Abstract
This preliminary study investigated how cognitive-linguistic status in multiple sclerosis (MS) is reflected in two speech tasks (i.e. oral reading, narrative) that differ in cognitive-linguistic demand. Twenty individuals with MS were selected to comprise High and Low performance groups based on clinical tests of executive function and information processing speed and efficiency. Ten healthy controls were included for comparison. Speech samples were audio-recorded and measures of global speech timing were obtained. Results indicated predicted differences in global speech timing (i.e. speech rate and pause characteristics) for speech tasks differing in cognitive-linguistic demand, but the magnitude of these task-related differences was similar for all speaker groups. Findings suggest that assumptions concerning the cognitive-linguistic demands of reading aloud as compared to spontaneous speech may need to be re-considered for individuals with cognitive impairment. Qualitative trends suggest that additional studies investigating the association between cognitive-linguistic and speech motor variables in MS are warranted.
Keywords: acoustic analysis, dysarthria, adults
Introduction
Cognitive-linguistic and motor execution processes are often conceptualized as separate stages in models of spoken language (e.g. Levelt, Roelofs, & Meyers, 1999; van der Merwe, 2009; Yorkston, Beukelman, Strand, & Hakel, 2011). However, there is growing appreciation from studies of healthy individuals as well as various clinical populations that cognitive-linguistic and speech motor processes influence one another or interact in spoken language (e.g. Dromey & Bates, 2005; Dromey & Benson, 2003; Huber & Darling, 2011; Kent, 2004; Lowit, Brendel, Dobinson, & Howell, 2006; Maner, Smith, & Grayson, 2000; Sadagopan & Smith, 2008). As discussed in the following paragraphs, multiple sclerosis (MS) is another clinical population for whom the interaction of cognitive-linguistic and speech motor variables is of interest.
Nature of cognitive and motor speech impairment in MS
At least 50% of individuals with MS experience impairment in the domains of memory, conceptual reasoning, processing speed, attention, concentration or executive function (Benedict & Zivadinov, 2011; Langdon, 2011). Because cognitive impairment in MS may be subtle (Schiffer, 1999), results from cognitive screening tools must be interpreted with caution (Rao, 1995). Rather, tests comprising The Brief Repeatable Battery of Neuropsychological Tests (Rao and the Cognitive Function Study Group of the National Multiple Sclerosis Society, 1990) or the Minimal Assessment of Cognitive Function in MS (MACFIMS; Benedict et al., 2002) are considered to be ideally suited to assess cognitive function in MS (Strober et al., 2009).
Slowed information processing speed, or time required for mental processing, is one of the most common cognitive deficits in MS (Benedict & Bobholz, 2007). Working memory impairment and reduced information processing efficiency are also common (Benedict & Bobholz, 2007). Information processing efficiency reflects the capacity of working memory and rate or speed of information processing (Archibald & Fisk, 2000; Benedict, Morrow, Weinstock-Guttman, Cookfair, & Schretlen, 2010). Thus, reduced information processing efficiency suggests slowed neural transmission of information and/or restricted working memory capacity (Archibald & Fisk, 2000; Benedict et al., 2010). Executive function, defined as higher level cognitive processes such as reasoning, planning and organizing behavior, is also impaired with considerable frequency in MS (Beatty & Monson, 1996; Benedict & Bobholz, 2007). Finally, cognitive impairment in MS may also manifest as a subtle non-aphasic, higher level language deficit (Arnott, Jordan, Murdoch, & Lethlean, 1997; Beatty, Goodkin, Monson, & Beatty, 1989; Henry & Beatty, 2006; Kujala, Portin, & Ruutiainen, 1996; Lethlean & Murdoch, 2000; Murdoch & Lethlean, 2000; Wallace & Holmes, 1993; Yorkston, Klasner, & Swanson, 2001).
Motor speech impairment, in the form of dysarthria, also affects about 40% of individuals with MS (Hartelius, Runmarker, & Andersen, 2000; Yorkston et al., 2003). The nature of the dysarthria is classically described as having spastic and/or ataxic components (Duffy, 2005). Dysarthria severity, often indexed by quantitative measures of speech intelligibility (Weismer, Jeng, Laures, Kent, & Kent, 2001), varies from mild to severe.
Cognitive-linguistic and speech motor interactions in MS
An important consideration in the clinical management of any neurodegenerative disease, including MS, is how impairment in one functional system interacts with or influences other functional systems (Baylor, Yorkston, Bamer, Britton, & Amtmann, 2010). Despite the frequency with which cognitive-linguistic impairment and/or dysarthria occurs in MS, little is known about the potential interaction or association of these variables. Yorkston et al. (2003) surveyed over 700 individuals with MS and found that speech changes were likely to occur in concert with physical, cognitive and psychosocial alterations. Smith and Arnett (2007) explored cognitive–speech motor interaction in MS using rapid syllable repetitions (i.e. diadochokinesis, DDK) and standard cognitive or neuropsychological tests requiring a rapid verbal response. When DDK rate was statistically controlled, cognitive test scores for persons with MS and healthy controls became more similar. Similar findings were reported by Arnett, Smith, Barwick, Benedict, and Ahlstrom (2008). Although these studies seem to suggest that speech motor impairment may contribute to the appearance of cognitive impairment in MS, the use of DDK in this study should be considered carefully (Westbury & Dembowski, 1993). Finally, Mackenzie and Green (2009) reported that higher scores on the Arizona Battery for Communication Disorders of Dementia (Bayles & Tomoeda, 1993) for individuals with MS were associated with higher sentence intelligibility (Yorkston & Beukelman, 1984).
Although these studies are a good start, it is apparent that much remains to be learned regarding the potential association among cognitive-linguistic and motor speech variables in MS. As discussed below, the topic has been approached in Parkinson’s disease (PD) by investigating global speech timing (i.e. speech rate, pause characteristics).
Global speech timing in reading and spontaneous speech
Reading is characterized as having a reduced cognitive-linguistic load compared to spontaneous speech (e.g. Conrad & Schonle, 1979; Goldman-Eisler, 1968; Kempler & Van Lancker, 2002; Tasko & McClean, 2004; Van Lancker Sidtis et al., 2004; Walker, 1988). Measures of global speech timing, including speech rate and pause characteristics, further appear to be sensitive to the different online processing demands of reading and spontaneous speech. Speech rate is defined as the number of output units per unit time including pauses, while articulation rate is defined similarly but excludes pausing (Tsao, Weismer, & Iqbal, 2006). Silent pauses within or between words may or may not coincide with inhalation. In contrast, a filled pause is a nonlexical one syllable vocalization (e.g. um, er) (Clark & Fox Tree, 2002; Goldman-Eisler, 1961). Clark and Fox Tree (2002) further suggest that filled pauses are planned by speakers to signal an imminent delay. Thus, a filled pause indicates that an individual can predict an upcoming speech delay. The frequency of filled pauses also increases with increased language complexity (Lay & Paivio, 1969; Reynolds & Paivio, 1968). Interjections, defined as words or phrases that do not add information to an utterance (e.g. well, like), may also be used to signal a future delay in speaking necessary for planning (Clark & Fox Tree, 2002).
Speech rate is slower for spontaneous speech than reading (e.g. Hoit & Hixon, 1987; Huber & Darling, 2011; Walker, 1988). This does not appear to be attributable to a slowed rate of speech sound production, as overall articulation rate for spontaneous speech tends to be faster than for reading aloud (Jacewicz, Fox, O’Neill, & Salmons, 2009; Jacewicz, Fox, & Wei, 2010). Rather, increased pause time explains the slower speech rate of spontaneous speech, which provides time needed for formulation of thought or cognitive-linguistic processing (Goldman-Eisler, 1968; Rochester, 1971; Walker, 1988). Spontaneous speech also has a higher proportion of grammatically inappropriate pauses compared to reading (Wang, Green, Nip, Kent, & Kent, 2010; Winkworth, Davis, Adams, & Ellis, 1995; Winkworth, Davis, Ellis, & Adams, 1994).
