Abstract
Purpose
Previous studies of human retinal pigment epithelium (RPE) morphology found spatial differences in density: a high density of cells in the macula, decreasing peripherally. Because the RPE sheet is not perfectly regular, we anticipate that there will be differences between conditions and when and where damage is most likely to begin. The purpose of this study is to establish relationships among RPE morphometrics in age, cell location, and disease of normal human and AMD eyes that highlight irregularities reflecting damage.
Methods
Cadaveric eyes from 11 normal and 3 age-related macular degeneration (AMD) human donors ranging from 29 to 82 years of age were used. Borders of RPE cells were identified with phalloidin. RPE segmentation and analysis were conducted with CellProfiler. Exploration of spatial point patterns was conducted using the “spatstat” package of R.
Results
In the normal human eye, with increasing age, cell size increased, and cells lost their regular hexagonal shape. Cell density was higher in the macula versus periphery. AMD resulted in greater variability in size and shape of the RPE cell. Spatial point analysis revealed an ordered distribution of cells in normal and high spatial disorder in AMD eyes.
Conclusions
Morphometrics of the RPE cell readily discriminate among young vs. old and normal vs. diseased in the human eye. The normal RPE sheet is organized in a regular array of cells, but AMD exhibited strong spatial irregularity. These findings reflect on the robust recovery of the RPE sheet after wounding and the circumstances under which it cannot recover.
XX.1 Introduction
The retinal pigment epithelium (RPE) layer is located between the neurosensory retina and the choroid. Its main functions are to supply the highly metabolically active retina with nutrients and remove waste products from the photosensory processes of the cones and rods. To correctly function, the RPE layer must remain intact without any holes in the cell layer (Rizzolo 2014). The RPE layer robustly compensates for some damage or death of RPE cells until a certain point (Negi and Marmor 1984; Kalnins et al. 1995; Nagai and Kalnins 1996), but in the advanced stages of some retinal and macular diseases, the RPE layer can break down, leaving empty spaces (Ambati and Fowler 2012; Bhutto and Lutty 2012; van Lookeren Campagne et al. 2014). Toxic products are generated near the RPE layer in many eye diseases, such as age-related macular degeneration (AMD) and Stargardt’s. As RPE cells age, toxic metabolites continue to accumulate, causing the RPE cells to die (Liang and Godley 2003). With extensive RPE cell death, the epithelial sheet loses its overall stability (Chrenek et al. 2012; Jiang et al. 2013; Jiang et al. 2014), which leads to RPE dysfunction and impaired functioning and damage to the retina, such as that seen in AMD. Epithelial sheets are in general resilient and resistant to damage (Roider et al. 1992), and they maintain barrier function by tiling across the sheet (Jiang et al. 2013). In this study, we hypothesized increased variability in the shape and size of RPE cells and increased spatial irregularity by: (1) region-, (2) age-, and (3) disease.
To test this hypothesis, we analyzed RPE cell shape and size from human cadaveric eyes. Here we report the initial findings from both normal (undiseased) and AMD eyes across a broad age range. We found that RPE cell properties vary fairly consistently according to the region in the eye, age at death, and disease status.
XX.2 Methods
Cadaveric human donor eyes (n=14) harvested <7 h postmortem were dissected to obtain a strip of RPE from the optic nerve through the macula to the ora. We adhered to ARVO guidelines, and the Emory IRB approved the study.
The RPE was flatmounted, stained with AF635-phalloidin, and then imaged using confocal microscopy (Chrenek et al. 2012; Jiang et al. 2013; Jiang et al. 2014). Images (typically 200 to 400 images, each image with hundreds of cells) were photomerged using Autopano Pro v2.5 (Kolor, Montmélian, France). RPE segmentation and analysis were amassed with CellProfiler (Lamprecht et al. 2007). Exploration of spatial point patterns was conducted using the “Spatstat” package (Baddeley and Turner 2005) of R.
XX.3 Results
XX.3.1 Preliminary findings
In the normal eye, cell density was higher at the macula compared to the far periphery. All parameters showed trends toward more variability in size and shape from macula to periphery. By region, irrespective of age, the macula and mid-periphery exhibited an isometric, small RPE cell, while the far periphery had a less uniform and larger RPE cell.
