Abstract
Bacteria stimulate macrophages as part of normal host defense. However, when this response is not limited, vascular smooth muscle may also be activated to express “vasoactive” genes (e.g., cyclooxygenase), leading to vascular collapse and septic shock. In macrophages, Toll-like receptors (TLRs) 4 and 2 transduce responses to Gram-negative and Gram-positive bacteria, respectively. However, the role of these TLRs in sensing bacteria in vascular smooth muscle is unclear. To address this question, we have cultured vascular smooth muscle cells from mice deficient in TLR4 (TLR4–/– mice), mice deficient in TLR2 (TLR2–/– mice), or control mice. Cells cultured from control or TLR2–/– mice, but not from TLR4–/– mice, expressed cyclooxygenase-2 and released increasing levels of prostaglandin E2 after stimulation with whole Escherichia coli bacteria; the combination of IL-1β plus TNF-α induced cyclooxygenase-2 in cells cultured from all three groups of animals. By contrast, Staphylococcus aureus affected cyclooxygenase-2 expression in two distinct ways. First, S. aureus induced a transient inhibition of cyclooxygenase-2 expression, which was overcome with time, and increased protein expression was noted. The effects of S. aureus on cyclooxygenase-2 expression were TLR2- and not TLR4-dependent. Thus, we show that Gram-positive and Gram-negative bacteria induce cyclooxygenase-2 in vascular smooth muscle with differing temporal profiles but with appropriate TLR2-versus-TLR4 signaling. These data have important implications for our understanding of the innate immune response in vascular cells and how it may impact vascular disease.
When exposed to bacteria, leukocytes become stimulated to kill and remove the invading pathogens as part of normal host defense. However, when this response is not limited, tissues within the cardiovascular system also become activated. It is now increasingly recognized that pathogen activation of vascular tissue influences cardiovascular disease directly or indirectly. In the case of systemic infections, pathogen-induced activation of the cardiovascular system, particularly the vascular smooth muscle, may result in sepsis followed by septic shock. Bacterial sepsis can be caused by Gram-positive or Gram-negative bacteria, although the type of immune response and cardiovascular dysfunction induced may differ dramatically.
Vascular smooth muscle, which is normally synthetically quiescent, displays elements of an innate immune response when stimulated in vitro with the active component of the Gram-negative bacteria LPS. For example, LPS induces the release of vasoactive inflammatory mediators, including cyclooxygenase products from vascular smooth muscle cells (1, 2) and intact blood vessels, in culture (3). Other inflammatory and vasoactive genes induced by LPS in vascular cells include nitric oxide synthase (4) and endothelin-1 (5). The production of vasoactive inflammatory mediators by vascular smooth muscle is thought not only to mediate vascular dysfunction in septic shock, but also to possibly contribute to the chronic inflammatory response associated with atherosclerosis (6).
In murine macrophages, Gram-negative LPS is recognized, or “sensed,” by the Toll-like receptor (TLR)4 pathway (7–10). Most recently, evidence has suggested that TLR2 may also be involved in the recognition of Gram-negative LPS in its unpurified form (11–13). Nevertheless, it seems clear that TLR4 is the principal cell-surface receptor activated by purified Gram-negative LPS in macrophages (9). It is less clear, however, how Gram-negative LPS is sensed in nonmyeloid cells, including those of the vasculature. Specifically, using human cells in culture as experimental models, Andreakos et al. (14) suggest that, although TLR4 is the essential link between LPS and activation of myloid cells (macrophages), it is not involved in activation of all nonmyloid cells (vascular endothelium or fibroblasts). Further evidence in vivo suggests that pathways other than TLR4 are activated in early immune responses. Specifically, TLR4 was shown not to mediate an early neutrophil recruitment phenomenon and bacterial clearing pathway in genetically modified mice (15). Alternative candidates for the cell surface sensing of LPS or whole Gram-negative bacteria are the subject of debate. However, intracellular NOD receptors can be activated by “contaminating” peptidoglycan in unpurified LPS (16).
Much less is known about how Gram-positive pathogens activate cells. However, it is suggested that TLR2s are involved in transducing the effects of Gram-positive pathogens. Gram-positive sepsis is now a serious problem in intensive care wards. Indeed, a recent study demonstrates that the number of cases of Gram-positive sepsis now outweigh those of Gram-negative sepsis (17). It is therefore important that we begin to study how different pathogens active cells in parallel. Vascular smooth muscle cells are particularly important to study in this respect because they are a key site of pathological events in sepsis and septic shock. We suggest the identification of similarities and differences in the essential pathogen-sensing mechanisms of vascular smooth muscle cells and immune cells as a key approach to the future treatment of sepsis and, perhaps, other vascular diseases.
