Abstract
The oxetane ring serves as an isostere of the carbonyl moiety, suggesting that oxetan-3-ol may be considered as a potential surrogate of the carboxylic acid functional group. To investigate this structural unit, as well as thietan-3-ol and the corresponding sulfoxide and sulfone derivatives, as potential carboxylic acid bioisosteres, a set of model compounds has been designed, synthesized, and evaluated for physicochemical properties. Similar derivatives of the cyclooxygenase inhibitor, ibuprofen, were also synthesized and evaluated for inhibition of eicosanoid biosynthesis in vitro. Collectively, the data suggest that oxetan-3-ol, thietan-3-ol, and related structures hold promise as isosteric replacements of the carboxylic acid moiety.
Keywords: Oxetan-3-ol, thietan-3-ol, carboxylic acid bioisostere, cyclooxygenase, lipoxygenase, dual inhibitors
The (bio)-isosteric replacement of the carboxylic acid moiety of biologically active compounds is a common strategy in medicinal chemistry that is frequently employed to improve/modify the pharmacokinetic and/or pharmacodynamic properties of compounds of interest.1−3 Although carboxylic acid isosteres are typically designed to mimic the carboxylic acid functional group, it is often the difference in structure and physicochemical properties of the isosteric replacement relative to the carboxylic acid that is critical to the success of this strategy. For this reason, and in consideration of the fact that the success of any isosteric replacement is typically context dependent, the evaluation and development of alternative surrogate structures that could complement and expand the existing set of bioisosteres continues to be a promising area of research.4−9
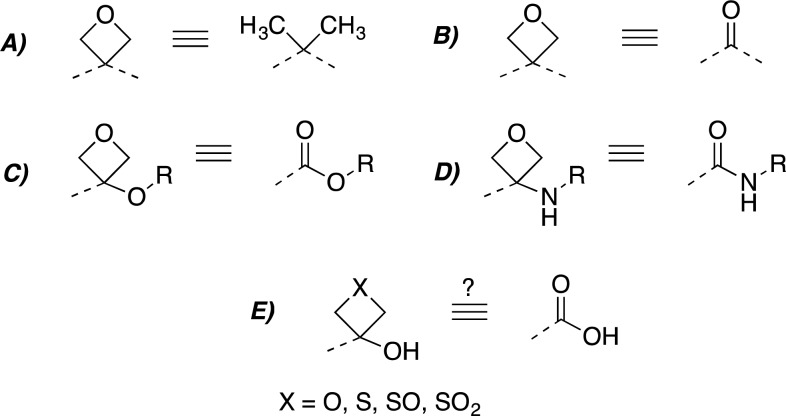
In recent years the oxetane ring has attracted considerable attention in medicinal chemistry10 due to the fact that this four-membered ring heterocycle can be used to modulate important physicochemical properties of molecules, including aqueous solubility,11 lipophilicity, and metabolic stability.12 A series of publications12−15 illustrated the potential of the oxetane ring as an isosteric replacement of the gem-dimethyl and the carbonyl group (Figure 1A,B). Importantly, 3-substituted oxetanes have been proposed as potential replacements of carboxylic esters and amides (Figure 1C,D).14,16−19 These findings indicate that the oxetan-3-ol could be a potentially promising replacement of the carboxylic acid. Thus far, however, an evaluation of this fragment as a carboxylic acid surrogate has not been reported (Figure 1E).
Figure 1.
Oxetane ring holds promise as an isosteric replacement of the gem-dimethyl (A), and the carbonyl group in the context of ketones (B), esters (C), and amides (D); similar replacements may be of interest in the context of carboxylic acids (E).