Studies of PD illustrate how measures of global speech timing may provide insight regarding the relations among cognitive-linguistic and speech motor execution processes. Huber and Darling (2011) found that speakers with PD had a slower speech rate than healthy controls for a spontaneous speech task but not a reading task. The PD group but not controls also produced longer breath pauses in spontaneous speech compared to reading. Furthermore, spontaneous speech for the PD group contained fewer filled pauses compared to the control group. Results were interpreted to suggest that individuals with PD have difficulty integrating language formulation and respiratory support for extemporaneous speech. The finding of fewer filled pauses in the spontaneous speech of individuals with PD also is consistent with the idea that these speakers had difficulty predicting upcoming delays in speech (Clark & Fox Tree, 2002) and/or used reduced language complexity (Lay & Paivio, 1969; Reynolds & Paivio, 1968). Although it was suggested that the cognitive status of the PD group likely contributed to these findings, cognition was not directly tested beyond administration of a screening tool.
Lowit et al. (2006) also compared global speech timing for a reading passage and sentences produced by speakers with PD and healthy controls. The PD group was further divided into Low and High subgroups on the basis of cognitive testing. The Low PD group and controls tended to differ on measures of global speech timing, while the High PD group and controls performed more similarly. While noting several methodological limitations, results were interpreted to suggest that cognition plays a more substantive role in speech than often assumed.
Summary and purpose
Cognitive-linguistic impairment and/or speech motor impairment are common in MS, yet the potential association of these variables is only beginning to be understood. The present study was an initial effort to examine how cognitive-linguistic status in MS affects global speech timing for oral reading and spontaneous speech. Healthy controls were studied for comparison. Ten speakers with MS performing within normal limits on clinical tests of information processing speed and efficiency as well as executive function comprised an operationally defined high-performing group (High MS Group) and 10 speakers with MS exhibiting relatively poorer scores comprised an operationally defined low-performing group (Low MS Group). Participants with MS had intact sentence intelligibility so that the association of cognitive-linguistic status and global speech timing could be examined without the complication of impaired intelligibility.
It was hypothesized that all groups would demonstrate a slower speech rate, faster articulation rate, increased frequency of pauses, increased average silent pause duration and a reduced proportion of grammatically appropriate pauses for the spontaneous speech task as compared to the reading task (e.g. Huber & Darling, 2011; Rochester, 1971; Walker, 1988; Wang et al., 2010). Another hypothesis evaluated was that task-related differences in speech rate, pause frequency, average silent pause duration and proportion of grammatically appropriate pauses would be magnified for the Low MS Group, as the greater cognitive-linguistic demands of spontaneous speech would presumably prove most challenging for this group. Relatedly, it was hypothesized that the Low MS Group would produce fewer filled pauses and interjections in spontaneous speech compared to the other speaker groups. This follows from the idea that speakers with relatively poorer cognitive-linguistic status would be expected to be less efficient in predicting and signaling delays in speech (Clark & Fox Tree, 2002) and may have reduced language complexity (Lay & Paivio, 1969; Reynolds & Paivio, 1968).
Method
Participants
Thirty speakers, including 20 community-dwelling individuals with MS and 10 age- and sex-matched healthy controls, participated. Participants with MS were selected from a larger database (N = 50) on the basis of performance on standard clinical neuropsychological tests described in more detail below. The mean age of control participants was 49 years (range = 37–60 years). Mean ages for the Low and High Performance MS groups were 49 years (range = 42–60 years) and 47 years (range = 28–62 years), respectively. Mean years post-diagnosis for the Low and High Performance MS groups were 12 years (range = 4–27 years) and 11 years (range = 2–26 years), respectively. For both the Low and High Performance MS groups, the mean number of years of education was 15 years. The mean number of years of education for the Control group was 16 years. All groups included five men and five women. Table 1 reports additional characteristics for participants with MS.
Table 1.
Characteristics and average sentence intelligibility test (SIT) scores are reported for participants with MS who were classified as being either high or low functioning according to neuropsychological tests.
| Participant code | Function level | Disease course | Deviant perceptual characteristics of speech | SIT (%) |
|---|---|---|---|---|
| MSF3 | High | RR | – | 98 |
| MSF5 | High | RR | – | 97 |
| MSF22 | High | SP | Harsh/hoarse voice, intensity decay, variable rate, imprecise consonants | 96 |
| MSF23 | High | SP | – | 97 |
| MSF33 | High | SP | – | 99 |
| MSM1 | High | RR | – | 97 |
| MSM5 | High | RR | – | 97 |
| MSM9 | High | SP | Slow rate, imprecise consonants | 93 |
| MSM17 | High | SP | – | 98 |
| MSM23 | High | RR | – | 96 |
| MSF10 | Low | SP | Strained voice, variable rate, imprecise consonants | 94 |
| MSF20 | Low | SP | – | 96 |
| MSF25 | Low | RR | Harsh/hoarse voice, excess and equal stress, slow and variable rate | 97 |
| MSF30 | Low | SP | Breathy voice, slow rate, imprecise consonants, excess and equal stress | 96 |
| MSF32 | Low | RR | Harsh/hoarse voice, slow rate, imprecise consonants, excess and equal stress | 96 |
| MSM7 | Low | RR | Harsh voice, imprecise consonants, slow and variable rate | 96 |
| MSM11 | Low | SP | – | 98 |
| MSM13 | Low | SP | Slow rate, reduced loudness | 98 |
| MSM14 | Low | RR | Harsh/strained voice, imprecise consonants, slow rate, excess and equal stress | 94 |
| MSM16 | Low | SP | Imprecise consonants, slow rate, excess and equal stress | 95 |
Note. SIT scores reflect the combined judgments of three speech-language pathologists. Participant code: multiple sclerosis (MS), female (F) and male (M). Disease course code: relapsing remitting (RR) and secondary progressive (SP).
All participants spoke standard American English and reported no history of or current substance abuse, no history of speech therapy, were free from past or present use of antipsychotic medication and did not use a hearing aid. Participants with MS reported no other major medical disorder or history of neuropsychiatric disease and no use of corticosteroids for the relapse of MS within 8 weeks of testing. Hearing was screened at 500, 1000, 2000 and 4000 Hz at 40 dB, bilaterally. All participants passed the hearing screening in at least one ear.
The Darley, Aronson, and Brown (1969) prominent, deviant perceptual characteristics in Table 1 were obtained to further describe the speech of participants with MS and to supplement sentence intelligibility scores discussed in the following section. Perceptual characteristics reflect the consensus judgment of three speech-language pathologists (SLPs) based on audio recordings of DDK, the SIT (Yorkston, Beukelman, & Tice, 1996), the Grandfather passage (Duffy, 2005) and a brief spontaneous speech sample. Stimuli were presented to SLPs in a quiet room via loudspeaker. Prior to listening to the stimuli, SLPs were informed of the speaker’s age, gender, years of education, years post diagnosis and disease course.