Thus, the purpose of this study was to investigate how Gram-negative (Escherichia coli) versus Gram-positive (Staphylococcus aureus) bacteria influence the expression of cyclooxygenase-2 in vascular smooth muscle. We focused our work on cyclooxygenase-2 because, unlike some vasoactive genes, it is readily induced in human as well as rodent cells (2, 3). Specifically, we have investigated the respective roles of TLR2 and TLR4 in responses of vascular smooth muscle cells to bacteria. There are currently no specific pharmacological tools to determine the roles of TLR2 or TLR4. Furthermore, molecular manipulation of cells in vitro to increase or decrease TLR function is likely to result in an altered phenotype. For these reasons, we have used primary cells cultured from either wild-type mice or those deficient in TLR2 or TLR4.
Methods
Mice. Breeding pairs of genetically modified mice where TLR2 or TLR4 receptors had been deleted were kindly supplied by O. Takeuchi (Osaka University, Osaka) (18). These mice were imported and bred commercially by B & K Universal (East Yorkshire, U.K.). Mice were genotyped during the initial breeding program, and random testing was then performed. Mice used in this study were homozygotes of either TLR2–/– or TLR4–/– genotype, back-bred for more than seven generations with C57BL/6 mice. Cells cultured from C57BL/6 mice expressed a complete TLR2+/+ and TLR4+/+ profile (as measured by genomic RT-PCR; data not shown) and were used as controls.
Culture of Vascular Smooth Muscle Cells. Murine vascular smooth muscle was cultured by using an explant method. Briefly, adult male animals (15–31 weeks) were anesthetized with i.p. pentobarbitone and killed by cervical dislocation. The descending aorta was excised, immediately immersed into sterile PBS, cleared of connective tissue, and cut longitudinally. The intimal surface was gently scraped with forceps to remove endothelial cells. The remaining media of the aorta was cut into one 2-mm square, five to six pieces of which were set apart and put onto 35-mm culture plates. To maintain the tissues close to the plate, each was placed under a glass coverslip with 5-mm sides. Tissues were then cultured in 1 ml of DMEM containing 20% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), amphotericin (2.5 μg/ml), and nonessential amino acids in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Culture medium was exchanged every 4 days. After 10–14 days in culture, the outgrowth of the cells was seen to form a subconfluent monolayer beyond the border of the covering glass. Cells were then harvested after treatment with trypsin (0.05%)–EDTA (0.2 mM), and 1 × 105 cells were placed into a noncoated 35-mm dish and cultured in 4 ml of DMEM containing 10% FBS until ≈90% subconfluent. After serial subcultures, experiments were performed using cells of passage numbers 4–10. It should be noted, however, that at high passage numbers, the effects of LPS on cyclooxygenase activity tended to diminish or were lost (J.A.M. and T.D.W., unpublished data). Vascular smooth muscle cells were identified by immunohistochemical analysis by using a murine monoclonal antibody against α-smooth muscle actin and a goat anti-mouse antibody (1A4:N1584, Sigma). In some experiments (“time course”), clonal vascular smooth muscle cells (rodent, contractile phenotype; supplied by T.D.W.) were cultured by using standard techniques (as above).
Experimental Procedures. Cells were grown in DMEM containing 10% FBS and supplemented with antibiotic/mycotic mix. Cells from wild-type, TLR2-deficient, or TLR4-deficient mice were grown on 96-well or 75-cm2 plates until confluent with serum being withdrawn for 24 h before experimentation. After addition of bacteria, cytokines and/or drugs cells were incubated as described, after which time conditioned medium was removed and either stored at –80°C or analyzed immediately. Prostaglandin E2 (PGE2) levels were measured by RIA as described in ref. 19. For time-course experiments, cells, bacteria, and/or cytokines were added to the cells for 0.5–48 h. Where samples were being collected for Western blots, medium was removed and cells were lysed as described below. Where cyclooxygenase activity was being measured, archidonic acid (30 μM; ref. 19) was added for 30 min, after which time the medium was removed from the measurement of PGE2 by RIA, as described above.
