Abstract
Purpose
To quantify adrenocorticotropin (ACTH) and cortisol secretion after epidural glucocorticoid injection.
Methods
Eight men (ages 25–63 yr) were studied at baseline, 1, 4, and 12 wk after triamcinolone (80 mg) injection epidurally. ACTH (pg/mL) and cortisol (μg/dL) were measured every 10 min for 4 h, and after ACTH-releasing hormone (CRH) (1 μg/kg) injection.
Results
Epidural triamcinolone markedly suppressed: (1) pre-CRH injection ACTH (from 18 ± 3.1 to 4.8 ± 0.4: P<0.01) and cortisol (from 12.2 ± 1.6 to 1.6 ± 0.3: P<0.0001) at wk 1, with recovery at 4 wk, and (2) CRH-stimulated summed ACTH (from 633 ± 116 to 129 ± 10 pg/mL/min, P<0.0001), and summed cortisol at wk 1 (from 385 ± 29 to 56 ± 22 μg/dL/min, P<0.0001) and 4 wk (284 ± 53; P<0.01). Serum cortisol was <18 μg/dL in 8 of 8 men at 4 wk, and 6 of 8 men at wk 12. Urinary free cortisol (μg/24 hr) remained low at wk 12: baseline (60 ± 6.5); wk 1 (9.0 ± 1.3, P<0.01); wk 4 (36 ± 8.6) and wk 12 (38 ± 4.1). Urinary cortisol/cortisone ratios rose at wk 4 only. Serum triamcinolone peaked at wk 1 (16/16 samples), declining at wk 4 (13/16 samples) and wk 12 (6/16 samples).
Limitations
Relatively small group.
Conclusion
Epidural triamcinolone suppresses unstimulated and CRH-stimulated ACTH and cortisol secretion for 1–4 wk but urinary free cortisol ≥12 wk. Suppression of ACTH and cortisol after glucocorticoid treatment is thus complex.
Keywords: glucocorticoid, ACTH, cortisol, human, inhibition
Introduction
Corticotrope-adrenal function is suppressed by parenteral, oral, topical, rectal and inhaled glucocorticoids [1]. ACTH-adrenal suppression is less well characterized after glucocorticoid injection into confined compartments, such as epidural, intrathecal, or joint spaces. The extent of corticotrope-adrenal inhibition presumably reflects the type, dose, frequency and site of glucocorticoid injection, as well as its subsequent efflux and metabolism [2]. Theoretically, cortisol suppression by injected steroid could arise via any of several hypothalamic-pituitary-adrenal (HPA) routes. Indeed, case reports of secondary adrenal insufficiency after intrathecal steroid administration [3] and emergence of Cushing’s syndrome after extradural [4], paraspinal [5], and epidural [6] glucocorticoid injections could reflect both central and peripheral exposure.
The use of epidural glucocorticoid injections for chronic pain management has increased exponentially over the past decade [7]. An untoward clinical sequel is suppression of the corticotropic axis, inferred initially from markedly decreased cortisol concentrations one wk after administration of a single dose of epidural methylprednisolone [8]. Other measurements of corticotropic and adrenal function, such as ACTH, urinary free cortisol, and salivary cortisol also manifest inhibition within 24 to 48 h [9–12]. Suppression can occur as early as 15 min for ACTH and 30 min for cortisol after epidural triamcinolone administration [13]. Moreover, suppression of insulin-induced hypoglycemia-stimulated cortisol secretion occurs after epidural injection of triamcinolone in dogs [14], and impairment of adrenal cortisol secretion occurs in response to synthetic ACTH stimulation after extradural methylprednisolone and epidural prednisone administration in humans [9, 15]. The duration of suppression after paraspinal glucocorticoid injections is less well defined, possibly up to 21 days after a single glucocorticoid dose [10], and 3 months after repeated dosing [13]. However, in mechanistic assessments, measurements of glucocorticoid concentrations in spinal fluid, blood and urine have not yielded conclusive results [3, 9, 16]. Moreover, ACTH responses to CRH are largely unknown in the settings.
