Abstract
We report here the main characteristics of ‘Blautia phocaeensis’ strain Marseille-P3441 sp. nov. and ‘Lachnoclostridium edouardi’ strain Marseille-P3397 sp. nov., that were isolated from a faecal specimen of a 42-year-old female Saudi Bedouin. We used a bacterial culturomics approach combined with taxono-genomics.
Keywords: ‘Blautia phocaeensis’, gut microbiota, ‘Lachnoclostridium edouardi’, new species, taxono-genomics
In 2016 we isolated two bacteria, using a bacterial culturomics approach in the human gut microbiota, that could not be identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a Microflex spectrometer (Bruker Daltonics, Bremen, Germany) [1], [2]. These species were isolated from the stool of a Saudi Arabia Bedouin woman. The donor of the stool has signed informed consent and the study has been validated by the Ethics Committee of the IFR48 Federative Research Institute under number 09-022. Both strains failed to be identified by MALDI-TOF MS and their 16S rRNA genes were sequenced using fD1-rP2 primers as described previously using a 3130-XL sequencer (Applied Biosciences, Saint Aubin, France) [3].
The stool sample was pre-incubated for 1 day at 37°C in a blood culture bottle (BD Diagnostics, Le Pont-de-Claix, France) supplemented with 2 mL of rumen fluid filter-sterilized through a 0.2-μm pore filter (Thermo Fisher Scientific, Villebon sur Yvette, France) and 2 mL of sheep blood (bioMérieux, Marcy l'Etoile, France).
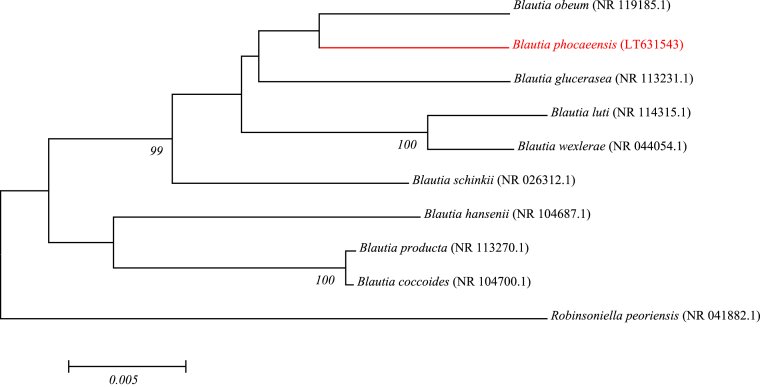
Strain Marseille-P3441 was isolated after initial growth of 2 days on Columbia agar supplemented with 5% sheep blood at 37°C under strict anaerobic conditions. The colonies appeared ochre, non-haemolytic, motile, spore forming and were 1 mm in size. The cells were Gram-positive, cocci-shaped with a 1-μm diameter. The strain did not show catalase or oxidase activity. The strain Marseille-P3441 had a 16S rRNA gene sequence identity of 95.53% with Blautia glucerasea strain JCM 17039 (NR_113231), the phylogenetically closest species with standing in nomenclature (Fig. 1). Strain HFTH-1T isolated from dog faeces, was Gram-positive, anaerobic, oval spore forming, rod-shaped, lecithinase-negative and lipase-negative [4]. The similarity was <98.65%, which led us to putatively classify Marseille-P3441T as a new member in the Lachnospiraceae family of the Clostridiales order of the phylum Firmicutes [5]. Therefore we propose the creation of the new species ‘Blautia phocaeensis’ (pho.ca.een'sis NL adj fem, to refer to Phocaea, the Latin name of Phocea, the city from whence came the founders of Marseille, France, where the strain was isolated). Marseille-P3441T is the type strain of the species ‘Blautia phocaeensis’.
Fig. 1.
Phylogenetic tree showing the position of ‘Blautia phocaeensis’ strain Marseille-P3441T relative to other phylogenetically close neighbours. Sequences were aligned using ClustalW, and phylogenetic inferences were obtained with Kimura two-parameter models using the maximum-likelihood method within the MEGA software. Numbers at the nodes are percentages of bootstrap values obtained by repeating the analysis 1000 times to generate a majority consensus tree. The scale bar indicates a 0.5% nucleotide sequence divergence.