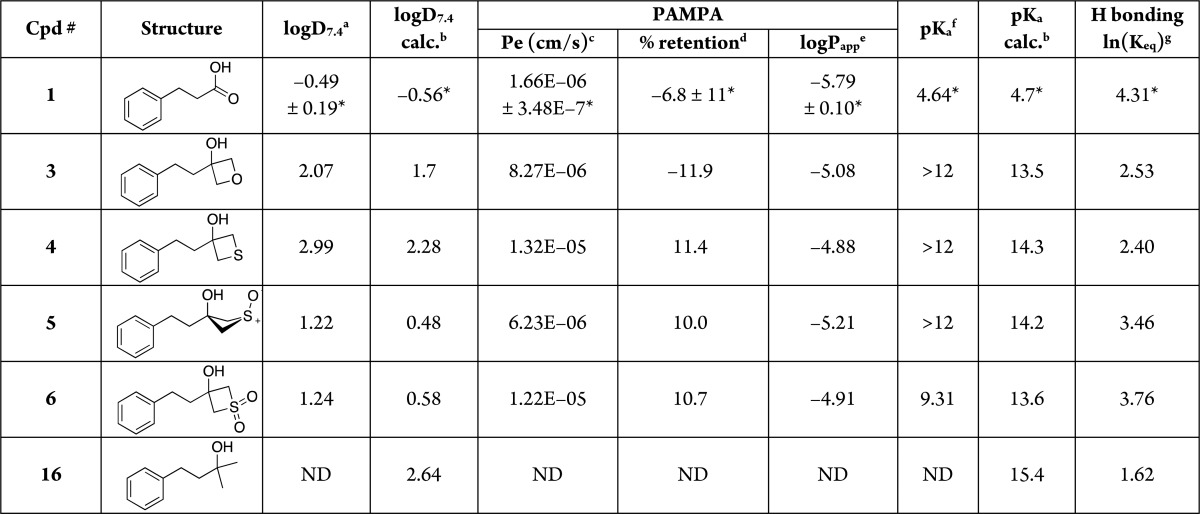
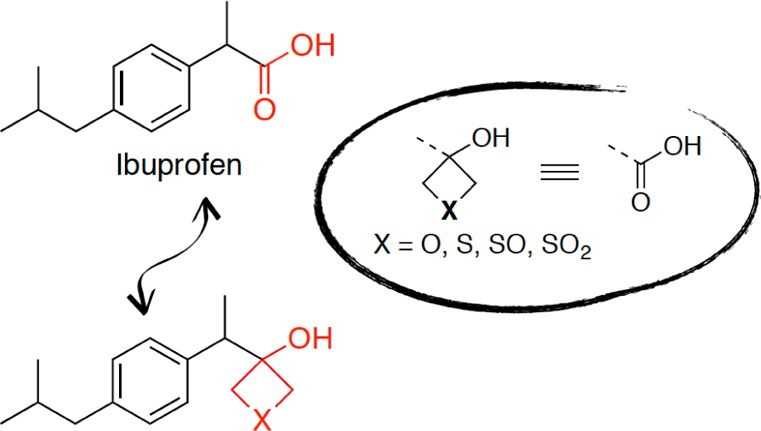
Given our ongoing interest in the area of carboxylic acid bioisosteres,6−8,20,21 we set out to investigate a range of physicochemical properties of the oxetan-3-ol, as well as the thietan-3-ol and the corresponding sulfoxide and sulfone structural units. To enable a more informative and rigorous comparison of the properties of these fragments relative to those of carboxylic acids and other known carboxylic acid bioisosteres, we constructed and evaluated a focused set of derivatives of the phenylpropionic acid (1, Table 1), as this carboxylic acid was already employed as a template structure in the synthesis and evaluation of a series of analogues comprising a wide selection of known carboxylic acid surrogates.20 The properties evaluated here include acidity (pKa), lipophilicity (logD7.4), and permeability in the parallel artificial membrane permeability assay (PAMPA). Hydrogen-bonding studies were also conducted to evaluate these fragments as hydrogen bond (HB) donors. In addition, to evaluate the potential of oxetan-3-ol, thietan-3-ol, and related sulfoxide and sulfone derivatives as replacements of the carboxylic acid moiety in the context of biologically active compounds, a small set of derivatives of the cyclooxygenase (COX) inhibitor, ibuprofen (2, Table 2), was designed, synthesized, and evaluated as inhibitors of eicosanoid formation in rat basophilic leukemia (RBL-1) cells.