The present study quantifies HPA disruption after epidural triamcinolone injection by measuring plasma ACTH and serum cortisol concentrations every 10 min for 4 h on each of 4 successive occasions (0 baseline, 1, 4 and 12 wk) along with serum and urinary triamcinolone concentrations, as well as 24-hr urinary free cortisol, and the urinary cortisol/cortisone ratio by mass spectrometry. CRH was injected to evaluate corticotrope suppression.
Material and Methods
Subjects
Eight men ages 25–63 yr with a clinical indication (back pain due to degenerative lumbar disc disease in 2 patients, and lumbosacral spondylosis in 6 men) for epidural glucocorticoid administration completed the study, which was approved by the Salem Veterans Affairs Medical Center Institutional Review Board. Participant voluntarily signed informed consent. Subjects were excluded with history of diabetes mellitus (DM), chronic lung disease, and/or psychiatric disorders, use of glucocorticoids by any route within 6 months, prior epidural, intrathecal or any other central nervous system (CNS) glucocorticoid administration, chronic use of anticonvulsants or opiates, substance abuse including ethanol, and hemoglobin less than 13 g/dL. Subjects were recruited over a 5-year period, and the study was conducted at the Salem V.A. Medical Center, Salem, VA USA.
Protocol
Each subject was studied after an overnight fast on 4 separate occasions: baseline (wk 0), as well as 1, 4, and 12 wk after epidural injection of 80 mg triamcinolone Kenalog (Bristol-Meyers Squibb, Princeton, NJ), which is a standard epidural dose for pain management. Sessions started at 0900 h, and continued for a period of 4 h of blood sampling at 10-min intervals. Synthetic ovine corticotropic hormone (CRH) (1 ug/kg) was injected at 1000 h. Urine was collected for 24 h just prior to each session.
Assays
ACTH and cortisol concentrations were measured in each blood sample collected at 10-min intervals over the 4-h study period by Immulite 2000 (Siemens Healthcare Diagnostics, Flanders, NJ). The assay for cortisol has a detection range of 0.2–50 μg/dL, with intra- and interassay coefficients of variation of 7.2–9.4% and 6.3–9.4% at respective concentrations of 3.8–44 and 3.7–41 μg/dL. The ACTH assay has a detection range of 5–1250 pg/mL, with intra- and interassay coefficients of variation of 6.1–8.2% and 4.4–5.7% at respective concentrations of 32–417 and 30–446 pg/mL [17]. Urinary free cortisol was assayed by radioimmunoassay (RIA) using reagents from DiaSorin (Stillwater, Mn). Serum (4-h pool) and urine (aliquot of the 24-h collection) triamcinolone concentrations were measured by mass spectrometry, as described [18]. Urinary cortisol/cortisone rations were also measured by mass spectrometry in the 24-hr collections [19].
Deconvolution Analysis
Plasma ACTH and cortisol time series were subjected to deconvolution analysis using a Matlab-implemented maximum-likelihood methodology [20]. The two-component cortisol half-life model was 2.4 and 56 min (63% slow decay), and that of ACTH was 3.5 and 18 min (63% slow component) [21]. Outcome variables were basal (nonpulsatile), pulsatile and total (sum of basal plus pulsatile) secretion, and the mass (concentration units), number (per study period), and duration (mode) of ACTH and cortisol secretory bursts [20, 22].
Statistical analysis
Repeated-measures analysis of variance (ANOVA) was used to assess time-dependent effects across the 4 treatment sessions [23]. Dunnett’s test was used for post hoc comparisons. Primary outcomes were summed 1-h pre-CRH and summed 3-h post-CRH plasma ACTH and serum cortisol concentrations, measured 24-h urinary free cortisol concentrations, and deconvolution-estimated ACTH and cortisol secretion rates. Data are presented as the mean ± SEM. Significance was construed for experiment-wise P<0.05.
