Abstract
Human activities have increased N availability dramatically in terrestrial and aquatic ecosystems. Extensive research demonstrates that local plant species diversity generally declines in response to nutrient enrichment, yet the mechanisms for this decline remain unclear. Based on an analysis of >900 species responses from 34 N-fertilization experiments across nine terrestrial ecosystems in North America, we show that both trait-neutral and trait-based mechanisms operate simultaneously to influence diversity loss as production increases. Rare species were often lost because of soil fertilization, randomly with respect to traits. The risk of species loss due to fertilization ranged from >60% for the rarest species to 10% for the most abundant species. Perennials, species with N-fixing symbionts, and those of native origin also experienced increased risk of local extinction after fertilization, regardless of their initial abundance. Whereas abundance was consistently important across all systems, functional mechanisms were often system-dependent. As N availability continues to increase globally, management that focuses on locally susceptible functional groups and generally susceptible rare species will be essential to maintain biodiversity.
Keywords: functional traits, metaanalysis, productivity, random loss, rarity
Net primary production in terrestrial temperate ecosystems is generally limited by N availability (1). Theory predicts that, above a certain level of primary productivity, local species diversity declines as production increases (2–4). Observational studies across N deposition gradients (5) and numerous N-fertilization experiments (6, 7) provide support for this theory. After land use change, N deposition and climate change have been predicted to be major drivers of diversity loss (8). Given that human activity has doubled available N (9) and that net primary production is increasing globally (10), a more mechanistic understanding of diversity decline due to resource enhancement is needed to develop strategies that minimize the potential loss of biodiversity.
Explanations for the decline in species diversity as productivity increases range from random extinctions of locally rare species (11, 12) to functional trade-offs between aboveversus below-ground competitive ability (3, 13–15). The random-loss hypothesis predicts that increased competition causes community-level thinning, decreasing density because of the death of small individuals of all species. Rare species would be most at risk of loss as a consequence of their small population size. Studies have found either strong (16) or partial (12, 17) support for this hypothesis. In contrast, functional-based hypotheses predict that fertilization allows species with traits that are advantageous under the changed conditions to exclude other species. Most often, exclusion is tied to competition, in which the overall intensity of competition increases because of fertilization (2) or a switch occurs to mainly above-ground competition when soil resources are abundant but shading is intense (15). This second set of hypotheses assumes the importance of functional traits; however, tests of the importance of particular traits at the community level are rare.
We use functional traits to test mechanistic links between diversity loss and fertilization (18). Traits of individuals reflect evolutionarily derived strategies of resource capture and interactions among species, both of which influence community structure and ecosystem processes (19–23). Analysis of responses of functional groups to fertilization can discern functional mechanisms related to the pattern of diversity decline. However, adequate replication of functional trait diversity rarely occurs within a single experimental study because of area limitations on local species diversity and environmental filters (18). In addition, investigations of species responses in one system cannot distinguish between dynamics that are environmentally dependent and those that are general across systems (23). Broad generality can only be achieved by combining data from multiple, independent experiments carried out in different ecosystems.
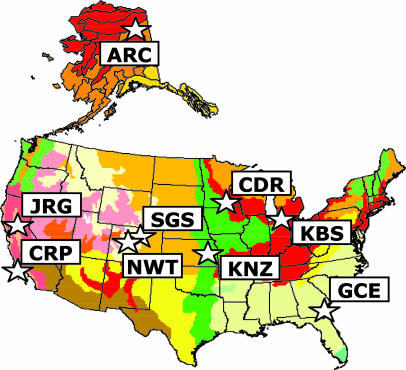
We assembled functional trait data for 967 plant species records in 34 N-fertilization experiments across nine sites in North America to gain a more mechanistic understanding of diversity decline due to resource enhancement. Ecosystems included arctic and alpine tundra, grasslands, abandoned agricultural fields, and coastal salt marshes (Fig. 1).
Fig. 1.