Table 1. Calculated and Experimental Properties of Test Compounds.
Distribution coefficient between n-octanol and aqueous buffer (pH 7.4) determined by LC/MS (experiment run by WuXi AppTech).
Calculated values using ChemAxon.
Effective permeability (PAMPA assay run by Analiza, Inc.).
Membrane retention.
Log of the apparent permeability coefficient.
pKa values determined by capillary electrophoresis (experiment run by Analiza, Inc.).
Equilibrium constants (Keq) determined from a colorimetric assay that monitors the blue-shift of the maximum wavelength of a fluorescent pyrazinone HB acceptor upon complexation with the HB donor analyte. *Data previously reported.20 ND = not determined.
Table 2. IC50 Values of Test Compounds in the PGE2/PGD2 and LTB4 Assays.
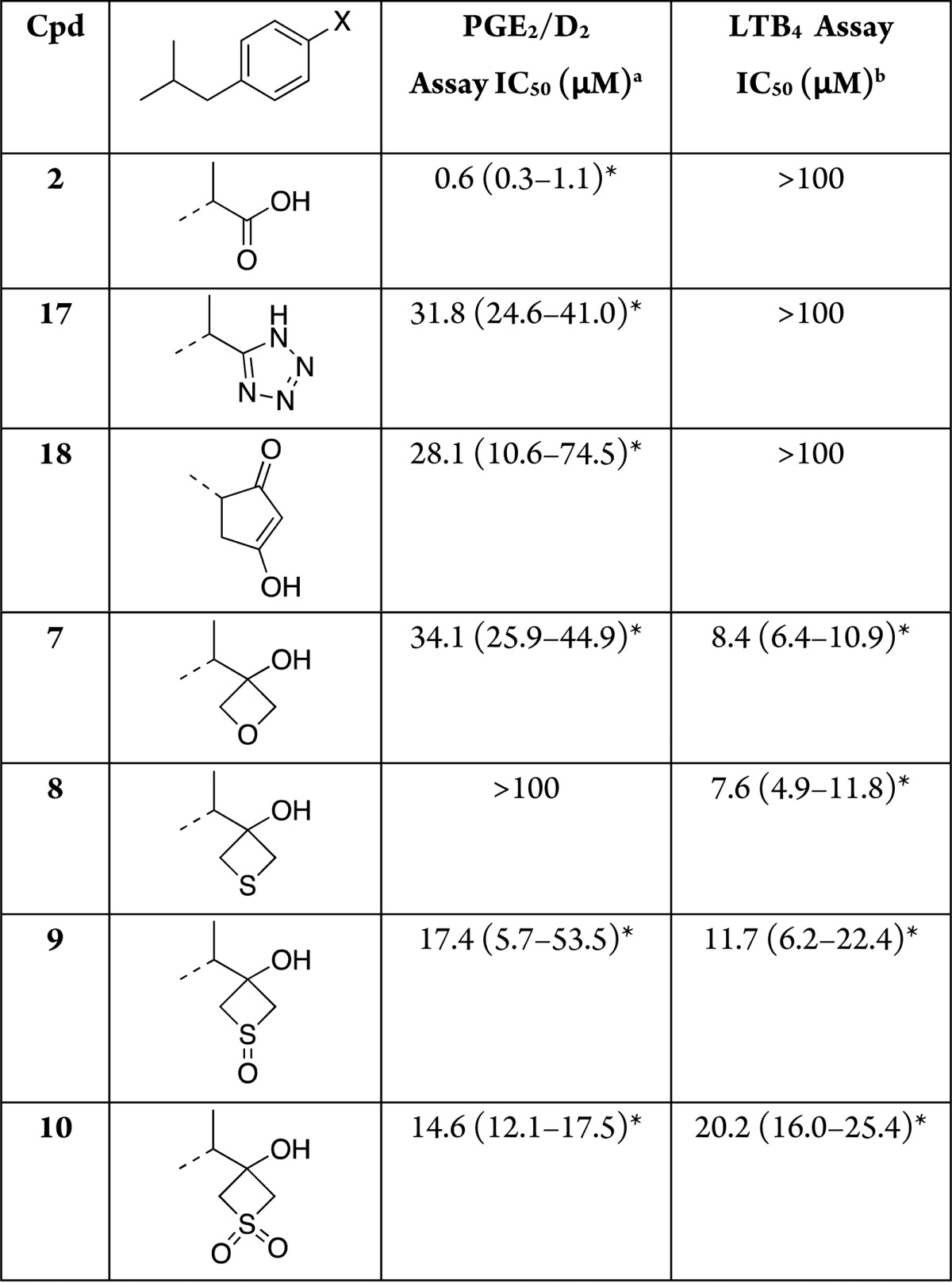
Inhibition of COX pathway as determined by LC–MS/MS analyses of the combined production of COX-derived PGD2 and PGE2 in RBL-1 cells upon stimulation with arachidonic acid in the presence or absence of test compounds.