Results
Mean (± SEM) 10-min sampled ACTH and cortisol concentration time series in all 8 subjects are illustrated in Figure 1 (A & B). ANOVA revealed significant overall suppression and recovery of summed 60-min pre-CRH-injected plasma ACTH and serum cortisol concentrations at respective P-values of 0.0002 and <0.0001. Epidural triamcinolone administration decreased (60-min mean) ACTH (from 18 ± 3.1 to 4.8 ± 0.4; P<0.0001) and cortisol (from 12 ± 1.6 to 1.6 ± 0.3; P<0.0001) at wk 1, with recovery to values not significantly different from the baseline at wk 4 (Figure 2A and 2B). As a group, the ACTH response to injected CRH was blunted 1 wk after triamcinolone administration; viz., 3-h post-CRH summed ACTH concentrations fell from 633 ± 116 to 129 ± 10 pg/mL/min (P<0.0001) and summed cortisol concentrations from 385 ± 29 to 56 ± 22 μg/dL/min (P<0.0001). Whereas the ACTH response to CRH normalized at wk 4, the adrenal cortisol response was still suppressed (284 ± 53 vs 385 ± 29: P<0.01), but recovered fully at wk 12. Moreover, in individual subjects, for a normal serum cortisol cut-off > 18 μg/dL, 30 min post-CRH values remained suppressed in all 8 subjects at wk 4, and in 6 of 8 individuals at wk 12. At min 60 post-CRH, cortisol concentrations continued to be lower than 18 μg/dL in 4 and 2 men, respectively at weeks 4 and 12. Figure 3 demonstrates decreases in 24-h urinary free cortisol concentrations (μg/day) from 60 ± 6.5 to 9.0 ± 1.3 at wk 1 (P<0.0001), rising somewhat at wk 4 (36 ± 8.6) and also at wk 12 (38 ± 4.1). One and 3-mo values remained significantly below baseline (P<0.05). Urinary ratios of cortisol to cortisone at wks 0, 1, 4 and 12 were 4.94 ± 0.50, 4.76 ± 0.67, 5.74 ± 0.53 and 4.14 ± 0.38, respectively (P=0.0014 for wk 4 vs wk 12 contrast).
Figure 1.
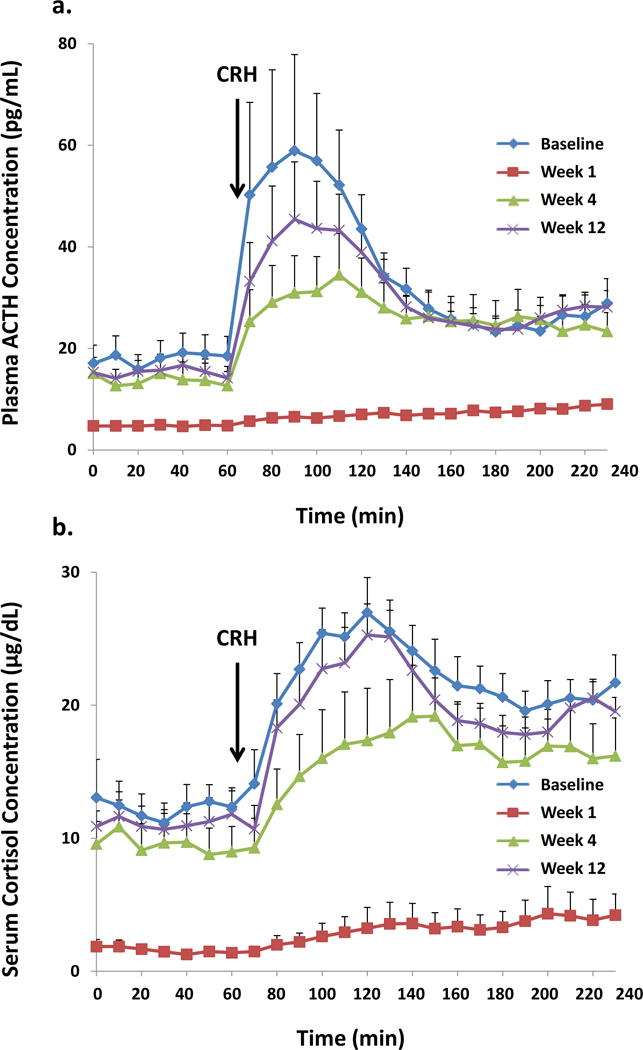
Mean (± SEM) plasma ACTH (Panel A) and serum cortisol (Panel B) concentrations sampled at 10-min intervals for 4 h in 8 volunteers. CRH was injected immediately after the 60-min blood sample. Data were obtained at baseline (wk 0) and again after 1, 4 and 12 wk.