Species responses to N fertilization were analyzed from 34 experiments at nine sites representing major herbaceous ecosystems in temperate North America. ARC, Toolik Lake, AK, arctic tundra; CRP, Carpenteria, CA, Pacific salt marsh; CDR, Cedar Creek, MN, sand prairie/old field; GCE, Sapelo Island, GA, Atlantic salt marsh; JRG, Jasper Ridge, CA, annual grassland; KBS, Kellogg Biological Station, old field; KNZ, Konza Prairie, KS, tallgrass prairie; NWT, Niwot Ridge, CO, alpine tundra; and SGS, Central Plains Experimental Range, CO, shortgrass steppe. Map delineations are Holdridge life zones.
We use these data to address two commonly hypothesized mechanisms driving the productivity–diversity relationship. They are not mutually exclusive. First, as productivity is enhanced, the chance that a species is lost is proportional to its initial abundance, and therefore, diversity decline is driven primarily by the loss of rare species (trait random loss). Second, as productivity increases, species losses are a function of changes in the traits optimal for resource use (functional trait-based loss). Specifically, species that are able to tolerate low levels of below-ground resources in unfertilized conditions (e.g., species that are perennial or have a C4 photosynthetic pathway), effectively obtain below-ground resources (e.g., species that support N-fixing symbionts or are clonal), or constrained in their use of above-ground resources (e.g., short-stature species) should be more likely to be excluded in fertilized conditions (24, 25). To test these hypotheses, we quantified simple functional traits for all species in all experiments. We determined the degree to which the decline in diversity in the 34 N-fertilization experiments depended on increased productivity and/or functional-group turnover. We then examined what types of species were being lost in these experiments and whether initial abundance or particular traits of these species predicted the risk of loss due to fertilization.
Materials and Methods
We used data from experimental studies of N fertilization from nine ecosystems in North America (Fig. 1). Although these systems were all largely dominated by herbaceous vegetation, they encompassed a broad range of growth form representation and productivity (see Table 2, which is published as supporting information on the PNAS web site). Across the nine ecosystems, we include 34 N-fertilization experiments. In all experiments, we compared unfertilized (no N added) and fertilized (N added) treatments.
Trait Characterization. We chose broad functional categories, some of which overlap with previously suggested core plant traits (26, 27), rather than many specific quantitative traits for several reasons. First, detailed quantitative traits are rarely available for the North American flora, unlike the published databases for the European flora (28, 29). Second, recent studies incorporating both detailed plant measurements and broader life history traits have found relatively equal usefulness of the two approaches (24, 30). Third, general traits can be more easily used by those charged with managing land to maintain species diversity in the face of anthropogenic N inputs.
Based on our predictions, we selected six different functional groupings to use in our analyses. First, graminoid species were grouped by C3 or C4 photosynthetic pathway. Second, forb species were classified as to whether or not they were associated with an N-fixing symbiont. Third, life history was categorized as either annual/biennial or perennial. Fourth, species were classified according to their height relative to the canopy (bottom third, middle third, and upper third) in control conditions for each experiment. Because there were few differences between responses of species in the middle and upper third of the canopy, those categories were subsequently combined. Fifth, species were classified based on whether they were nonclonal (no vegetative spread), caespitose (densely packed ramets such as tussocks or phalanx growth form), or rhizomatous (widely spaced ramets or guerilla growth form). Again, because we found few differences in the response of caespitose and rhizomatous species, we combined these groups in the analyses presented here. Last, we classified species based on whether they were native to North America or nonnative based on information in the U.S. Department of Agriculture plants database (http://plants.usda.gov). Analyses based on family taxonomic groupings were not possible because of the highly unbalanced nature of family representation in the database: >75% of the 67 families in the data set were represented by <10 species, whereas two families (Poaceae and Asteraceae) were both represented by >80 species records.
Response Metrics. We used species density (number of species per unit area) as our measure of species diversity (7). By following standard metaanalytic approaches (31, 32), we calculated the species diversity response as species density in fertilization treatment divided by species density in control (unfertilized) treatment, with values of 1 indicating no change in response due to fertilization. A similar metric was calculated to describe the change in above-ground primary production due to fertilization, based on standing biomass measures.