Neuropsychological testing
Three tests of the MACFIMS, including the Delis-Kaplan Executive Function System (D-KEFS), the Paced Auditory Serial Addition Test – three second version (PASAT3) and the Symbol Digit Modalities Test (SDMT), were used to index cognitive ability. The D-KEFS (Delis, Kaplan, & Kramer, 2001) measures higher executive function. Scores from the Sorting Test were normalized using a regression-based procedure yielding z-scores (see Parmenter, Testa, Schretlen, Weinstock-Guttman, & Benedict, 2010). A mean z-score was calculated for the number of correct card sorts and the description accuracy of the sorting concept. The PASAT3 (Gronwall, 1977) and SDMT (Smith, 1982) measure cognitive processing speed and working memory. Scores from the PASAT3 and the SDMT were also normalized using the same regression-based normative procedure referenced above (Parmenter et al., 2010). Z-scores from the PASAT3 and the SDMT were subsequently averaged to provide a composite measure of information processing speed and efficiency (Benedict et al., 2010).
As summarized in Table 2, MS speakers comprised Low and High Performance groups on the basis of measures of cognitive test scores. Table 2 also reports the descriptive statistics for the Expanded Disability Status Scale (EDSS; Kurtzke, 1983), a clinical metric of overall disease severity for MS as well as sentence intelligibility scores. These sentences were audio-recorded at the same time as experimental speech materials following procedures described below. Intelligibility scores reflect the combined judgments of three SLPs. Three different SLPs judged the perceptual characteristics for individuals with MS reported in Table 1, because these were obtained several months following intelligibility judgments. Each clinician independently scored intelligibility using the computerized scoring program for the SIT.
Table 2.
Mean z-scores and standard deviations are reported for neuropsychological and Sentence Intelligibility Testing (SIT) for each group.
| Group | SIT | EDSS | PASAT3 & SDMT information processing | Mean D-KEFS executive function |
|---|---|---|---|---|
| MS high function | 97 (1.62) | 3.30 (2.28) | −0.18 (0.52) | 0.20 (0.30) |
| MS low function | 96 (1.41) | 5.00 (1.18) | −2.09 (0.46) | −2.17 (0.31) |
| Control | 98 (0.84) | – | −0.13 (0.57) | −0.07 (0.59) |
Note. Neuropsychological testing code: Expanded Disability Status Scale (EDSS), Paced Auditory Serial Addition Test-three second version (PASAT3), Symbol Digit Modalities Test (SDMT) and Delis-Kaplan Executive Function System Sorting Test (D-KEFS). SIT scores reflect the combined judgments of three speech-language pathologists. These SLPs were different than those SLPs who provided perceptual judgments in Table 1.
One-way ANOVA confirmed group differences for the D-KEFS [F(2, 27) = 95.18, p < 0.05] and the information processing index [F(2, 27) = 46.83, p < 0.05]. Bonferroni post hoc tests indicated no difference in test scores for the High Performance MS group and control group for either the D-KEFS or the information processing index (p > 0.05). However, the Low Performance MS group demonstrated significantly poorer D-KEFS scores compared to the High Performance MS group (p < 0.001) and the control group (p < 0.001). In addition, the Low Performance MS group demonstrated significantly poorer performance on the information processing index compared to the High Performance MS group (p < 0.001) and controls (p < 0.001). An independent samples t-test comparing EDSS scores for the MS groups further indicated a significant difference in overall disease severity [t(18) = −2.098, p < 0.05], with greater overall disease severity for the Low MS group. Finally, although ANOVA indicated a significant main effect of Group for sentence intelligibility [F(2, 27) = 3.600, p < 0.05], this difference was not considered to be meaningful as all groups demonstrated 96% or greater average sentence intelligibility.
Speech procedures
Participants were audio-recorded producing a variety of speech tasks. An Isomax ear-mounted microphone (model # E610P5L2) was positioned 6 cm from the center of the speaker’s upper lip. The speech signal was pre-amplified and digitized at a sampling rate of 22.05 kHz using TF32 (Milenkovic, 2002).
Experimental speech tasks
Speech materials of interest included the Grandfather passage and a spontaneous speech sample (i.e. Narrative). Speakers were given a typed printed script of the Grandfather passage to read aloud. For the Narrative, speakers were asked to talk about a topic of interest for about 2 min. A list of suggested topics was provided including a pet, favorite meal, memorable vacation or trip, hobbies, job and favorite sports team. The order in which speakers performed the speech tasks was randomized.
Global speech timing measures were obtained for the initial 45 s of each Narrative and the entire reading passage. This duration corresponds to the average duration of the Grandfather passage for all speakers in a larger laboratory database (N = 75). The initial portion of the Narrative was selected for study as it was desirable to identify a continuous stretch of spontaneous speech that did not contain speech from an investigator, which occurred when speakers ran out of things to talk about on the topic they selected. The first speech run of each Narrative was taken as the onset of the 45-s sample, with a speech run defined as stretch of speech bound by a silent pause of more than 200 ms (Tjaden & Wilding, 2004; Turner & Weismer, 1993). The sample was terminated at the onset of the first run when the time of the sample was approximately 45 s. If the 45-s sample ended in the middle of a run, the sample was terminated at the onset of the next run. Each Narrative was transcribed orthographically by an investigator and verified by a second investigator. Discrepancies were resolved through discussion until consensus was achieved.
Acoustic analyses
Speech samples were segmented into speech runs and pauses using TF32 (Milenkovic, 2002). Standard acoustic criteria were used to identify run onsets and offsets (see Tjaden & Wilding, 2004). Speech run durations for each task and speaker were summed to yield total articulation time. The number of syllables for each speech run was also obtained. A filled pause was defined as a nonlexical one syllable filler vocalization or a sound of hesitation of any length (Clark & Fox Tree, 2002; Goldman-Eisler, 1961). Filled pause onsets and offsets were also identified using conventional acoustic criteria. Filled pauses of interest to the current study were “uh” and “um” as these are commonly used in connected speech (Clark & Fox Tree, 2002).
Speech rate and articulatory rate
For each speaker and task, speech rate in syllables per second was calculated by dividing the total number of syllables by the total sample duration (i.e. articulation time and pause time). Articulatory rate in syllables per second was also calculated by counting the total number of syllables and dividing by the total articulation time.
Pause type, pause duration and grammatical appropriateness of pauses
Silent pause durations for each task were averaged to yield an average silent pause duration for each speaker (a silent pause was defined as greater than 200 ms). Filled pauses and silent pauses were also tallied to obtain a total number of filled pauses and silent pauses. The grammatical appropriateness of silent and filled pauses was also determined (see Goldman-Eisler, 1968; Henderson, Goldman-Eisler, & Skarbek, 1965; Tjaden & Wilding, 2011; Wang et al., 2010). For the Grandfather passage, a pause was deemed to be grammatical if it occurred at a punctuation mark, prior to a conjunction or prior to a phrase or clause. For the Narrative, a pause was deemed to be grammatical if the pause occurred at a natural juncture (e.g. the end of a complete sentence), prior to a conjunction, prior to a phrase or clause or after an indirect or implied question. For both speech tasks, a pause was judged to be ungrammatical if it occurred within a word, in the middle of a phrase, between repeated words and phrases or in the middle of a verbal compound (Goldman-Eisler, 1968). For each speaker and task, the proportion of grammatically appropriate pauses was calculated. Silent and filled pauses were combined for this calculation because filled pauses were infrequent and usually immediately preceded or followed a silent pause, such that both types of pauses were either grammatical or ungrammatical.
Interjections
For each speaker, the number of interjections (e.g. I mean, well, like) for the Narrative task was tallied. Interjections further contributed toward the syllable count for articulation rate.
Reliability
Intrajudge and interjudge reliability of acoustic measures were calculated for approximately 10% of speech samples. Thus, six samples were selected (i.e. three Grandfather passage samples and three Narrative speech samples) and measures were repeated. Average absolute measurement and standard deviation were used to index reliability. Pearson product correlation coefficients were also obtained (see also Tjaden, 2003; Tjaden & Wilding, 2004).