Inhibition of 5-LOX pathway as determined by LC–MS/MS analyses of the production of 5-LOX-derived LTB4 in RBL-1 cells upon stimulation with arachidonic acid in the presence or absence of test compounds. *The data represent the calculated IC50 values and associated 95% confidence intervals as determined from triplicate samples at each concentration after a sigmoidal curve fit using GraphPad Prism software.
Collectively, the data generated from these studies provide a characterization of a range of physicochemical properties of oxetan-3-ol, thietan-3-ol, and related structures and, in turn, exemplify the possible utility of these fragments as replacements of the carboxylic acid functional group in drug design.
The synthesis of oxetane and thietane derivatives 3–6 and 7–10 was conducted as highlighted in Scheme 1. Model compounds 3–6 were constructed starting from phenethylmagnesium chloride 11 and the appropriate oxetan-3-one (12) or thietan-3-one (13) to give alcohols 3 and 4, respectively. Treatment of the thietan-3-ol derivative 4 with urea-hydrogen peroxide complex (UHP) in acetic acid led to the corresponding sulfoxide as a mixture (6:4) of cis- and trans-isomers, as determined by 1H NMR. When m-CPBA was used for the oxidation step, the cis/trans ratio was >98:2. Recrystallization of this mixture led to the formation of crystals of the cis-isomer 5 that were suitable for X-ray diffraction analysis (Scheme 1). Alternatively, oxidation of 4 with 2.2 equiv of oxone led to the formation of the fully oxidized sulfone derivative 6, a crystalline material that enabled X-ray diffraction analysis (see Supporting Information). In a similar fashion, the synthesis of ibuprofen derivatives 7–10 was carried out starting from known alkyl chloride 14, which was prepared in two steps from commercially available ketone 15. Grignard addition to 12 or 13 yielded the oxetan-3-ol derivative 7 and thietan-3-ol derivative 8, respectively. Oxidation of the latter compound with m-CPBA led to the sulfoxide derivative 9 (cis/trans ratio >20:1), while oxidation of 8 with oxone (2.2 equiv) resulted in sulfone derivative 10.
Scheme 1.
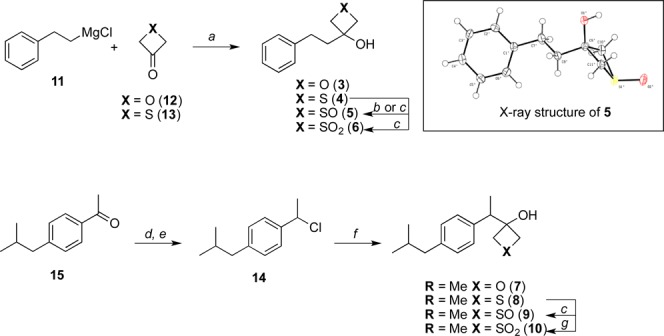
Reagents and reaction conditions: (a) THF, −78 °C, 15 min, then rt, 1 h; (b) UHP, AcOH, rt, 14 h; (c) m-CPBA, CH2Cl2, −78 °C, 1 h; (d) NaBH4, MeOH, 0 °C to rt, 30 min; (e) SOCl2, CH2Cl2, 0 °C to rt, 18 h; (f) (i) Mg, LiCl, ZnCl2, THF, rt, 6 h, (ii) 12 or 13, THF, rt, 17 h; (g) oxone, acetone/H2O, 0 °C to rt, 20 h.