To describe functional group responses, we summed the mean relative abundance (of replicates within each experiment) for species comprising each functional grouping. The ratio of relative functional group abundance in fertilized and control plots [lnRR = ln(RAfertilized/RAcontrol)], where RA is relative abundance of a functional group), was used as our response metric. Ratios were natural log-transformed to meet normality assumptions. Thus, a positive lnRR value indicates that fertilization increased that relative abundance of the functional group, a value near zero indicates little change, and a negative value indicates that fertilization decreased the relative abundance of a functional group.
Functional group turnover was calculated as the average of the absolute value of response ratios across all n functional groups in each experiment:
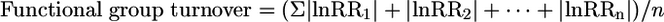 |
where lnRRn is the response to fertilization of the nth functional group (Table 3, which is published as supporting information on the PNAS web site, for a calculation example). Functional group turnover is a general index of the degree to which fertilization produces a shift in functional group abundance between control and fertilized plots. Because functional group turnover is based on the relative abundance of many species within each functional group, we do not expect a statistical correlation with species losses. There were only two cases in the data set in which fertilization caused the complete loss of a functional group; these were omitted from this analysis.
We classified species as lost from an experiment if they were present in control plots but absent in all fertilized plots. This distinction does not take into account the original composition of a particular plot but is conservative in that a species has to be absent from every replicate fertilized plot to be considered lost. In one experiment that had time-series data from the start of the experiment (CDR; Table 2), our classifications based on presence/absence of species in control and fertilized plots predicted >80% of the species that were actually lost over time from fertilized plots. For all analyses, we excluded experiments that did not initially contain species with both trait types being compared (e.g., both annuals and perennials had to be present for an analysis based on life history functional groupings) and experiments that did not experience at least one species loss.
Data Analyses. We used three different logistic regression approaches to test the importance of initial abundance and trait groupings on the probability of species loss in fertilized plots. First, to account for the overall influence of abundance and traits on loss, we ran logistic regressions of species loss likelihood for different functional groups, including all species from all sites. We analyzed each functional grouping separately and did not combine functional groupings (e.g., we did not compare two-trait categories such as perennial exotic or tall N-fixer). Second, to examine the generality across all sites, we conducted similar analyses separately for each site. To determine whether particular sites strongly influenced the overall analysis, we also ran the overall analyses while excluding sites that we identified as having strong effects when they were analyzed separately. Third, to examine the potential influence of trait correlations, we conducted similar analyses on multivariate descriptions (principle component axes) of trait variability rather than just the single trait categories. Life history, origin, and clonality had high loadings on the first axis, which described 26% of the variation in trait groupings. The second and third axes examined an additional 18% and 14% of the variation, respectively. Photosynthetic type and height had high loadings on the second axes, and N-fixation and clonality had high loadings on the third axis. All analyses were based on the likelihood of species losses and included both initial abundance and trait groupings as factors.
Results and Discussion
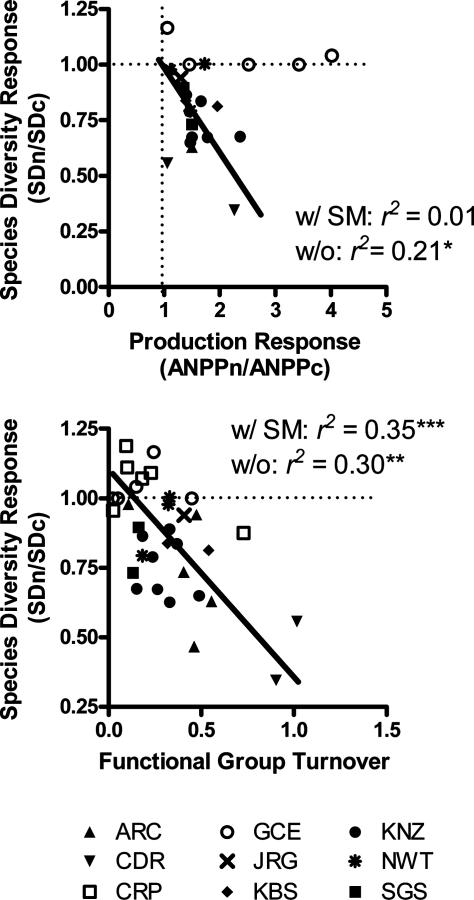
N fertilization increased net primary production and decreased species richness in all terrestrial experiments (r2 = 21, n = 19, P = 0.03) but not in salt-marsh experiments (Fig. 2 Upper). Salt marshes are highly productive and generally support only three or four plant species per m2 under natural conditions, constraining possible diversity responses to fertilization. Functional group turnover (see Materials and Methods) was stronger than productivity as a predictor of diversity decline (Fig. 2 Lower). Large shifts in functional group abundance (e.g., increase in C3 grasses, decrease in legumes, etc.) were related to large losses in diversity both with (r2 = 0.35, n = 34, P < 0.001) and without (r2 = 0.30, n = 24, P = 0.004) salt marsh sites. Shifts in functional group abundance were not related to changes in production (r2 = 0.04, n = 19, P = 0.42) nor initial levels of production (r2 = 0.05, n = 34, P = 0.84). Other factors that differed across experiments, such as experiment length and amount of N added, did not explain diversity decline.