For intrajudge reliability, absolute average measurement error and standard deviations were 16.04 ms (SD 34.79 ms) and 0.05 ms (SD 0.11 ms) for pause duration (i.e. silent and filled) and articulatory rate, respectively. All intra-judge correlations were 0.99. Ninety-nine percent of pauses judged to be grammatical or ungrammatical for the original measurement were judged similarly the second time while 91% of interjections (i.e. 10 of 11 interjections) identified during the original measurement were identified similarly upon remeasurement.
For interjudge reliability, absolute average measurement error and standard deviations were 18.11 ms (SD 31.43 ms) and 0.18 ms (SD 0.88 ms) for pause duration (i.e. silent and filled) and articulatory rate, respectively. Interjudge correlations were 0.99 and 0.92 for pause duration (i.e. silent and filled) and articulatory rate, respectively. One hundred percent of pauses judged to be grammatical or ungrammatical for the first measure were judged to be the same for the second measure. Ninety percent of interjections (i.e. 18 of 20) identified by one investigator during the initial measurement were identified similarly by a second investigator upon remeasurement.
Statistical analysis
A two-factor mixed-model analysis of variance (ANOVAs) with repeated measures was used to analyze measures of speech rate, articulatory rate, total pause frequency (i.e. number of silent and filled pauses), silent pause frequency, average silent pause duration and proportion of grammatically appropriate pauses. The Within subject factor was Speech Task (i.e. Grandfather passage and Narrative) and the Between subject factor was Group (i.e. Control, High MS and Low MS groups). The model included main effects of Speech Task and Group as well as the interaction of Speech Task × Group. One-way ANOVA was used to analyze filled pause frequency and interjection frequency for the Narrative task, as filled pauses and interjections did not occur in the Grandfather passage. A measure of effect size – partial eta squared (η2), the proportion of the total variance, plus error was obtained for significant main effects and interactions. Dependent measures were further characterized using descriptive statistics (i.e. means and standard deviations). Statistical analyses were performed using SPSS version 19. A nominal α level of 0.05 was used for all hypothesis testing.
Results
Articulatory rate and speech rate
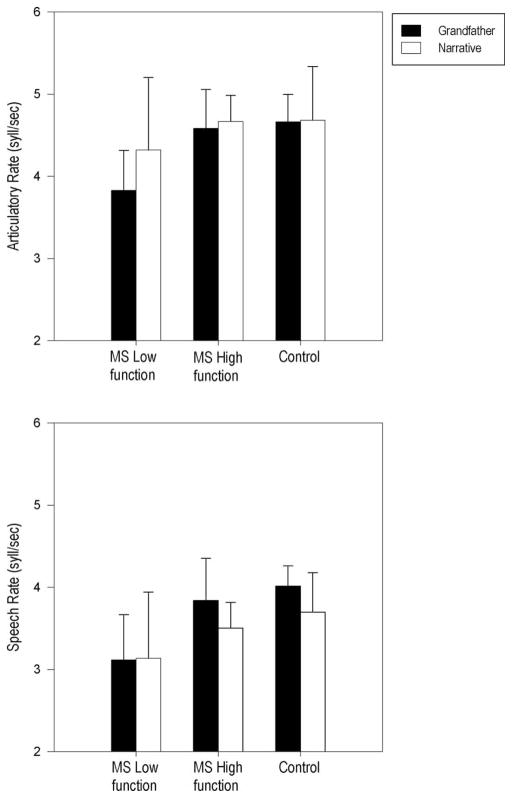
Figure 1 reports mean articulatory rates and speech rates as well as standard deviations for the control and MS groups as a function of speech task. The upper panel of Figure 1 indicates that, on average, all speaker groups used a faster articulatory rate in the Narrative compared to the Grandfather passage. The statistical analysis further indicated significant main effects of Task, F(1,27) = 4.33, p = 0.047, partial η2 = 0.14 and Group, F(2,27) = 4.52, p = 0.020, partial η2 = 0.25. The Group × Task interaction was not significant. Post hoc tests indicated a significant difference between the Low MS and Control groups (p = 0.035) with a slower overall articulation rate for the Low MS group. Individual speaker data further indicated that 9 of 10 speakers and 6 of 10 speakers in the Low MS group produced slower articulatory rates compared to the Control group mean for the Grandfather passage and the Narrative task, respectively.
Figure 1.
Mean and standard deviation bars are reported for Articulatory rate (upper panel) and Speech rate (lower panel) in syllables per second for the MS Low functioning, MS High functioning and Control groups for Grandfather reading and Narrative speech tasks.
The lower panel of Figure 1 also indicates that, on average, the High MS and control groups had a slower speech rate in the Narrative as compared to the Grandfather passage. The Low MS group demonstrated similar speech rates for the two speech tasks. The statistical analysis further indicated a significant effect of Task, F(1,27) = 5.99, p = 0.021, partial η2 = 0.18 and Group, F(2,27) = 6.78, p = 0.004, partial η2 = 0.33. The Group × Task interaction was not significant. Post hoc tests indicated that compared to both the High MS and the Control groups, speech rate was slower for the Low MS group, p < 0.05. Individual speaker data further indicated that 7 of 10 speakers in the Low MS group produced slower speech rates for the Grandfather passage and 9 of 10 speakers in the Low MS group produced slower speech rates for the Narrative task relative to the High MS group mean. Relative to the Control group mean, 9 of 10 speakers in the Low MS group and 8 of 10 speakers in the Low MS group also produced slower speech rates for the Grandfather passage and the Narrative, respectively.
Pauses and interjections
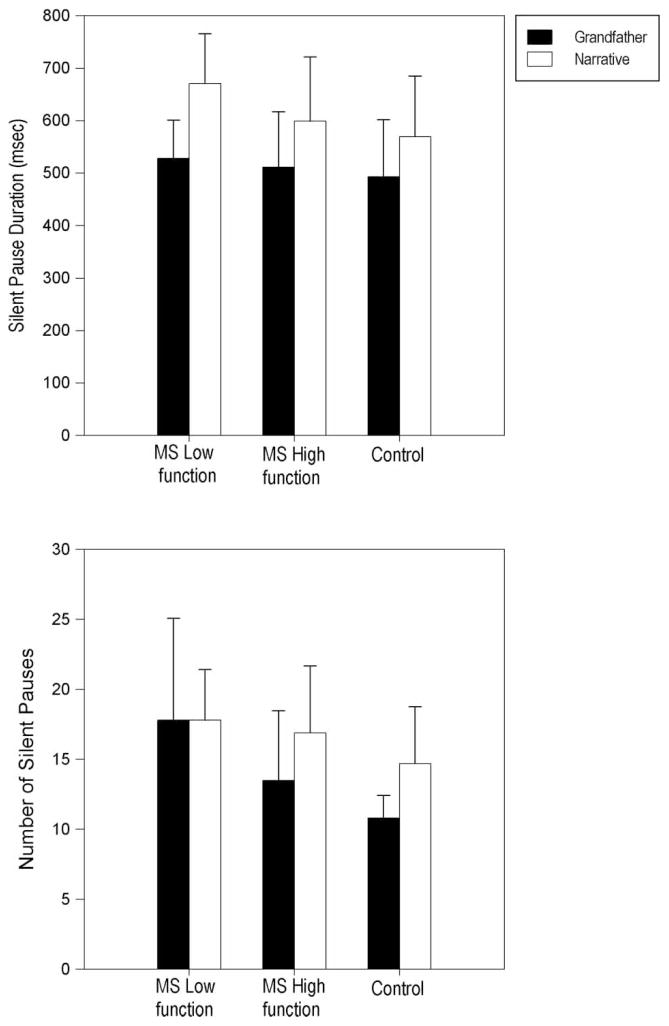
Figure 2 reports mean silent pause durations and silent pause frequency as well as standard deviations for all speaker groups. The upper panel of Figure 2 indicates that, on average, all speaker groups tended to produce longer silent pauses in the Narrative as compared to the Grandfather passage. Moreover, relative to other groups, the upper panel of Figure 2 suggests a trend for the Low MS group to demonstrate a greater difference in mean silent pause duration for the two speech tasks. Specifically, the difference in mean silent pause duration for the Narrative versus the reading task was 142, 87 and 76 ms for the Low MS, High MS and Control group, respectively. Despite these trends, the statistical analysis for silent pause duration revealed a significant main effect of Task, F(1,27) = 22.68, p < 0.001, partial η2 = 0.46. The main effect of Group and Group × Task interaction was not significant.
