Abstract
Although baculovirus has been used as a safe and convenient gene delivery vector in mammalian cells, baculovirus-mediated transgene expression is less effective in various mammalian cell lines. Identification of the negative regulators in host cells is necessary to improve baculovirus-based expression systems. Here, we performed high-throughput shRNA library screening, targeting 176 antiviral innate immune genes, and identified 43 host restriction factor genes in a human A549 lung carcinoma cell line. Among them, suppression of receptor interaction protein kinase 1 (RIP1, also known as RIPK1) significantly increased baculoviral transgene expression without resulting in significant cell death. Silencing of RIP1 did not affect viral entry or cell viability, but it did inhibit nuclear translocation of the IRF3 and NF-κB transcription factors. Also, activation of downstream signaling mediators (such as TBK1 and IRF7) was affected, and subsequent interferon and cytokine gene expression levels were abolished. Further, Necrostatin-1 (Nec-1)—an inhibitor of RIP1 kinase activity—dramatically increased baculoviral transgene expression in RIP1-silenced cells. Using baculovirus as a model system, this study presents an initial investigation of large numbers of human cell antiviral innate immune response factors against a “nonadaptive virus.” In addition, our study has made baculovirus a more efficient gene transfer vector for some of the most frequently used mammalian cell systems.
Keywords: baculovirus, cell defense, RIP1, transgene, antiviral response, gene therapy
Introduction
Baculoviruses are a group of pathogens that specifically infect invertebrates, primarily lepidopterans. Among them, Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is one of the most frequently used baculoviruses for foreign gene expression because of its numerous advantages. First, baculovirus is safe for human application because of its inability to either replicate or integrate its genome into the chromosomes of vertebrate cells.1, 2 Therefore, it can be produced with high titers in biosafety level 1 facilities by infecting its natural insect hosts.3 Second, baculovirus has a large genome (∼134 kb) and, hence, possesses a cloning capacity as large as 38 kb.4 Third, it is better than other protein expression systems, such as E. coli and yeast, in terms of its high levels of gene expression and proper post-translational modifications of the engineered protein.5 These characteristics make baculovirus a desirable system for protein production.
Baculovirus has been used as a gene delivery vector for a wide variety of applications, including multigene delivery for in vivo creation of virus-like particles,6 in cancer gene therapy7 and regenerative medicine, and as vaccine vectors.8, 9 In the last decade, baculovirus has been widely used as a safe and convenient tool for foreign gene delivery into mammalian cells.10 Despite its potential as a safe and useful tool, the levels of transgene expression mediated by baculovirus vectors are significantly restricted by host immune systems in mammalian cells.
Upon virus infection, host cells respond with a strong antiviral immune response. Pathogenic viruses can counteract certain host cell defenses through host-pathogen co-evolution, so a complete picture of host responses is not yet clear. However, baculovirus is not an established pathogen against mammalian cells, and thus it provides a unique opportunity for us to study the immune response to a DNA virus that is not adapted to infecting mammalian cells. Baculovirus has been shown to stimulate some innate immune responses in mammalian cells, including human mesenchymal stem cells (MSCs) 11, 12 and mouse embryonic fibroblasts (MEFs).13 A global analysis of host immune responses against baculovirus would also provide a comprehensive view of host defenses against a “nonadaptive” viral agent.