Western Blotting. Vascular smooth muscle cells were seeded in separate 75-cm2 flasks and treated for designated times with bacteria or cytokines (10 ng/ml each). The medium was then removed, and the cells were lysed with Tris buffer (50 mM, pH 7.4) containing 1% (vol/vol) Triton X-100, EDTA (10 mM), PMSF (1 mM), pepstatin (0.05 mM), and leupeptin (0.2 mM). Cell extracts were boiled at a 1:1 ratio with Tris (50 mM, pH 6.8) containing 4% (wt/vol) SDS, 10% (vol/vol) glycerol, 4% (vol/vol) 2-mercaptoethanol, and 2 mg/ml bromophenol blue. Samples of equal protein were loaded onto 7.5% Tris-glycine SDS gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were probed with a specific cyclooxygenase-2 antibody (Cayman Chemical, Ann Arbor, MI) raised in rabbit. The blots were then incubated with anti-rabbit IgG (raised in goat), conjugated to horseradish peroxidase, and developed by enhanced chemiluminescence (Amersham Biosciences). Rainbow markers (14,000–200,000 kDa, Amersham Biosciences) were used for molecular mass determinations.
Materials. Human recombinant IL-1β and TNF-α were purchased from Roche (East Sussex, U.K.), TRIzol reagent and antibiotic/mycotics were from Invitrogen, Western blot luminescent detection reagents and horseradish peroxidase-conjugated anti-rabbit IgG were from Santa Cruz Biotechnology and Autogen Bioclear (Calne, U.K.), and radiolabeled PGE2 was obtained from Amersham Biosciences. Rofecoxib was supplied by Merck Frosst (Pointe Claire, PQ, Canada), and all other reagents were from Sigma–Aldrich.
Bacterial Components and Bacteria. All bacteria were stored as frozen glycerol stocks and streaked onto agar plates before inoculation of single colonies into RPMI medium 1640 with 10% FCS and glutamine. Cultures were incubated overnight and then centrifuged at 800 × g to pellet bacteria. Bacteria were then washed twice in sterile saline, and pellets were resuspended in sterile saline. Aliquots of the bacterial suspension were serially diluted and plated onto agar to quantify the cell density. The bacterial suspensions were then heat-treated for 45 min at 70°C to kill all bacteria; sterility was confirmed by plating of the resultant suspension. Suspensions were adjusted to 1010 to 1012 colony-forming units (CFU)/ml and then frozen with 20% glycerol in aliquots before use in cell culture experiments. E. coli reference strain 0111.B4 was used, in addition to a clinical blood culture S. aureus isolate, H380. Bacterial LPS from E. coli 0111.B4 was purchased from Sigma.
Data Analysis. All experiments were performed in at least three separate wells or plates of cells. Where murine cells were used, cells were cultured from at least three separate mice for each group. Data are shown as the mean ± SEM for the number of experiments.
Results
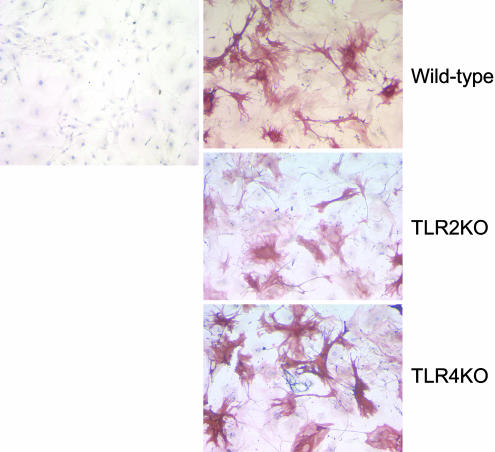
Morphology of Mouse Aortic Vascular Smooth Muscle Cells. Vascular smooth muscle cells cultured from TLR2–/– or TLR4–/– mice appeared similar in morphology to cells from wild-type animals, having standard smooth muscle phenotype and staining positive for smooth muscle α-actin (Fig. 1).
Fig. 1.
Morphology of mouse aortic smooth muscle cells. Primary cultures from control, TLR2–/–, and TLR4–/– mice exhibited the characteristic hill-and-valley morphology of vascular smooth muscle cells. Immunostaining shows smooth muscle α-actin with counterstaining for the nuclei. Staining in the absence of primary antibodies is shown in Left.