Figure 2.
Mean and standard deviation bars are reported for Silent Pause Durations in milliseconds (upper panel) and Number of Silent Pauses (lower panel) for the MS Low functioning, MS High functioning and Control groups for Grandfather reading and Narrative speech tasks.
All speaker groups demonstrated more total pauses (i.e. silent and filled pauses) in the Narrative compared to the Grandfather passage. The statistical analysis further indicated a significant main effect of Task, F(1, 27) = 25.83, p < 0.001, partial η2 = 0.49, for total number of pauses. The main effect of Group and Group × Task interaction was not significant.
Silent pause frequency data in the lower panel of Figure 2 suggest that, on average, all groups demonstrated the same or more silent pauses in the Narrative compared to the Grandfather passage. Statistical analyses for silent pause frequency indicated a significant main effect of Task, F(1, 27) = 5.94, p = 0.022, partial η2 = 0.18 and a significant main effect of Group, F(2,27) = 4.36, p = 0.023, partial η2 = 0.24. The Group × Task interaction was not significant. Post hoc comparisons further indicated a significant difference between the Low MS group and the Control group (p = 0.019). That is, 9 of 10 speakers in the Low MS group produced more silent pauses compared to the Control group mean for the Grandfather passage and 8 of 10 speakers in the Low MS group also produced more silent pauses in the Narrative compared to the Control group mean. The statistical analysis for filled pause frequency revealed no significant differences.
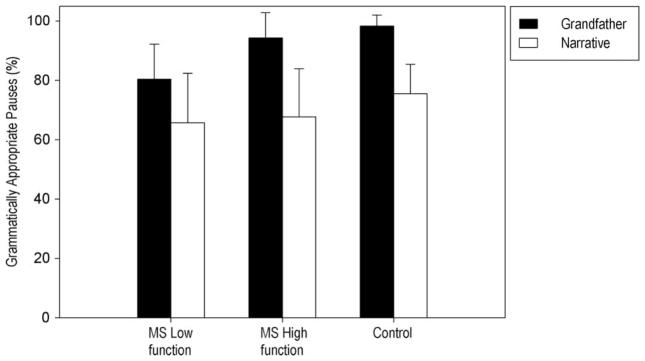
As indicated in Figure 3, on average, all groups produced a lower percentage of grammatically appropriate pauses in the Narrative. The statistical analysis further indicated a significant main effect of Task, F(1,27) = 54.43, p < 0.001, partial η2 = 0.66 and a main effect of Group, F (2,27) = 6.08, p = 0.007, partial η2 = 0.31. The Group × Task interaction was not significant. Post hoc tests indicated that the Low MS group demonstrated a significantly lower proportion of grammatically appropriate pauses compared to the Control group (p = 0.005). Individual speaker data further indicated that 9 of 10 and 8 of 10 speakers in the Low MS group produced a lower percentage of grammatically appropriate pauses relative to the Control group mean for the Grandfather passage and the Narrative, respectively. Finally, on average, the Low MS group produced the fewest number of interjections in the Narrative relative to other groups. ANOVA indicated no difference in interjection frequency among the three groups, however. Individual speaker data further indicated that 4 of 10 speakers in the Low MS group produced no interjections in the Narrative. In contrast, only 2 of 10 speakers in the High MS group and 1 of 10 speakers in the Control group produced no interjections in the Narrative.
Figure 3.
Mean and standard deviation bars are reported for the Percentage of Grammatically Appropriate Pauses for the MS Low functioning, MS High functioning and Control groups for Grandfather reading and Narrative speech tasks.
Discussion
The present study was an initial effort to examine how or even if cognitive-linguistic status in MS impacts global speech timing for oral reading and spontaneous speech. Healthy controls were studied for comparison. The hypothesis that all speaker groups would demonstrate a slower speech rate, faster articulation rate, increased frequency of pauses, longer silent pause duration and a lower percentage of grammatically appropriate pauses in the Narrative task as compared to the Grandfather passage was supported. The hypothesis that differences in measures of global speech timing for the reading and spontaneous speech tasks would be magnified for the Low MS group, and that this group also would demonstrate the least number of filled pauses and interjections for the Narrative was not supported by the statistical analyses. Findings and implications are considered further in the following sections.
Hypothesis 1: task differences
Consistent with the studies of neurologically normal talkers (Jacewicz et al., 2009, 2010), articulation rate was significantly faster for the Narrative task compared to the Grandfather passage. A faster rate of articulation for extemporaneous speech has been interpreted to suggest a more casual manner of talking compared to oral reading (see discussion in Jacewicz et al., 2009). That is, syllable durations are reduced when a more casual manner of talking is adopted, with an associated increase in articulatory rate. The idea that spontaneous speech is a more casual manner of talking is also supported by studies reporting prosodic differences for read and spontaneous speech (Howell & Kadi-Hanifi, 1991). The current study extends these findings to MS and further suggests that even individuals with MS with impaired executive function as well as reduced information processing speed and efficiency maintain the typical speech style distinction for reading and spontaneous speech, as indexed by articulation rate.
The finding that speech rate was significantly slower for the Narrative compared to the Grandfather passage is also in agreement with previous studies of healthy talkers as well as studies of PD (e.g. Huber & Darling, 2011; Walker, 1988). Given the findings for articulation rate, the overall slower speech rate for the Narrative can be attributed to increased pause time, which in turn provides time needed physiologically for breathing as well as time for planning and formulating speech (Goldman-Eisler, 1968; Rochester, 1971; Walker, 1988).
Task differences also were found for pause variables. On average, significantly more total pauses (i.e. filled and silent), fewer grammatically appropriate pauses, and longer and more frequent silent pauses were found for the Narrative compared to the Grandfather passage. As previously noted, these types of task-related differences in pausing follow from the greater online language processing demands of extemporaneous speech (Goldman-Eisler, 1968; Rochester, 1971; Walker, 1988). Wang et al. (2010) further suggest that the tendency for extemporaneous speech to have a reduced proportion of grammatically appropriate breath pauses may reflect increased difficulty coordinating pausing with a less predictable grammatical structure.