Evaluation of physicochemical properties of model compounds 3–6 revealed that replacement of the carboxylic acid of 1 with these four-membered ring heterocycles results in a drastic reduction in acidic character (see Table 1). This is evident from the fact that sulfone derivative 6 exhibits a pKa value of ∼9.3, while all other derivatives were found to have pKa values >12 (i.e., above the range of the assay), with calculated values as high as ∼14. However, an evaluation of the hydrogen bond (HB) acidity of these compounds, which was conducted employing a previously reported colorimetric assay,22 suggested that these replacements cause a far less dramatic reduction in the HB acidity scale relative to 1. For example, compound 3 exhibited at least eight orders of magnitude lower acidity relative to 1, but less than two orders of magnitude weaker binding via hydrogen bonding to the fluorescent HB acceptor used in the assay (see equilibrium constant, Keq, values of test compounds 3–6, Table 1). Also of interest, the Keq values of 3–6 are significantly higher than that of alcohol 16, confirming that the four-membered ring heterocycles found in compounds 3–6 play an important role in determining the HB-donating ability of the hydroxyl moiety. These observations, combined with prior studies that established the remarkable HB basicity of the oxetane ring,23 suggest that the oxetan-3-ol 3 and related structures 4–6 have significant HB capacity.
The combination of limited acid character with the ability to establish HB may be a desirable feature of these structural units with respect to possible applications in drug design, especially in those circumstances where the presence of a negatively ionizable acid in a drug candidate may be responsible for an insufficient passive diffusion across biological membranes. Indeed, consistent with the relatively high pKa values and with these molecules being mostly neutral at physiological pH, all of the model compounds were found to be comparatively more lipophilic and permeable in PAMPA compared to 1. Furthermore, a comparison of physicochemical properties of model compounds 3–6 with 33 other phenylpropionic acid derivatives in which the carboxylic acid moiety was replaced by known carboxylic acid surrogates reveals that the four-membered ring heterocycles 3–6 are among the most permeable derivatives within the entire set (see Supplemental Figure 1, Supporting Information).
Evaluation of ibuprofen derivatives was conducted employing a modified RBL-1 cell assay that was previously developed for the evaluation of 5-lipoxygenase (5-LOX) inhibition,24 which we adapted to monitor for compound inhibition of both COX and 5-LOX biosynthetic pathways. In addition to oxetane and thietane derivatives (7–10), ibuprofen analogues bearing a tetrazole25 (17) and a cyclopentane-1,3-dione (18) were also constructed and tested for comparison. Typical assay conditions involved the coincubation of RBL-1 cells in 24-well plates with different concentrations of test compounds for 2 h, followed by the addition of the calcium ionophore, A23187 (12 μM), for 15 min to induce arachidonic acid production. Culture supernatants were subsequently collected and assessed for COX-derived prostaglandins (PGs) and 5-LOX-derived leukotrienes (LTs) by LC–MS/MS, as described in the Supporting Information. In initial concentration–response testing (Table 2), LTB4 and combined PGD2 and PGE2, which coeluted under the chromatographic conditions, were quantified. In subsequent studies, a refined LC–MS/MS protocol was employed that permitted separate analyses of PGD2 and PGE2, as well as LTB4 and LTC4 (see Supporting Information).