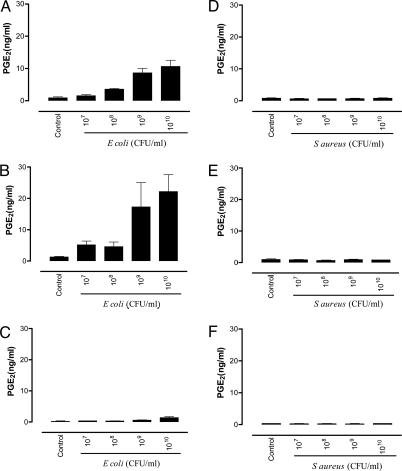
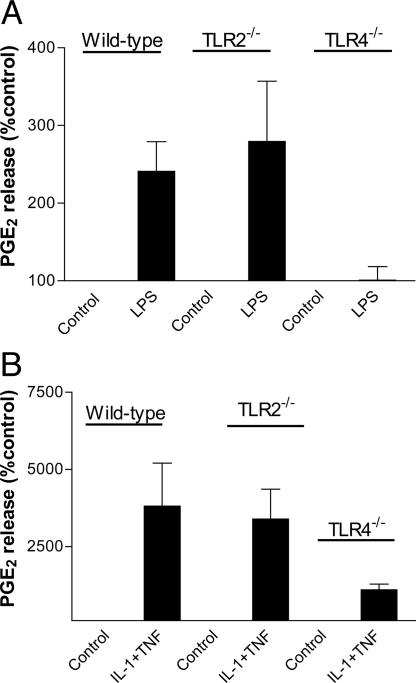
Effect of Gram-Negative or Gram-Positive Bacteria on Cyclooxygenase Activity in Murine Vascular Smooth Muscle Cells Derived from Control and TLR4–/– or TLR2–/– Animals. Gram-negative E. coli (107 to 1010 CFU/ml) induced a concentration-dependent increase in PGE2 release by vascular smooth muscle cells cultured from the aortae of wild-type control animals or from animals deficient in TLR2s (Fig. 2). By contrast, E. coli had little or no effect on PGE2 release by cells grown from the aortae of TLR4–/– animals (Fig. 2). S. aureus (107 to 1010 CFU/ml) had no effect on PGE2 release in cells grown from wild-type, TLR4–/–, or TLR2–/– animals (Fig. 2). In line with observations made with whole heat-killed bacteria, LPS (E. coli, serotype 0111:B4) also increased PGE2 release by cells cultured from control and TLR2–/– vessels but not from vessels removed from TLR4–/– mice (Fig. 3). In contrast, lipoteichoic acid (derived from S. aureus, up to 100 μg/ml) had no effect on PGE2 release by vascular smooth muscle cells cultured from control, TLR2–/–, or TLR4–/– mice or on cyclooxygenase-2 expression (data not shown). In vascular smooth muscle cells cultured from mice of any group, increased levels of PGE2 release were measured when cells were stimulated by either IL-1β (10 ng/ml), TNF-α (10 ng/ml) (data not shown), or, to a greater extent, the combination of IL-1β and TNF-α (Fig. 3). In contrast to data obtained with E. coli or LPS, the combination of IL-1β plus TNF-α was still able to induce PGE2 release in vascular smooth muscle cells cultured from TLR4–/– mice. There was a tendency for the amount of cyclooxygenase activity induced by cytokines to be lower in TLR4–/– mice than in control or TLR2–/– mice, but this difference did not reach statistical significance (one-way ANOVA followed by Dunnett's multiple comparison test; Fig. 3).
Fig. 2.
Effect of Gram-negative and Gram-positive bacteria on cyclooxygenase activity in murine smooth muscle cells from wild-type, TLR2–/–, and TLR4–/– mice. (A and B) E. coli (107 to 1010 CFU/ml) induced a concentration-dependent increase in the activity of cyclooxygenase in vascular smooth muscle cells derived from the aortae of control (A) or TLR2–/– (B) animals. (C) However, E. coli had a little or no effect on the production of PGE2 in vascular smooth muscle cells cultured from TLR4–/– mice. (D–F) S. aureus (107 to 1010 CFU/ml) had no effect on cyclooxygenase activity in cells cultured from control (D), TLR2–/– (E), or TLR4–/– (F) mice. Data shown are mean ± SEM for three separate wells of cells. Similar observations (using bacteria at 109 CFU/ml) were made in two other experiments.
