| Summary: |
The invention in this patent application relates to
five-membered heteroarylcarboxamide derivatives represented generally
by formula I. These compounds are plasma kallikrein inhibitors and
may be used for the treatment and/or prophylaxis of diabetic complications,
particularly retinal vascular permeability associated with diabetic
retinopathy and diabetic macular edema. |
| Plasma
kallikrein (KLKB1) is a trypsin-like serine protease. It is secreted
by the hepatocytes in the liver as an inactive precursor named plasma
prekallikrein (PK). PK remains in the plasma either as a free zymogen
or as a heterodimeric complex with the high molecular weight kininogen.
This heterodimeric complex is activated by factor XII (FXII) to generate
the active plasma kallikrein; this activation affects the release
of kinins (including bradykinin) from kininogens. Kinins are potent
mediators of inflammation that act through G protein-coupled receptors
such as bradykinin receptors. |
| Available data
implicate plasma kallikrein activities in a number of inflammatory
disorders such as diabetic macular edema (DME) and hereditary angioedema
(HAE) among many others. The high levels of blood sugar can damage
blood vessels in the retina of diabetes patients causing them to leak
fluids into the retina, which is a disorder known as diabetic retinopathy.
DME occurs when fluids from the damaged blood vessels leak into the
macula. This disorder is a main cause of vision impairment or loss
in diabetes patients. HAE is a rare disease that is caused by an inherited
deficiency or mutation of the C1-inhibitor protein. This protein is
a physiological inhibitor of the plasma kallikrein protease. The disease
occurs because of excessive production of bradykinin in a process
mediated by plasma kallikrein. It is characterized by recurrent painful
edema that affect different parts of the body such as the hands, feet,
face, and abdomen. |
| The inventors mentioned
a very long list of additional inflammatory disorders that may be
caused by the activities of plasma kallikrein including proliferative
and nonproliferative retinopathy, clinically significant macular edema
(CSME), cystoid macular edema (CME), CME following cataract extraction,
CME induced by cryotherapy, CME induced by uveitis, endophthalmitis,
CME following vascular occlusion (e.g., central retinal vein occlusion,
branch retinal vein occlusion, or hemiretinal vein occlusion), retinal
edema, complications related to cataract surgery in diabetic retinopathy,
hypertensive retinopathy, retinal trauma, dry and wet aged-related
macular degeneration (AMD), polypoidal choroidal vasculopathy (PCV),
intracerebral hemorrhage, hemorrhagic transformation of ischemic stroke,
cerebral trauma associated with injury or surgery, brain aneurysm,
and many more. |
| Therefore, the inhibition
of plasma kallikrein may be a useful therapeutic target to develop
medicaments for the treatments of many of these disorders, particularly
those associated with edema formation such as edema related to ischemic
reperfusion injuries, DME, HAE, and brain edema. Plasma kallikrein
inhibitors may particularly be useful in the treatment of retinopathy,
e.g., retinopathy associated with diabetes and/or hypertension, and
in the treatment of macular edema, e.g., macular edema associated
with diabetes and/or hypertension. |
| While
there are plasma kallikrein inhibitors already known in the art; there
exists a need for inhibitors with enhanced potency and bioavailability,
high metabolic and/or chemical stability, high selectivity and tolerability,
desirable plasma protein binding, and improved pharmacokinetic profiles.
