Abstract
Patients treated with platinum-based chemotherapy frequently experience neurotoxic symptoms, which may lead to premature discontinuation of therapy. Despite discontinuation of platinum drugs, these symptoms can persist over a long period of time. Cisplatin and oxaliplatin, among all platinum drugs, have significant neurotoxic potential. A distal dose-dependent symmetrical sensory neuropathy is the most common presentation of platinum neurotoxicity. DNA damage-induced apoptosis of dorsal root ganglion (DRG) neurons seems to be the principal cause of neurological symptoms. However, DRG injury alone cannot explain some unique symptoms such as cold-aggravated burning pain affecting distal extremities that is observed with oxaliplatin administration. In this article, we briefly reviewed potential mechanisms for the development of platinum drugs-associated neurological manifestations.
Keywords: Cisplatin, Dorsal root ganglion, Mechanism, Oxaliplatin, Neurotoxicity, Neuropathic pain, Sodium channel
Core tip: Platinum drug-based chemotherapies may lead to intolerable neuropathic symptoms, preventing their administration at the optimal effective doses and duration. A better understanding of potential mechanisms underlying these symptoms can help clinicians better manage patients experiencing acute and/or cumulative neurotoxicity during treatment with platinum-containing chemotherapy.
INTRODUCTION
Platinum drugs, including cisplatin (cis-diamminedichloroplatinum II), carboplatin (cis-diammine-1, 1-cyclobutane dicarboxylate platinum II), and oxaliplatin (trans-R,R-cyclohexane-1,2-diamineoxalatoplatinum II) have become an important part of the combination chemotherapy regimens used to treat different types of solid tumors. Despite their favorable anti-tumor properties, platinum drugs can cause serious side effects such as neurotoxicity[1-3].
Carboplatin neurotoxicity is negligible compared with that of cisplatin and oxaliplatin, however, it can develop, particularly high doses are administered[3,4]. Exposure of rat sensory neurons in culture to cisplatin, oxaliplatin or carboplatin in vitro caused a concentration-dependent increase in cell death and apoptotic cells[5]. However, carboplatin required a 10-fold higher drug concentration than cisplatin to induce a similar degree of cytotoxic effect. In addition, both cisplatin and oxaliplatin led to increased reactive oxygen species production and 8-oxoguanine DNA damage, but carboplatin did not[5]. These preclinical observations may partly explain why carboplatin has less neurotoxic effects.
Conversely, conventional-dose cisplatin- or oxaliplatin-based therapies can sometimes lead to intolerable neuropathic symptoms, preventing their administration at the optimal effective doses and duration. Large-diameter sensory nerve fibers appear to be the most affected by platinum drugs, leading to symmetrical glove and stocking type of sensory loss, numbness, tingling, pain, and burning sensation[4]. Some of these symptoms may persist for months or even years. Furthermore, in some cases, they may continue to worsen even after treatment cessation, a phenomenon known as “coasting”[6].
Platinum-induced neurologic symptoms become evident when certain cumulative drug doses have been administered. Cumulative doses of cisplatin and oxaliplatin of 350 mg/m2 and 550 mg/m2, respectively, have been considered as the threshold values for neurotoxicity development[6]. Some clinical and genetic features of patients may make them more susceptible to developing severe neurotoxicity during treatment with platinum drugs. A recent study by Velasco et al[7] found that among patients treated with oxaliplatin-based chemotherapy, male patients, patients experiencing more severe acute neuropathic symptoms, patients with abnormal findings on mid-treatment nerve conduction velocity studies, and patients receiving higher cumulative oxaliplatin doses have an increased risk of developing significant neuropathic symptoms. Several recent pharmacogenomics studies have suggested that patients with polymorphisms in the Glutathione S-transferases genes (GSTM1, GSTT1, and GSTP1) are more likely to develop grade 3-4 cumulative neuropathy during oxaliplatin treatment due to decreased drug detoxification[8].
Oxaliplatin may also cause acute dose-independent neurotoxicity, which can occur in approximately 90% of patients during or shortly after infusion, and is characterized by transient cold-induced paresthesias and dysesthesias affecting the distal extremities, and perioral and pharyngolaryngeal regions[9,10].
A better understanding of the potential mechanisms underlying cisplatin or oxaliplatin neurotoxicity will certainly help clinicians identify the optimal clinical management of this side effect. The aim of this review was, therefore, to summarize the current knowledge on the neuronal events induced during platinum-based therapy.