XX.3.2 Aging in the normal RPE
There was a transition at about 60 years old (yo), when the normal RPE sheet began to deteriorate. The deterioration was location specific. The macula and the far periphery showed significant changes. However, the mid-periphery exhibited no major changes between the younger vs., older eyes (data not shown). In the macula, there was more variability in sidedness (comparing <60 yo to >60 yo), reflected by a reduced percentage of hexagonal cells (43.5% vs. 38.0 % respectively, p=0.01).
XX.3.3 The AMD Eye
In cadaveric eyes from AMD patients, the disrupted RPE showed great variability in both the size and shape of cells. In the macula of AMD eyes, the RPE exhibited patches of very large cells (Fig. XX.1). Where the RPE was atrophic, the surrounding RPE cells had an aberrant elevated rim. When soft drusen were present, adjacent RPE cells were often larger and stretched.
Fig. XX.1.
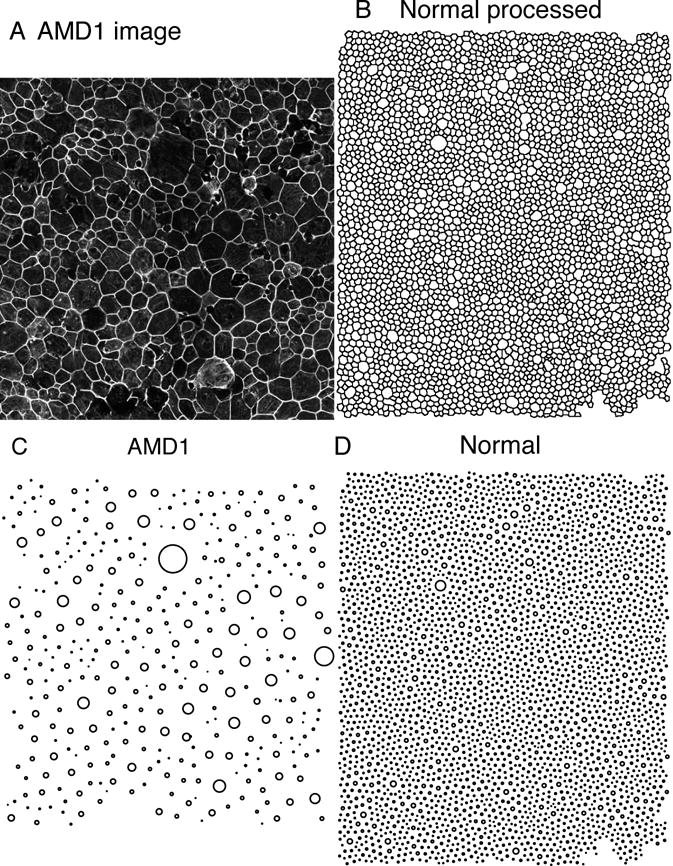
Normal and AMD representative images of the RPE sheet. A: An image from an AMD eye in the macula. B: A partially processed image from the macula of a normal individual. The cell borders are now outlined and transformed so that CellProfiler can process the image. C: Spatial point patterns from the AMD eye in A. D: Spatial point pattern from the normal eye in B. Sizes of the circles in C and D represent the size of each cell.
XX.3.4 Regularity in AMD and normal RPE sheets
Regularity in spacing was clearly evident in the RPE sheet, with much more uniformity in the normal RPE sheet than in the macula of the AMD patient. Images of each RPE sheet are illustrated in Fig. XX.1. In Fig. XX.2, the cumulative distribution function of the nearest-neighbor distance (the G-function) is compared among the RPE pattern of an AMD eye (red), normal eye (green), and the control: the hexagonal cell lattice with the averaged cell size in the normal eye (blue). We also randomized the normal and the hexagonal lattice (dashed lines) to show the broadening of the distribution function. The plot indicated that the normal RPE pattern is remarkably similar to a hexagonal lattice of cells with a narrow distribution (tight size range within 8–12 microns), while the AMD pattern shows a much broader distribution and a strong shift to the right. The latter indicates that the AMD RPE pattern has a larger minimum size, and a much reduced regularity.