Figure 2.
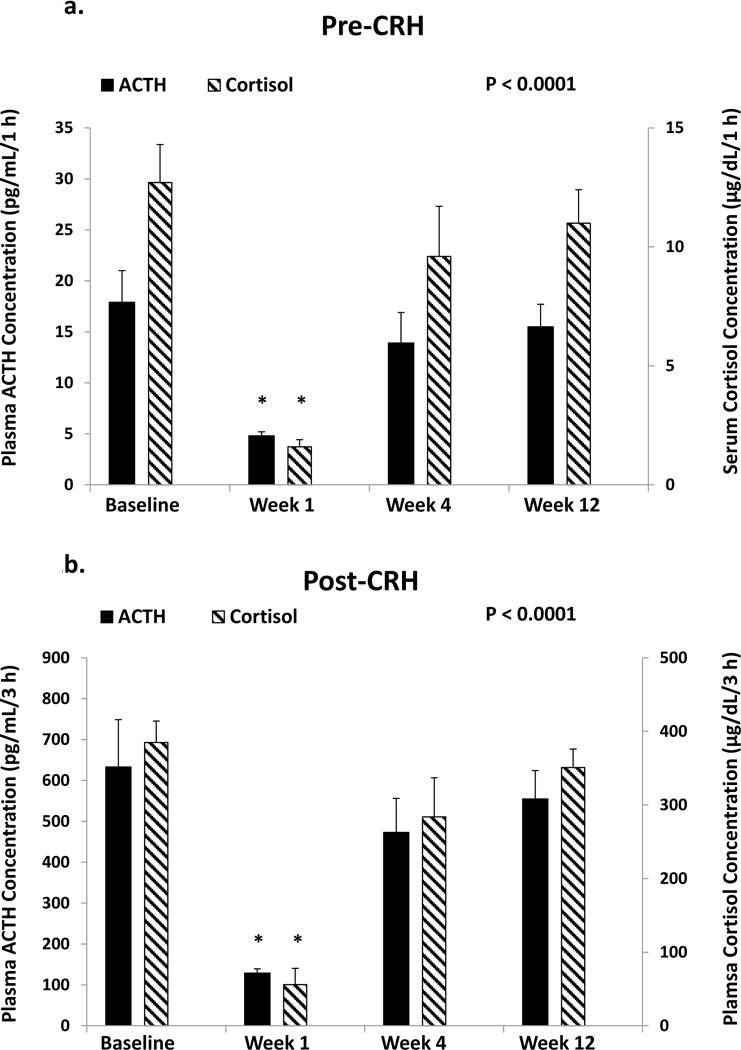
Mean (± SEM) plasma ACTH concentrations obtained over 1 h before (pre-CRH, Panel A) and 3 h after (post-CRH, Panel B) CRH injection (solid bars, left axis label) and matching cortisol concentrations (checkered bars, right axis) in 8 subjects studied as described in Figure 1. Asterisks denote P<0.01 vs all other data on post hoc testing. The overall P value of <0.0001 denotes the time effect by repeated-measures ANOVA.
Figure 3.
