
- 25.Soh CR, Ng SB, Lim SL. Dorsal nerve block. Paediatr Anaesth. 2003;13(4):329–33. doi: 10.1046/j.1460-9592.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 26.Taddio A, Pollock N, Gilbert-MacLeod C, et al. Combined analgesia and local anesthesia to minimize pain during circumcision. Arch Pediatr Adolesc Med. 2000;154(6):620–3. doi: 10.1001/archpedi.154.6.620. [DOI] [PubMed] [Google Scholar]
- 27.Kirya C, Werthmann MW. Neonatal circumcision and penile dorsal nerve block: a painless procedure. J Pediatr. 1978;92:988–1000. doi: 10.1016/s0022-3476(78)80386-2. [DOI] [PubMed] [Google Scholar]
- 28.Lehr VT, Cepeda E, Frattarelli DA, et al. Lidocaine 4% cream compared with lidocaine 2. 5% or dorsal penile block for circumscision. Am J Perinatol. 2005;22(5):231–7. doi: 10.1055/s-2005-871655. [DOI] [PubMed] [Google Scholar]
- 29.Choi WY, Irwin MG, Hui TW, et al. EMLA cream versus dorsal penile nerve block for postcircumcision analegsia in children. Anesth Analg. 2003;96(2):396–9. doi: 10.1097/00000539-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Emsen IM. Catastrophic complication of the circumcision that carried out with local anesthesia contained adrenaline. J Trauma. 2006;60(5):1150. doi: 10.1097/01.ta.0000217273.26149.d8. [DOI] [PubMed] [Google Scholar]
- 31.Abaci A, Makay B, Unsal E, et al. An unusual complication of dorsal penile nerve block for circumscision. Paediatr Anaesth. 2006;16(10):1094–5. doi: 10.1111/j.1460-9592.2006.01941.x. [DOI] [PubMed] [Google Scholar]
- 32.Cherian J, Rao AR, Thwaini A, et al. Medical and surgical management of priapism. Postgrad Med J. 2006;82:89. doi: 10.1136/pgmj.2005.037291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina CA. Clitoral priapism: a rare condition presenting as a cause of vulvar pain. Obstet Gynecol. 2002;100:1089. doi: 10.1016/s0029-7844(02)02084-7. [DOI] [PubMed] [Google Scholar]
- 34.Eland IA, van der Lei J, Stricker BH, et al. Incidence of priapism in the general population. Urology. 2001;57:970. doi: 10.1016/s0090-4295(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 35.Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259. doi: 10.1016/s0022-5347(17)34928-5. [DOI] [PubMed] [Google Scholar]
- 36.Harmon WJ, Nehra A. Priapism: diagnosis and management. Mayo Clin Proc. 1997;72(4):350–5. doi: 10.4065/72.4.350. Department of Urology, Mayo Clinic Rochester. [DOI] [PubMed] [Google Scholar]
- 37.Champion HC, Bivalacqua TJ, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim NN, Kim JJ, Hypolite J, et al. Altered contractility of rabbit penile corpus cavernosum smooth muscle by hypoxia. J Urol. 1996;155:772. [PubMed] [Google Scholar]
- 39.Roberts . Urologic procedures. 5. Chapter 55. Elsevier; 2009. Clinical procedures in emergency medicine. [Google Scholar]
- 40.Minevich E. Genitourinary emergencies in children. Minerva Pediatr. 2009;61(1):53–65. [PubMed] [Google Scholar]
- 41.Van der horst C, et al. Priapism—etiology, pathophysiology and management. Int Braz J Urol. 2003;29(5):391–400. doi: 10.1590/s1677-55382003000500002. [DOI] [PubMed] [Google Scholar]
- 42.Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag. 2008;4:1315. doi: 10.2147/tcrm.s3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantadakis E, Ewalt DH, Cavender JD. Outpatient penile aspiration and epinephrine irrigation for young patients with sickle cell anemia and prolonged priapism. Blood. 2000;95(1):78–82. Division of Hematology-Oncology, Department of Pediatrics, The University of Texas Southwestern Medical Center at Dallas. [PubMed] [Google Scholar]
