| Summary: |
The invention in this patent application relates to
substituted benzothiadiazine derivatives represented generally by
formula I. These compounds are inhibitors of CD73 and may potentially
be useful for the treatment of cancer, precancerous syndromes, and
other diseases associated with CD73 activities, such as AIDS, autoimmune
diseases, infections, atherosclerosis, and ischemia-reperfusion injury. |
| Conditions such as infection, tissue injury, ischemia,
or intervention-induced tumor cell death cause sharp elevation of
local extracellular adenosine triphosphate (ATP). Increased level
of ATP serves as a danger signal to alert the immune system to initiate
multiple pro-inflammatory events, including the recruitment of macrophages
and dendritic cells. The extracellular ectonucleotidases CD73 and
CD39 dephosphorylate extracellular ATP to lower its levels and replace
it with extracellular adenosine. This results in sharp increase of
the concentration of adenosine from a low homeostatic level of 20–200 to 1000–10000 nM. Elevated adenosine concentrations engage the immunosuppressive
actions of adenosine A2A and A2B receptors on the infiltrating lymphocytes
to shield the cells from excessive inflammatory response and thereby
provide a self-limiting mechanism to resolve the immune response.
It was observed that hypoxia can increase the adenosine levels in
a solid tumor by 10–20-fold
compared to normal cell levels. It is believed that such elevated
level of adenosine will maintain a chronic suppression of the innate
immune response, which leads to immune tolerance and subsequently
to uncontrolled malignant growth. |
| The cluster
of differentiation 73 (CD73), also known as 5′-ribonucleotide
phosphohydrolase, is a glycophosphatidylinositol-anchored di-Zn2+ metallo-phosphatase specific for the dephosphorylation of
purine and pyrimidine ribo- and deoxyribonucleoside monophosphates
to the corresponding nucleosides. CD73 has high affinity to adenosine
monophosphate (AMP) and catalyzes its conversion to the bioactive
adenosine. It is believed that this process is the major contributor
to extracellular adenosine formation in the tumor microenvironment.
Expression of CD73 is directly regulated by the hypoxia-inducible
factor 1α (HIF1α), which explains the observed increase
in extracellular adenosine under hypoxic conditions. CD73 was found
