Abstract
Antenatal brain hypoxia-ischemia, which occurs in cerebral palsy, is considered a significant cause of motor impairments in children. The mechanisms by which antenatal hypoxia-ischemia causes brain injury and motor deficits still need to be elucidated. Tetrahydrobiopterin is an important enzyme cofactor that is necessary to produce neurotransmitters and to maintain the redox status of the brain. A genetic deficiency of this cofactor from mutations of biosynthetic or recycling enzymes is a well-recognized factor in the development of childhood neurological disorders characterized by motor impairments, developmental delay, and encephalopathy. Experimental hypoxia-ischemia causes a decline in the availability of tetrahydrobiopterin in the immature brain. This decline coincides with the loss of brain function, suggesting this occurrence contributes to neuronal dysfunction and motor impairments. One possible mechanism linking tetrahydrobiopterin deficiency, hypoxia-ischemia, and neuronal injury is oxidative injury. Evidence of the central role of the developmental biology of tetrahydrobiopterin in response to hypoxic ischemic brain injury, especially the development of motor deficits, is discussed.
Keywords: Hypertonia, Neuronal nitric oxide synthase, Reactive oxygen species, Dopamine, Fetal brain MRI, Sepiapterin
Graphical abstract
Highlights
-
•
Tetrahydrobiopterin is needed for normal brain development.
-
•
Fetal brain hypoxia is a conceivably cause of motor disorders.
-
•
Hypoxia-ischemia decrease tetrahydrobiopterin in brain regions.
-
•
NOS uncoupling is mediated by tetrahydrobiopterin deficiency.
-
•
Brain ROS level are increased in fetal brain hypoxia-ischemia.
1. Introduction
Cerebral palsy (CP), which is the most common cause of childhood motor disability, refers to a group of disorders characterized by abnormalities of movement, posture, and balance. CP is most often associated with prenatal brain injury that results in motor impairments of variable severity that affect different parts of the body [1], [2]. The clinical presentations of CP can be spastic, ataxic, and athetoid or dyskinetic, but most subjects show a combination of symptoms that are believed to indicate the involvement of different brain region(s) and degree of injury [3].
Epidemiological studies have identified several risk factors for developing CP, which in combination indicate complex and multiple interactions in the antenatal period in the development of the disease [2], [4], [5]. These risk factors are divided into manifestations of complicated fetal development and conditions affecting the normal progress of pregnancy [6], [7], [8], [9]. and include prematurity, low birthweight, and multiparity. Complications of pregnancy such as placental abruption, maternal exposure to toxic substances, and viral and bacterial infection also have a strong association [10].
Hypoxia-ischemia (HI) of the fetal brain that originates from or is associated with antenatal complications is a significant cause of brain injury leading to mental and/or motor impairment [3]. Still the exact pathophysiological mechanisms of CP need to be elucidated [11]. Progress toward prevention and effective treatment of CP have been hindered by lack of mechanistic understanding, difficulty early diagnoses and the lack of markers of critical brain injury.
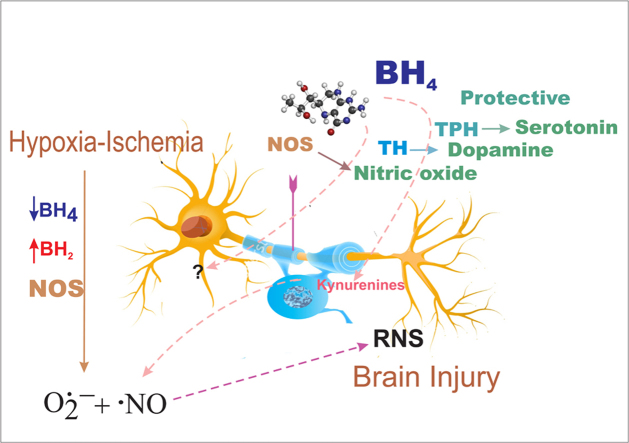
Tetrahydrobiopterin (BH4) is an obligatory cofactor of the enzymes in the biosynthetic pathway of monoamine neurotransmitters (Fig. 1) [12]. Insufficiency in brain BH4 due to genetic defects is a known cause of pediatric motor impairments sometimes accompanied by other central nervous system complications [13]. Little is known about BH4 regulation in the normal fetal brain during development and after brain injury. Herein, the BH4-regulated biochemical pathways and, emerging evidence indicating that BH4 is an important target of injury and a possible therapeutic intervention to ameliorate antenatal brain injury are discussed.
Fig. 1.
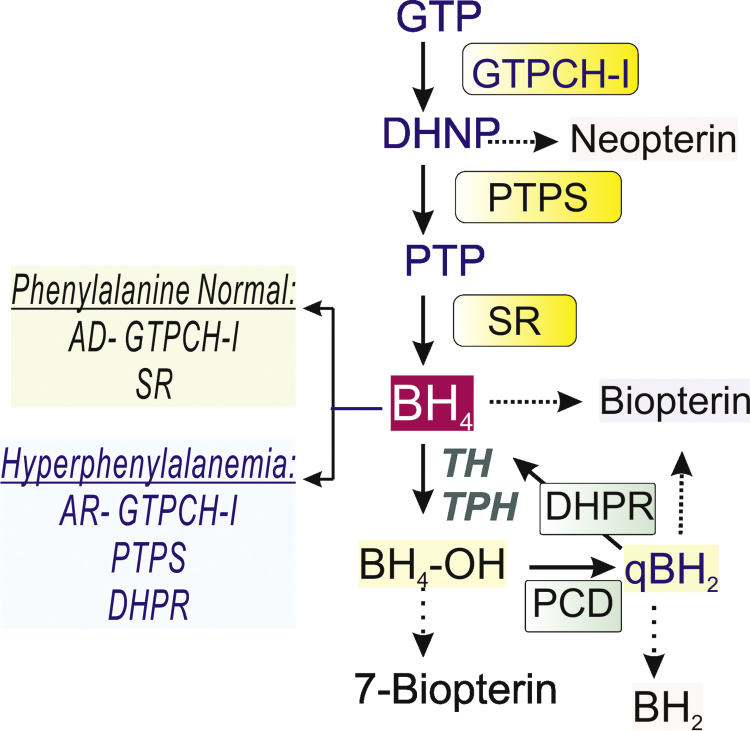
Biochemical markers of BH4 deficiency. Neopterin and biopterin are measured in cerebral spinal fluid for differential diagnosis of enzyme deficiencies. The blood phenylalanine levels are also used in the screening for AD-GTPCHI (autosomal dominant deficiency, GTPCH-I); AR-GTPCHI (autosomal recessive deficiency, GTPCH-I); SR (sepiapterin reductase); PTPS (pyruvoyl-6-tetrahydropterin synthase); PCD (Pterin 4-alpha-carbinolamine dehydratase); DHPR (dihydropterin reductase).