Nuclear DNA damage in dorsal root ganglion neurons
The accumulation of platinum compounds and their metabolites in the dorsal root ganglion (DRG) after their systemic administration and formation of platinum-DNA adducts are considered key steps in neurotoxicity development (Figure 1)[2,11]. The presence of an abundant fenestrated capillary network and the absence of blood-brain barrier in DRG allow platinum drugs to preferentially accumulate in DRG with easy access to sensory neurons[2,11,12].
Figure 1.
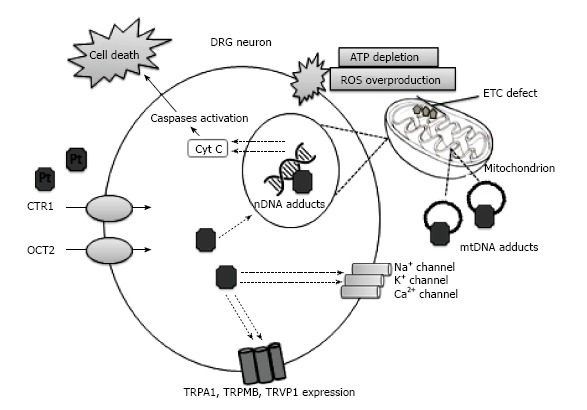
Proposed mechanisms of platinum-induced neurotoxicity. Dorsal root ganglion (DRG) is the main target of platinum drugs that preferentially accumulate in DRG neurons. Membrane transporters, copper transporter-1 (CTR1) and organic cation transporter-2 (OCT2), can facilitate the cellular uptake of platinum drugs. Platinum-DNA adducts inhibit replication and transcription, which results in caspase activation and subsequent cell death. Neuronal mitochondrial damage leads to cellular ATP depletion and increased reactive oxygen species (ROS) production. The voltage-gated sodium (Na+), potassium (K+) and calcium (Ca2+) channels dysfunction, and the enhanced expression and responsiveness of transient receptor potential channels (TRPA1, transient receptor potential ankyrin-1; TRPM8, transient receptor potential melastatin 8; TRPV1, transient receptor potential vanilloid 1) play an important role in the development of platinum-induced neurotoxicity.
Recently, it was demonstrated that the uptake of platinum drugs into DRG neurons may be facilitated by two different types of neuronal membrane transporters: Copper transporter-1 (CTR1) and organic cation transporter-2 (OCT2)[13-15]. The overexpression of these transporters in neurons, therefore, can contribute to the development or aggravation of neurotoxicity. For example, a 16- to 35-fold increase in the cellular oxaliplatin uptake was observed in neurons overexpressing mouse OCT2 or human OCT2, and this process resulted in significantly increased DNA platination and neurotoxicity[15].
Once the platinum drugs reach the neuronal cell nucleus, they attack the nuclear DNA to form adducts. They usually form same types of adducts on the same DNA sites, including 1,2-intrastrand d (GpG) (between adjacent guanine bases on the same DNA strand) and 1,2-intrastrand d (ApG) (between adenine and adjacent guanine bases on the same DNA strand) crosslinks. A correlation between adduct levels and the degree of neurotoxicity has been reported[16]. The platinum-DNA adduct levels produced by cisplatin were found to be approximately three times higher than those generated by equimolar oxaliplatin doses. Concordantly, in vitro cisplatin caused significantly more neuronal cell death than oxaliplatin[16]. DNA repair ability of DRG neurons for adducts (primarily performed by the nucleoid excision repair) is an important factor determining neurotoxicity severity[17]. Chronic cisplatin administration resulted in an accelerated accumulation of unrepaired platinum-DNA adducts in DRG neurons of DNA repair-deficient mice, which induced early neurophysiological alterations and led to an increase in neuronal cell death[17].
Inhibition of the global transcriptional activity of DRG neurons is one of the major consequences of DNA adduct formation[18]. DRG neurons need a high level of active transcription to sustain their large size, high metabolism, and long axons. Therefore, platinum-induced DNA damage leads to neuronal atrophy and disruption of their distant axonal connections[18].