Fig. 2.
The decline in species diversity is related to the magnitude of production increase on plots fertilized with N (Upper) and the magnitude of functional-group turnover (Lower). Functional-group turnover is the mean of the responses of all functional groups to fertilization (see Materials and Methods) within an experiment. Each symbol represents one fertilization experiment. Regression coefficients are given when all experiments are included (w/SM) and when salt marshes are excluded (w/o). Open symbols indicate salt marshes. *, P < 0.05; **, P < 0.01; ***, P < 0.001; SDn and SDc, species density (no. of species per area) in fertilized and control plots, respectively; ANPPn and ANPPc, above-ground net primary production in fertilized and control plots, respectively. For site definitions, see the legend to Fig. 1.
The general relationship of diversity loss with changes in productivity and functional group abundance suggests that both numerical (abundance-based) and functional (trait-based) mechanisms are related to the decline in diversity as local productivity increases. To address these mechanisms in more detail, we sought to determine whether initial abundance or particular functional traits predict the probability that a species would be lost as resource availability increased above-ground production.
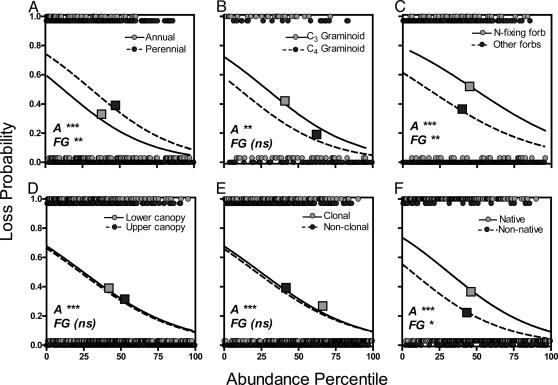
The random-loss hypothesis predicts that rare species account for most of the decline in species diversity with fertilization due to random loss of individuals as plant size increases and density declines (11, 12, 33). Our data partially support this hypothesis. Initial abundance was significantly associated with the likelihood of loss due to fertilization (Fig. 3); the rarest species had >60% chance of loss due to fertilization, whereas the most abundant species had only a 10% chance. However, local extinctions occurred even among the most abundant species, indicating that loss was not wholly due to rarity.
Fig. 3.
Likelihood of local extinction in plots with added N for six trait groupings as a function of initial abundance. Circles indicate abundance of species that were lost (1) or not lost (0) because of fertilization. Squares indicate the average abundance and proportional loss for each functional group. Relative abundance measures are expressed in percentile, with 100 being the most abundant species in the data set and 0 being the least abundant species in the data set. Logistic regressions on species loss as a function of abundance in unfertilized (control) plots. A, abundance; FG, functional grouping. ***, P < 0.001; **, P < 0.01; *, P < 0.05; and ns (not significant), P > 0.05.