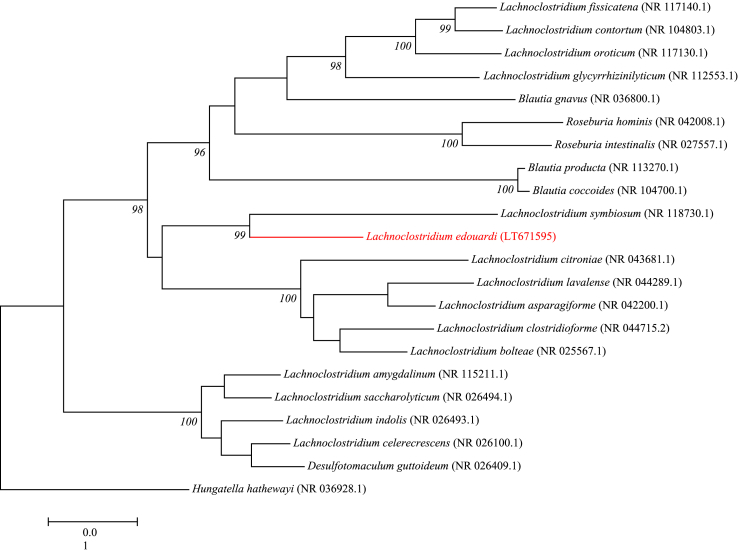
Strain Marseille-P3397 was isolated after an initial growth of 3 days on Columbia agar supplemented with 5% sheep blood at 37°C under strict anaerobic conditions. The colonies appeared beige, non-haemolytic, motile, non-spore forming and were 2 mm in size. The cells were Gram-positive, rod-shaped and ranged from 5 μm and 0.7 μm. The strain did not show catalase or oxidase activity. The strain Marseille-P3397 had a 16S rRNA gene sequence identity of 94.26% with Clostridium symbiosum strain ATCC 14940 (NR_118730), the phylogenetically closest species with standing in nomenclature (Fig. 2). This similarity of ≈95% leads us to putatively classify Marseille-P3397 as a new member in the Clostridiaceae family of the Clostridiales order of the Firmicutes phylum [5]. Therefore we propose the creation of the new species ‘Lachnoclostridium edouadi’. Marseille-P3397T is the type strain of the species ‘Lachnoclostridium edouardi’ (e.dou.ar’di L. adj. neut. Edouardi in honour of the microbiologist Sophie Edouard, Marseille, France).
Fig. 2.
Phylogenetic tree showing the position of ‘Lachnoclostridium edouardi’ strain Marseille-P3397T relative to other phylogenetically close neighbours. Sequences were aligned using ClustalW, and phylogenetic inferences were obtained with Kimura two-parameter models using the maximum-likelihood method within the MEGA software. Numbers at the nodes are percentages of bootstrap values obtained by repeating the analysis 1000 times to generate a majority consensus tree. The scale bar indicates a 1% nucleotide sequence divergence.
MALDI-TOF MS spectra accession numbers
The MALDI-TOF MS spectra of these species are available at http://mediterranee-infection.com/article.php? laref=256&titre=urms-database.
Nucleotide sequence accession numbers
The 16S r RNA gene sequence was deposited in GenBank under accession numbers: ‘Blautia phocaeensis’ strain Marseille-P3441T (LT631543) and ‘Lachnoclostridium edouardi’ strain Marseille-P3397T (LT671595).
Deposit in a culture collection
The strains were deposited in the Collection de Souches de l’Unité des Rickettsies (CSUR, WDCM 875) under numbers P3441 (‘Blautia phocaeensis’ strain Marseille-P3441T) and P3397 (‘Lachnoclostridium edouardi’ strain Marseille-P3397T).
Transparency declaration
None to declare.
Funding
This work was funded by Fondation Mediterrannée-Infection.
References
- 1.Lagier J.C., Hugon P., Khelaifia S., Fournier P.E., La S.B., Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28:237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagier J.C., Khelaifia S., Alou M.T., Ndongo S., Dione N., Hugon P. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. doi: 10.1038/nmicrobiol.2016.203. [DOI] [PubMed] [Google Scholar]
- 3.Drancourt M., Bollet C., Carlioz A., Martelin R., Gayral J.P., Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000;38:3623–3630. doi: 10.1128/jcm.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuya H., Ide Y., Hamamoto M., Asanuma N., Hino T. Isolation of a novel bacterium, Blautia glucerasei sp. nov., hydrolyzing plant glucosylceramide to ceramide. Arch Microbiol. 2010;192:365–372. doi: 10.1007/s00203-010-0566-8. [DOI] [PubMed] [Google Scholar]
- 5.Kim M., Oh H.S., Park S.C., Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]