2. Tetrahydrobiopterin deficiency and pediatric neurological disorders
The best-known function of BH4 in the brain is its cofactor role in tryptophan hydroxylase (TPH, EC 1.14.16.4), tyrosine hydroxylase (TH, EC 1.14.16.2, tyrosine-3-monooxygenase), and nitric oxide synthase (NOS, EC 1.14.13.39). TPH and TH produce serotonin, and dopamine, respectively. BH4 deficiency is suspected when symptoms such as motor impairments, encephalopathy, and developmental delays are evident. Paradoxically, neurotransmitters levels are not the first-line assay in diagnosing diseases suspected to be associated with the loss of BH4 [14], [15]. It is not an uncommon finding that cerebral spinal fluid (CSF) neurotransmitter levels are not markedly different from age-matched control values in certain conditions. Thus, a definitive diagnosis of BH4 deficiency is generally based on clinical, biochemical, pharmacological, and genetic analyses [16], [17], [18].
Genetic alteration of the BH4 pathway enzymes (Table 1) is considered the paramount mechanism of BH4 deficiency [19]. Novel evidence, however, indicates that regulation of BH4 may also respond to changes in free radicals and nucleophilic oxidants such as hydrogen peroxide and peroxynitrite [20]. Cytochrome c also influence BH4 content in cells by promoting oxidation [21]. Some of these reactions contribute to the loss of BH4 and function in neurodegenerative disorders [22].
Table 1.
Biochemical characteristics of the BH4 biosynthetic enzymes.
| Enzyme | EC number | Size (kDa)/subunit | Chromosomal location |
|---|---|---|---|
| GTPCH−1 | 3.5.4.16 | 27.9/10 | 14q22.1–22.2 |
| PTS | 4.6.1.10 | 16.4/6 | 11q22.3–23.3 |
| SR | 1.1.1.153 | 28.0/2 | 2p13 |
| PCD | 4.2.1.96 | 11.9/4 | 10q22 |
| DHPR | 1.6.99.7 | 25.8/2 | 4p15.31 |
Although BH4 deficiency is the common end-point of several different genetic enzyme deficiencies, important biochemical distinctions indicate that these deficiencies are not equivalent [23]. This could be explained by different degrees of deficiency, regional changes and a differential impact in dopamine and serotonin production [24].
3. GTP cyclohydrolase-I deficiency
Autosomal dominant GTPCH-I deficiency causes hereditary progressive dystonia with marked diurnal fluctuation (dopa responsive dystonia [DRD], Segawa disease, or DYT5). A high mutation rate causes reduction of GTPCH-I activity in various degrees [25]. GTPCH-I quaternary structure shows two dimers of pentamers [12]. In autosomal dominant GTPCH-I deficiency, is proposed that lack of activity correspond to formation of homodecamer-hybrids, i.e., protein containing both mutant subunits and subunits encoded by normal alleles [26]. The resultant mutant hybrid protein shows reduced activity that is explained by the inability to stabilize homodecamer structure [27]. This concept is however controversial, as low GTPCH protein is considered to better explain reduced enzyme activity.
Clinical biochemical CSF findings indicative of the disease include low concentrations of neopterin, a metabolite derived from 7,8-dihydroneopterin phosphate, and low biopterin, a metabolite derived from BH4 oxidation (Fig. 1). Homovallinic acid (HVA) and 5-hydroxyindole acetic acid (5-HIAA) levels may also be decreased. TH appears to be the most affected enzyme in GTPCH-I deficiency due to decreased BH4 regional distribution and destabilization of TH in conditions of low cofactor availability [28]. This observation has lent support to the idea that some symptoms of BH4 deficiency are indicative of the dependence of dopaminergic development on the availability of the cofactor.
The discovery that asymptomatic carriers sharing the same GTPCH-I mutation as symptomatic subjects supports the hypothesis that additional mechanisms are involved in BH4 regulation in the brain. It is estimated that subjects with deficiency of <20% of normal values of BH4 in CSF will develop neurological symptoms such as dystonia of the legs and postural instability [13]. Children show marked diurnal fluctuation of symptoms worsening during the day. A marked response to L-dopa/carbidopa treatment is characteristic. Early clinical signs of disease such as postural dystonia, action dystonia (retrocollis and oculogyric crisis), and imbalance generally appear at approximately six years, but it is not rare to have a late development, >15 years of age. Adult onset shows postural tremor, writer's cramp, and gait disturbance due to generalized rigidity. Whether the timing of symptom development stems from a continuum of GTPCH-I loss of activity in the brain is an intriguing possibility.
There is evidence that GTPCH-I is a sensitive target of electrophiles such as the lipid peroxidation end-product 4-hydroxynonenal [29] and oxidants such as peroxynitrite, which target the protein for proteasomal degradation. This mechanism could result in critical GTPCH-I inactivation and loss of BH4 aggravating disease in carriers of ‘mild’ genetic deficiency. Also, an enhanced capability to inhibit oxidant accumulation in the brain may explain asymptomatic carriers that may better preserve GTPCH above critical threshold activity. Oxidant accumulation in the brain from uncoupled superoxide radical anion (O2•–) production from NOS [30] is one possible mechanism increasing oxidant injury in the brain, which together with diminished basal NO could further accelerate lipid peroxidation in the brain.