Several preclinical studies have reported that platinum-induced DNA damage also induces apoptosis and neuron loss in DRG both in vivo and in vitro[19-22]. Cisplatin has been shown to initiate several apoptotic events in neuronal cells, including p53 activation, Bax translocation, mitochondrial cytochrome c release, and activation of caspase-3 and caspase-9. Gill and Windebank demonstrated that following exposure to cisplatin, DRG neurons attempt to re-enter the cell cycle from G0 phase, and this event can be a prelude to triggering neuronal cell death[22].
Mitochondrial DNA damage
Mitochondrial dysfunction in DRG neurons was first described as a potential mechanism for platinum drugs neurotoxicity by Podratz et al[23]. They demonstrated that cisplatin also directly binds to mitochondrial DNA with similar binding affinity as nuclear DNA. Cisplatin-mitochondrial DNA adducts inhibit mitochondrial DNA transcription and replication, and cause morphological changes in the mitochondria. This can lead to disruption of the electron transport chain, loss of adenosine triphosphate (ATP) generation, energy failure, and overproduction of reactive oxygen species. All these events cause the opening of mitochondrial permeability transition pores, mitochondrial membrane depolarization, intracellular calcium accumulation, and expression of apoptotic proteins.
Cisplatin may also impair mitochondrial transport dynamics in neurons[24]. Proper mitochondrial transport in neurons is critical to cellular homeostasis. A new study in Drosophila has shown that cisplatin can significantly reduce mitochondrial movement frequency in axons[24]. This is probably caused by both ATP depletion and cellular calcium accumulation.
Some studies have demonstrated that cisplatin can alter the expression of mitochondrial fusion and fission proteins in peripheral nerves[25]. These proteins regulate mitochondrial shape, size, and number. Bobylev et al[25] detected a significant decrease in the mitochondrial fusion protein mitofusin 2 expression levels in DRG and tibial nerves of cisplatin-treated mice, resulting in mitochondrial swelling and vacuolization.
Voltage-gated ion channels dysfunction (channelopathies)
Oxaliplatin exhibits a tetrodotoxin-like inhibitory effect on the neuron voltage-gated sodium (Na+) channels[26-30]. It remarkably slows their inactivation and reduces the peak Na+ current, leading to an increase in the duration of the relative refractory period of sensory neurons. Oxaliplatin may also affect the Na+ channels indirectly via the chelation of extracellular calcium ions by its metabolite oxalate (diaminocyclohexane-platinum-C2O4)[26]. Because of Na+ channel dysfunction, sensory neurons become hyperexcitable and eventually generate spontaneous ectopic discharges.
Oxaliplatin can display isoform-specific effects on voltage-gated Na+ channels leading to the development of unique neuropathy symptoms such as cold-aggravated peripheral pain[31,32]. It has been suggested that oxaliplatin-induced Nav1.6 dysfunction may play a role in cold allodynia development[33,34]. Cooling in the presence of oxaliplatin increased Nav1.6-mediated persistent and resurgent Na+ currents in large-diameter DRG neurons and resulted in the generation of action potential burst firing[31].
Peripheral nerve axonal excitability studies performed before and immediately after oxaliplatin administration have confirmed the above mentioned in vitro findings and revealed acute abnormalities in sensory nerve function related to Na+ channel dysfunction, including decreased refractoriness and increased superexcitability[35]. Interestingly, it was shown that these excitability abnormalities can be detected in the initial oxaliplatin treatment cycles and may serve as a predictive tool to identify patients who are more likely to develop moderate or severe neurotoxicity.
Kagiava et al[33] suggested that altered voltage-gated potassium channel activity may be involved in oxaliplatin-induced neurotoxicity development. In their study, the effects of oxaliplatin on the compound action potential of rat sciatic nerve were observed to be similar to those with the potassium channel blockers 4-aminopyridine and tetraethylammonium. Oxaliplatin was found to cause broadening of action potentials and repetitive firing, suggesting its antagonistic effect on neuronal fast and slow potassium channels. This finding is indirectly supported by Sittl et al[34]. They showed that enhancement of axonal potassium conductance by flupirtine may reduce oxaliplatin-induced peripheral nerve hyperexcitability.
Conversely, voltage-gated potassium channels are unlikely to be the primary target for oxaliplatin because patch-clamp studies failed to show any effect of oxaliplatin on Shaker-type potassium channels[36]. Kagiava et al[37] found some evidence indicating that potassium channel dysfunction during oxaliplatin treatment can occur due to malfunction of the gap junction (GJ) channels and hemichannels in myelinated fibers. According to their findings, oxaliplatin causes prolonged opening of GJ channels and hemichannels, leading to excessive potassium accumulation in the periaxonal space and its osmotic swelling. This event is likely to have a disturbing effect on the voltage-gated potassium channel function.