Functional mechanisms have also been invoked to explain shifts in diversity after fertilization. One commonly hypothesized mechanism is a shift from below-ground competition for nutrients to above-ground competition for light as resources are enhanced (12, 34, 35). This hypothesis predicts that species that support N-fixing bacteria and are poor competitors for light would decline because of fertilization. After accounting for the contribution of initial abundance to the likelihood of loss, N-fixing forbs were more susceptible to loss than forbs that did not support N-fixing bacteria (Fig. 3 and Table 1). This decline supports the expectation that competition for soil N decreases in fertilized areas. Also, species in the lower canopy, the species likely to experience more intense competition for light (36), were more likely to be lost due to fertilization than species in the upper canopy. However, functional mechanisms cannot be invoked in this latter case: species in the lower canopy were also more likely to be less abundant before fertilization. When initial abundances were accounted for, species lower in the canopy did not have a higher risk of local extinction than taller species (Fig. 3D).
Table 1. Influence of initial abundance and functional groupings on the likelihood of species loss across all sites, for each site, and with site deletion to test for the influence of particular sites.
| Functional grouping
|
|||||||
|---|---|---|---|---|---|---|---|
| Species set | Abundance | Life history (annual, perennial) | Photosynthetic pathway (C3, C4) | N fixation (N-fix, non) | Height (lower, upper) | Clonality (clonal, non) | Origin (native, non) |
| Overall | t761 = -9.4*** | t591 = -2.9** | t160 = 1.6 | t466 = 2.9** | t738 = 0.39 | t711 = 0.71 | t481 = 2.6** |
| ARC | t90 = -3.5*** | — | — | t27 = 0.01 | t89 = 0.48 | t96 = 0.17 | t17 = -0.01 |
| CDR | t61 = -3.1** | t59 = -0.16 | t17 = -0.98 | t40 = 0.45 | t57 = 2.0* | t60 = 1.3 | t59 = 2.6** |
| CRP | t16 = -1.7+ | — | — | — | t16 = 0.50 | — | — |
| KBS | t131 = -3.6*** | t117 = -1.7+ | t33 = 2.4 | t131 = 0.04 | t117 = 0.03 | t120 = -1.1 | t108 = 0.58 |
| KNZ | t331 = -5.7*** | t325 = -1.7+ | t93 = 0.38 | t226 = 3.4*** | t329 = -1.5 | t325 = -0.68 | t325 = -0.90 |
| NWT | t56 = -0.77 | — | — | t45 = -0.01 | t59 = 1.3 | t56 = -0.03 | — |
| SGS | t60 = -3.9*** | t59 = 0.78 | t17 = 0.39 | t31 = -1.9+ | t59 = 1.5 | t59 = 1.2 | t57 = 0.81 |
| Site deletions | — | — | — | t239 = -0.11† | t681 = 0.25‡ | — | t422 = 1.6‡ |
Results for functional grouping account for initial abundance in logistic regression model. There were no species losses due to fertilization in any of the experiments at GCE, and models for JRG failed tolerance tests because of small sample size. tn, t ratio statistic from the logistic regression model, with n indicating no. of species records. +, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001; —, data did not meet criteria for analysis (i.e. both categories within a grouping were not represented, no species were lost, or there was no site effect for deletion). Bold indicates significant factors. Site names are as described in the legend to Fig. 1.
Deletion of KNZ species records.
Deletion of CDR species records.
Another hypothesized mechanism is a shift from conservative to acquisitive resource-use strategies (25, 37, 38). Species with quick returns on investments of nutrients (i.e., rapid potential growth rates, high leaf N content) are often thought to be able to better use pulses of available resources. Although we do not have resource-uptake measures for all of the species in this database, we did classify species based on their life history. Assuming that life history is a broad surrogate for resource-use strategies, perennials should be more conservative in their resource use and decline as resources are enhanced. After accounting for differences in initial abundance, species with perennial life history were more likely to be lost than annual species (Fig. 3A).
Nonnative species can be successful invaders because of a myriad of traits (39, 40). However, it has been suggested that invasion can be enhanced by increases in resource availability (14, 41, 42). Although native and nonnative invasion rates into fertilized plots were equivalent (K.N.S., unpublished data), native species had a higher probability of local extinction as resource availability increased (Fig. 3F). Many native species were also perennial and clonal. Multivariate analyses indicate that this suite of traits (covariation among origin, life history, and clonality) is related to loss due to fertilization (n = 715, t = 2.33, P = 0.02).