Fig. 3.
Effect of LPS (10 μg/ml) (A) or the combination of the cytokines TNF-α and IL-1β (both at 10 ng/ml) (B) on PGE2 production in primary cultures of murine smooth muscle cells from control, TLR2–/–, and TLR4–/– mice, respectively. The release of PGE2 is calculated as percentage of control. Data are means ± SEM for 5–11 determinations with cells from three separate aortae.
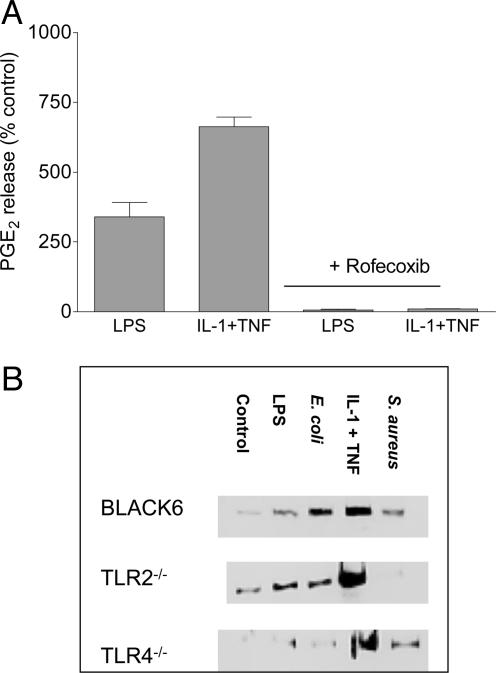
Characterization of Cyclooxygenase-2 Induced in Murine Vascular Smooth Muscle Cells. The increased production of PGE2 induced by LPS (10 μg/ml), E. coli (109 CFU/ml), or cytokine treatment was abolished by the cyclooxygenase-2-selective inhibitor rofecoxib (100 nM; Fig. 4). Furthermore, the increase in PGE2 release induced by E. coli, LPS, or cytokine treatment in cells from control animals or TLR2–/– animals was associated with increased expression of cyclooxygenase-2 protein (immunoreactivity at ≈70 kDa; Fig. 4). Similarly, cells cultured from TLR4–/– mice expressed increased levels of cyclooxygenase-2 protein when cells were treated with IL-1β plus TNF-α. However, cells from TLR4–/– mice did not express increased levels of cyclooxygenase-2 protein when treated with either LPS or E. coli. Interestingly, cells cultured from control or TLR4–/– animals, but not from TLR2–/– animals, expressed increased amounts of cyclooxygenase-2 protein after simulation with S. aureus (Fig. 4).
Fig. 4.
Characterization of cyclooxygenase-2 activity in primary cultures of murine smooth muscle cells from control, TLR4–/–, and TLR2–/– mice. (A) Inhibition by rofecoxib (100 nM) of the increase in PGE2 production induced by LPS (10 μg/ml) or the combination of TNF-α plus IL-1β (10 ng/ml each) for 48 h. Data are means ± SEM for three experiments. (B) Western blot analysis of cyclooxygenase-2 protein expression in whole cell extracts from wild-type control, TLR2–/–, and TLR4–/– mice. Lanes contain samples of unstimulated cells from control, TLR2–/–, and TLR4–/– mice and cells stimulated with LPS (10 μg/ml), E. coli (109 CFU/ml), the combination of TNF-α and IL-1β (10 ng/ml each), or S. aureus (109 CFU/ml) for 48h. Similar results were obtained by using cells cultured from three separate aortae.
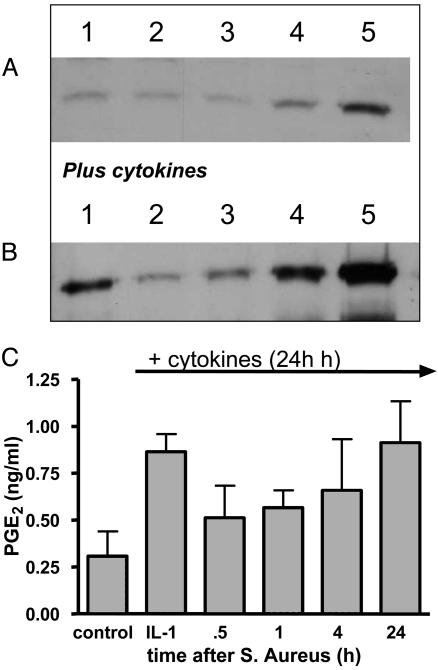
Temporal Analysis of the Effects of S. aureus on Cyclooxygenase-2 Expression in Rodent and Human Vascular Smooth Muscle Cells. As noted above, cyclooxygenase-2 in different cultures of control cells was consistently expressed under control culture conditions (Fig. 5). As mentioned above, the addition of cytokines induced increased levels of cyclooxygenase-2 protein.