- 44.Volkmer BG, Nesslauer T, Kraemer SC, et al. Prepubertal high flow priapism: incidence, diagnosis and treatment. J Urol. 2001;166:1018. [PubMed] [Google Scholar]
- 45.McGregor TB, Pike JG, Leonard MP. Pathologic and physiologic phimosis: approach to the phimotic foreskin. Can Fam Physician. 2007;53(3):445–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Bromage SJ, Crump A, Pearce I. Phimosis as a presenting feature of diabetes. BJU Int. 2008;10(30):338–40. doi: 10.1111/j.1464-410X.2007.07274.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsen HF, Morgenstern H, Mack T, et al. Risk factors for penile cancers: results of a population-based case-control study in Los Angeles County (United States) Cancer Causes Control. 2001;12(3):267–77. doi: 10.1023/a:1011266405062. [DOI] [PubMed] [Google Scholar]
- 48.Thiruchelvam N, Nayak P, Mostafid H. Emergency dorsal slit for balanitis with retention. J R Soc Med. 2004;97(4):205–6. doi: 10.1258/jrsm.97.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borsellino A, Spagnoli A, Vallasciani S, et al. Surgical approach to concealed penis: technical refinements and outcomes. Urology. 2007;69(6):1195–8. doi: 10.1016/j.urology.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 50.Chu CC, Chen YH, Diau GY, et al. Preputial flaps to correct buried penis. Pediatr Surg Int. 2007;23(11):1119–21. doi: 10.1007/s00383-007-2003-x. [DOI] [PubMed] [Google Scholar]
- 51.Chloe JM. Paraphimosis: current treatment options. Am Fam Physician. 2000;62(12):2623–6. [PubMed] [Google Scholar]
- 52.Koenig LM, Carnes M. Body piercing medical concerns with cutting-edge fashion. J Gen Intern Med. 1999;14(6):379–85. doi: 10.1046/j.1525-1497.1999.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raman SR, Kate V, Ananthakrishnan N. Coital paraphimosis causing penile necrosis. Emerg Med J. 2008;25:454. doi: 10.1136/emj.2007.054601. [DOI] [PubMed] [Google Scholar]
- 54.William JC, Morrison PM, Richardson JR. Paraphimosis in elderly men. Am J Emerg Med. 1995;13(3):351–3. doi: 10.1016/0735-6757(95)90217-1. [DOI] [PubMed] [Google Scholar]
- 55.Rangarajan M, Jayakar SM. Paraphimosis revisited: is chronic paraphimosis a predominantly third world condition? Trop Doct. 2008;38(1):40. doi: 10.1258/td.2007.006212. [DOI] [PubMed] [Google Scholar]
- 56.McCollough M, Sharieff GQ. Abdominal surgical emergencies in infants and young children. Emerg Med Clin North Am. 2003;21:909. doi: 10.1016/s0733-8627(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 57.Mackway-Jones K, Teece S. Best evidence topic reports. Ice, pins or sugar to reduce paraphimosis. Emerg Med J. 2004;21(1):77–8. [PMC free article] [PubMed] [Google Scholar]
- 58.Little B, White M. Treatment options for paraphimosis. Int J Clin Pract. 2005;59(5):591–3. doi: 10.1111/j.1742-1241.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal MM, Singh SK, Sharma DK, et al. Fracture of the penis: a radiological or clinical diagnosis? A case series and literature review. Can J Urol. 2009;16(2):4568–75. [PubMed] [Google Scholar]