4. Pyruvoyltetrahydropterin synthase deficiency
PTPS deficiency is an autosomal recessive disorder resulting in decreased levels of BH4, serotonin, and dopamine. Diagnosis is based on CSF HVA and 5-HIAA levels, and patients can be severely (>80%) or mildly affected (<20%) [31]. Hyperphenylalaninemia (HPA) is frequently diagnosed in the neonatal period, and a higher neopterin-to-biopterin ratio is characteristic. PTPS deficiency is the most common enzyme defect of the BH4 pathway. Generally, affected neonates are small for gestational age, premature, and have low birthweight and microcephaly. These characteristics strongly suggest an antenatal component in the development of disease. Children develop several neurological symptoms such as dystonia, hypokinesia, rigidity, tremor, oculogyric crises, irritability, and developmental delays [31], [32]. Although rare, some severely deficient PTPS patients have been misdiagnosed with CP based on their mild HPA [33], [34]. Thus not only early diagnosis and treatment of the disease are important in the overall prognosis of the disease but also genetic confirmation. Treatment of PTPS deficiency includes high doses of levodopa, 5-hydroxytryptophan, and BH4 with the goal of reversing the hyperphenylalaninemia.
5. Sepiapterin reductase deficiency
SR catalyzes the final steps in the synthesis of BH4 [35]. Genetic SR deficiency is an autosomal recessive disorder that causes motor and cognitive delays. The early motor impairments hypotonia and dystonia as well as oculogyric crisis are common findings, while tremor and chorea are less prevalent [36]. This enzyme deficiency is one of the most recent to be documented and, it was noted that several children symptomatic for SR deficiency have been mistakenly diagnosed with CP [37], [38]. A differential genetic screening for SR is now recommended in patients with CP.
The mouse model of SR deficiency shows significant growth retardation, less locomotion, tremor-like symptoms in forelegs, and bradykinesia with age [39], [40]. Brain BH4 levels are significantly decreased, in the homozygous knockout (SR-/-) while in the heterozygous (SR+/-) are not significantly different from those in the wild-type animals. Brain dopamine levels in these animals parallel the trend seen with changes in BH4 levels, i.e., SR-/- showing low levels compared to SR+/- and wild type. This relationship may be linked to a marked decrease in TH protein levels in the SR-/- animals.
A significant accumulation of biopterin and sepiapterin, a nonenzymatic oxidation product of 1′-hydroxy-2′-oxotetrahydropterin, with normal levels of neopterin is found in human CSF. The neurotransmitter profile that shows decreased HVA and 5-HIAA in CSF without phenylalaninemia. Retention of some SR activity generally leads to mild phenotypes, which have been shown to correspond with SR splicing defect [41]. SR deficiency responds to treatments with levodopa, carbidopa, 5-hydroxytryptophan, and BH4.
6. Dihydropterin reductase deficiency
DHPR deficiency is associated with severe neurological symptoms in children. Onset of disease is in the neonatal period or early childhood. Children develop feeding difficulties, truncal and limb hypertonia, and delayed motor and cognitive development. Hyperphenylalaninemia is characteristic [42], with decreased CSF HVA, 5-HIAA, and folate; increased levels of biopterin; and unchanged neopterin levels [43]. Changes in CSF BH4 level are not always detected.
DHPR catalyzes the recycling of quinoid BH2 generated from TH and TPH reactions. This product is unstable and, in the absence of a reducing enzyme, will be converted to BH2 (Fig. 1). In DHPR deficiency, it is not exactly clear whether BH2 accumulation and/or limiting concentrations of BH4 fully explain the decreases in serotonin and dopamine. Additional changes in the brain's redox state, consequent to BH2 accumulation, are also considered to play a role in the pathophysiological mechanisms of disease.
The possibility that BH2 interferes with folate metabolism was examined in the QdPR-/- mice, a model of DHPR deficiency, which shows elevated concentrations of BH2 in the brain [44]. Kinetic studies demonstrated that high concentrations of BH2 are needed to compete with the reduction of folate by dihydrofolate reductase (DHFR) with Ki of 88.2 μM. Thus, inhibition of DHFR-folate reactions by BH2 requires the accumulation of BH2 at much higher concentrations than those found in the brain tissue of QdPR-/- animals. Metabolomic analysis of QdPR-/- however showed increased expression of mediators of cell redox balance such as glutathione, gamma-glutamine-cysteine, and taurine, among others [44]. Although the reasons for this increase are unclear, one speculation is that increased BH2 accumulation in the brain can increase oxidative injury via stimulated O2•– release from uncoupled nitric oxide synthases (NOS) activity. This is highly significant since the QdPR-/- brain could be the first model demonstrating the impact of NOS-uncoupling in the mechanisms of brain disease.
Subjects of DHPR deficiency are treated with BH4 and, in severe cases, dietary restriction of phenylalanine is required [45]. Treatment with BH4 is nonetheless controversial as it could increase BH2 in the brain. However, this increase is unlikely as the oral doses of BH4-supplemented to regulate phenylalanine metabolism (~20 mg/kg/day) are mostly retained in the liver. Levodopa and 5-hydroxytryptophan together with monoamine oxidase inhibitors are also frequently required. Treatment with folinic acid is also recommended to ameliorate the loss of folate [46] and prevent 5-methyltetrahydrofolate deficiency.
7. Reactive species and BH4
Neuronal nitric oxide synthase (nNOS) regulate several functions in the developing brain. nNOS was first isolated from the rat cerebellum [47]. nNOS and endothelial NOS require BH4 as a cofactor and, are activated by calcium-calmodulin [48]. One of the best-known functions of diffusible NO involves the production of cyclic guanosine monophosphate (cGMP), leading to activation of protein kinase G and several other targets promoting diverse physiological effects. From the developmental viewpoint, NO has been shown to control proliferation and promote differentiation of brain neuronal progenitor cells [49], influencing neurogenesis in the adult brain, density of mature oligodendrocytes, and myelin content in the immature rat brain.