Hypothesis 2: task × group interaction
The Task × Group interaction was not significant for any of the dependent variables. Modest participant numbers aside, one interpretation is that measures of global speech timing are not sensitive to cognitive-linguistic status in MS, at least when cognitive status is indexed in a binary manner (i.e. within normal limits versus impaired). It is also possible that the degree of cognitive impairment for the Low MS Group was not severe enough to reveal the hypothesized effects. However, measures of global speech timing have been shown to be sensitive to cognitive-linguistic variables for individuals with other progressive neurological diseases (Huber & Darling, 2011; Lowit et al., 2006), and there is no a priori reason why similar findings should not hold for MS. In addition, the poorer neuropsychological test scores for individuals in the Low MS group are not only statistically different from those for controls and the High MS group, but are also clinically meaningful (Benedict et al., 2002; Parmenter et al., 2010). As discussed in the following paragraphs, one alternative explanation for the lack of a significant Task × Group interaction is that the reading task was associated with a greater cognitive-linguistic load than previously assumed for the Low MS group, and the manner in which the narrative was elicited minimized the cognitive-linguistic demands of the task. Thus, presumed differences in cognitive-linguistic demand for reading aloud and spontaneous speech may have been effectively reduced for the Low MS group.
First, consider trends for articulation rate illustrated in the upper panel of Figure 1. Average articulation rates for all groups are quite similar for the narrative task, while average articulation rate for the Low MS group is notably reduced relative to other speaker groups for the reading task. This trend could reflect a more formal speech style for individuals in the Low MS group or could be a compensatory strategy used to maintain intelligibility when reading aloud. That is, Table 1 suggests that the majority of speakers in the Low MS group had prominent deviant perceptual characteristics suggestive of dysarthria. If these speakers increased effort during reading as a way to maintain intelligibility, this may have resulted in a slower average articulation rate. It is unclear, however, why only the Low MS group would adopt a more formal speaking style when reading aloud or would slow articulation rate to maintain intelligibility only for reading but not spontaneous speech. Moreover, the similar articulation rates in the Narrative task for all three speaker groups suggest that the trend for a slowed articulation rate for the Low MS group in the reading passage is not likely explained by motor limitations. A more plausible explanation, in our view, is that the less overlearned speech task of reading has a greater cognitive-linguistic load than previously assumed for individuals with cognitive impairment. That is, the trend for the Low MS group to use a slower articulation rate when reading aloud relative to other speaker groups may reflect the relatively poorer cognitive status for these speakers or could be a compensatory strategy for overcoming slowed neural transmission of information, which is a prominent feature of cognitive impairment in MS (Benedict & Bobholz, 2007). Further support for the idea that the reading passage was more demanding for the Low MS group as compared to other speaker groups comes from the fact that the Low MS group also produced the same number of silent pauses and almost identical speech rates for both speech tasks. Thus, reading a passage aloud may tax the cognitive-linguistic resources of individuals with MS with cognitive impairment in a different way or to a different extent than individuals with normal cognition.
The manner in which the Narrative task was elicited may also have contributed to the lack of a significant Group × Task interaction. Speakers were provided with a list of common, everyday topics and were allowed to talk about a topic of their choosing. This approach may have rendered the task less demanding or at least less demanding than previously thought, in comparison to the reading task. Studies examining different methods for eliciting narratives are needed to evaluate this suggestion. For example, requiring individuals to talk about an unfamiliar topic or provide procedural information to complete a novel task would be one way to investigate this issue.
This is not to say that there was no evidence of the hypothesized Task × Group interaction. The Low MS group, for example, demonstrated the greatest difference in mean silent pause duration for the Narrative task and Grandfather passage. This trend was due to the relatively greater lengthening of silent pauses in the Narrative (see upper panel of Figure 2). Although the statistical analysis for interjection frequency was not significant, visual inspection of the data indicated that the Low MS group, on average, produced the lowest number of interjections in the Narrative in comparison to other speaker groups. These findings hint at the idea that speakers in the Low MS group had a reduced ability to predict an upcoming delay during speech. Rather than being concerned with monitoring speech for upcoming delays, speakers in the Low MS group may have placed emphasis on other aspects of spoken language, such as phonetic and syntactic characteristics. As in studies investigating cognitive-speech motor associations in PD (Huber & Darling, 2011), language characteristics of narratives were not evaluated, but the trend for interjections may also suggest that the Low MS group used language that was less complex relative to other groups (Lay & Paivio, 1969; Reynolds & Paivio, 1968).
The current study was not intended to address causal relationships. However, it seems likely that some of the speech hesitation characteristics (i.e. silent pauses, interjections) noted for the Low MS group may be compensatory in nature, while others were more directly related to disease processes. For example, longer and more frequent silent pauses in the Narrative could be used to compensate for slowed information processing speed, poor planning, formulation or word retrieval difficulties. Alternatively, as previously noted, the majority of speakers in the Low MS group had perceptual characteristics suggestive of dysarthria despite intact sentence intelligibility. Thus, in addition to cognitive-linguistic status, it is plausible that speech motor variables contributed to some of the findings. Indeed, the main effect of group in some of the analyses could reflect differences in cognitive-linguistic status, speech motor variables or some combination thereof. A trend for fewer interjections in Narratives produced by the Low MS group, however, is likely not compensatory, but probably is a direct reflection of deficits in information processing speed and efficiency and executive function. More studies are needed to tease apart variables that are reflective of cognitive-linguistic function, dysarthria or both in MS. Atypical verbal fluency patterns, as suggested by the speech hesitation characteristics for Narratives produced by the Low MS group as well as deviant perceptual characteristics reported in Table 1, also have been shown to contribute to perceptions of reduced speaker competence (MacGregor, Corley, & Donaldson, 2010) which in turn has implications for quality of life. Determining the perceptual relevance of the types of global speech timing patterns for spontaneous speech produced by speakers with MS exhibiting cognitive impairment is an important topic for future studies.
Limitations and conclusions
As in any study, the current investigation has several limitations. Although subject numbers would seem suitable for a preliminary study, future studies should employ larger numbers of participants to provide a more favorable statistical environment to evaluate variables of interest. For greater ecological validity, extemporaneous speech tasks that are more representative of everyday communication should be employed, such as two individuals engaged in a dialog. In this manner, the extemporaneous task would more readily tap into variables such as determining what the communication partner already knows, monitoring the conversation for breakdown in understanding and signaling the communication partner to hold the floor (Brown, Currie, & Kenworthy, 1980). In addition, speech recording and neuropsychological testing were often executed on the same day. Individuals with MS exhibit fatigue approximately 90% of the time (Krupp, Alvarez, LaRocca, & Scheinberg, 1988). Thus, fatigue may have contributed to cognitive and speech performance for participants with MS (Kinsinger, Lattie, & Mohr, 2010; Yorkston et al., 2003). The two MS groups also differed in overall disease severity, as indexed by the EDSS.
In conclusion, the results of this preliminary study suggest the assumption that reading aloud is associated with lower cognitive-linguistic demand as compared to spontaneous speech may need to be re-considered for individuals with deficits in executive function as well as reduced information processing speed and efficiency. While the statistical analyses did not support the hypothesis that differences in measures of global speech timing for reading and spontaneous speech would be magnified for individuals with MS with cognitive impairment, several qualitative trends in the data suggest the influence of cognitive-linguistic variables on measures of global speech timing. Future studies with larger numbers of participants are needed to more firmly establish the association between cognitive-linguistic and speech motor variables in MS.
Acknowledgments
A portion of this research was conducted to complete a pre-doctoral dissertation project. We thank Heather Sheppard of the Motor Speech Disorders Laboratory at the University at Buffalo and Marietta Hoogs at Buffalo General Hospital for assistance with various aspects of this study. We also thank Dr. Christina Kuo for her comments on previous versions of the manuscript. Portions of this study were presented at the 6th International Conference on Motor Speech.
Footnotes
Declaration of Interest: This research was supported by NIH-NIDCD R01DC004689-08S. The authors report no conflict of interest.