Fig. XX.2.
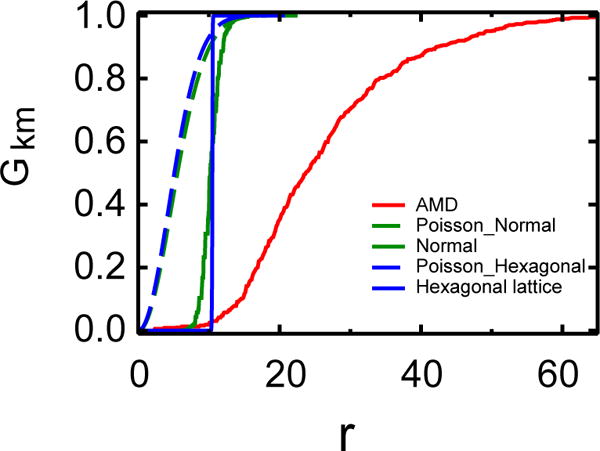
Spatial point analysis by cumulative nearest neighbor distance distribution function. The key point is that G(r) for normal RPE is markedly different from that of AMD RPE, suggesting that the spatial patterns are more regular in normal eyes and becomes irregular in AMD RPE sheets.
XX.4 Discussion
XX.4.1 The context of our findings
The fraction of hexagonal cells on a surface is an indication of the mechanical stability of a tissue: Hexagonal tiling is the most efficient way to cover a plane with a monolayer of cells of equal area with the least total perimeter per cell (Thompson 1942). Sharp deviations from this tiling pattern indicate mechanical stress or a dynamic environment not near equilibrium. Cell death, cell division, and regional differences can affect the regular tiling and these defects compromise the strength and durability of the RPE sheet, and make these spots more vulnerable to neovascularization or the initiation of atrophic lesions (Shirinifard et al. 2012). Tiling defects occur in age-related loss of RPE cells (Watzke et al. 1993), inherited mouse retinal diseases (Jiang et al. 2013), and regional differences (foveal RPE versus equatorial) (Gao and Hollyfield 1992). We have initiated computer simulations of RPE cell death. The simulations seem to reveal testable hypotheses on loss of regularity in spatial point patterns according to a loss of inhibition in cell growth required to fill in holes in the RPE sheet.
XX.4.2 The normal eye and aging
The regular shape of RPE cells in the macula implies small and balanced external forces that pull or tug on each cell. The far-peripheral RPE cells were more irregular, suggesting forces causing uneven tension. The far periphery may be subjected to different amounts of strain due to the nearby ciliary body and the non-uniform, intermittent stress from the ciliary muscles. Alternatively, the RPE cells in the far periphery may be different from those in the macula or mid-periphery (Burke and Hjelmeland 2005). RPE in the macula and far-periphery showed changes in both shape and size with age while the mid-periphery did not. Drusen and basal laminar deposits tend to occur in the macula and far periphery. These are signs of RPE stress; we hypothesize there is more RPE stress in the macula and far periphery than mid-periphery. These models need to be tested that consider differences (e.g., metabolic demands of overlying photoreceptor cells and incidences of hard drusen) among these three locations versus other models of inherently different classes of RPE cells (Burke and Hjelmeland 2005) in these three locations.
XX.4.2 The AMD eye
Understanding normal RPE morphology helps to better understand RPE pathology by discriminating between the effects of age (the most important risk factor of AMD) versus other risk factors (genetics, smoking, type of drusen, and environmental factors). Our preliminary evaluations here may imply that clustered outliers in size and shape of RPE cells are risks for or may initiate progression of AMD. Our studies may help to identify breakdown in the RPE at an earlier stage allowing for more prompt evaluation and treatment.
XX.4.4 Spatial point patterning
We have found regularity in the spatial patterning of RPE cells that may reflect intercellular interactions. Future studies will help delineate when this regularity occurs/develops, and how it is lost in AMD, including the impact of druse, which distort the pattern in the RPE sheet.