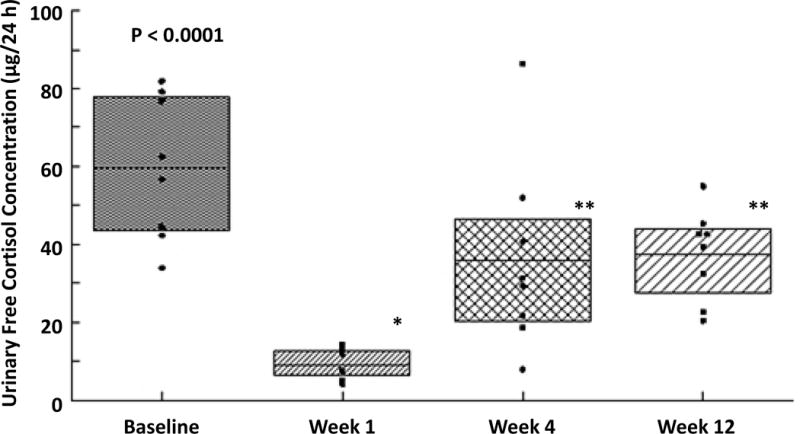
Twenty-four h mean (± 95% CI: shaded area; horizontal mean line; dots are individual data) urinary free cortisol concentrations at baseline (wk 0), and 1, 4, and 12 wk after triamcinolone injection. *P<0.01 and **P<0.05 vs baseline (wk 0) data. Overall ANOVA P<0.0001.
By deconvolution analysis, total secretion of ACTH (pg/mL/4 h) and cortisol (μg/dL/4 h) decreased markedly 1 wk after triamcinolone administration (ACTH secretion fell from 447 ± 83 to 92 ± 6.8; cortisol secretion from 92 ± 5.8 to 14 ± 5.2, P<0.0001). Triamcinolone inhibited both basal and pulsatile ACTH and cortisol secretion (all P values <0.005). The latter was due to suppressed ACTH and cortisol secretory-burst mass (P<0.005). Total (pulsatile plus basal) secretion rates remained lower at wk 4 than at wk zero for ACTH (312 ± 55 vs 447 ± 83: P<0.05) and cortisol (67 ± 14 vs 92 ± 5.8: P<0.01): Table 1A. Secretion recovered fully at 12 wk.
Table 1A.
Deconvolution-estimated 4 h ACTH and cortisol secretion at baseline and 1, 4 and 12 wk after epidural administration of triamcinolone
| ACTH (pg/mL/4h) | Cortisol (μg/dL/4h) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Study session | Basal sec | Pulsatile sec | Total sec | Mass/bur st | Basal sec | Pulsatile sec | Total sec | Mass/bur st |
| baseline | 247±47 | 200±54 | 447±83 | 67±19 | 45±4.7 | 47±5.4 | 92±5.8 | 17±3.0 |
| week 1 | 64±10* | 28±9.3* | 92±6.8* | 5.9±1.7* | 4.0±0.6 | 10±5.8** | 14±5.2 | 3.6±1.9* |
| ** | ** | * | ||||||
| 312±55 | 22±8.0* | 67±14* | ||||||
| week 4 | 167±47 | 145±24 | ** | 33±6.0** | * | 45±10 | * | 13±3.1 |
| week 12 | 238±31 | 137±31 | 375±44 | 51±8.2 | 39±9.5 | 47±5.3 | 86±7.7 | 19±2.3 |
|
|
||||||||
| <0.000 | ||||||||
| P value | 0.0007 | 0.004 | <0.0001 | 0.0024 | 0.0001 | 0.0006 | 1 | 0.0003 |
|
|
||||||||
Boldface data are significant at
P<0.01,
P<0.05: compared with baseline by Dunnett’s test (within-column test).
Data are the mean ± SEM. For mass/burst, units are concentration.
Overall P value was estimated by repeated-measures ANOVA.
Triamcinolone concentrations peaked 1 wk after epidural injection both in serum (0.295 ± 0.077 μg/dL) and urine (0.892 ± 0.196 μg/dL), and then decreased significantly to 0.053 ± 0.019 (serum) and 0.292 ± 0.101 (urine) at 4 wk, and to 0.002 ± 0.001 (serum) and 0.024 ± 0.01 (urine) at 12 wk [P<0.001]: Table 1B.
Table 1B.