Interestingly, the RBL-1 cell assay results revealed that replacement of the carboxylic acid moiety of 2 with the comparatively less acidic and more permeable four-membered ring structures results in analogues (7–10) that, unlike 2 as well as the tetrazole (17) and the cyclopentane-1,3-dione (18) derivatives, inhibit 5-LOX-mediated synthesis of LTB4. Furthermore, with the exception of thietan-3-ol derivative 8, analogues 7, 9, and 10 were found to inhibit the formation of both COX- and 5-LOX-derived eicosanoids with 9 and 10 exhibiting balanced inhibition activity in the micromolar range (see Figure 2). Although the RBL-1 assay does not permit us to unambiguously determine the enzymes in the arachidonic acid cascade that are inhibited by the test compounds, the observation that 7, 9, and 10 effectively reduce the formation of multiple PGs and LTs (see supplemental Table 1, Supporting Information) suggests that such inhibition is likely to take place at the COX and 5-LOX enzymes. These findings appear to be generally consistent with prior reports showing that selected bioisosteric replacements of the carboxylic acid moiety of different nonsteroidal anti-inflammatory drugs (NSAIDs), including 2, can result in inhibitors of the 5-LOX pathway or multitargeted derivatives capable of inhibiting concurrently multiple enzymes in the COX- and 5-LOX biosynthetic pathways.26,27
Figure 2.
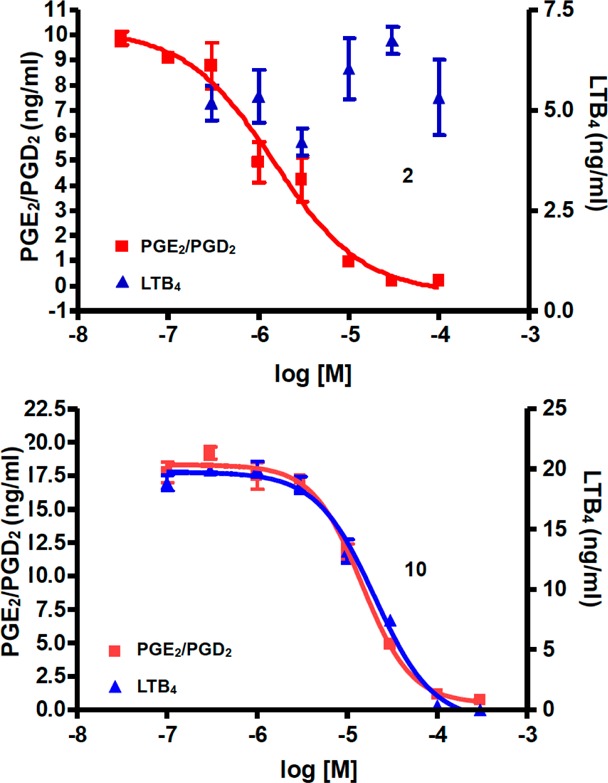
Concentration–response analyses of inhibition of 5-LOX-derived LTB4 and COX-derived PGE2/PGD2 by compounds 2 (top) and 10 (bottom). Error bars represent standard error of the mean from triplicate samples.
Taken together, our results clearly suggest that oxetan-3-ol as well as thietan-3-ol and related sulfoxide and sulfone derivatives may be considered as alternative bioisosteres of the carboxylic acid functional group. Given the relatively low acidity and high permeability, these fragments may be considered especially in the context of CNS drug design, when isosteric replacement of the carboxylic acid is often needed to improve the brain penetration of a candidate compound.
Acknowledgments
Financial support for this work has been provided in part by the NIH/NIA (Grant AG044332-01), the NSF Grant CHE-0840438 (X-ray facility), the Woods Charitable Foundation, and the Cenci Bolognetti Foundation (LM).
Glossary
ABBREVIATIONS
- PG
prostaglandin
- LT
leukotriene
- HB
hydrogen bond
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00212.
