Highlights
-
•
A case of grade 2 endometrial adenocarcinoma in a young woman desiring fertility-sparing treatment
-
•
Successful conservative management of refractory endometrial adenocarcinoma with dual progestin therapy
-
•
A brief review of conservative management in endometrial adenocarcinoma
1. Background
Although the majority of women diagnosed with endometrial carcinoma are postmenopausal and have completed childbearing, some endometrial cancers will occur in women under the age of 40. Most young women will be diagnosed with early stage and low grade disease, conferring a five-year survival rate of 99% (Siegel et al., 2016). The current standard of care is surgical, including total hysterectomy with or without bilateral salpingo-oophorectomy (SGO Clinical Practice Endometrial Cancer Working Group et al., 2014). However, this standard of care presents a unique challenge in young women interested in retaining childbearing potential. While conservative fertility-sparing therapy with single agent progesterone has been described in patients with endometrial hyperplasia and grade 1 endometrial cancer (SGO Clinical Practice Endometrial Cancer Working Group et al, Gunderson et al., 2012, Ramirez et al., 2004), use of dual progesterone therapy in those failing single agent therapy has not been well described. Further, support for use of conservative treatment in higher grade tumors has been limited (Perri et al., 2011). Reported here is a case of successful fertility-sparing therapy in a young woman with grade 2 endometrial adenocarcinoma refractory to single agent progestin treatment.
2. Case
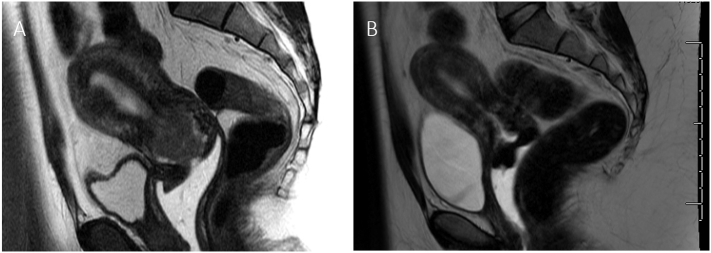
A 25 year old nulliparous patient presented to her primary gynecologist with abnormal uterine bleeding, which was becoming persistently heavier. Her past medical history was significant for a diagnosis of polycystic ovarian syndrome (PCOS) and obesity (BMI 37 kg/m2). Transvaginal ultrasound revealed a possible cervical fibroid, and she was taken to the operating room for hysteroscopy, dilation and curettage (D&C). At the time of surgery, she was noted to have abundant thickened endocervical tissue with increased vascularity and a normal intrauterine cavity. Pathologic evaluation of this tissue was consistent with FIGO grade 1 endometrial adenocarcinoma, resulting in referral to a gynecologic oncologist for further treatment. MRI was significant for a 2.8 × 3.8 cm endocervical mass infiltrating > 50% of the cervix and < 50% of the myometrium (Fig. 1A). Additionally, a prominent right external iliac lymph node was noted. There was no evidence of intra-abdominal disease. Further evaluation with a PET-CT scan confirmed presence of a mass in the lower uterine segment and cervix with no evidence of nodal involvement.
Fig. 1.
Imaging findings before and after levonorgestrol intrauterine system treatment. (A) Initial MRI showing large endocervical mass involving the myometrium (B) Resolution of endocervical mass with residual heterogeneity after 3 months of levonogestrol intrauterine system treatment.
Given the patient's strong desire for fertility preservation, interventional radiology performed biopsy of the right external iliac node, which was negative for malignancy. She was taken to the operating room for cone biopsy of the cervix to assess for cervical extension of disease, as well as repeat hysteroscopy and D&C. The cone biopsy was negative for cervical involvement, but the endometrial sample was positive for grade 2 endometrial adenocarcinoma. Immunohistochemistry testing of the tumor demonstrated positive expression of MLH1, MSH2, MSH6, and PMS2 proteins, ruling out Lynch Syndrome.
After extensive discussion of the risks and benefits of hysterectomy versus fertility preservation, the patient ultimately elected for fertility-sparing treatment with the strict caveat this was outside standard of care. She met with a reproductive endocrinologist for discussion of fertility preservation should conservative management fail.
A levonogesterel intrauterine system (IUS) was placed with plan for close surveillance with endometrial sampling and imaging. Three months after IUS placement, MRI demonstrated resolution of the mass with some residual heterogeneity in the endocervical region (Fig. 1B). Endometrial sampling showed residual grade 2 adenocarcinoma. The patient was again offered surgical management, but she requested continued conservative management. At her next visit, about seven months after IUS placement, the patient noted vaginal spotting, and endometrial sampling revealed a focus of persistent grade 2 endometrial adenocarcinoma. At this time, oral megestrol acetate, 80 mg twice a day, was added to her regimen with plans for repeat sampling. At three months, her biopsy returned negative for adenocarcinoma. Repeat sampling and MRI at 6 and 9 months post dual therapy initiation revealed continued complete response. The patient is now planning to attempt conception. Endometrial biopsies will be obtained at 3–6 month intervals until conception is achieved with hysterectomy planned at the completion of child-bearing.