Serum and urinary triamcinolone concentrations in 8 men, before baseline and at wk 1, 4 and 12 after epidural administration of 80 mg triamcinolone
| Serum triamcinolone (μg/ML) | Urinary triamcinolone (μg/dL) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Study subjects | baseline | week 1 | week 4 | week 12 | baseline | week 1 | week 4 | week 12 |
| 1 | 0 | 0.112 | 0 | 0 | 0 | 0.337 | 0.049 | 0 |
| 2 | 0 | 0.190 | 0.071 | 0.009 | 0 | 0.797 | 0.484 | 0.063 |
| 3 | 0 | 0.207 | 0.126 | 0 | 0 | 0.249 | 0.895 | 0.068 |
| 4 | 0 | 0.282 | 0.108 | 0 | 0 | 0.729 | 0.381 | 0.039 |
| 5 | 0 | 0.279 | 0.021 | 0.006 | 0 | 1.5 | 0.177 | 0.026 |
| 6 | 0 | 0.819 | 0 | 0 | 0 | 1.89 | 0.079 | 0 |
| 7 | 0 | 0.218 | 0.096 | 0 | 0 | 0.669 | 0.152 | 0 |
| 8 | 0 | 0.249 | 0 | 0 | 0 | 0.938 | 0.118 | 0 |
|
|
||||||||
Drug levels were determined by mass spectrometry. Zero denotes undetectable.
Discussion
Epidural triamcinolone administration markedly suppressed multiple measures of the corticotropic axis, viz., both pre- and post-CRH stimulated plasma ACTH and serum cortisol concentrations, basal and pulsatile (and thus total) ACTH secretion rates, and 24-h urinary free cortisol concentrations. Triamcinolone-induced suppression of ACTH and cortisol concentrations after CRH stimulation reflected marked inhibition of pulsatile ACTH and cortisol secretion. HPA activity was maximally blunted at wk one after triamcinolone administration. However, inhibition of CRH-stimulated ACTH and cortisol secretion persisted for 4 wk, and of 24-h urinary free cortisol concentrations for over12 wk. The latter could indicate lack of full recovery of HPA axis free cortisol levels in blood, possibly due to suppressed hypothalamic CRH release, which was not tested in this study, or altered dynamics of cortisol binding to transcortin (CBG). Urinary free cortisol is a product of glomerular filtration of plasma protein-unbound cortisol.
Modulation of corticotropic function after glucocorticoid injection into paraspinal and epidural spaces has been investigated sporadically over the past decades [3, 8–13, 15]. However, dynamic testing of the HPA axis in this setting is quite limited. The present study used CRH to evaluate the corticotrope suppression after epidural administration of triamcinolone. The CRH stimulation test was used to test pituitary ACTH inhibition by the injected glucocorticoid. Reduced ACTH secretion in response to CRH would reflect direct inhibition of anterior pituitary ACTH secretion. Maximal inhibition of ACTH occurred at 1 wk, 30% inhibition of cortisol at 4 wk, and full recovery of both ACTH and cortisol at wk 12. Serum cortisol 30 min after CRH injection was <18 μg/dL in 8 of 8 men at wk 3, and 6 of 8 men at wk 12, denoting reduced responsitivity of the HPA axis. At 1 hr after CRH injection, serum cortisol failed to rise to >18 μg/dL in 4 of 8 patients at wk 4, and 2 of 8 patients at wk 12.
Mass spectrometry was used to quantify injected glucocorticoid concentrations. Earlier studies have not been conclusive [3, 9, 16]. One reported persistent prednisolone concentrations in cerebrospinal fluid (CSF) after 6 days, and another after 2 mo, following spinal methylprednisolone injection [3, 24]. In other analyses, serum prednisolone was not measurable after extradural steroid injection [9], whereas urinary synthetic glucocorticoid was detectable [16]. In the present study, triamcinolone was measurable in the blood and urine after epidural administration, with maximal concentrations after 1 wk, decreased values after 4 wk, and low to undetectable levels after 12 wk. A presumptively slower rise in serum triamcinolone after epidural than intraarticular injection, inferable from the literature, may reflects greater absorptive capacity of the joint than the epidural tissues.