Experimental details; NMR spectra of test compounds; X-ray crystal structures; Supplemental Table 1; Supplemental Figure 1; docking studies (PDF)
Author Contributions
The manuscript was written by C.B., K.R.B., and A.B.S. and was reviewed by all authors. P.L., K.O., V.M., V.T., L.M., K.V., L.H., M.J.J., and M.K. made experimental contributions; C.B. and K.R.B. directed research; and V.M.-Y.L. and J.Q.T. provided resources. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ballatore C.; Huryn D. M.; Smith A. B. III Carboxylic acid (bio)isosteres in drug design. ChemMedChem 2013, 8, 385–395. 10.1002/cmdc.201200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- Herr R. J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. 10.1016/S0968-0896(02)00239-0. [DOI] [PubMed] [Google Scholar]
- Duncton M. A. J.; Murray R. B.; Park G.; Singh R. Tetrazolone as an acid bioisostere: application to marketed drugs containing a carboxylic acid. Org. Biomol. Chem. 2016, 14, 9343–9347. 10.1039/C6OB01646D. [DOI] [PubMed] [Google Scholar]
- Borhade S. R.; Svensson R.; Brandt P.; Artursson P.; Arvidsson P. I.; Sandstrom A. Preclinical characterization of acyl sulfonimidamides: potential carboxylic acid bioisosteres with tunable properties. ChemMedChem 2015, 10, 455–460. 10.1002/cmdc.201402497. [DOI] [PubMed] [Google Scholar]
- Ballatore C.; Soper J. H.; Piscitelli F.; James M.; Huang L.; Atasoylu O.; Huryn D. M.; Trojanowski J. Q.; Lee V. M.; Brunden K. R.; Smith A. B. III Cyclopentane-1,3-dione: a novel isostere for the carboxylic acid functional group. application to the design of potent thromboxane (A2) receptor antagonists. J. Med. Chem. 2011, 54, 6969–6983. 10.1021/jm200980u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C.; Gay B.; Huang L.; Robinson K. H.; James M. J.; Trojanowski J. Q.; Lee V. M. Y.; Brunden K. R.; Smith A. B. III Evaluation of the cyclopentane-1,2-dione as a potential bio-isostere of the carboxylic acid functional group. Bioorg. Med. Chem. Lett. 2014, 24, 4171–4175. 10.1016/j.bmcl.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Liu L.; Huang L.; Herbst-Robinson K.; Cornec A.-S.; James M. J.; Sugiyama S.; Bassetto M.; Brancale A.; Trojanowski J. Q.; Lee V. M. Y.; Smith A. B. III; Brunden K. R.; Ballatore C. Potent, long-acting cyclopentane-1,3-dione thromboxane (A2)-receptor antagonists. ACS Med. Chem. Lett. 2014, 5, 1015–1020. 10.1021/ml5002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton N.; Graden H.; Evertsson E.; Bratt E.; Lepistö M.; Johannesson P.; Svensson P. H. Synthesis and functionalization of cyclic sulfonimidamides: a novel chiral heterocyclic carboxylic acid bioisostere. ACS Med. Chem. Lett. 2012, 3, 574–578. 10.1021/ml3000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J. A.; Croft R. A.; Davis O. A.; Doran R.; Morgan K. F. Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem. Rev. 2016, 116, 12150–12233. 10.1021/acs.chemrev.6b00274. [DOI] [PubMed] [Google Scholar]
- Skoda E. M.; Sacher J. R.; Kazancioglu M. Z.; Saha J.; Wipf P. An uncharged oxetanyl sulfoxide as a covalent modifier for improving aqueous solubility. ACS Med. Chem. Lett. 2014, 5, 900–904. 10.1021/ml5001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuitschik G.; Rogers-Evans M.; Müller K.; Fischer H.; Wagner B.; Schuler F.; Polonchuk L.; Carreira E. M. Oxetanes as promising modules in drug discovery. Angew. Chem., Int. Ed. 2006, 45, 7736–7739. 10.1002/anie.200602343. [DOI] [PubMed] [Google Scholar]
- Wuitschik G.; Carreira E. M.; Wagner B. R.; Fischer H.; Parrilla I.; Schuler F.; Rogers-Evans M.; Müller K. Oxetanes in drug discovery: structural and synthetic insights. J. Med. Chem. 2010, 53, 3227–3246. 10.1021/jm9018788. [DOI] [PubMed] [Google Scholar]