to be overexpressed in leukemia and in multiple solid tumor types,
including aggressive and difficult to treat tumors, such as glioblastoma
and ovarian tumors. Studies have shown that T-regulatory cells (TReg) (both circulating and tumor associated) express both CD73
and CD39 in patients with head and neck squamous cell carcinoma (HNSCC).
Thus, there is a mechanism to convert ATP to adenosine that depends
only on TReg cells. Multiple studies employing small-interfering
ribonucleic acids (siRNA), transgenic knockouts, and overexpression
models have confirmed the involvement of CD73 in the generation of
adenosine and promotion of immune tolerance. Therefore, inhibitors
of CD73 may potentially be able to relieve the adenosine-mediated
immunosuppression of the tumor microenvironment and may provide a
viable treatment for cancer, either alone or in combination with other
agents. |
| As a consequence of decreasing extracellular
adenosine by CD73 inhibitors, they may also be useful for the treatment
of other diseases mediated by adenosine and its action on adenosine
receptors. Thus, it is possible to use CD73 inhibitors for enhancing
immune responses, enhancing immunization, and increasing inflammatory
responses, as well as for the treatment of a wide range of conditions
including neurological, neurodegenerative, and CNS diseases, including
depression, Parkinson’s disease, cerebral and cardiac ischemic
diseases, sleep disorders, and fibrosis. |
| Important Compound Classes: |
 |
| Key Structures: |
The inventors
described the structures and syntheses of 187 examples of formula
I, including the following representative examples: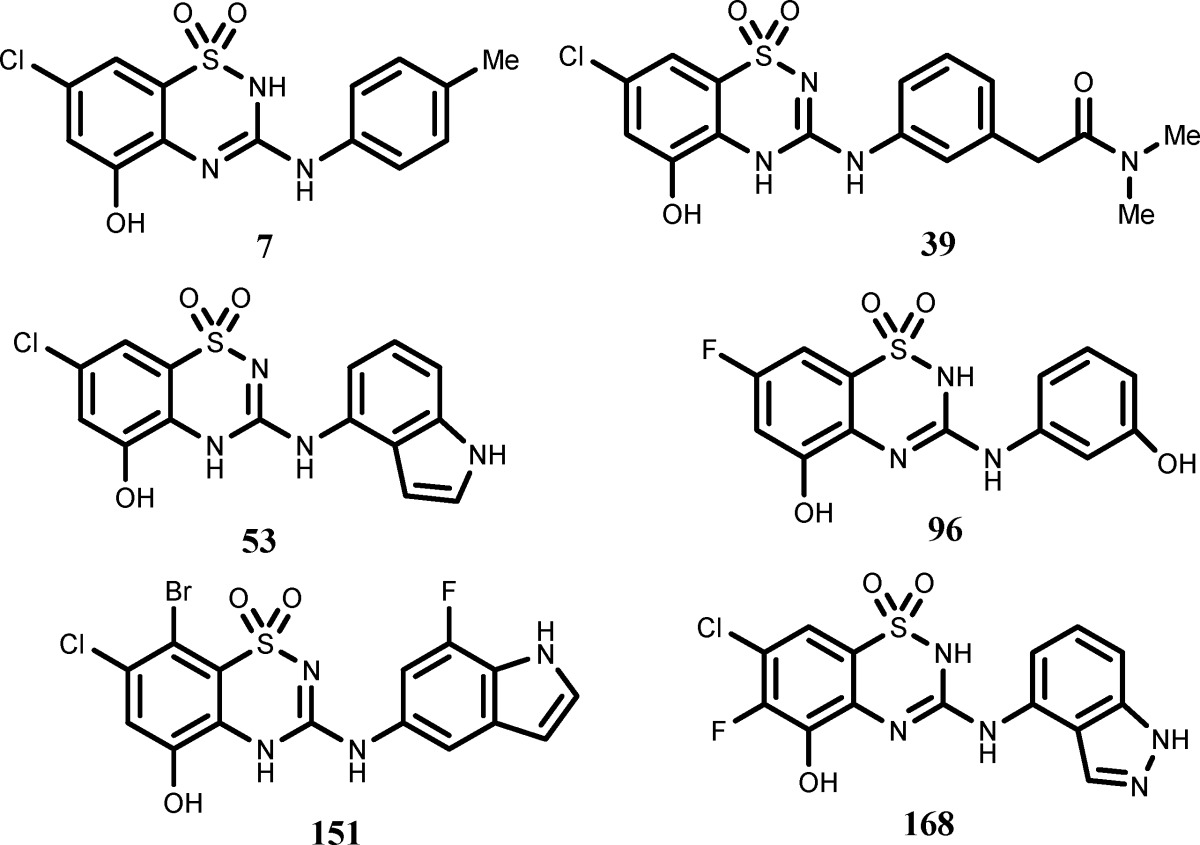
|
| Biological Assay: |
CD73 Assay |
| Biological Data: |
The
compounds of formula I were tested using the CD73 enzyme assay, and
in at least one experimental run in each case, they exhibited pIC50 values between 5 to 8.4 against CD73. The reported pIC50 values for the above representative examples are listed
in the following table:
|
| Recent Review
Articles: |
1. Dahan R.; Ravetch J. V.. Cancer
Cell 2016, 30 ( (3), ), 369–371. |
| 2. Allard B.; Turcotte M.; Stagg J.. Expert Opin. Ther. Targets 2014, 18 ( (8), ), 863–881. |
| 3. Beavis P. A.; Stagg J.; Darcy P.
K.; Smyth M.
J.. Trends Immunol. 2012, 33 ( (5), ), 231–237. |