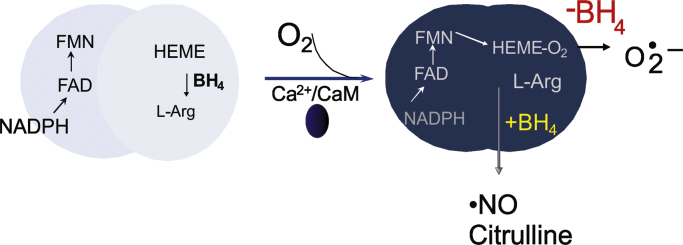
The nNOS oxygenase domain binds BH4 (Fig. 2), which remains tightly bound to the enzyme during catalysis. During normal enzyme turnover, BH4 undergoes redox cycling during the different catalytic cycles without dissociating from the enzyme [50]. This is one major difference compared to BH4 in catalytic cycle of amino acid hydroxylases, where BH4 undergoes hydroxylation and dissociation from enzyme. Also, binding of BH4 to NOS [48] promotes heme binding but not enzyme dimerization [51]. Conversely, BH4 aids in the normal folding of TH, and BH4 deficiency may influence TH misfolding [52].
Fig. 2.
Nitric oxide synthase products are regulated by BH4 cofactor. (Left) Resting state of NOS. Upon binding of calcium calmodulin, electron flow from NADPH to heme-Fe3+ is established. (Right) The heme-Fe2+ reacts with oxygen to form heme-Fe-O2 species that can generate superoxide radical anion (O2•–) conditions of low BH4. The production of O2•– from NOS is known as NOS-uncoupling. When BH4 is bound to the enzyme the reaction proceeds to generate NO and citrulline.
Early studies on nNOS activity indicated that the enzyme produces ROS [53], [54]. Applying EPR spin trapping studies, we definitively demonstrated that BH4 deficient enzyme produces O2•– upon activation with calcium calmodulin. The release of O2•– was shown to be dose-dependently inhibited upon reconstitution of the enzyme with BH4 [30] (Fig. 2). These studies also showed that the reductase domain of NOS does not contribute to oxidant generation. In combination, this information established that BH4 deficiency increases O2•– from the heme-iron bound to the oxygenase domain of the enzyme, and that BH4 regulates the coupling of NADPH to L-arginine oxidation in the oxygenase domain. Both L-arginine and BH4 are required for optimal NO production from NOS (Fig. 2). Splice variants of nNOS (nNOS-α and nNOS-µ) have been found to be highly expressed in the brain. These isoforms show similar enzyme activity (NO-formation), and BH4-inhibitable O2•– formation, although nNOS-α appears to have increased ROS activity when overexpressed in cells [55]. A consequence of the increased ROS signaling from NOS is that hydrogen peroxide and peroxynitrite can propagate oxidant injury via protein S-oxidation [56], S-guanylation [57], and tyrosine-nitration [58].
8. Rabbit model of HI injury
Part of the problem with the development of much-needed therapies for perinatal HI and CP is the lack of animal models that are appropriate to the clinical condition reproducing several of the key aspects involved in the human disease development. We have studied the developmental brain responses to HI in a fetal rabbit model. Rabbits are perinatal brain developers like humans, which is an advantage. The rabbit model of HI also is advantageous in that it provides a platform that closely emulates in utero brain responses to HI at different developmental ages, corresponding to 70–92% gestation in rabbits, and mimicking placental abruption—a human condition where there is an interruption of oxygen and nutrients from the mother to the fetus. After birth, newborn survivors of HI present with pronounced motor deficits such as impaired locomotion, reflex motor activity and coordination of suck and swallow. Other behavioral changes closely mimic those of human CP [59], [60], [61], including postural changes and hypertonia [59]. Using a human classification of tone disorders, hypertonia in rabbits is mostly dystonic and rarely spastic.
HI at 70% gestation injury is characterized by postnatal hypertonia. Imaging findings indicate that HI causes concomitant white matter injury affecting mostly the internal capsule as determined by postnatal magnetic resonance imaging (MRI), and corticospinal tract injury may explain some of the hypertonia [60]. HI in the near-term rabbit, at 92% gestation, demonstrated additional injury to the basal ganglia–thalamus–brain stem and, to a lesser extent, cortex and periventricular white matter injury. These lesions are strongly associated with the severity of the postnatal motor deficits [62]. In this age group, we can demonstrate a short hypotonic phase before the manifestation of hypertonia, a progression that is comparable to the manifestation in most human newborns that results in CP. In the younger ages, presumably the hypotonic phase occurs in utero, and we are only able to observe the hypertonia after birth. Human newborns can also present at birth with long-lasting hypertonia, further supporting the view that etiological insults causing CP are mostly antenatal in origin.
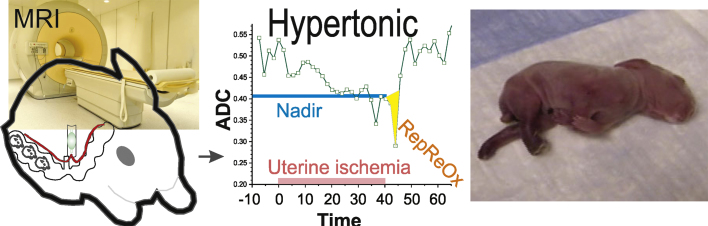
The other unique feature of the rabbit HI model is that MRI during HI at 72% gestation reveals distinct patterns of changes of apparent diffusion coefficient (ADC) by diffusion-weighted imaging, which predicts which fetuses in the litter will become hypertonic (Fig. 3). Most litters present with fetal subpopulations that have almost normal motor function as well as severe postural changes and hypertonia. Both subpopulations can be predicted by MRI biomarkers [63]. Furthermore, there is a subpopulation that manifests detectable reperfusion-deoxygenation injury on MRI. This reperfusion-reoxygenation injury occurs with increased superoxide formation [64].