Outcomes of this investigation must be interpreted in the context of strengths and limitations. Strengths are precise estimates of unstimulated and CRH-stimulated corticotropic and adrenal secretion (rather than just direct stimulation of adrenal function with ACTH injection) achieved by repetitive 10-min sampling for 4 h; estimation of ACTH and cortisol secretory rates by deconvolution; quantification of 24-h urinary free cortisol concentrations and quantification of triamcinolone in blood and urine at 0, 1, 4 and 12 wk. Limitations include sample size, and lack of spinal-fluid triamcinolone measurements. Peak triamcinolone concentrations, and times to suppression and recovery of the HPA axis could be established more precisely by more frequent surveillance.
Conclusion
In conclusion, epidural administration of triamcinolone markedly suppresses the HPA axis within 1 wk, followed by a gradual return of function over 4–12 wk. Although unstimulated mean blood ACTH and cortisol concentrations recover within 4 wk, at least 3 mo are required for full recovery of urinary free cortisol and clearance of injected glucocorticoid. The latter findings have significant clinical implications, particularly if the procedure is used repeatedly, which could further prolong the time to recovery. These data may aid physicians in interpreting ACTH and cortisol measurements after epidural glucocorticoid treatment.
Statement of Human Rights and Animal Rights.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Statement of Informed Consent.
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
We thank Jill Smith for support of manuscript preparation and the Mayo Immunochemical Laboratory for assistance with mass spectrometry (RJS).
Funding: The Mayo Immunochemical Laboratory provided assistance with mass spectrometry (RJS). Supported in part via R01 DK073148 (JDV) from the National Institutes of Health (Bethesda, MD), and the Salem Research Institute of the Salem VA Medical Center (AI). Contents are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
Funding Statement: This study was funded in part by the National Institutes of Health in Bethesda, MD [R01 DK073148 (JDV)], and the Salem Research Institute of the Salem VA Medical Center (AI).
Abbreviations
- ACTH
adrenocorticotropin
- ANOVA
analysis of variance
- CI
confidence interval
- CNS
central nervous system
- CRH
corticotropin-releasing hormone
- CSF
cerebrospinal fluid
- DM
diabetes mellitus
- HPA
hypothalamic-pituitary-adrenal
- RIA
radioimmunoassay
- SEM
structure equation of modeling
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Author Statement: All authors have had access to the data and a role in writing the manuscript.
Author Contributions: Conception and design of research (AI, JDV, DG); performed experiments (AI, DG); analyzed data (JDV); interpreted results of experiments (AI, JDV); drafted manuscript (AI, JDV); prepared figures (AI, JDV); edited and revised manuscript (AI, JDV); approved final version of manuscript (AI, DG, RS, JDV).
Reference List
- 1.Chrousos, Pavlaki AN, Magiakou MA. In: Glucocorticoid Therapy and Adrenal Suppression. de Groote L, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. South Dartmouth, MA: MDText.com, Inc; 2000. Online Textbook. Endotext ( www.endotext.org) [Google Scholar]
- 2.Osterlund C, Spencer RL. Corticosterone pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis activity via multiple actions that vary with time, site of action, and de novo protein synthesis. J Endocrinol. 2011;208:311–322. doi: 10.1530/JOE-10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chernow B, Vigersky R, O’Brian JT, Georges LP. Secondary adrenal insufficiency after intrathecal steroid administration. J Neurosurg. 1982;56:567–570. doi: 10.3171/jns.1982.56.4.0567. [DOI] [PubMed] [Google Scholar]
- 4.Knight CL, Burnell JC. Systemic side-effects of extradural steroids. Anaesthesia. 1980;35:593–594. doi: 10.1111/j.1365-2044.1980.tb03858.x. [DOI] [PubMed] [Google Scholar]