Cisplatin does not appear to have a prominent effect on the neuronal sodium or potassium channel function. Initial studies using whole cell patch-clamp electrophysiological technique reported that cisplatin decreases the calcium channel currents, particularly in small-diameter neurons of rat DRG[38]. However, a new study revealed an increase in calcium influx through N-type calcium channels in rat DRG neurons after exposure to cisplatin[39]. This was mainly caused by the upregulation of the N-type calcium channels. Increased intracellular calcium levels led to caspase-3 activation and apoptosis induction.
Enhanced responsiveness of thermosensitive transient receptor potential ion channels
Sensory neurons express various types of transient receptor potential (TRP) channels, including TRPA1, TRPM8, and TRVP1, which all play an important role in the generation and sensation of inflammatory and neuropathic pain[40-45].
Nassini et al[40] showed that oxaliplatin- and cisplatin-induced mechanical and cold hyperalgesia in rats are mediated by transient receptor potential ankyrin-1 (TRPA1), and TRPA1 activation is most likely caused by glutathione-sensitive molecules. Subsequently, Zhao et al[44] reported that oxaliplatin-induced cold hyperalgesia could be related to increased responsiveness of TRPA1. Pretreatment of the cultured DRG neurons with oxaliplatin resulted in an increase in the number of allyl-isothiocyanate (a TRPA1 agonist)-sensitive neurons.
The results of a recent study suggested that aluminum accumulation in DRG may augment oxaliplatin-induced neuropathic pain through activation of TRPA1 and stimulation of apoptotic cell death[46]. In this study, aluminum concentration of in DRG was greater in mice treated with aluminum chloride and oxaliplatin than in those treated with aluminum chloride alone.
Gauchan et al[43] revealed that oxaliplatin treatment increased the cold receptor transient receptor potential melastatin 8 (TRPM8) expression in rat DRG neurons, which resulted in enhanced sensitivity to cooling stimulation. Capsazepine, a blocker of both TRMP8 and TRPV1 channels, but not the selective TRV1 blocker 5’-Iodoresiniferatoxin, was able to inhibit oxaliplatin-induced cold allodynia. These findings suggested that TRPM8 plays a role in cold allodynia caused by oxaliplatin.
Ta et al[41] showed that mice DRG neurons treated with cisplatin or oxaliplatin displayed an increase in transient receptor potential vanilloid 1 (TRPV1), TRPA1, and TRMP8 mRNA expression. Trigeminal ganglion neurons from the cisplatin-treated animals showed increased TRPV1 and TRPA1 mRNA expression, and this was associated with enhanced heat and mechanical hypersensitivity. Conversely, oxaliplatin affected only TRPA1 expression, which induced cold and mechanic hypersensitivity.
Glial activation
Di Cesare Mannelli et al[47,48] first suggested a link between oxaliplatin-induced neuropathic pain and glial activation. In a rat model with oxaliplatin-induced peripheral neuropathy, they showed a transient activation of microglia and astrocytes in the spinal cord and supraspinal areas involved with pain modulation accompanied by a decrease in mechanical and thermal pain thresholds following intraperitoneal oxaliplatin administration[48]. Intrathecal co-administration of microglial inhibitor minocycline was able to prevent microglial activation, but had no effect on the response of astrocytes. The astrocytic activation could be inhibited by intrathecal injection of fluorocitrate, an astrocyte specific metabolic inhibitor. Fluorocitrate did not influence oxaliplatin-induced microglial activation. Both drugs increased pain tolerance, but fluorocitrate produced greater pain relief than minocycline. However, neither minocycline nor fluorocitrate prevented oxaliplatin-dependent morphological alterations in DRG neurons[48]. These findings provide some evidence for the participation of glial cells in oxaliplatin-induced neuropathy.
Involvement of nicotinic receptors
Oxaliplatin treatment was found to induce down regulation of alpha7 nicotinic acetylcholine receptor (nAChR) in the rat sciatic nerve, DRG, and spinal cord[49]. The administration of the selective alpha7 nAChR agonists (R)-ICH3 and PNU-282987 could prevent receptor down regulation and increase the pain threshold by oxaliplatin. These two agonists also could inhibit oxaliplatin-induced morphological changes in DRG and peripheral nerves, and upregulate glial cell density in the spinal cord, thalamus, and somatosensory area 1. CDP-choline, the other selective alpha7 nAChR agonist, was also found to be effective in reducing oxaliplatin-induced mechanical hyperalgesia when administered into the cerebral ventricles[50]. These findings suggested a neuroprotective role of alpha7 nAChR during oxaliplatin treatment.