The compounds of formula I in this patent application are described
to possess these properties and may potentially provide needed useful
treatments for many of the above-mentioned disorders, particularly
in reducing retinal vascular permeability associated with diabetic
retinopathy and DME retinopathy or edema-associated diseases. Kallikrein
inhibitors may treat additional diabetes complications such as cerebral
hemorrhage, nephropathy, cardiomyopathy, and neuropathy. |
| Important Compound Classes: |
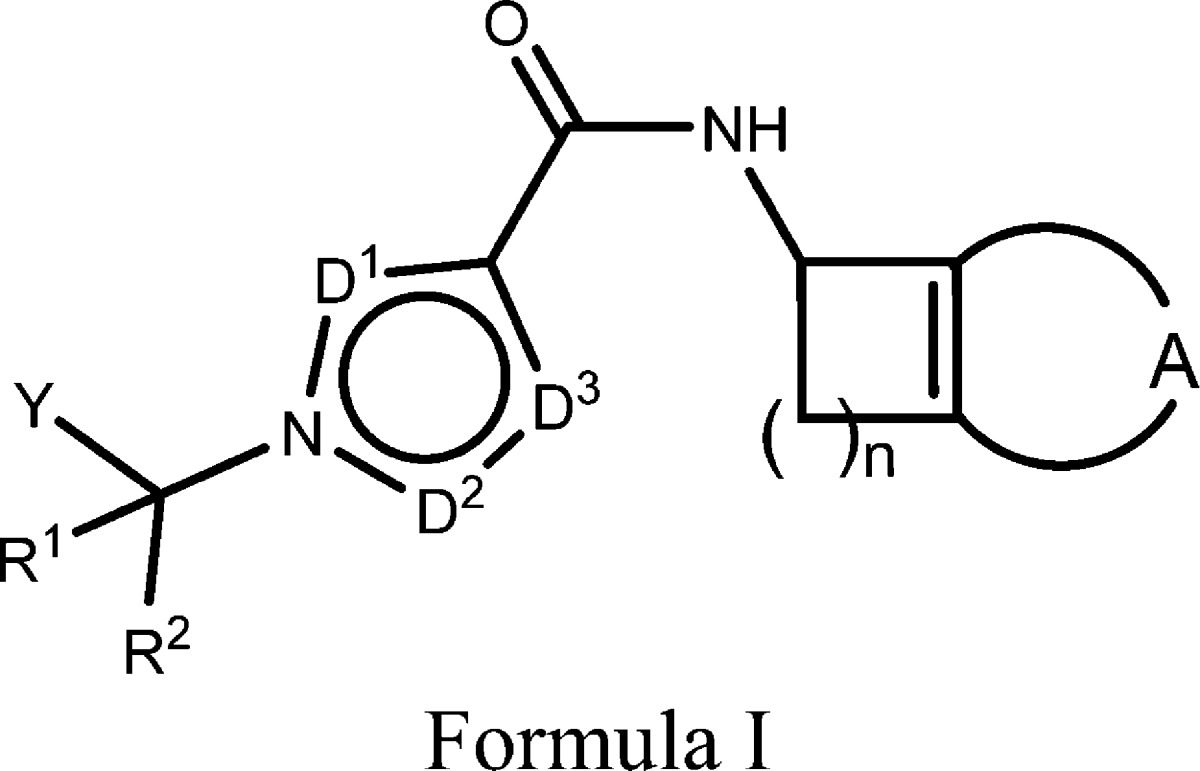 |
| Key Structures: |
The inventors
described 16 examples of formula I including the following representative
examples: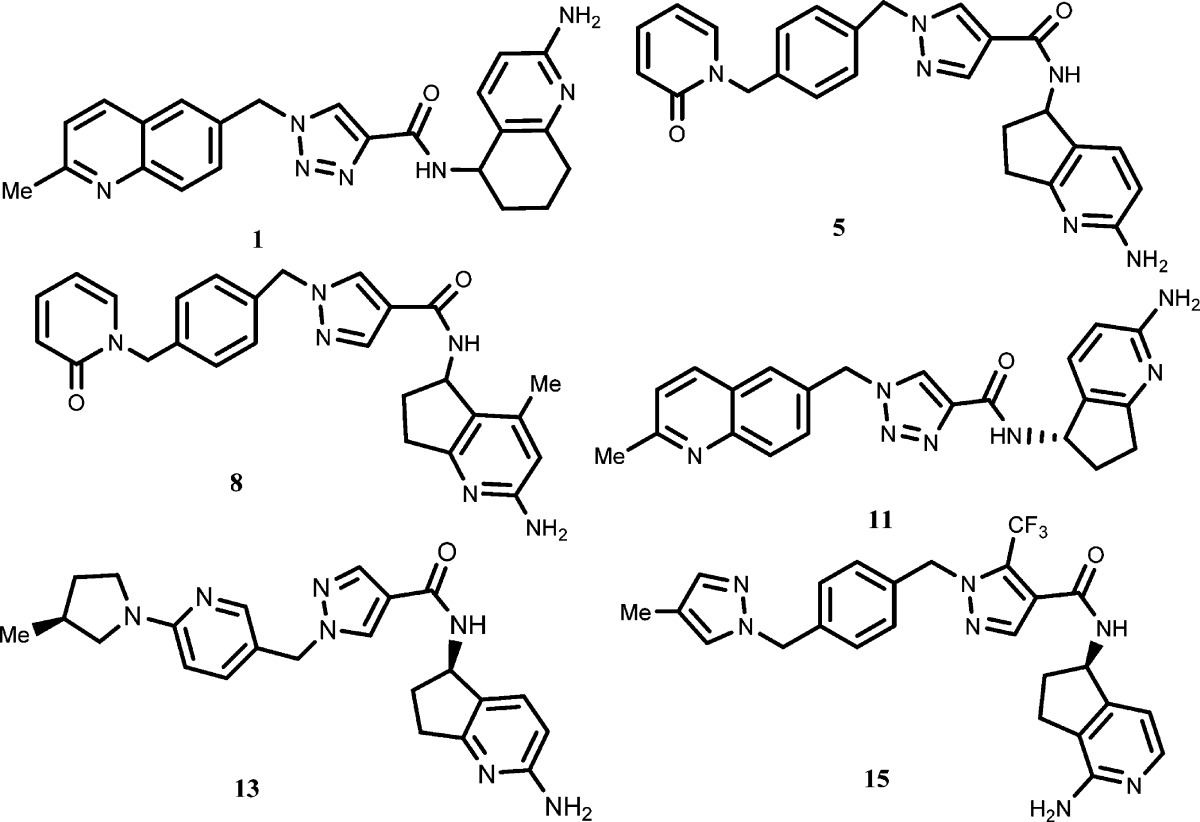
|
| Biological Assay: |
The following are some of the assays reported by the