References
- Archibald CJ, Fisk JD. Information processing efficiency in patients with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 2000;22(5):686–701. doi: 10.1076/1380-3395(200010)22:5;1-9;FT686. [DOI] [PubMed] [Google Scholar]
- Arnett PA, Smith MM, Barwick FH, Benedict RHB, Ahlstrom BP. Oralmotor slowing in multiple sclerosis: Relationship to neuropsychological tasks requiring an oral response. Journal of the International Neuropsychological Society. 2008;14:454–462. doi: 10.1017/S1355617708080508. [DOI] [PubMed] [Google Scholar]
- Arnott WL, Jordan FM, Murdoch BE, Lethlean JB. Narrative discourse in multiple sclerosis: An investigation of conceptual structure. Aphasiology. 1997;11(10):969–991. [Google Scholar]
- Bayles KA, Tomoeda CK. The Arizona battery for communication disorders of Dementia. Phoenix: Canyonlands Publishing, Inc; 1993. Normed edition. [DOI] [PubMed] [Google Scholar]
- Baylor C, Yorkston K, Bamer A, Britton D, Amtmann D. Variables associated with communicative participation in people with multiple sclerosis: A regression analysis. American Journal of Speech-Language Pathology. 2010;19:143–153. doi: 10.1044/1058-0360(2009/08-0087). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Goodkin DE, Monson N, Beatty PA. Cognitive disturbances in patients with relapsing remitting multiple sclerosis. Archives in Neurology. 1989;46:1113–1119. doi: 10.1001/archneur.1989.00520460103020. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Monson N. Problem solving by patients with multiple sclerosis: Comparison of performance on the Wisconsin and California Card Sorting Test. Journal of the International Neuropsychological Society. 1996;2:134–140. doi: 10.1017/s1355617700000989. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Bobholz JH. Multiple sclerosis. Seminars in Neurology. 2007;27:78–85. doi: 10.1055/s-2006-956758. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Fischer JS, Archibald CJ, Arnett PA, Beatty WW, Bobholz J, Chelune GJ, Fisk JD, Langdon DW, Caruso L, Foley F, LaRocca NG, Vowels L, Weinstein A, DeLuca J, Rao SM, Munschauer F. Minimal neuropsychological assessment of MS patients: A consensus approach. The Clinical Neuropsychologist. 2002;16:381–397. doi: 10.1076/clin.16.3.381.13859. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Morrow SA, Weinstock-Guttman B, Cookfair D, Schretlen DJ. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. Journal of the International Neuropsychological Society. 2010;16:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nature Reviews Neurology. 2011;7:332–342. doi: 10.1038/nrneurol.2011.61. [DOI] [PubMed] [Google Scholar]
- Brown G, Currie KL, Kenworthy J. Questions of intonation. London: Croom Helm; 1980. [Google Scholar]
- Clark HH, Fox Tree J. Using uh and um in spontaneous speaking. Cognition. 2002;84:73–111. doi: 10.1016/s0010-0277(02)00017-3. [DOI] [PubMed] [Google Scholar]
- Conrad B, Schonle P. Speech and respiration. Archiv fur Psychiatrie und Nervenkrankheiten. 1979;226:251–268. doi: 10.1007/BF00342238. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research. 1969;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Dromey C, Bates E. Speech interactions with linguistic, cognitive, and visuomotor tasks. Journal of Speech, Language, and Hearing Research. 2005;48:295–305. doi: 10.1044/1092-4388(2005/020). [DOI] [PubMed] [Google Scholar]
- Dromey C, Benson A. Effects of concurrent motor, linguistic, or cognitive tasks on speech motor performance. Journal of Speech, Language, and Hearing Research. 2003;46:1234–1246. doi: 10.1044/1092-4388(2003/096). [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. 2. St. Louis: Elsevier Science; 2005. [Google Scholar]
- Goldman-Eisler F. The distributions of pause durations in speech. Language and Speech. 1961;4:232–237. [Google Scholar]
- Goldman-Eisler F. Psycholinguistics: Experiments in spontaneous speech. New York: Academic Press; 1968. [Google Scholar]
- Gronwall DMA. Paced auditory serial addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hartelius L, Runmarker B, Andersen O. Prevalence and characteristics of dysarthria in multiple-sclerosis cohort: Relation to neurological data. Folia Phoniatrica et Logopaedica. 2000;52:160–177. doi: 10.1159/000021531. [DOI] [PubMed] [Google Scholar]
- Henderson A, Goldman-Eisler F, Skarbek A. Temporal patterns of cognitive activity and breath control in speech. Language and Speech. 1965;8(4):236–242. doi: 10.1177/002383096500800405. [DOI] [PubMed] [Google Scholar]
- Henry JD, Beatty WW. Verbal fluency deficits in multiple sclerosis. Neuropsychologia. 2006;44:1166–1174. doi: 10.1016/j.neuropsychologia.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ. Age and speech breathing. Journal of Speech and Hearing Research. 1987;30:351–366. doi: 10.1044/jshr.3003.351. [DOI] [PubMed] [Google Scholar]
- Howell P, Kadi-Hanifi K. Comparison of prosodic properties between read and spontaneous speech material. Speech Communication. 1991;10:163–169. [Google Scholar]
- Huber JE, Darling M. Effect of Parkinson’s disease on the production of structured and unstructured speaking tasks: Respiratory physiologic and linguistic considerations. Journal of Speech, Language, and Hearing Research. 2011;54:33–46. doi: 10.1044/1092-4388(2010/09-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacewicz E, Fox RA, O’Neill C, Salmons J. Articulation rate across dialect, age, and gender. Language Variation & Change. 2009;21(2):233–256. doi: 10.1017/S0954394509990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacewicz E, Fox RA, Wei L. Between-speaker and within-speaker variation in speech tempo of American English. Journal of Acoustical Society of America. 2010;128:839–850. doi: 10.1121/1.3459842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler D, Van Lancker D. The effect of speech task on intelligibility in dysarthria: A case study of Parkinson’s disease. Brain and Language. 2002;80:449–464. doi: 10.1006/brln.2001.2602. [DOI] [PubMed] [Google Scholar]
- Kent RD. Models of speech motor control: Implication from recent developments in neurophysiological and neurobehavioral science. In: Maassen B, editor. Speech motor control in normal and disordered speech. New York: Oxford University Press; 2004. pp. 3–28. [Google Scholar]
- Kinsinger SW, Lattie E, Mohr DC. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology. 2010;5:573–580. doi: 10.1037/a0019222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Archives of Neurology. 1988;45:435–437. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- Kujala P, Portin R, Ruutiainen J. Language functions in incipient cognitive decline in multiple sclerosis. Journal of the Neurological Sciences. 1996;141:79–86. doi: 10.1016/0022-510x(96)00146-3. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Langdon DW. Cognition in multiple sclerosis. Current Opinion in Neurology. 2011;24:244–249. doi: 10.1097/WCO.0b013e328346a43b. [DOI] [PubMed] [Google Scholar]
- Lay CH, Paivio A. The effects of task difficulty and anxiety on hesitations in speech. Canadian Journal of Behavioral Science. 1969;1:25–27. [Google Scholar]
- Lethlean JB, Murdoch BE. Subgroups of multiple sclerosis patients based on language function. In: Theodoros DG, editor. Speech and language disorders in multiple sclerosis. London: Whurr Publishers; 2000. pp. 155–194. [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22:1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Lowit A, Brendel B, Dobinson C, Howell P. An investigation into the influences of age, pathology and cognition on speech production. Journal of Medical Speech-Language Pathology. 2006;12:253–262. [PMC free article] [PubMed] [Google Scholar]