Induction of host immune responses by pathogens is mediated by activation of pattern recognition receptors (PRRs). There are four major groups of mammalian PRRs: Toll-like receptors (TLRs), retinoic acid-inducible protein I (RIG-I)-like receptors (RLRs), nucleotide oligomerization domain (NOD)-like receptors (NLRs), and the DNA-sensing receptor ZBP1.14, 15 All of these receptors are responsible for recognition of viral nucleic acids and viral factors in the cytosol. In human MSCs, TLR3, a receptor that generally recognizes double-stranded RNA, has been shown to be upregulated by baculovirus transduction, triggering the production of interleukin-6 (IL-6) and IL-8, but not β-IFN or other inflammatory cytokines.16 Transduction of baculovirus in chondrocytes elicited IFN-α/β expression, which repressed transgene expression in a dose-dependent manner.17 In MEFs, baculovirus has been shown to induce the secretion of inflammatory cytokines and type I IFNs through both TLR-dependent and -independent pathways.13 Furthermore, transgene expression of recombinant baculovirus was enhanced in MEFs deficient for innate immune signaling pathway genes, including STING, TBK1, IRF3, and IPS-1.18 These results show that the innate immune responses induced by baculovirus transduction attenuate transgene expression in mammalian cells. Thus, it is of great interest to decipher the relationship between baculoviral transgene expression and antiviral mechanisms in mammalian cells. This information will be invaluable for developing baculovirus gene therapies or when using baculovirus as a mammalian gene transfer vector.
Human lung cancer A549 cells have been commonly used in influenza virus vaccine-related studies, including for viruses H7N919 and H1N1,20 and to discern the molecular mechanisms involved in the pathogenicity of avian influenza virus (H5N1 or H9N2).20, 21, 22 In cell-based assays, a high level of transgene expression by recombinant baculovirus is necessary to attain detection sensitivity. Although baculovirus can efficiently transduce most mammalian cell types without any replication, transgenes are not very efficiently expressed in some cells, including A549.10 The reason for the low expression may be host resistance mediated by innate immune responses induced by baculovirus transduction.18
In this study, we transduced A549 cells with baculovirus and performed an intensive and iterative cell screen with a short hairpin RNA (shRNA) library highly enriched for human antiviral response pathways, including TLRs, RLRs, NLRs, and cytosolic DNA-sensing pathways. Of the 176 genes assayed, knockdown of 102 genes resulted in elevated gene expression driven by the cytomegalovirus immediate early enhancer (CMV) promoter, whereas downregulation of gene expression by the same promoter occurred in 31 genes. Among them, RIP1 knockdown enhanced baculoviral transgene expression 10-fold and was the only one of these 102 genes for which cell viability was not significantly affected upon knockdown of the target genes. We found that increased luciferase activity in RIP1 knockdown A549 cells was not due to higher levels of baculovirus entry into the cells but, instead, arose through increased activity of the promoter.
We present here a relatively complete picture of baculovirus-induced antiviral immunity in mammalian cells. Because a pathogenic virus can evade its natural host’s defense mechanism for successful infection, it is difficult to observe and examine uninterrupted host immune responses. However, because baculovirus is not infectious to mammalian cells, it provides a unique opportunity to study host immune responses against nonadaptive invading viral agents. Our experiments also provide informative results that can considerably improve transgene expression using baculovirus as an efficient vector in mammalian cells.
Results
Screening of an shRNA Library Targeting Innate Immune-Responsive Genes Reveals Significant Antiviral Candidates, as Reflected by Baculoviral Transgene Expression
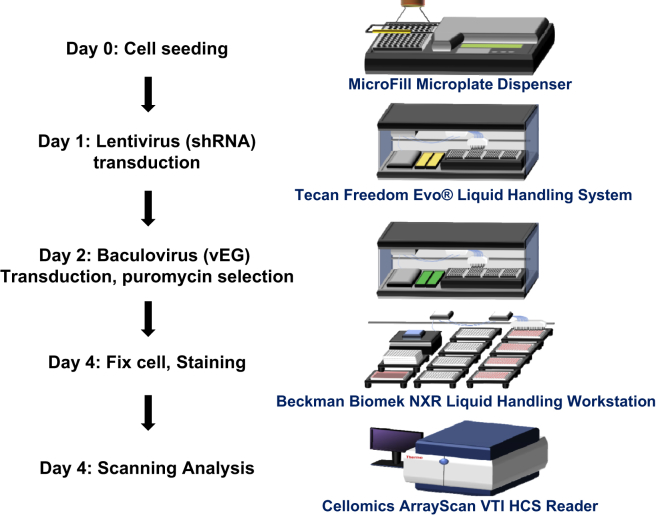
We performed high-throughput screening of a lentivirus-based shRNA library targeting innate immune-related genes to identify host proteins that restrict baculovirus transgene expression in an A549 lung carcinoma cell line. We screened a total of 880 shRNA lentivirus clones targeting 176 genes chosen from the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database and specifically involved in the four major antiviral innate immune pathways (TLRs, RLRs, NLRs, and cytosolic DNA-sensing) to knock down their transcripts by an RNAi mechanism. Some of the genes were found to be involved in more than one immune signaling pathway. Each gene was targeted at five different regions by transduction with individual shRNA lentivirus clones. A schematic of our high-throughput screening methodology is illustrated in Figure 1.