inventors: |
Evaluation of the inhibition of KLKB1 using an end point assay Evaluation of the inhibition of human KLKB1
in Dextransulfat activated human PPP Evaluation of the inhibition of KLKB1 (Ki) Evaluation of the inhibition of FXIIa
(Ki) |
| Biological Data: |
The IC50 values for the inhibition of KLKB1 obtained from testing
the above representative examples are listed in the following table: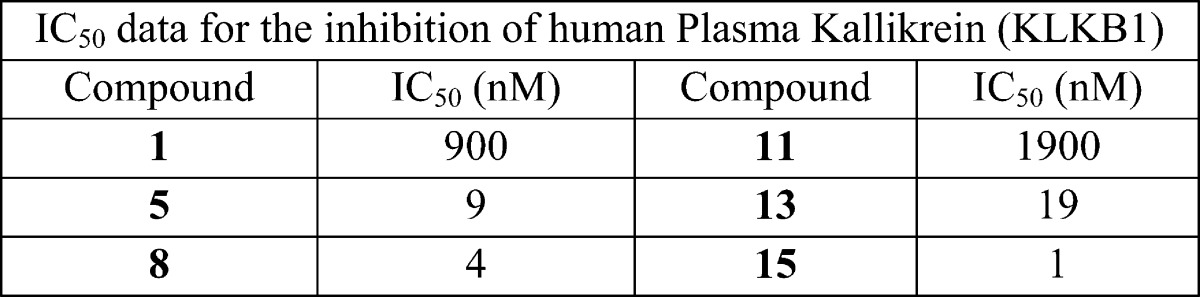
|
| Recent Review Articles: |
1 Kolte D.; Shariat-Madar Z.. Cardiol. Rev. 2016, 24 ( (3), ), 99–109. |
| 2 Murakami T.Diabetes 2015, 64 ( (10), ), 3350–3352. |
| 3 Masuda T.; Shimazawa M.; Hara H.. Eur. J. Pharmacol. 2015, 749, 161–163. |
| 4 Feener E. P.; Zhou Q.; Fickweiler W.. Thromb. Hemost. 2013, 110 ( (3), ), 434–441. |