We cannot determine the ultimate causes of increased extinction risk for functional groups that also tended to be rare, such as short-stature and nonclonal plants and C3 graminoids (Fig. 3). Although these traits were associated with loss, we assume that these traits contributed to lower abundance and abundance-based mechanisms directly influenced the increased risk of loss for these groups. Controlled manipulations of functional group abundance and fertilization could test this assumption.
We conducted similar logistic regression analyses within each site to identify whether the patterns of loss that we identified in the overall analysis were influenced by dynamics in particular environments. Although abundance was consistently an important predictor of loss likelihood in all systems, functional mechanisms were often system-specific (Table 1). In particular, the disproportionate loss of N-fixers (30 of 70 N-fixing species were lost) due to fertilization occurred predominately at the tallgrass prairie (KNZ; Table 1), and the disproportionate loss of native species (146 of 372 native species were lost) occurred largely at the successional sand prairie (CDR; Table 1). In both cases, when the influential site was removed from the overall analysis, the trait grouping no longer described the likelihood of species loss in the remaining sites. Sites vary in frequency of given trait groups, frequency of species losses, and general species richness; these factors could all statistically influence these results. However, we suspect that these results have a biological basis: functional traits appear to be better predictors of loss at smaller spatial scales in response to local environmental contingencies.
Species diversity declined as production increased in all but the salt marshes and one examined alpine tundra community (Fig. 2 Upper). Diversity loss was most severe when fertilization strongly increased production or shifted functional group dominance. Rare species, regardless of their traits, were more susceptible to local extinction. Rajaniemi (17) speculated that random loss would be strongest in communities of nonclonal plants; however, we found support for the random-loss hypothesis in virtually every system that we examined.
Functional mechanisms related to traits also predicted loss. Although abundance-based mechanisms were nearly universal, functional mechanisms were often system-specific. This result has two important implications. First, it suggests that traits (in the broad sense used here) may not influence above-ground and below-ground competition effects on diversity consistently across environments. Secondly, it suggests that a single functional group may not be a global bellwether for changes in plant biodiversity. Rather than a focus on particular indicator functional type, we suggest managing to enhance the abundance of rare species (to slow numerical losses) and to stabilize functional group dominance (to slow functional losses). Management that counteracts production increases, particularly of the abundant species (e.g., grazing, cutting, or carbon addition; refs. 36 and 43–45), can be used to achieve these objectives and conserve species at risk.
Extensive observational and experimental evidence shows that plant species diversity declines with resource enhancement in mesic ecosystems worldwide (5, 7, 46, 47). Net primary production is increasing globally because of anthropogenic N deposition, altered precipitation regimes and longer growing seasons (9, 10, 48). Together, these global environmental changes portend a continued loss of biodiversity (49), which may have significant consequences for ecosystem functioning (50). Our ability to predict which species will be lost as a consequence of these global changes has been hampered by a limited understanding of the mechanisms that drive the relationship between production and diversity. Our analyses demonstrate that species losses can be predicted from a combination of abundance- and functional-based mechanisms, but that these mechanisms operate at different spatial scales. A focus on susceptible functional groups at local scales and rare species at larger scales will be an essential conservation strategy as primary production increases globally.
Supplementary Material
Acknowledgments
We thank individual sites and the investigators W. Bowman, R. Callaway, C. Field, W. Lauenroth, H. Mooney, T. Seastedt, G. Shaver, J. Simpson, and D. Tilman for providing data; D. Liptzin, A. Ruiz, and M. Walsh for assistance with data compilation; and F. S. Chapin, III, P. Grime, S. Harpole, S. Lavorel, M. Leibold, S. Naeem, T. Rajaniemi, G. Shaver, D. Tilman, and two anonymous reviewers for useful comments on the manuscript. This work was supported by the National Science Foundation-funded Long-Term Ecological Research Network Office and the Niwot Ridge Long-Term Ecological Research Program.