Figure 1.
Schematic of Our High-Throughput Screening Approach
Shown is a flowchart (left) of the methodology carried out in the A549 cell line and (right) the machines used at each step of the screening process.
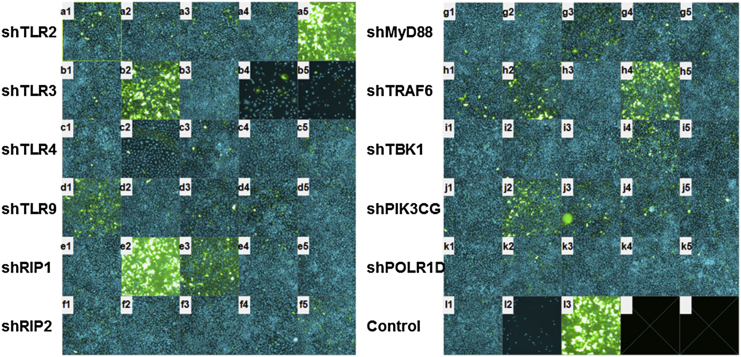
The cells were first transduced with individual shRNA lentivirus clones at an MOI of 10 for 24 hr, followed by transduction with recombinant baculovirus (vAcE) expressing a CMV promoter-driven EGFP23 for 48 hr under puromycin for selection of cells harboring shRNA. The fluorescence intensity (FI) of EGFP-positive cells reflects the transgene expression level resulting from knockdown of the particular gene (Figure 2; the pictures represent only a few genes). As a negative control, transduction of cells with a non-target shRNA sequence (shCtrl) was used throughout the experiment. FI was averaged across the number of EGFP-positive cells in the particular wells and further normalized with the average FI obtained in the shCtrl well to obtain the relative fold changes in baculovirus transgene expression levels. Simultaneously, the effects of gene knockdown on cell viability were examined. The numbers of Hoechst dye-stained cells left after washing and fixing during the screening procedure were considered viable cells. To narrow down the genes of interest, we adopted stringent selection criteria. The EGFP expression level must be enhanced 2-fold compared with the negative control from at least three of five individual shRNA lentivirus clones targeted to the particular transcript, and at least one of the shRNA lentivirus clones must enhance gene expression by 5-fold or more. We found 43 candidates that met these criteria, but cell survival rates were variable (Table 1). Baculovirus transgene expression was almost 10-fold greater under knockdown of five genes: IL-1β, TLR3, RIP1, CD14, and IKBKB. Of these five candidate genes, RIP1 knockdown did not have severe effects on cell viability (93%, clone e2; Figure 2). Considering that cell viability is a crucial factor in baculovirus transgene expression technology, we then focused on studying the biological role of RIP1 in baculovirus transgene expression.
Figure 2.
Analysis of Baculovirus Transgene Expression upon shRNA Lentivirus Clones Acting on Antiviral Genes
Shown are representative images resulting from knockdown of antiviral genes by five independent shRNA clones targeting different regions of a gene. For simplicity, only a few genes are shown. Nuclei were stained with Hoechst 33342 (blue). (A1–A5) TLR2. (B1–B5) TLR3. (C1–C5) TLR4. (D1–D5) TLR9. (E1–E5) RIP1. (F1–F5) RIP2. (G1–G5) MyD88. (H1–H5) TRAF6. (I1–I5) TBK1. (J1–J5) PIK3CG. (K1–K5) POLR1D. (L1) shCtrl, negative control. (L2) Cell-only control. (L3) shTLR2, positive control.