XX.4.5 Future directions
In the near future, we will report full quantitative analysis of changes (per cell and in organization) in RPE with age, region, and disease state. We will correlate en-face metrics with histopathology of the same location cut in cross sections. These analyses should provide insight into the basic biology underlying transition from isometric cells to those that vary widely in shape, size, and function.
Acknowledgments
Support provided by Research to Prevent Blindness; NIH R01EY021592, P30EY06360, R01EY016470, R01EY014026, UL1TR000454, and TL1TR000456; Abraham J. and Phyllis Katz Foundation; USAMRAA DOD W81XWH-12-1-0436; The Emory Neurosciences Initiative.
References
- Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Turner R. spatstat: An R Package for Analyzing Spatial Point Patterns. Journal of statistical software. 2005;12:1–42. [Google Scholar]
- Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Molecular aspects of medicine. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Hjelmeland LM. Mosaicism of the retinal pigment epithelium: seeing the small picture. Molecular interventions. 2005;5:241–249. doi: 10.1124/mi.5.4.7. [DOI] [PubMed] [Google Scholar]
- Chrenek MA, Dalal N, Gardner C, et al. Analysis of the RPE sheet in the rd10 retinal degeneration model. Advances in Experimental Medicine and Biology. 2012;723:641–647. doi: 10.1007/978-1-4614-0631-0_81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investigative Ophthalmology & Visual Science. 1992;33:1–17. [PubMed] [Google Scholar]
- Jiang Y, Qi X, Chrenek MA, et al. Functional principal component analysis reveals discriminating categories of retinal pigment epithelial morphology in mice. Investigative Ophthalmology & Visual Science. 2013;54:7274–7283. doi: 10.1167/iovs.13-12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Qi X, Chrenek MA, et al. Analysis of Mouse RPE Sheet Morphology Gives Discriminatory Categories. Advances in Experimental Medicine and Biology. 2014;801:601–607. doi: 10.1007/978-1-4614-3209-8_76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins VI, Sandig M, Hergott GJ, et al. Microfilament organization and wound repair in retinal pigment epithelium. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1995;73:709–722. doi: 10.1139/o95-079. [DOI] [PubMed] [Google Scholar]
- Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. BioTechniques. 2007;42:71–75. doi: 10.2144/000112257. [DOI] [PubMed] [Google Scholar]
- Liang F-Q, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Experimental eye research. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Nagai H, Kalnins VI. Normally occurring loss of single cells and repair of resulting defects in retinal pigment epithelium in situ. Exp Eye Res. 1996;62:55–61. doi: 10.1006/exer.1996.0007. [DOI] [PubMed] [Google Scholar]
- Negi A, Marmor MF. Healing of photocoagulation lesions affects the rate of subretinal fluid resorption. Ophthalmology. 1984;91:1678–1683. doi: 10.1016/s0161-6420(84)34084-2. [DOI] [PubMed] [Google Scholar]
- Rizzolo LJ. Barrier properties of cultured retinal pigment epithelium. Experimental eye research. 2014 doi: 10.1016/j.exer.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Roider J, Michaud NA, Flotte TJ, et al. Response of the retinal pigment epithelium to selective photocoagulation. Archives of ophthalmology. 1992;110:1786–1792. doi: 10.1001/archopht.1992.01080240126045. [DOI] [PubMed] [Google Scholar]
- Shirinifard A, Glazier JA, Swat M, et al. Adhesion failures determine the pattern of choroidal neovascularization in the eye: a computer simulation study. PLoS Computational Biology. 2012;8:e1002440. doi: 10.1371/journal.pcbi.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DW. On growth and form. 1942. [Google Scholar]
- van Lookeren Campagne M, LeCouter J, Yaspan BL, et al. Mechanisms of age-related macular degeneration and therapeutic opportunities. The Journal of pathology. 2014;232:151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- Watzke RC, Soldevilla JD, Trune DR. Morphometric analysis of human retinal pigment epithelium: correlation with age and location. Current eye research. 1993;12:133–142. doi: 10.3109/02713689308999481. [DOI] [PubMed] [Google Scholar]