DETECTION AND ASSESSMENT OF PLATINUM-INDUCED NEUROTOXICITY
Currently, no standard clinical method for the early detection and comprehensive assessment of platinum-induced neurotoxicity is known. The use of self-reporting questionnaires developed by the United States National Cancer Institute and European Organization for Research and Treatment of Cancer throughout the treatment course has been recommended as a simple clinical tool for determining and grading a pre-existing or new neuropathy[51,52]. These questionnaires contain items that evaluate the occurrence, severity, degree of distress, and frequency of neuropathic symptoms and their negative impacts on the patient daily activities.
Among neurophysiological techniques, nerve conduction velocity studies and electromyography remain the gold standard technique for detecting the location and extent of neuronal damage due to treatment with platinum drugs[1,6]. Nerve excitability studies performed before and immediately after oxaliplatin infusion have emerged as novel non-invasive tests for early identification of patients at high risk for severe neurotoxicity[35,53].
PREVENTION AND TREATMENT STRATEGIES
A recent Cochrane review examined the effects of the potential chemo-protective agents against neurotoxicity of platinum analogs[54]. This review included 29 randomized controlled trials (RCTs) and analyzed data from 2906 participants who received platinum-containing chemotherapy (cisplatin, carboplatin, or oxaliplatin) alone or in combination with a potential chemo-protectant, including amifostine, calcium/magnesium infusion, glutathione, Org 2766, acetylcysteine, oxcarbazepine, or vitamin E[54]. The data obtained in this study were found to be insufficient to recommend any particular agent to prevent or limit platinum drug neurotoxicity.
In 2014, the American Society of Clinical Oncology convened an expert panel to develop a clinical practice guideline for the prevention and treatment of chemotherapy-induced neuropathies in adult cancer survivors[55]. The experts reviewed 48 RCTs that investigated the efficacy of pharmacological agents, including antiepileptic drugs (carbamazepine and oxcarbazepine), antidepressants (amitriptyline, nortriptyline, venlafaxine and duloxetine), vitamins/minerals (calcium/magnesium infusions, vitamin E, and glutamine), and antioxidants (glutathione, N-acetylcysteine, and amifostine) against neuropathic pain caused by platinum compounds, paclitaxel or vinca alkaloids. They concluded that enough evidence to support routine clinical implementation of these agents for the prevention of platinum-induced peripheral neurotoxicity was not found. Conversely, duloxetine was found potentially useful for treating oxaliplatin-induced neuropathic pain.
CONCLUSION
The apoptotic loss of DRG neurons plays a central role in the initiation and progression of platinum-induced neurotoxicity. Recent evidence suggests that secondary mitochondrial dysfunction can mediate and aggravate cisplatin-mediated neuronal damage. Impaired activity of voltage-gated ion channels and/or increased sensitivity of TRP channels in sensory neurons seem to be the major events leading to the development of oxaliplatin-induced acute neurological side effects, including cold-induced paresthesias and painful dysesthesias. The potential roles of glial cells and nAChRs in platinum-induced neurotoxicity deserve further investigation to explore new strategies to prevent and to treat this side effect.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 12, 2017
First decision: May 22, 2017
Article in press: July 3, 2017
P- Reviewer: Chen CJ, Levine JD, Lotti M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, Peters GJ. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411–432. doi: 10.1634/theoncologist.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. doi: 10.1155/2011/843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiorazzi A, Semperboni S, Marmiroli P. Current view in platinum drug mechanisms of peripheral neurotoxicity. Toxics. 2015;3:304–321. doi: 10.3390/toxics3030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley MR, Jiang Y, Guo C, Reed A, Meng H, Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One. 2014;9:e106485. doi: 10.1371/journal.pone.0106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 7.Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, Frigeni B, Cacciavillani M, Lonardi S, Cortinovis D, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014;85:392–398. doi: 10.1136/jnnp-2013-305334. [DOI] [PubMed] [Google Scholar]