Table 1.
List of Genes Obtained from Screening Innate Immune Pathways that Were Significantly Affected by Baculovirus Transgene Expression in an A549 Cell Line
| Gene Symbol | Fold Gene Activationa | Cell Viability (%)b | TLR | RIG-I | NOD | Cytosolic DNA |
|---|---|---|---|---|---|---|
| IL1B | 14.4 | 38.2 | ✓ | ✓ | ✓ | ✓ |
| TLR3 | 11.6 | 55.6 | ✓ | |||
| RIP1 | 11.2 | 93.6 | ✓ | ✓ | ✓ | |
| CD14 | 10 | 59.3 | ✓ | |||
| IKBKB | 10 | 49.3 | ✓ | ✓ | ✓ | ✓ |
| XIAP | 9.8 | 55.7 | ✓ | |||
| IRF5 | 9.4 | 43.6 | ✓ | |||
| IFNA7 | 9.3 | 22.4 | ✓ | ✓ | ✓ | |
| CXCL1 | 8.8 | 62.2 | ✓ | |||
| TLR2 | 8.7 | 80.4 | ✓ | |||
| PIK3CG | 8.4 | 71.2 | ✓ | |||
| LY96 | 8.2 | 60.6 | ✓ | |||
| TOLLIP | 7.9 | 65.5 | ✓ | |||
| CCL4 | 7.4 | 74.5 | ✓ | ✓ | ||
| ZBP1 | 7.3 | 33.8 | ✓ | |||
| ICAM1 | 7.3 | 42 | ✓ | |||
| MAP2K6 | 7.2 | 69.9 | ✓ | |||
| TBK1 | 6.9 | 59.9 | ✓ | ✓ | ✓ | |
| POLR3C | 6.6 | 63.3 | ✓ | |||
| PIK3R2 | 6.5 | 65 | ✓ | |||
| MAPK3 | 6.4 | 77.2 | ✓ | ✓ | ||
| IFNA8 | 6.4 | 60.2 | ✓ | ✓ | ✓ | |
| ERBB2IP | 6.3 | 91 | ✓ | |||
| SUGT1 | 6.2 | 64.7 | ✓ | |||
| MAP2K7 | 6.2 | 48.8 | ✓ | |||
| CCL5 | 6.2 | 80.9 | ✓ | ✓ | ✓ | |
| MAP2K6 | 6.1 | 67.8 | ✓ | |||
| TLR9 | 6.1 | 68.7 | ✓ | |||
| TRAF6 | 5.9 | 77 | ✓ | ✓ | ✓ | |
| CXCL10 | 5.9 | 70.1 | ✓ | ✓ | ✓ | |
| CASP8 | 5.9 | 43.7 | ✓ | ✓ | ✓ | |
| PIN1 | 5.7 | 71.5 | ✓ | |||
| CHUK | 5.7 | 71.4 | ✓ | ✓ | ✓ | ✓ |
| IKBKE | 5.7 | 42.6 | ✓ | ✓ | ✓ | |
| TLR7 | 5.6 | 65.9 | ✓ | |||
| LBP | 5.5 | 28.5 | ✓ | |||
| IL8 | 5.5 | 59.7 | ✓ | ✓ | ✓ | |
| IFNB1 | 5.5 | 49 | ✓ | ✓ | ✓ | |
| IFNW1 | 5.3 | 31.7 | ✓ | |||
| DHX58 | 5.3 | 73.9 | ✓ | |||
| CTSK | 5.3 | 61.2 | ✓ | |||
| IFNK | 5.3 | 58 | ✓ | |||
| MAP3K7 | 5.2 | 79.5 | ✓ | ✓ | ✓ |
This table shows the genes involved in innate immune signaling pathways (resource: KEGG pathway database), and most of these genes function in more than one signaling pathway.
Fold gene activation represents the average values of the FIs of the number of EGFP-positive cells relative to the negative control obtained upon silencing the genes by lentivirus shRNA clones.