- 60.Miller S, McAninch JW. Penile fracture and soft tissue injury. In: McAninch JW, editor. Traumatic and reconstructive urology. Philadelphia: W.B. Saunders; 1996. pp. 693–8. [Google Scholar]
- 61.Ferhany AF, Angermeier KW, Montague DK. Review of Cleveland Clinic experience with penile fracture. Urology. 2003;61:1259. doi: 10.1016/s0090-4295(99)00115-6. [DOI] [PubMed] [Google Scholar]
- 62.Roy M, Matin M, Alam M, et al. Fracture of the penis with urethral rupture. Mymensingh Med J. 2008;17(1):70–3. [PubMed] [Google Scholar]
- 63.Sack GS, Garraway I, Reznichek R, et al. Current treatment options for penile fractures. Rev Urol. 2004;6(3):114–20. [PMC free article] [PubMed] [Google Scholar]
- 64.Evan AP, Willis LR, Connors B, et al. Shock wave lithotripsy–induced renal injury. Am J Kidney Dis. 1991;17:445–50. doi: 10.1016/s0272-6386(12)80639-1. [DOI] [PubMed] [Google Scholar]
- 65.Evan AP, Willis LR, Lingeman JE, et al. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998;78:1–8. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz Marcellan FJ, Ibarz Servio L. Evaluation of renal damage in extracorporeal lithotripsy by shock waves. Eur Urol. 1986;12:73–5. doi: 10.1159/000472585. [DOI] [PubMed] [Google Scholar]
- 67.Chaussy C, Schmiedt E. Extracorporeal shock wave lithotripsy (ESWL) for kidney stones: an alternative to surgery? Urol Radiol. 1984;6:80–7. doi: 10.1007/BF02923707. [DOI] [PubMed] [Google Scholar]
- 68.Krambeck AE, Rohlinger AL, Lohse CM, et al. Long term effects of shock wave lithotripsy for nephrolithiasis: a nineteen year study. J Endourol. 2005;19(Suppl):A33. [Google Scholar]
- 69.Piper NY, Dalrymple N, Bishoff JT. Incidence of renal hematoma formation after ESWL using the new Dornier Doli-S lithotriptor. J Urol. 2001;165:377. [Google Scholar]
- 70.Dhar NB, Thornton J, Karafa MT, et al. A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urol. 2004;172(Pt 1):2271–4. doi: 10.1097/01.ju.0000143459.03836.2d. [DOI] [PubMed] [Google Scholar]
- 71.Knapp PM, Kulb TB, Lingeman JE, et al. Extracorporeal shock wave lithotripsy–induced perirenal hematomas. J Urol. 1988;139:700–3. doi: 10.1016/s0022-5347(17)42604-8. [DOI] [PubMed] [Google Scholar]
- 72.Peterson JC, Finlayson B. Effects of ESWL on blood pressure. In: Gravenstein JS, Peter K, editors. Extracorporeal shock wave lithotripsy for renal stone disease: technical and clinical aspects. Boston: Butterworths; 1986. [Google Scholar]
- 73.Williams CM, Kaude JV, Newman RC, et al. Extracorporeal shock-wave lithotripsy: long-term complications. AJR Am J Roentgenol. 1988;150:311–5. doi: 10.2214/ajr.150.2.311. [DOI] [PubMed] [Google Scholar]
- 74.Orestano F, Caronia N, Gallo G, et al. Functional aspects of the kidney after shock wave lithotripsy. In: Lingeman JE, Newman DM, editors. Shock wave lithotripsy 2: urinary and biliary lithotripsy. New York: Plenum Press; 1989. pp. 15–7. [Google Scholar]
- 75.Brito CG, Lingeman JE, Newman DM. Long-term follow-up of renal function in ESWL treated patients with a solitary kidney. J Urol. 1990;143(Suppl):299. [Google Scholar]
- 76.Chaussy C, Fuchs G. Current state and future developments of noninvasive treatment of human urinary stones with extracorporeal shock wave lithotripsy. J Urol. 1989;141(Pt 2):782–9. doi: 10.1016/s0022-5347(17)41010-x. [DOI] [PubMed] [Google Scholar]