- 8.Cavaletti G, Alberti P, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity in the era of pharmacogenomics. Lancet Oncol. 2011;12:1151–1161. doi: 10.1016/S1470-2045(11)70131-0. [DOI] [PubMed] [Google Scholar]
- 9.Pasetto LM, D’Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol. 2006;59:159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Lucchetta M, Lonardi S, Bergamo F, Alberti P, Velasco R, Argyriou AA, Briani C, Bruna J, Cazzaniga M, Cortinovis D, et al. Incidence of atypical acute nerve hyperexcitability symptoms in oxaliplatin-treated patients with colorectal cancer. Cancer Chemother Pharmacol. 2012;70:899–902. doi: 10.1007/s00280-012-2006-8. [DOI] [PubMed] [Google Scholar]
- 11.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. 2008;4:10. doi: 10.1186/1744-8069-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavaletti G, Ceresa C, Nicolini G, Marmiroli P. Neuronal drug transporters in platinum drugs-induced peripheral neurotoxicity. Anticancer Res. 2014;34:483–486. [PubMed] [Google Scholar]
- 14.Ciarimboli G. Membrane transporters as mediators of Cisplatin effects and side effects. Scientifica (Cairo) 2012;2012:473829. doi: 10.6064/2012/473829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprowl JA, Ciarimboli G, Lancaster CS, Giovinazzo H, Gibson AA, Du G, Janke LJ, Cavaletti G, Shields AF, Sparreboom A. Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc Natl Acad Sci USA. 2013;110:11199–11204. doi: 10.1073/pnas.1305321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Dzagnidze A, Katsarava Z, Makhalova J, Liedert B, Yoon MS, Kaube H, Limmroth V, Thomale J. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci. 2007;27:9451–9457. doi: 10.1523/JNEUROSCI.0523-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan F, Liu JJ, Ip V, Jamieson SM, McKeage MJ. Role of platinum DNA damage-induced transcriptional inhibition in chemotherapy-induced neuronal atrophy and peripheral neurotoxicity. J Neurochem. 2015;135:1099–1112. doi: 10.1111/jnc.13355. [DOI] [PubMed] [Google Scholar]
- 19.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Alaedini A, Xiang Z, Kim H, Sung YJ, Latov N. Up-regulation of apoptosis and regeneration genes in the dorsal root ganglia during cisplatin treatment. Exp Neurol. 2008;210:368–374. doi: 10.1016/j.expneurol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Cavaletti G, Tredici G, Petruccioli MG, Dondè E, Tredici P, Marmiroli P, Minoia C, Ronchi A, Bayssas M, Etienne GG. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer. 2001;37:2457–2463. doi: 10.1016/s0959-8049(01)00300-8. [DOI] [PubMed] [Google Scholar]
- 22.Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998;101:2842–2850. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41:661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podratz JL, Lee H, Knorr P, Koehler S, Forsythe S, Lambrecht K, Arias S, Schmidt K, Steinhoff G, Yudintsev G, et al. Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve. Neurobiol Dis. 2017;97:60–69. doi: 10.1016/j.nbd.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobylev I, Joshi AR, Barham M, Neiss WF, Lehmann HC. Depletion of Mitofusin-2 Causes Mitochondrial Damage in Cisplatin-Induced Neuropathy. Mol Neurobiol. 2017 doi: 10.1007/s12035-016-0364-7. Jan 21; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25–32. doi: 10.1016/s0014-2999(00)00667-1. [DOI] [PubMed] [Google Scholar]
- 27.Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PLoS One. 2011;6:e18469. doi: 10.1371/journal.pone.0018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin and axonal Na+ channel function in vivo. Clin Cancer Res. 2006;12:4481–4484. doi: 10.1158/1078-0432.CCR-06-0694. [DOI] [PubMed] [Google Scholar]