Fig. 3.
MRI diagnostic of hypertonia in brain hypoxia-ischemia reperfusion-reoxygenation. Apparent diffusion coefficient (ADC) changes involving decrease below a nadir and further fall during uterine reperfusion (RepReOx) can be used to predict fetal population that will develop hypertonia.
9. HI and oxidant injury
Early studies indicate that fetal brain HI injury varies with timing and brain regions, from resistant to highly susceptible cells. Cells presenting with critical injury are observed to die days after injury, while others appear to recover from damage [65]. In fetuses manifesting evidence of reperfusion-reoxygenation injury as indicated by MRI, fetal brains show fewer healthy neuronal cells and oligodendrocytes [64]. This observation is relevant to the human pattern of brain injury, which does not generally manifest the full range of motor deficits immediately after insult, but generally is delayed for 18–24 months for CP, a long enough period to lose the ability to discern possible etiological factors.
Neurotransmitter release can be variable in the perinatal brain following HI injury. Following acute hypoxic exposure in postnatal day 4 (P4) rats, both dopamine content and density of dopamine receptors are decreased in the cerebellum [66]. Moreover, HI led to preferential loss of TH neurons [67]. In contrast, striatal dopamine release in awake P5 rats was increased following hypoxia [68].
10. Tetrahydrobiopterin and fetal brain HI
We showed, in normal developing fetal rabbit brain, a rapid gain of function with respect to increases in biopterin and regional BH4 in the brain from 70% to 92% gestational development [69]. The thalamus and basal ganglia show the greatest developmental increase of BH4 between 70% and 92% gestation, which significantly decreases upon in vivo HI injury. Low BH4 was verified in the thalamus, after HI [59], [70]. Brain ascorbate was also reduced by HI although the brain region affected is more diffuse than BH4.
Different survival rates are found in ex vivo primary neuronal cultures from the basal ganglia, cortex, and thalamus following in vivo HI. Thalamic cells from 70% gestation exhibit the lowest survival rate in a BH4-free milieu, in control and HI groups [71]. HI but not control cells are amenable to rescue with supplemented ex vivo BH4, suggesting a causal relationship between HI injury and BH4-dependent pathways. At 92% gestational age, only cortical neurons show some improved survival. This interaction of regional susceptibility, timing, and embryonic age is key in the overall pathogenesis of brain injury.
11. Low developmental BH4 levels in brain HI injury
The degree of HI-injury outcomes coincides with developmentally low levels of BH4. Increasing BH4 in the fetal brain prior to HI prevents or ameliorates brain injury. Maternal treatment with low dose of the BH4 precursor sepiapterin (0.6 mg/kg/day for five days) increases BH4 in maternal and fetal tissues [69]. Brain BH4 however improved preferentially in the thalamus, and after HI, BH4 were higher than the levels in naïve controls, while other brain regions show no change with respect to controls [69]. Increasing BH4 levels in the fetal brain above a critical threshold of depletion better preserves intact motor functions following HI injury. The neurobehavioral scoring reveals fewer newborn kits showing severe hypertonia and dystonia, while improving locomotion and righting reflex.
Also, increasing BH4 offset dopamine loss in the basal ganglia after HI, while fully preserving serotonin levels in the thalamus. Whether loss of neurotransmitters explains motor dysfunction in CP is however debatable. A recent clinical trial testing the benefits of levodopa in dystonic CP showed no benefits of the treatment in patients who had compromised upper limb movement [72]. This study was limited by a small sample size, but suggested that global neurotransmitter modulation has a limited role in ameliorating motor deficits of advanced CP. Treatment at early ages, however, has shown improvement. Thus, more studies are needed to better define a window of therapeutic opportunity. Another confounding aspect is that BH4 depletion increases oxidant formation, which may play a role [64], [73].
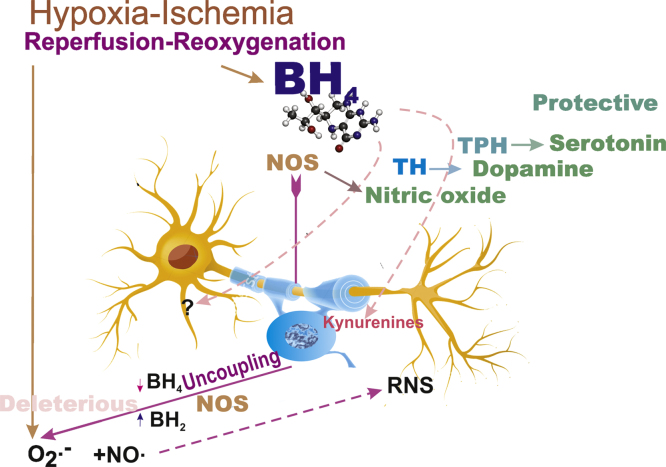
The fetal rabbit model indicates that BH4 pathway is a target of HI (Fig. 4), and a two-hit model likely explain susceptibility to injury [71]. The first condition necessary is low developmental, like in prematurity, BH4 levels. Even at term gestation, BH4 concentration appear to be lower than in adults. This sets the stage for HI causing a depletion below the critical threshold for critical injury. BH4 supplementation counteracts HI injury, probably because the levels in the brain increase above a critical threshold even after HI. This threshold may be different depending on the molecular function and location given the fact that BH4 is a cofactor for several critical enzyme pathways. Whether supplementation with BH4 is a significant strategy for prevention of HI-brain injury in pregnancies at risk or as an early treatment option is a possibility to be considered.
Fig. 4.
HI and reperfusion reoxygenation target BH4. Changes in BH4 bioavailability in the immature fetal brain may have significant consequences in neuronal survival, neurotransmitter synthesis, and redox homeostasis via NOS uncoupling.