- 77.Doran O, Foley B. Acute complications following extracorporeal shock-wave lithotripsy for renal and ureteric calculi. Emerg Med Australas. 2008;20:105–11. doi: 10.1111/j.1742-6723.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 78.Awsare NS, Krishnam J, Boustead GB, et al. Complications of vasectomy. Ann R Coll Surg Engl. 2005;87(6):406–10. doi: 10.1308/003588405X71054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kendrick J, Gonzales B, Huber DH, et al. Complications of vasectomies in the United States. J Fam Pract. 1987;25:245–8. [PubMed] [Google Scholar]
- 80.Pant PR, Sharma J, Subba S, et al. Scrotal haematoma: the most common complication of no-scalpel vasectomy. Kathmandu Univ Med J (KUMJ) 2007;5(2):279–80. [PubMed] [Google Scholar]
- 81.Romero Perez P, Merenciano Cortina FJ, Rafie Mazketli W, et al. Vasectomy: study of 300 interventions. Review of the national literature and of its complications. Actas Urol Esp. 2004;28(3):175–214. doi: 10.1016/s0210-4806(04)73061-2. [DOI] [PubMed] [Google Scholar]
- 82.Tandon S, Sabanegh E., Jr Chronic pain after vasectomy: a diagnostic and treatment dilemma. BJU Int. 2008;102(2):166–9. doi: 10.1111/j.1464-410X.2008.07602.x. [DOI] [PubMed] [Google Scholar]
- 83.Selikowitz S, Schned AR. A late post-vasectomy syndrome. J Urol. 1985;134:494. doi: 10.1016/s0022-5347(17)47256-9. [DOI] [PubMed] [Google Scholar]
- 84.Viddeleer AC, Lycklama A, Nijeholt GA. Lethal Fournier’s gangrene following vasectomy. J Urol. 1992;147:1613–4. doi: 10.1016/s0022-5347(17)37645-0. [DOI] [PubMed] [Google Scholar]
- 85.Nangia AK, Myles JL, Thomas AJ. Vasectomy reversal for the postvasectomy pain syndrome: a clinical and histological evaluation. J Urol. 2000;164:1939–42. [PubMed] [Google Scholar]
- 86.Davis BE, Noble MJ, Wigel JW, et al. Analysis and management of chronic testicular pain. J Urol. 1990;143:936–9. doi: 10.1016/s0022-5347(17)40143-1. [DOI] [PubMed] [Google Scholar]
- 87.Schuman LM, Coulson AH, Mandel JS, et al. Health status of American men—a study of post-vasectomy sequelae. J Clin Epidemiol. 1993;46:697–958. doi: 10.1016/0895-4356(93)90180-9. [DOI] [PubMed] [Google Scholar]
- 88.Barone MA, Hutchinson PL, Johnson CH, et al. Vasectomy in the United States, 2002. J Urol. 2006;176(1):232–6. doi: 10.1016/S0022-5347(06)00507-6. [DOI] [PubMed] [Google Scholar]
- 89.Adams CE, et al. Risks and complications of vasectomy. Urol Clin North Am. 2009;36:331–6. doi: 10.1016/j.ucl.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Kuznetsov DD, Kim HL, Patel RV, et al. Comparison of artificial urinary sphincter and collagen for the treatment of postprostatectomy incontinence. Urology. 2000;56:600–3. doi: 10.1016/s0090-4295(00)00723-8. [DOI] [PubMed] [Google Scholar]
- 91.Montague DK, Angermeier KW. Postprostatectomy urinary incontinence: the case for artificial urinary sphincter implantation. Urology. 2000;55:2–4. doi: 10.1016/s0090-4295(99)00413-6. [DOI] [PubMed] [Google Scholar]
- 92.Dalkin BL, Wessells H, Cui H. A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol. 2003;169:237–9. doi: 10.1016/S0022-5347(05)64076-1. [DOI] [PubMed] [Google Scholar]
- 93.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206–8. [PubMed] [Google Scholar]
- 94.Gundian JC, Barrett DM, Parulkar BG. Mayo Clinic experience with use of the AMS800 artificial urinary sphincter for urinary incontinence following radical prostatectomy. J Urol. 1989;142:1459–61. doi: 10.1016/s0022-5347(17)39126-7. [DOI] [PubMed] [Google Scholar]