Fig. 5.
Temporal effects of S. aureus (108 CFU/ml) on cyclooxygenase-2 expression in wild-type cells. (A) A representative Western blot for cyclooxygenase-2 with samples from control cells (lane 1) or cells treated with S. aureus for 30 min (lane 2), 1 h (lane 3), 4 h (lane 4), or 24 h (lane 5). (B) A representative blot in which cells were treated with cytokines alone for 24 h (lane 1) or with cytokines plus S. aureus for 30 min (lane 2), 60 min (lane 3), 4 h (lane 4), or 24 h (lane 5). Twenty-five micrograms of protein was loaded in each well. Similar data were obtained by using three other separate cell cultures. In each experiment, the inhibitory effects of S. aureus on expression were significant. It should be noted, however, that the duration of inhibition of cyclooxygenase induction varied in time from between 4 and 24 h. (C) The inhibitory effects of S. aureus on cyclooxygenase activity [measured as PGE2 for 30 min after treatments in the presence of substrate (30 μM)].
The basal expression of cyclooxygenase-2 was transiently inhibited by S. aureus (108 CFU/ml; Fig. 5). At 24–48 h after addition of S. aureus, the inhibitory effects were overcome, and increased levels of cyclooxygenase-2 protein were detected. Similarly, S. aureus inhibited cyclooxygenase-2 protein induced by cytokines. This effect, like that seen on basal cyclooxgyenase-2 expression, was also transient and lasted from between 0.5 and 24 h (Fig. 5). In line with its effects on cyclooxygenase-2 protein, S. aureus inhibited the increase in cyclooxygenase activity (measured as PGE2 in the presence of arachidonic acid; 30 μM) induced by cytokines (Fig. 5).
Discussion
This study shows that Gram-negative and Gram-positive bacteria differ in their ability to induce cyclooxygenase-2 in vascular smooth muscle cells and that their actions are mediated exclusively by TLR4 and TLR2, respectively.
Activation of the innate immune response is critical to health. However, when this system is overwhelmed, blood vessels are inappropriately activated, and cardiovascular dysfunction ensues. It is now clear that vascular smooth muscle is an important site within the vessel for pathogen activation. For instance, both human and rodent cultures of vascular smooth muscle are activated by LPS to express a number of vasoactive genes, such as cyclooxygenase (1, 3), normally only seen in the endothelium (20). Once this activation has occurred, cells release large and unregulated amounts of vasoactive mediators, which render the vessel dysfunctional. In the current study, we confirm in cultured vascular smooth muscle cells that LPS induces the expression of cyclooxygenase-2 protein and that this expression is associated with an increased production of PGE2, which is sensitive to inhibition by the selective cyclooxygenase-2 inhibitor rofecoxib.
In vascular smooth muscle cells cultured from TLR4–/– mice, neither LPS nor whole E. coli induced significant expression of cyclooxygenase-2 protein or an increase in PGE2 production. Our results could simply show that cells cultured from TLR4–/– mice have a fundamental defect in the cyclooxygenase pathway that is incidental to the TLR4 gene. However, we show that, despite having no response to LPS, cells cultured from TLR4–/– mice can be activated to express increased levels of cyclooxygenase-2 and produce PGE2 by exposure to the combination of IL-1β plus TNF-α. PGE2 release from TLR4–/– cells under these conditions tended to be lower than that from wild-type control cells in matched experiments, although this release did not reach statistical significance. Indeed, the level of cyclooxygenase-2 protein expressed in TLR4–/– cells after incubation with IL-1β plus TNF-α was comparable to that seen in cells cultured from wild-type animals. IL-1β and TNF-α activate cells, including vascular smooth muscle, to express cyclooxygenase-2 (1, 21) via pathways that should not directly involve the TLR pathway. Our data support this hypothesis and provide an essential control for our conclusion.