12. Conclusions
Loss of BH4 in the fetal brain decreases neuronal function. The pathophysiology of genetic versus HI-induced loss of BH4, however, is dissimilar in that HI-induced loss of BH4 occurs concomitantly with oxidant injury. Although BH4 supplementation prevents HI-injury, a full understanding of the immediate and long-term results of BH4 supplementation in the prevention and treatment of childhood disorders warrants further investigation.
Conflicts of interest
Nothing to report.
Acknowledgments
The authors are grateful to Janice E. Brunstrom-Hernandez, MD, for her insightful contribution to this work. Research in this publication was supported by the National Institutes of Health under Award Number R01 NS081936. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Jeannette Vasquez-Vivar, Email: jvvivar@mcw.edu.
Sidhartha Tan, Email: sidharthatan@gmail.com.
References
- 1.Coq J.O., Delcour M., Massicotte V.S., Baud O., Barbe M.F. Prenatal ischemia deteriorates white matter, brain organization, and function: implications for prematurity and cerebral palsy. Dev. Med. Child Neurol. 2016;58(Suppl 4):7–11. doi: 10.1111/dmcn.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diagnosis O.'Shea T.M. Treatment, and prevention of cerebral palsy in near-term/term infants. Clin. Obstet. Gynecol. 2008;51:816–828. doi: 10.1097/GRF.0b013e3181870ba7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham H.K., Rosenbaum P., Paneth N. Cerebral palsy. Nat. Rev. Dis. Prim. 2016;2:15082. doi: 10.1038/nrdp.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parolin Schnekenberg R., Perkins E.M., Miller J.W. De novo point mutations in patients diagnosed with ataxic cerebral palsy. Brain. 2015;138:1817–1832. doi: 10.1093/brain/awv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J.L., Hvidtjørn D., Basso O. Parental infertility and cerebral palsy in children. Human. Reprod. (Oxf., Engl.) 2010;25:3142–3145. doi: 10.1093/humrep/deq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa J., Toyokawa S., Ikenoue T. Relevant obstetric factors for cerebral palsy: fromfrom the nationwide obstetric compensation system in Japan. PLoS ONE. 2016;11:e0148122. doi: 10.1371/journal.pone.0148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tronnes H., Wilcox A.J., Lie R.T., Markestad T., Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev. Med. Child Neurol. 2014;56:779–785. doi: 10.1111/dmcn.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark S.M., Ghulmiyyah L.M., Hankins G.D. Antenatal antecedents and the impact of obstetric care in the etiology of cerebral palsy. Clin. Obstet. Gynecol. 2008;51:775–786. doi: 10.1097/GRF.0b013e3181870994. [DOI] [PubMed] [Google Scholar]
- 9.Colver A., Fairhurst C., Pharoah P.O. Cerebral palsy. Lancet. 2014;383:1240–1249. doi: 10.1016/S0140-6736(13)61835-8. [DOI] [PubMed] [Google Scholar]
- 10.Miller J.E., Pedersen L.H., Streja E. Maternal Infections during pregnancy and cerebral palsy: a population-based cohort study. Paediatr. Perinat. Epidemiol. 2013;27:542–552. doi: 10.1111/ppe.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longo M., Hankins G. Defining cerebral palsy: pathogenesis, pathophysiology and new intervention. Minerva Ginecol. 2009;61:421–429. [PubMed] [Google Scholar]
- 12.Thony B., Auerbach G., Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Segawa M. Hereditary progressive dystonia with marked diurnal fluctuation. Brain Dev. 2011;33:195–201. doi: 10.1016/j.braindev.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Bonafe L., Thony B., Leimbacher W., Kierat L., Blau N. Diagnosis of dopa-responsive dystonia and other tetrahydrobiopterin disorders by the study of biopterin metabolism in fibroblasts. Clin. Chem. 2001;47:477–485. [PubMed] [Google Scholar]
- 15.Moreno-Medinilla E.E., Mora-Ramirez M.D., Calvo-Medina R., Martinez-Anton J. [Autosomal recessive GTPCH 1 deficiency: the importance of the analysis of neurotransmitters in cerebrospinal fluid] Rev. Neurol. 2016;62:502–506. [PubMed] [Google Scholar]
- 16.Opladen T., Hoffmann G.F., Kuhn A.A., Blau N. Pitfalls in phenylalanine loading test in the diagnosis of dopa-responsive dystonia. Mol. Genet. Metab. 2013;108:195–197. doi: 10.1016/j.ymgme.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Shintaku H. Disorders of tetrahydrobiopterin metabolism and their treatment. Curr. Drug Metab. 2002;3:123–131. doi: 10.2174/1389200024605145. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z.F., Zhang Y.H., Guo J.F. Genetic diagnosis of two dopa-responsive dystonia families by exome sequencing. PLoS One. 2014;9:e106388. doi: 10.1371/journal.pone.0106388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner E.R., Blau N., Thony B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem. J. 2011;438:397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic. Biol. Med. 2009;47:1108–1119. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capeillere-Blandin C., Mathieu D., Mansuy D. Reduction of ferric haemoproteins by tetrahydropterins: a kinetic study. Biochem. J. 2005;392:583–587. doi: 10.1042/BJ20050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K., Bindokas V.P., Kowlessur D. Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J. Biol. Chem. 2001;276:34402–34407. doi: 10.1074/jbc.M103766200. [DOI] [PubMed] [Google Scholar]
- 23.Ng J., Papandreou A., Heales S.J., Kurian M.A. Monoamine neurotransmitter disorders--clinical advances and future perspectives. Nat. Rev. Neurol. 2015;11:567–584. doi: 10.1038/nrneurol.2015.172. [DOI] [PubMed] [Google Scholar]