- Burkhard J. A.; Wuitschik G.; Rogers-Evans M.; Müller K.; Carreira E. M. Oxetanes as versatile elements in drug discovery and synthesis. Angew. Chem., Int. Ed. 2010, 49, 9052–9067. 10.1002/anie.200907155. [DOI] [PubMed] [Google Scholar]
- Croft R. A.; Mousseau J. J.; Choi C.; Bull J. A. Structurally Divergent Lithium Catalyzed Friedel–Crafts Reactions on Oxetan-3-ols: Synthesis of 3,3-Diaryloxetanes and 2,3-Dihydrobenzofurans. Chem. - Eur. J. 2016, 22, 16271–16276. 10.1002/chem.201604031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M.; Yazaki R.; Fessard T. C.; Carreira E. M. Oxetanyl peptides: novel peptidomimetic modules for medicinal chemistry. Org. Lett. 2014, 16, 4070–4073. 10.1021/ol501590n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. P.; Müller S.; Wolfstädter B. T.; Wolfrum S.; Schepmann D.; Wünsch B.; Carreira E. M. Oxetanyl amino acids for peptidomimetics. Org. Lett. 2017, 19, 2510–2513. 10.1021/acs.orglett.7b00745. [DOI] [PubMed] [Google Scholar]
- Beadle J. D.; Knuhtsen A.; Hoose A.; Raubo P.; Jamieson A. G.; Shipman M. Solid-Phase Synthesis of Oxetane Modified Peptides. Org. Lett. 2017, 19, 3303–3306. 10.1021/acs.orglett.7b01466. [DOI] [PubMed] [Google Scholar]
- Powell N. H.; Clarkson G. J.; Notman R.; Raubo P.; Martin N. G.; Shipman M. Synthesis and structure of oxetane containing tripeptide motifs. Chem. Commun. 2014, 50, 8797–8800. 10.1039/C4CC03507K. [DOI] [PubMed] [Google Scholar]
- Lassalas P.; Gay B.; Lasfargeas C.; James M. J.; Tran V.; Vijayendran K. G.; Brunden K. R.; Kozlowski M. C.; Thomas C. J.; Smith A. B. III; Huryn D. M.; Ballatore C. Structure property relationships of carboxylic acid isosteres. J. Med. Chem. 2016, 59, 3183–3203. 10.1021/acs.jmedchem.5b01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper J. H.; Sugiyama S.; Herbst-Robinson K.; James M. J.; Wang X.; Trojanowski J. Q.; Smith A. B. III; Lee V. M.; Ballatore C.; Brunden K. R. Brain-penetrant tetrahydronaphthalene thromboxane A2-prostanoid (TP) receptor antagonists as prototype therapeutics for Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 928–940. 10.1021/cn3000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh P. N. H.; Walvoord R. R.; Kozlowski M. C. Rapid Quantification of the activating effects of hydrogen-bonding catalysts with a colorimetric sensor. J. Am. Chem. Soc. 2012, 134, 15621–15623. 10.1021/ja3050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot M.; Besseau F.; Laurence C. The hydrogen-bond basicity pKHB scale of peroxides and ethers. Eur. J. Org. Chem. 1998, 1998, 925–931. . [DOI] [Google Scholar]
- Tries S.; Neupert W.; Laufer S. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2. Inflammation Res. 2002, 51, 135–143. 10.1007/PL00000285. [DOI] [PubMed] [Google Scholar]
- Valenti P.; Rampa A.; Fabbri G.; Giusti P.; Cima L. Tetrazole analogs of ibuprofen and flurbiprofen. Arch. Pharm. (Weinheim, Ger.) 1983, 316, 752–755. 10.1002/ardp.19833160904. [DOI] [PubMed] [Google Scholar]
- Elkady M.; Niess R.; Schaible A. M.; Bauer J.; Luderer S.; Ambrosi G.; Werz O.; Laufer S. A. Modified acidic nonsteroidal anti-inflammatory drugs as dual inhibitors of mPGES-1 and 5-LOX. J. Med. Chem. 2012, 55, 8958–8962. 10.1021/jm3010543. [DOI] [PubMed] [Google Scholar]
- Boschelli D. H.; Connor D. T.; Hoefle M.; Bornemeier D. A.; Dyer R. D. Conversion of NSAIDS into balanced dual inhibitors of cyclooxygenase and 5-lipoxygenase. Bioorg. Med. Chem. Lett. 1992, 2, 69–72. 10.1016/S0960-894X(00)80657-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.