A limited number of studies have been published addressing the role of TLR4 in LPS signaling in vascular cells. TLR4 has been identified on endothelial cells in human coronary vessels and appears important in LPS-induced neutrophil–endothelial cell interactions (22, 23). However, there are a significant number of studies implicating a role of TLR4 in atherosclerosis (24, 25), and, in support of this, a functional TLR4 is expressed in human adventitial fibroblasts, macrophages (22), and vascular smooth muscle (26).
Interestingly, we found that not only “crude” LPS activated vascular cells via the TLR4 pathway, but the same was true for whole E. coli bacteria. Crude LPS and whole bacteria contain peptidoglycan, which, as discussed above, can activate cells via TLR2 or possibly via intracellular NOD receptors. Thus, our experiments suggest that the overriding sensing pathway for Gram-negative pathogens in vascular smooth muscle cells is TLR4. In line with this hypothesis, we found that both LPS and E. coli stimulated cyclooxygenase-2 activity and expression in vascular cells grown from the aortae of TLR2–/– mice. In fact, responses to LPS or E. coli, but not IL-1 plus TNF, tended to be greater in cells from TLR2–/– mice than those observed in cells cultured from control mice.
In the current study, we found that S. aureus increased cyclooyxgenase-2 protein expression after 24–48 h, an effect that was found to be TLR2-mediated; similar observations have been made using macrophages and peptidoglycan from S. aureus (27). However, increased activity in the form of PGE2 release was not observed. Moreover, we identified an interesting inhibitory property of the Gram-positive pathogen on cyclooxygenase-2 expression and activity. Specifically, S. aureus induced a transient inhibitory effect on cyclooxygenase-2 expression that lasted between 4 and 24 h. This inhibitory effect resulted in a delay in the time course of cyclooxygenase-2 induced and a predicted reduction in the amount of PGE2 release accumulated in the course of the experiment. This inhibitory effect on cyclooxygenase-2 may have some relevance to the different profile of immune and cardiovascular profiles observed in sepsis. It should be noted, however, that we cannot rule out additional inhibitory effects of S. aureus (compared with E. coli) on PGE2 production posttranscriptionally or at the level of substrate availability (by means of low phospholipase A2 activity).
In conclusion, we have demonstrated that in vascular smooth muscle cells, which are critical target cells for pathogens and septic shock, Gram-negative and Gram-positive bacteria are sensed and result in cyclooxygeanse-2 expression, which depend on TLR4 and TLR2, respectively. It should be noted, however, that our observations do not exclude the possibility that alternative pathways of activation may exist in vascular smooth muscle that act in synergy with TLR4 or TLR2 in the induction of cyclooxygenase-2 by whole bacteria. Furthermore, we show that the induction of cyclooxygenase-2 by Gram-positive S. aureus is delayed because of an initial “inhibitory” phase. These observations define a potential demarcation in the effects of Gram-positive versus Gram-negative bacteria on vascular genes and subsequent insult. Activation of vascular cells by bacteria as well as other pathogens is implicated in both chronic and acute cardiovascular disease. This study highlights the importance to cardiovascular health of understanding how vascular tissue senses pathogens. As similarities in and differences between pathways used by vascular tissue and essential immune cells are recognized and identified, selective interventions to stimulate or protect vascular tissue may be possible.
Acknowledgments
We thank Prof. Timothy Evans for his invaluable help and advice. This work was funded by grants from the Medical Research Council, the Spanish government, the National Heart and Lung Institute, and the British Heart Foundation.
Abbreviations: TLR, Toll-like receptor; PGE2, prostaglandin E2; CFU, colony-forming units.