- 29.Wu SN, Chen BS, Wu YH, Peng H, Chen LT. The mechanism of the actions of oxaliplatin on ion currents and action potentials in differentiated NG108-15 neuronal cells. Neurotoxicology. 2009;30:677–685. doi: 10.1016/j.neuro.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br J Pharmacol. 2005;146:1027–1039. doi: 10.1038/sj.bjp.0706407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc Natl Acad Sci USA. 2012;109:6704–6709. doi: 10.1073/pnas.1118058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, Vetter I. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain. 2013;154:1749–1757. doi: 10.1016/j.pain.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagiava A, Tsingotjidou A, Emmanouilides C, Theophilidis G. The effects of oxaliplatin, an anticancer drug, on potassium channels of the peripheral myelinated nerve fibres of the adult rat. Neurotoxicology. 2008;29:1100–1106. doi: 10.1016/j.neuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Sittl R, Carr RW, Fleckenstein J, Grafe P. Enhancement of axonal potassium conductance reduces nerve hyperexcitability in an in vitro model of oxaliplatin-induced acute neuropathy. Neurotoxicology. 2010;31:694–700. doi: 10.1016/j.neuro.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Park SB, Lin CS, Kiernan MC. Nerve excitability assessment in chemotherapy-induced neurotoxicity. J Vis Exp. 2012;(62):3439. doi: 10.3791/3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broomand A, Jerremalm E, Yachnin J, Ehrsson H, Elinder F. Oxaliplatin neurotoxicity--no general ion channel surface-charge effect. J Negat Results Biomed. 2009;8:2. doi: 10.1186/1477-5751-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagiava A, Theophilidis G, Sargiannidou I, Kyriacou K, Kleopa KA. Oxaliplatin-induced neurotoxicity is mediated through gap junction channels and hemichannels and can be prevented by octanol. Neuropharmacology. 2015;97:289–305. doi: 10.1016/j.neuropharm.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Tomaszewski A, Büsselberg D. Cisplatin modulates voltage gated channel currents of dorsal root ganglion neurons of rats. Neurotoxicology. 2007;28:49–58. doi: 10.1016/j.neuro.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Leo M, Schmitt LI, Erkel M, Melnikova M, Thomale J, Hagenacker T. Cisplatin-induced neuropathic pain is mediated by upregulation of N-type voltage-gated calcium channels in dorsal root ganglion neurons. Exp Neurol. 2017;288:62–74. doi: 10.1016/j.expneurol.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 41.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kono T, Satomi M, Suno M, Kimura N, Yamazaki H, Furukawa H, Matsubara K. Oxaliplatin-induced neurotoxicity involves TRPM8 in the mechanism of acute hypersensitivity to cold sensation. Brain Behav. 2012;2:68–73. doi: 10.1002/brb3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gauchan P, Andoh T, Kato A, Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nativi C, Gualdani R, Dragoni E, Di Cesare Mannelli L, Sostegni S, Norcini M, Gabrielli G, la Marca G, Richichi B, Francesconi O, et al. A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci Rep. 2013;3:2005. doi: 10.1038/srep02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH, Chae J, Roh K, Kil EJ, Lee M, Auh CK, Lee MA, Yeom CH, Lee S. Oxaliplatin-Induced Peripheral Neuropathy via TRPA1 Stimulation in Mice Dorsal Root Ganglion Is Correlated with Aluminum Accumulation. PLoS One. 2015;10:e0124875. doi: 10.1371/journal.pone.0124875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain. 2013;14:1585–1600. doi: 10.1016/j.jpain.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C. Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol. 2014;261:22–33. doi: 10.1016/j.expneurol.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Di Cesare Mannelli L, Pacini A, Matera C, Zanardelli M, Mello T, De Amici M, Dallanoce C, Ghelardini C. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation. Neuropharmacology. 2014;79:37–48. doi: 10.1016/j.neuropharm.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 50.Kanat O, Bagdas D, Ozboluk HY, Gurun MS. Preclinical evidence for the antihyperalgesic activity of CDP-choline in oxaliplatin-induced neuropathic pain. J BUON. 2013;18:1012–1018. [PubMed] [Google Scholar]
- 51.Alberti P, Rossi E, Cornblath DR, Merkies IS, Postma TJ, Frigeni B, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol. 2014;25:257–264. doi: 10.1093/annonc/mdt409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curcio KR. Instruments for Assessing Chemotherapy-Induced Peripheral Neuropathy: A Review of the Literature. Clin J Oncol Nurs. 2016;20:144–151. doi: 10.1188/16.CJON.20-01AP. [DOI] [PubMed] [Google Scholar]
- 53.Hill A, Bergin P, Hanning F, Thompson P, Findlay M, Damianovich D, McKeage MJ. Detecting acute neurotoxicity during platinum chemotherapy by neurophysiological assessment of motor nerve hyperexcitability. BMC Cancer. 2010;10:451. doi: 10.1186/1471-2407-10-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2014;(3):CD005228. doi: 10.1002/14651858.CD005228.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]