Author contributions: K.N.S., S.L.C., and L.G. designed research; K.N.S., S.L.C., L.G., C.C., E.E.C., K.L.G., D.G.M., and S.P. analyzed data and performed research; and K.N.S. and S.L.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vitousek, P. M., Hattenschwiler, S., Olander, L. & Allison, S. (2002) Ambio. 31, 97–101. [DOI] [PubMed] [Google Scholar]
- 2.Grime, J. P. (1973) Nature 242, 344–347. [Google Scholar]
- 3.Tilman, D. (1982) Resource Competition and Community Structure (Princeton Univ. Press, Princeton). [PubMed]
- 4.Gross, K. L., Willig, M. R., Gough, L., Inouye, R. & Cox, S. B. (2000) Oikos 89, 417–427. [Google Scholar]
- 5.Stevens, C. J., Dise, N. B., Mountford, J. O. & Gowing, D. J. (2004) Science 303, 1876–1879. [DOI] [PubMed] [Google Scholar]
- 6.Silvertown, J., Dodd, M. E., McConway, K., Potts, J. & Crawley, M. (1994) Ecology 75, 2430–2437. [Google Scholar]
- 7.Gough, L., Osenberg, C. W., Gross, K. L. & Collins, S. L. (2000) Oikos 89, 428–439. [Google Scholar]
- 8.Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L. F., Jackson, R. B., Kinzig, A., et al. (2000) Science 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- 9.Vitousek, P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. (1997) Science 277, 494–499. [Google Scholar]
- 10.Nemani, R., Keeling, C. D., Hashimoto, H., Jolly, W. M., Piper, S. C., Tucker, C. J., Myneni, R. B. & Running, S. W. (2003) Science 300, 1560–1563. [DOI] [PubMed] [Google Scholar]
- 11.Oksanen, J. (1996) J. Ecol. 84, 293–295. [Google Scholar]
- 12.Goldberg, D. E. & Miller, T. E. (1990) Ecology 71, 213–225. [Google Scholar]
- 13.Tilman, D. (1990) Oikos 58, 3–15. [Google Scholar]
- 14.Rajaniemi, T. K. (2003) Oikos 101, 449–457. [Google Scholar]
- 15.Newman, E. I. (1973) Nature 244, 310–310. [Google Scholar]
- 16.Stevens, M. H. H. & Carson, W. P. (1999) Ecology 80, 455–465. [Google Scholar]
- 17.Rajaniemi, T. K. (2002) J. Ecol. 90, 316–324. [Google Scholar]
- 18.Smith, T. M., Shugart, H. H. & Woodward, F. I. (1997) Plant Functional Types: Their Relevance to Ecosystem Properties and Global Change (Cambridge Univ. Press, Cambridge, U.K.).
- 19.Diaz, S. & Cabido, M. (2001) Trends Ecol. Evol. 16, 646–655. [DOI] [PubMed] [Google Scholar]
- 20.Grime, J. P. (2001) Plant Strategies, Vegetation Processes and Ecosystem Properties (Wiley, Chischester, West Sussex, U.K.).