References
- 1.Jourdan, K. B., Mitchell, J. A. & Evans, T. W. (1997) Biochem. Biophys. Res. Commun. 233, 668–672. [DOI] [PubMed] [Google Scholar]
- 2.Bishop-Bailey, D., Pepper, J. R., Larkin, S. W. & Mitchell, J. A. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1655–1661. [DOI] [PubMed] [Google Scholar]
- 3.Bishop-Bailey, D., Larkin, S. W., Warner, T. D., Chen, G. & Mitchell, J.A. (1997) Br. J. Pharmacol. 121, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse, R. & Mulsch A. (1990) FEBS Lett. 275, 87–90. [DOI] [PubMed] [Google Scholar]
- 5.Woods, M., Bishop-Bailey, D., Pepper, J. R., Evans, T. W., Mitchell, J. A. & Warner, T. D. (1998) J. Cardiovasc. Pharmacol. 31, S348–S350. [DOI] [PubMed] [Google Scholar]
- 6.Stemme, V., Swedenborg, J., Claesson, H. & Hansson, G. K. (2000) Eur. J. Vasc. Endovasc. Surg. 20, 146–152. [DOI] [PubMed] [Google Scholar]
- 7.Beutler, B. (2004) Mol. Immunol. 40, 845–859. [DOI] [PubMed] [Google Scholar]
- 8.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- 9.Beutler, B., Hoebe, K., Du, X. & Ulevitch, R. J. (2003) J. Leukocyte Biol. 74, 479–485. [DOI] [PubMed] [Google Scholar]
- 10.Aderem, A. & Ulevitch, R. J. (2000) Nature 406, 782–787. [DOI] [PubMed] [Google Scholar]
- 11.Tapping, R. I., Akashi, S., Miyake, K., Godowski, P. J. & Tobias P. S. (2000) J. Immunol. 165, 5780–5787. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld, M., Ma, Y., Weis, J. H., Vogel, S. N. & Weis, J. J. (2000) J. Immunol. 165, 618–622. [DOI] [PubMed] [Google Scholar]
- 13.Hirschfeld, M., Weis, J. J, Toshchakov, V., Salkowski, C. A., Cody, M. J., Ward, D. C., Qureshi, N., Michalek, S. M. & Vogel, S. N. (2001) Infect. Immun. 69, 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreakos, E., Sacre, S. M., Smith, C., Lundberg, A., Kiriakidis, S., Stonehon, T., Monaco, C., Feldmann, M. & Foxwell, B. M. (2004) Blood 103, 2229–2237. [DOI] [PubMed] [Google Scholar]
- 15.Haziot, A., Hijiya, N., Gangloff, S. C., Silver, J. & Goyert, S. M. (2001) J. Immunol. 166, 1075–1078. [DOI] [PubMed] [Google Scholar]
- 16.Camaillard, M., Girardin, S. E., Viala, J. & Philpott, D. J. (2003) Cell Microbiol. 5, 581–592. [DOI] [PubMed] [Google Scholar]
- 17.Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. (2003) N. Engl. J. Med. 348, 1546–1554. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi, O., Hoshino, K., Kawai, T., Sanjo, H., Takada, H., Ogawa, T., Takeda, K. & Akira, S. (1999) Immunity 11, 443–451. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell, J. A., Akarasereenont, P., Thiemermann, C., Flower, R. J. & Vane, J. R. (1993) Proc. Natl. Acad. Sci. USA. 90, 11693–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell, J. A. & Warner, T. D. (1999) Br. J. Pharmacol. 128, 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanford, S., Pepper, P. J. & Mitchell, J. A. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 835–838. [DOI] [PubMed] [Google Scholar]
- 22.Andonegui, G., Goyert, S. M. & Kubes, P. (2002) J. Immunol. 169, 2111–2119. [DOI] [PubMed] [Google Scholar]
- 23.Hijiya, N., Miyake, K., Akashi, S., Matsuura, K., Higuchi, Y. & Yamamoto, S. (2002) Pathobiology 70, 18–25. [DOI] [PubMed] [Google Scholar]
- 24.Pasterkamp, G., van Keulen, J. K. & Kleijn, P. V. (2004) Eur. J. Clin. Invest. 34, 328–334. [DOI] [PubMed] [Google Scholar]
- 25.Vink, A., Schoneveld, A. H., van der Meer, J. J., van Middelaar, B. J., Sluijter, J. P., Smeets, M. B., Quax, P. H., Lim, S. K., Borst, C., Pasterkamp, G. & de Kleijn, D. P. (2002) Circulation 106, 1985–1990. [DOI] [PubMed] [Google Scholar]
- 26.Sasu, S., La Verda, D., Qureshi, N., Golenbock, D. T. & Beasley, D. (2001) Circ. Res. 89, 244–250. [DOI] [PubMed] [Google Scholar]
- 27.Chen, B. C., Chang, Y. S., Kang, J. C., Hsu, M. J., Sheu, J. R., Chen, T. L., Teng, C. & Lin, C. H. (2004) J. Biol. Chem. 279, 20889–20897. [DOI] [PubMed] [Google Scholar]