- 5.Edmonds LC, Vance ML, Hughes JM. Morbidity from paraspinal depo corticosteroid injections for analgesia: Cushing’s syndrome and adrenal suppression. Anesth Analg. 1991;72:820–822. doi: 10.1213/00000539-199106000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Horani MH, Silverberg AB. Secondary Cushing’s syndrome after a single epidural injection of a corticosteroid. Endocr Pract. 2005;11:408–410. doi: 10.4158/EP.11.6.408. [DOI] [PubMed] [Google Scholar]
- 7.Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician. 2013;16:E349–E364. [PubMed] [Google Scholar]
- 8.Burn JM, Langdon L. Duration of action of epidural methyl prednisolone. A study in patients with the lumbosciatic syndrome. Am J Phys Med. 1974;53:29–34. [PubMed] [Google Scholar]
- 9.Jacobs S, Pullan PT, Potter JM, Shenfield GM. Adrenal suppression following extradural steroids. Anaesthesia. 1983;38:953–956. doi: 10.1111/j.1365-2044.1983.tb12025.x. [DOI] [PubMed] [Google Scholar]
- 10.Maillefert JF, Aho S, Huguenin MC, Chatard C, Peere T, Marquignon MF, Lucas B, Tavernier C. Systemic effects of epidural dexamethasone injections. Rev Rhum Engl Ed. 1995;62:429–432. [PubMed] [Google Scholar]
- 11.Younes M, Neffati F, Touzi M, Hassen-Zrour S, Fendri Y, Bejia I, Ben AA, Bergaoui N, Najjar MF. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74:472–476. doi: 10.1016/j.jbspin.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Chon JY, Moon HS. Salivary cortisol concentration changes after epidural steroid injection. Pain Physician. 2012;15:461–466. [PubMed] [Google Scholar]
- 13.Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary-adrenal axis in human subjects. Anesth Analg. 1994;79:501–505. doi: 10.1213/00000539-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Gorski DW, Rao TL, Glisson SN, Chinthagada M, El-Etr AA. Epidural triamcinolone and adrenal response to hypoglycemic stress in dogs. Anesthesiology. 1982;57:364–366. doi: 10.1097/00000542-198211000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Habib G, Jabbour A, Salman J, Hakim G, Haddad H. The effect of epidural methylprednisolone acetate injection on the hypothalamic-pituitary-adrenal axis. J Clin Anesth. 2013;25:629–633. doi: 10.1016/j.jclinane.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Lansang MC, Farmer T, Kennedy L. Diagnosing the unrecognized systemic absorption of intra-articular and epidural steroid injections. Endocr Pract. 2009;15:225–228. doi: 10.4158/EP.15.3.225. [DOI] [PubMed] [Google Scholar]
- 17.Iranmanesh A, Lawson D, Dunn B, Veldhuis JD. Glucose ingestion selectively amplifies ACTH and cortisol secretory-burst mass and enhances their joint synchrony in healthy men. J Clin Endocrinol Metab. 2011;96:2882–2888. doi: 10.1210/jc.2011-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs K, Wagley S, Quirk MT, Ceron OM, Silva PA, Singh RJ, Gukasyan HJ, Arroyo JG. Pharmacokinetic study of vitreous and serum concentrations of triamcinolone acetonide after posterior sub-tenon’s injection. Am J Ophthalmol. 2012;153:939–948. doi: 10.1016/j.ajo.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2002;48:1511–1519. [PubMed] [Google Scholar]
- 20.Veldhuis JD, Iranmanesh A, Roelfsema F, Aoun P, Takahashi PY, Miles JM, Keenan DM. Tripartite control of dynamic ACTH-cortisol dose-responsiveness by age, body mass index and gender in 111 healthy adults. J Clin Endocrinol Metab. 2011;96:2874–2881. doi: 10.1210/jc.2011-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bright GM. Corticosteroid-binding globulin influences kinetic parameters of plasma cortisol transport and clearance. J Clin Endocrinol Metab. 1995;80:770–775. doi: 10.1210/jcem.80.3.7883829. [DOI] [PubMed] [Google Scholar]
- 22.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29:823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar JH. Biostatistical analysis. 3rd. Upper Saddle River, NJ: Prentice Hall; 1996. [Google Scholar]
- 24.Sehgal AD, Tweed DC, Gardner WJ, Foote MK. Laboratory studies after intrathecal corticosteroids: determination of corticosteroids in plasma and cerebrospinal fluid. Arch Neurol. 1963;9:64–68. doi: 10.1001/archneur.1963.00460070074007. [DOI] [PubMed] [Google Scholar]