3. Comment
Endometrial adenocarcinoma presents a significant challenge when present in young women desiring retention of childbearing potential. Certainly, the standard of care limits opportunities for fertility preservation. This issue may continue to become more prevalent given many women currently delay child bearing until later in life. Furthermore, the incidence of obesity, a significant risk factor for endometrial adenocarcinoma, is rising in the US.
Conservative treatment of endometrial adenocarcinoma traditionally involved oral progestin therapy (Gunderson et al., 2012), although more recently treatment with the levonorgestrel IUS has been described (Perri et al., 2011, Amant et al., 2005). Two large meta-analyses have examined response to progesterone therapy. In these studies, the majority of patients were treated with oral regimens. The median time for response to progesterone therapy was 3–6 months. Despite initial response, approximately 25% of patients recurred over a median of 19–24 months (Gunderson et al., 2012, Ramirez et al., 2004). Other single institution studies have also demonstrated a recurrence rate of 8.3–62.5% with conservative management (Perri et al., 2011). Theoretically, increasing the progestin concentration to the target organ via the IUS may be more effective at regressing endometrial neoplasia than oral progestins and result in fewer side effects (Vereide et al., 2003). There are multiple ongoing studies to better explore this hypothesis (NCT00788671, NCT02397083, NCT02035787).
Conservative management of low grade superficially invasive endometrial adenocarcinoma carries risks of disease progression by delaying standard treatment. Most reports describing conservative management of endometrial adenocarcinoma stress this caveat as a cornerstone in patient counseling. Indeed, when either oral or intrauterine progestin therapy fails, most practitioners will recommend hysterectomy. Dual progestin therapy is rarely described, and it has only been described in women with grade 1 adenocarcinoma (Gungor et al., 2016).
Our patient ultimately preferred to attempt dual progestin therapy as a way to preserve fertility before proceeding with a hysterectomy. Though her cancer did not respond completely to single agent progestin therapy, there was ultimately a durable complete response to dual agent progestin therapy with the addition of oral progestin to the levonogesterel IUS. While we are optimistic given her response to dual progestin therapy, the need for continued surveillance is paramount in her treatment, given her risk for relapse.
Thus, management with dual progesterone therapy may be an additional option for young women who desire to retain their child bearing potential in the setting of progesterone-refractory endometrial adenocarcinoma, provided they are properly counseled and closely monitored. Indeed, Kim et al. have shown promising results with upfront dual progestin therapy in a small group of women with stage IA, grade 1 endometrial adenocarcinoma. They describe an overall remission rate of 87.5% with a recurrence rate of 12.5% (0% recurrence in women who had continued “maintenance” progestin therapy) (Kim et al., 2013). However, their small sample size makes it challenging to draw any conclusions from these results, and not all women will require dual progestin management. Our case offers another strategy to maintain fertility when single agent progestin therapy fails. Further studies are warranted to determine if this treatment has broad application for the growing population of young women with early stage, grade 1 and 2 endometrial adenocarcinoma.
Funding source
2P50CA098258-06 from the NIH SPORE in Uterine Cancer, P30CA016672 from the NIH MD Anderson Cancer Center Support Grant and the Andrew Sabin Family Fellowship.
References
- Amant F., Moerman P., Neven P., Timmerman D., Van Limbergen E., Vergote I. Endometrial cancer. Lancet. 2005 Aug 6;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Gunderson C.C., Fader A.N., Carson K.A., Bristow R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol. Oncol. 2012 May;125(2):477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Gungor T., Cetinkaya N., Yalcin H., Zergeroglu S., Erkaya S. Clinicopathologic characteristics and treatment features of women with the incidental diagnosis of endometrial adenocarcinoma during infertility follow-up in Ankara, Turkey. Taiwan J. Obstet. Gynecol. 2016 Jun;55(3):309–313. doi: 10.1016/j.tjog.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Seong S.J., Kim Y.S., Song T., Kim M.-L., Yoon B.S. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am. J. Obstet. Gynecol. 2013 Oct;209(4):358. doi: 10.1016/j.ajog.2013.06.031. e1-4. [DOI] [PubMed] [Google Scholar]
- Perri T., Korach J., Gotlieb W.H., Beiner M., Meirow D., Friedman E. Prolonged conservative treatment of endometrial cancer patients: more than 1 pregnancy can be achieved. Int. J. Gynecol. Cancer. 2011 Jan;21(1):72–78. doi: 10.1097/IGC.0b013e31820003de. [DOI] [PubMed] [Google Scholar]
- Ramirez P.T., Frumovitz M., Bodurka D.C., Sun C.C., Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol. Oncol. 2004 Oct;95(1):133–138. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- SGO Clinical Practice Endometrial Cancer Working Group, Burke W.M., Orr J., Leitao M., Salom E., Gehrig P. Endometrial cancer: a review and current management strategies: part II. Gynecol. Oncol. 2014 Aug;134(2):393–402. doi: 10.1016/j.ygyno.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016 Feb;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Vereide A.B., Arnes M., Straume B., Maltau J.M., Ørbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol. Oncol. 2003 Dec;91(3):526–533. doi: 10.1016/j.ygyno.2003.07.002. [DOI] [PubMed] [Google Scholar]