- 24.Sumi-Ichinose C., Urano F., Kuroda R. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin. A study from 6-pyruvoyltetrahydropterin synthase knockout mice. J. Biol. Chem. 2001;276:41150–41160. doi: 10.1074/jbc.M102237200. [DOI] [PubMed] [Google Scholar]
- 25.Hagenah J., Saunders-Pullman R., Hedrich K. High mutation rate in dopa-responsive dystonia: detection with comprehensive GCHI screening. Neurology. 2005;64:908–911. doi: 10.1212/01.WNL.0000152839.50258.A2. [DOI] [PubMed] [Google Scholar]
- 26.Hwu W.L., Wang P.J., Hsiao K.J., Wang T.R., Chiou Y.W., Lee Y.M. Dopa-responsive dystonia induced by a recessive GTP cyclohydrolase I mutation. Human. Genet. 1999;105:226–230. doi: 10.1007/s004390051093. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T., Ohye T., Inagaki H., Nagatsu T., Ichinose H. Characterization of wild-type and mutants of recombinant human GTP cyclohydrolase I: relationship to etiology of dopa-responsive dystonia. J. Neurochem. 1999;73:2510–2516. doi: 10.1046/j.1471-4159.1999.0732510.x. [DOI] [PubMed] [Google Scholar]
- 28.Homma D., Katoh S., Tokuoka H., Ichinose H. The role of tetrahydrobiopterin and catecholamines in the developmental regulation of tyrosine hydroxylase level in the brain. J. Neurochem. 2013;126:70–81. doi: 10.1111/jnc.12287. [DOI] [PubMed] [Google Scholar]
- 29.Whitsett J., Picklo M.J., Sr., Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 30.Vasquez-Vivar J., Hogg N., Martasek P., Karoui H., Pritchard K.A., Jr., Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J. Biol. Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 31.Leuzzi V., Carducci C.A., Carducci C.L. Phenotypic variability, neurological outcome and genetics background of 6-pyruvoyl-tetrahydropterin synthase deficiency. Clin. Genet. 2010;77:249–257. doi: 10.1111/j.1399-0004.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- 32.Kurian M.A., Gissen P., Smith M., Heales S., Jr., Clayton P.T. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–733. doi: 10.1016/S1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]
- 33.Demos M.K., Waters P.J., Vallance H.D. 6-pyruvoyl-tetrahydropterin synthase deficiency with mild hyperphenylalaninemia. Ann. Neurol. 2005;58:164–167. doi: 10.1002/ana.20532. [DOI] [PubMed] [Google Scholar]
- 34.Mak C.M., Ko C.H., Lam C.W. Phenylketonuria in Hong Kong Chinese: a call for hyperphenylalaninemia newborn screening in the Special Administrative Region, China. Chin. Med. J. 2011;124:2556–2558. [PubMed] [Google Scholar]
- 35.Auerbach G., Herrmann A., Gütlich M. The 1.25 A crystal structure of sepiapterin reductase reveals its binding mode to pterins and brain neurotransmitters. EMBO J. 1997;16:7219–7230. doi: 10.1093/emboj/16.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman J. Sepiapterin Reductase Deficiency. In: Pagon R.A., Adam M.P., Ardinger H.H., editors. GeneReviews(R) University of Washington, Seattle University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle; Seattle (WA): 1993. (All rights reserved) [Google Scholar]
- 37.Neville B.G., Parascandalo R., Farrugia R., Felice A. Sepiapterin reductase deficiency: a congenital dopa-responsive motor and cognitive disorder. Brain. 2005;128:2291–2296. doi: 10.1093/brain/awh603. [DOI] [PubMed] [Google Scholar]
- 38.Friedman J., Roze E., Abdenur J.E. Sepiapterin reductase deficiency: a treatable mimic of cerebral palsy. Ann. Neurol. 2012;71:520–530. doi: 10.1002/ana.22685. [DOI] [PubMed] [Google Scholar]
- 39.Yang S., Lee Y.J., Kim J.M. A murine model for human sepiapterin-reductase deficiency. Am. J. Human. Genet. 2006;78:575–587. doi: 10.1086/501372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takazawa C., Fujimoto K., Homma D. A brain-specific decrease of the tyrosine hydroxylase protein in sepiapterin reductase-null mice—as a mouse model for Parkinson's disease. Biochem. Biophys. Res. Commun. 2008;367:787–792. doi: 10.1016/j.bbrc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Arrabal L., Teresa L., Sanchez-Alcudia R. Genotype-phenotype correlations in sepiapterin reductase deficiency. A splicing defect accounts for a new phenotypic variant. Neurogenetics. 2011;12:183–191. doi: 10.1007/s10048-011-0279-4. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman S., Holtzman N.A., Milstien S., Butler L.J., Krumholz A. Phenylketonuria due to a deficiency of dihydropteridine reductase. N. Engl. J. Med. 1975;293:785–790. doi: 10.1056/NEJM197510162931601. [DOI] [PubMed] [Google Scholar]
- 43.Blau N., Kierat L., Matasovic A. Antenatal diagnosis of tetrahydrobiopterin deficiency by quantification of pterins in amniotic fluid and enzyme activity in fetal and extrafetal tissue. Clin. Chim. Acta; Int. J. Clin. Chem. 1994;226:159–169. doi: 10.1016/0009-8981(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 44.Xu F., Sudo Y., Sanechika S. Disturbed biopterin and folate metabolism in the Qdpr-deficient mouse. FEBS Lett. 2014;588:3924–3931. doi: 10.1016/j.febslet.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Ponzone A., Spada M., Ferraris S., Dianzani I., de Sanctis L. Dihydropteridine reductase deficiency in man: from biology to treatment. Med. Res. Rev. 2004;24:127–150. doi: 10.1002/med.10055. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein D.S., Hahn S.H., Holmes C. Monoaminergic effects of folinic acid, L-DOPA, and 5-hydroxytryptophan in dihydropteridine reductase deficiency. J. Neurochem. 1995;64:2810–2813. doi: 10.1046/j.1471-4159.1995.64062810.x. [DOI] [PubMed] [Google Scholar]
- 47.Bredt D.S., Hwang P.M., Snyder S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 48.Raman C.S., Li H., Martasek P., Kral V., Masters B.S., Poulos T.L. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 49.Phan Duy A., Pham H., Pansiot J., Gressens P., Charriaut-Marlangue C., Baud O. Nitric oxide pathway and proliferation of neural progenitors in the neonatal rat. Dev. Neurosci. 2015;37:417–427. doi: 10.1159/000375488. [DOI] [PubMed] [Google Scholar]