- 21.Lavorel, S. & Garnier, E. (2002) Funct. Ecol. 16, 545–556. [Google Scholar]
- 22.Bai, Y. F., Han, X. G., Wu, J. G., Chen, Z. Z. & Li, L. H. (2004) Nature 431, 181–184. [DOI] [PubMed] [Google Scholar]
- 23.Pakeman, R. J. (2004) J. Ecol. 92, 893–905. [Google Scholar]
- 24.Craine, J. M., Tilman, D., Wedin, D., Reich, P., Tjoelker, M. & Knops, J. (2002) Funct. Ecol. 16, 563–574. [Google Scholar]
- 25.Chapin, F. S. (1980) Annu. Rev. Ecol. Syst. 11, 233–260. [Google Scholar]
- 26.Weiher, E., van der Werf, A., Thompson, K., Roderick, M., Garnier, E. & Eriksson, O. (1999) J. Veg. Sci. 10, 609–620. [Google Scholar]
- 27.Chapin, F. S., Sala, O. E., Burke, I. C., Grime, J. P., Hooper, D. U., Lauenroth, W. K., Lombard, A., Mooney, H. A., Mosier, A. R., Naeem, S., et al. (1998) Bioscience 48, 45–52. [Google Scholar]
- 28.Diekmann, M. & Falkengren-Grerup, U. (2002) J. Ecol. 90, 108–120. [Google Scholar]
- 29.Pywell, R. F., Bullock, J. M., Roy, D. B., Warman, L. I. Z., Walker, K. J. & Rothery, P. (2003) J. Appl. Ecol. 40, 65–77. [Google Scholar]
- 30.Reich, P. B., Wright, I. J., Cavender-Bares, J., Craine, J. M., Oleksyn, J., Westoby, M. & Walters, M. B. (2003) Int. J. Plant Sci. 164, S143–S164. [Google Scholar]
- 31.Hedges, L. V., Gurevitch, J. & Curtis, P. S. (1999) Ecology 80, 1150–1156. [Google Scholar]
- 32.Osenberg, C. W., Sarnelle, O. & Goldberg, D. E. (1999) Ecology 80, 1103–1104. [Google Scholar]
- 33.Stevens, M. H. H. & Carson, W. P. (2002) Ecol. Lett. 5, 420–426. [Google Scholar]
- 34.Grace, J. B. (1999) Perspect. Plant Ecol. Evol. Syst. 2, 1–28. [Google Scholar]
- 35.Tilman, D. (1987) Ecol. Monogr. 57, 189–214. [Google Scholar]
- 36.Collins, S. L., Knapp, A. K., Briggs, J. M., Blair, J. M. & Steinauer, E. M. (1998) Science 280, 745–747. [DOI] [PubMed] [Google Scholar]
- 37.Diaz, S., Hodgson, J. G., Thompson, K., Cabido, M., Cornelissen, J. H. C., Jalili, A., Montserrat-Marti, G., Grime, J. P., Zarrinkamar, F., Asri, Y., et al. (2004) J. Veg. Sci. 15, 295–304. [Google Scholar]
- 38.Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., Cavender-Bares, J., Chapin, T., Cornelissen, J. H. C., Diemer, M., et al. (2004) Nature 428, 821–827. [DOI] [PubMed] [Google Scholar]
- 39.Levine, J. M., Vila, M., D`Antonio, C. M., Dukes, J. S., Grigulis, K. & Lavorel, S. (2003) Proc. R. Soc. London Ser. B 270, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, M. D. & Knapp, A. K. (2001) Int. J. Plant Sci. 162, 785–792. [Google Scholar]
- 41.Davis, M. A., Grime, J. P. & Thompson, K. (2000) J. Ecol. 88, 528–534. [Google Scholar]
- 42.Tilman, D. (2004) Proc. Natl. Acad. Sci. USA 101, 10854–10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakker, J. P. & Berendse, F. (1999) Trends Ecol. Evol. 14, 63–68. [DOI] [PubMed] [Google Scholar]
- 44.Baer, S. G., Blair, J. M., Collins, S. L. & Knapp, A. K. (2003) Ecology 84, 724–735. [Google Scholar]
- 45.Weiss, S. B. (1999) Conserv. Biol. 13, 1476–1486. [Google Scholar]
- 46.Waide, R. B., Willig, M. R., Steiner, C. F., Mittelbach, G., Gough, L., Dodson, S. I., Juday, G. P. & Parmenter, R. (1999) Annu. Rev. Ecol. Syst. 30, 257–300. [Google Scholar]
- 47.Mittelbach, G. G., Steiner, C. F., Scheiner, S. M., Gross, K. L., Reynolds, H. L., Waide, R. B., Willig, M. R., Dodson, S. I. & Gough, L. (2001) Ecology 82, 2381–2396. [Google Scholar]
- 48.Galloway, J. N. (1998) Environ. Pollut. 102, 15–24. [Google Scholar]
- 49.Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., Erasmus, B. F. N., de Siqueira, M. F., Grainger, A., Hannah, L., et al. (2004) Nature 427, 145–148. [DOI] [PubMed] [Google Scholar]
- 50.Loreau, M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J. P., Hector, A., Hooper, D. U., Huston, M. A., Rafaelli, D., Schmid, B., et al. (2001) Science 294, 804–808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.