- MacGregor LJ, Corley M, Donaldson DI. Listening to the sound of silence: Disfluent silent pauses in speech have consequences for listeners. Neuropsychologia. 2010;48:3982–3992. doi: 10.1016/j.neuropsychologia.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Mackenzie C, Green J. Cognitive-linguistic deficit and speech intelligibility in chronic progressive multiple sclerosis. International Journal of Language & Communication Disorders. 2009;44:401–420. doi: 10.1080/13682820802697879. [DOI] [PubMed] [Google Scholar]
- Maner KJ, Smith A, Grayson L. Influences of utterance length and complexity on speech motor performance in children and adults. Journal of Speech, Language, and Hearing Research. 2000;43:560–573. doi: 10.1044/jslhr.4302.560. [DOI] [PubMed] [Google Scholar]
- van der Merwe A. A theoretical framework for the characterization of pathological speech sensorimotor control. In: McNeil MR, editor. Clinical management of sensorimotor speech disorders. New York: Thieme; 2009. pp. 3–18. [Google Scholar]
- Milenkovic P. TF32 [Computer program] University of Wisconsin; Madison: 2002. [Google Scholar]
- Murdoch BE, Lethlean JB. Language disorders in multiple sclerosis. In: Theodoros DG, editor. Speech and language disorders in multiple sclerosis. London: Whurr Publishers; 2000. pp. 109–130. [Google Scholar]
- Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RHB. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) Journal of the International Neuropsychological Society. 2010;16:6–16. doi: 10.1017/S1355617709990750. [DOI] [PubMed] [Google Scholar]
- Rao SM. Neuropsychology of multiple sclerosis. Current Opinion in Neurology. 1995;8:216–220. doi: 10.1097/00019052-199506000-00010. [DOI] [PubMed] [Google Scholar]
- Rao SM the Cognitive Function Study Group of the National Multiple Sclerosis Society. A manual for the Brief, Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. New York: National Multiple Sclerosis Society; 1990. [Google Scholar]
- Reynolds A, Paivio A. Cognitive and emotional determinants of speech. Canadian Journal of Psychology. 1968;22:164–175. doi: 10.1037/h0082757. [DOI] [PubMed] [Google Scholar]
- Rochester SR. The significance of pauses in spontaneous speech. Journal of Psycholinguistic Research. 1971;2:51–81. doi: 10.1007/BF01067111. [DOI] [PubMed] [Google Scholar]
- Sadagopan N, Smith A. Developmental changes in the effects of utterance length and complexity on speech movement variability. Journal of Speech, Language, and Hearing Research. 2008;51:1138–1151. doi: 10.1044/1092-4388(2008/06-0222). [DOI] [PubMed] [Google Scholar]
- Schiffer RB. Cognitive loss. In: van den Noort S, Holland N, editors. Multiple sclerosis in clinical practice. New York: Demos Medical Publishing; 1999. [Google Scholar]
- Smith A. Symbol Digit Modalities Test (SDMT) manual (revised) Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Smith M, Arnett PA. Dysarthria predicts poorer performance on cognitive tasks requiring a speeded oral response in an MS population. Journal of Clinical and Experimental Neuropsychology. 2007;29(8):804–812. doi: 10.1080/13803390601064493. [DOI] [PubMed] [Google Scholar]
- Strober L, Englert J, Munschauer F, Weinstock-Guttman B, Rao S, Benedict RHB. Sensitivity of conventional memory tests in multiple sclerosis: Comparing the Rao Brief Repeatable Neuropsychological Batter and the Minimal Assessment of Cognitive Function in MS. Multiple Sclerosis. 2009;15:1077–1084. doi: 10.1177/1352458509106615. [DOI] [PubMed] [Google Scholar]
- Tasko SM, McClean MD. Variations in articulatory movement with changes in speech task. Journal of Speech, Language, and Hearing Research. 2004;47:85–100. doi: 10.1044/1092-4388(2004/008). [DOI] [PubMed] [Google Scholar]
- Tjaden K. Anticipatory coarticulation in multiple sclerosis and Parkinson’s disease. Journal of Speech, Language, and Hearing Research. 2003;46:990–1008. doi: 10.1044/1092-4388(2003/077). [DOI] [PubMed] [Google Scholar]
- Tjaden K, Wilding GE. Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research. 2004;47:766–783. doi: 10.1044/1092-4388(2004/058). [DOI] [PubMed] [Google Scholar]
- Tjaden K, Wilding GE. Effects of speaking task on intelligibility in Parkinson’s disease. Clinical Linguistics & Phonetics. 2011;25:155–168. doi: 10.3109/02699206.2010.520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao YC, Weismer G, Iqbal K. Interspeaker variation in habitual speaking rate: Additional evidence. Journal of Speech, Language, and Hearing Research. 2006;49:1156–1164. doi: 10.1044/1092-4388(2006/083). [DOI] [PubMed] [Google Scholar]
- Turner GS, Weismer G. Characteristics of speaking rate in the dysarthria associated with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1993;36:1134–1144. doi: 10.1044/jshr.3606.1134. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D, Hanson W, Jackson C, Lanto A, Kempler D, Metter EJ. Fundamental frequency (F0) measures comparing speech tasks in aphasia and Parkinson’s disease. Journal of Medical Speech-Language Pathology. 2004;12(4):207–212. [Google Scholar]
- Walker VG. Durational characteristics of young adults during speaking and reading tasks. Folia Phoniatrica et Logopaedica. 1988;40:12–20. doi: 10.1159/000265879. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Holmes S. Cognitive-linguistic assessment of individuals with multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 1993;74:637–643. doi: 10.1016/0003-9993(93)90163-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Green JR, Nip ISB, Kent RD, Kent JF. Breath group analysis for reading and spontaneous speech in healthy adults. Folia Phoniatrica et Logopaedica. 2010;62:297–302. doi: 10.1159/000316976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismer G, Jeng J, Laures JS, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica. 2001;53:1–18. doi: 10.1159/000052649. [DOI] [PubMed] [Google Scholar]
- Westbury JR, Dembowski J. Articulatory kinematics of normal diadochokinetic performance. Annual Bulletin of the Research Institute of Logopedics and Phoniatrics. 1993;1993:13–36. [Google Scholar]
- Winkworth AJ, Davis PJ, Adams RD, Ellis E. Breathing patterns during spontaneous speech. Journal of Speech and Hearing Research. 1995;38:124–144. doi: 10.1044/jshr.3801.124. [DOI] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ, Ellis E, Adams RD. Variability and consistency in speech breathing during reading: Lung volumes, speech intensity, and linguistic factors. Journal of Speech and Hearing Research. 1994;37:535–536. doi: 10.1044/jshr.3703.535. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Beukelman DR. Assessment of intelligibility of dysarthric speech. Austin, TX: PRO-ED; 1984. [Google Scholar]
- Yorkston KM, Beukelman DR, Strand EA, Hakel M. Management of motor speech disorders in children and adults. 3. Austin, TX: Pro-Ed; 2011. [Google Scholar]
- Yorkston K, Beukelman D, Tice R. Sentence intelligibility test. Lincoln, NE: Institute for Rehabilitation Science and Engineering at Madonna Rehabilitation Hospital; 1996. [Google Scholar]
- Yorkston KM, Klasner ER, Bowen J, Ehde DM, Gibbons LE, Johnson K, Kraft G. Characteristics of multiple sclerosis as a function of the severity of speech disorders. Journal of Medical Speech-Language Pathology. 2003;11:73–84. [Google Scholar]
- Yorkston KM, Klasner ER, Swanson KM. Communication in context: A qualitative study of the experiences in individuals with multiple sclerosis. American Journal of Speech-Language Pathology. 2001;10:126–137. [Google Scholar]