- 50.Ramasamy S., Haque M.M., Gangoda M., Stuehr D.J. Tetrahydrobiopterin redox cycling in nitric oxide synthase: evidence supports a through-heme electron delivery. FEBS J. 2016;283:4491–4501. doi: 10.1111/febs.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klatt P., Pfeiffer S., List B.M. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J. Biol. Chem. 1996;271:7336–7342. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 52.Thony B., Calvo A.C., Scherer T. Tetrahydrobiopterin shows chaperone activity for tyrosine hydroxylase. J. Neurochem. 2008;106:672–681. doi: 10.1111/j.1471-4159.2008.05423.x. [DOI] [PubMed] [Google Scholar]
- 53.Heinzel B., John M., Klatt P., Bohme E., Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem. J. 1992;281(Pt 3):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pou S., Keaton L., Surichamorn W., Rosen G.M. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J. Biol. Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 55.Ihara H., Kitamura A., Kasamatsu S. Superoxide generation from nNOS splice variants and its potential involvement in redox signal regulation. Biochem. J. 2017;474:1149–1162. doi: 10.1042/BCJ20160999. [DOI] [PubMed] [Google Scholar]
- 56.Brewer T.F., Garcia F.J., Onak C.S., Carroll K.S., Chang C.J. Chemical approaches to discovery and study of sources and targets of hydrogen peroxide redox signaling through NADPH oxidase proteins. Annu. Rev. Biochem. 2015;84:765–790. doi: 10.1146/annurev-biochem-060614-034018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akashi S., Ahmed K.A., Sawa T. Persistent activation of cGMP-dependent protein kinase by a nitrated cyclic nucleotide via site specific protein S-guanylation. Biochemistry. 2016;55:751–761. doi: 10.1021/acs.biochem.5b00774. [DOI] [PubMed] [Google Scholar]
- 58.Martinez A., Portero-Otin M., Pamplona R., Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. (Zur., Switz.) 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derrick M., Luo N.L., Bregman J.C. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J. Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derrick M., Drobyshevsky A., Ji X., Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 61.Tan S., Drobyshevsky A., Jilling T. Model of cerebral palsy in the perinatal rabbit. J. Child Neurol. 2005;20:972–979. doi: 10.1177/08830738050200120801. [DOI] [PubMed] [Google Scholar]
- 62.Derrick M., Englof I., Drobyshevsky A., Luo K., Yu L., Tan S. Intrauterine fetal demise can be remote from the inciting insult in an animal model of hypoxia-ischemia. Pediatr. Res. 2012;72:154–160. doi: 10.1038/pr.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drobyshevsky A., Derrick M., Prasad P.V., Ji X., Englof I., Tan S. Fetal brain magnetic resonance imaging response acutely to hypoxia-ischemia predicts postnatal outcome. Ann. Neurol. 2007;61:307–314. doi: 10.1002/ana.21095. [DOI] [PubMed] [Google Scholar]
- 64.Drobyshevsky A., Luo K., Derrick M. Motor deficits are triggered by reperfusion-reoxygenation injury as diagnosed by MRI and by a mechanism involving oxidants. J. Neurosci. 2012;32:5500–5509. doi: 10.1523/JNEUROSCI.5986-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derrick M., He J., Brady E., Tan S. The in vitro fate of rabbit fetal brain cells after acute in vivo hypoxia. J. Neurosci. 2001;21:RC138. doi: 10.1523/JNEUROSCI.21-07-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joseph B., Nandhu M.S., Paulose C.S. Dopamine D1 and D2 receptor functional down regulation in the cerebellum of hypoxic neonatal rats: neuroprotective role of glucose and oxygen, epinephrine resuscitation. Pharmacol. Res. 2010;61:136–141. doi: 10.1016/j.phrs.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Buller K.M., Wixey J.A., Pathipati P. Selective losses of brainstem catecholamine neurons after hypoxia-ischemia in the immature rat pup. Pediatr. Res. 2008;63:364–369. doi: 10.1203/PDR.0b013e3181659774. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima W., Ishida A., Takada G. Effect of anoxia on striatal monoamine metabolism in immature rat brain compared with that of hypoxia: an in vivo microdialysis study. Brain Res. 1996;740:316–322. doi: 10.1016/s0006-8993(96)00875-x. [DOI] [PubMed] [Google Scholar]
- 69.Vasquez-Vivar J., Whitsett J., Derrick M., Ji X., Yu L., Tan S. Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain. Ann. Neurol. 2009;66:323–331. doi: 10.1002/ana.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McQuillen P.S., Sheldon R.A., Shatz C.J., Ferriero D.M. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J. Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu L., Vasquez-Vivar J., Jiang R., Luo K., Derrick M., Tan S. Developmental susceptibility of neurons to transient tetrahydrobiopterin insufficiency and antenatal hypoxia-ischemia in fetal rabbits. Free Radic. Biol. Med. 2014;67:426–436. doi: 10.1016/j.freeradbiomed.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pozin I., Bdolah-Abram T., Ben-Pazi H. Levodopa does not improve function in individuals with dystonic cerebral palsy. J. Child Neurol. 2014;29:534–537. doi: 10.1177/0883073812473645. [DOI] [PubMed] [Google Scholar]
- 73.Tan S., Zhou F., Nielsen V.G., Wang Z., Gladson C.L., Parks D.A. Increased injury following intermittent fetal hypoxia-reoxygenation is associated with increased free radical production in fetal rabbit brain. J. Neuropathol. Exp. Neurol. 1999;58:972–981. doi: 10.1097/00005072-199909000-00007. [DOI] [PubMed] [Google Scholar]