- 95.Marks JL, Light JK. Management of urinary incontinence after prostatectomy with the artificial urinary sphincter. J Urol. 1989;142:302–4. doi: 10.1016/s0022-5347(17)38739-6. [DOI] [PubMed] [Google Scholar]
- 96.Kowalczyk JJ, Nelson R, Mulcahy JJ. Successful reinsertion of the artificial urinary sphincter after removal for erosion or infection. Urology. 1996;48:906–8. doi: 10.1016/s0090-4295(96)00245-2. [DOI] [PubMed] [Google Scholar]
- 97.Bell BB, Mulcahy JJ. Management of cuff erosion of the double cuff artificial urinary sphincter. J Urol. 2000;163:85–6. [PubMed] [Google Scholar]
- 98.Bryan DE, Mulcahy JJ, Simmons GR. Salvage procedure for infected noneroded artificial urinary sphincters. J Urol. 2002;168:2464–6. doi: 10.1016/S0022-5347(05)64169-9. [DOI] [PubMed] [Google Scholar]
- 99.Mulcahy JJ, Brant MD, Ludlow JK. Management of infected penile implants. Tech Urol. 1995;1:115–9. [PubMed] [Google Scholar]
- 100.Furlow WL, Barrett DM. The artificial urinary sphincter: experience with the AS 800 pump-control assembly for single-stage primary deactivation and activation—a preliminary report. Mayo Clin Proc. 1985;60:255–8. doi: 10.1016/s0025-6196(12)60318-8. [DOI] [PubMed] [Google Scholar]
- 101.Motley RC, Barrett DM. Artificial urinary sphincter cuff erosion. Experience with reimplantation in 38 patients. Urology. 1990;35:215–8. doi: 10.1016/0090-4295(90)80034-k. [DOI] [PubMed] [Google Scholar]
- 102.Raj GV, Peterson AC, Webster GD. Outcomes following erosions of the artificial urinary sphincter. J Urol. 2006;175:2186–90. doi: 10.1016/S0022-5347(06)00307-7. [DOI] [PubMed] [Google Scholar]
- 103.Debell M, Wessells H. Recurrent bulbar urethral stricture in the region of an artificial urinary sphincter. J Urol. 2001;166:1006–7. [PubMed] [Google Scholar]
- 104.Westney OL, Del Terzo MA, McGuire EJ. Balloon dilation of posterior urethral stricture secondary to radiation and cryotherapy in a patient with a functional artificial urethral sphincter. J Endourol. 1992;13:585–6. doi: 10.1089/end.1999.13.585. [DOI] [PubMed] [Google Scholar]
- 105.Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol. 2005;173:1143–6. doi: 10.1097/01.ju.0000155624.48337.a5. [DOI] [PubMed] [Google Scholar]
- 106.Magera JS, Jr, Elliot DS. Artificial urinary sphincter infection: causative organisms in a contemporary series. J Urol. 2008;180(6):2475–8. doi: 10.1016/j.juro.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 107.Venn SN, Greenwell TJ, Mundy AR, et al. The long-term outcome of artificial urinary sphincters. J Urol. 2000;164(3 Pt 1):702–6. doi: 10.1097/00005392-200009010-00020. [DOI] [PubMed] [Google Scholar]
- 108.Maillet F, Buzelin JM, Bouchot O, et al. Management of artificial urinary sphincter dysfunction. Eur Urol. 2004;46(2):241–5. doi: 10.1016/j.eururo.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 109.Wolf JS, Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379–90. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 110.Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol. 1996;156:1975–80. doi: 10.1016/s0022-5347(01)65408-9. [DOI] [PubMed] [Google Scholar]
- 111.Montague DK. The artificial urinary sphincter (AS 800): experience in 166 consecutive patients. J Urol. 1992;147:380–2. doi: 10.1016/s0022-5347(17)37242-7. [DOI] [PubMed] [Google Scholar]