Cell viability represents the number of living cells as determined by the AlamarBlue assay.
Previously, it has been reported18 that MEFs deficient for stimulator of IFN genes (STING), TANK binding kinase 1 (TBK1), IFN regulatory factor 3 (IRF3), or IFN-β promoter stimulator 1 (IPS-1) enhanced baculovirus transgene expression levels but not those deficient for IRF7, MyD88, or Z-DNA binding protein 1 (ZBP1)/DAI. Consistent with this observation, our screening results also showed that knockdown of TBK1, IRF3, and IPS-1/MAVS increased baculovirus transgene expression with different cell viability rates, but there was no significant effect in the case of IRF7 and MyD88 knockdown, indicating the reliability of our screening approach. The lentivirus shRNA clone targeting the STING gene was not available to us and so was excluded from our screening. Silencing of ZBP1 increased baculovirus transgene expression levels in our study, but with a reduced cell viability rate. We think that such discrepancies could be due to the different cell lines employed in the studies. Previous studies18 and our results demonstrate that some antiviral innate immune genes potentially hamper baculovirus transgene expression.
Baculovirus Transduction Elevates RIP1 Expression Levels, and RIP1 Knockdown Enhances Baculovirus Transgene Expression without Affecting Viral Entry
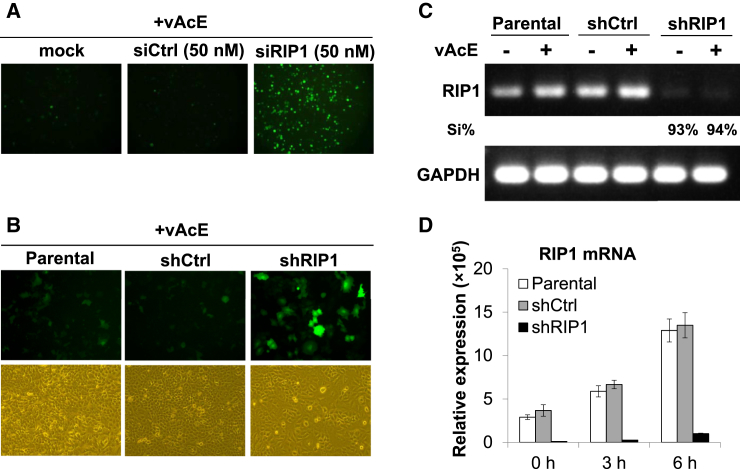
Our RNAi screen identified RIP1 as a candidate gene that, upon silencing, greatly enhances baculovirus transgene expression without severe effects on cell viability. Transient knockdown of RIP1 by siRNA transfections also indicated a significant increase in baculovirus transgene expression (Figure 3A). To investigate the functional role of RIP1 in baculovirus transduction, we established A549 cell clones stably harboring shRNA for RIP1 (shRIP1) using puromycin selection. To validate our results, we transduced parental A549, A549-shCtrl, and A549-shRIP1 cells with vAcE at an MOI of 50 for 48 hr and recorded the EGFP expression levels. As observed in the screening process, transgene expression levels were higher in shRIP1 cells compared with those in shCtrl or parental cells (Figure 3B). Transgene expression levels were similar between parental and shCtrl cells, ruling out an effect of the lentiviruses and/or puromycin treatment under our experimental conditions. We performed RT-PCR analysis on RNA extracted from cells transduced with or without recombinant baculovirus (vAcE) to examine the knockdown efficiency of RIP1. Quantification of band intensities in the agarose gels showed that more than 90% of the RIP1 gene was successfully knocked down in shRIP1 cells. GAPDH gene amplification served as a cellular internal control (Figure 3C). qRT-PCR analysis revealed very low amounts of RIP1 mRNA expression in shRIP1 cells, corroborating the RT-PCR result. We also observed induction of RIP1 gene expression in parental and shCtrl cells as a function of time in response to baculovirus transduction (Figure 3D).
Figure 3.
RIP1 Knockdown Enhances Baculovirus Transgene Expression
(A) Transient knockdown of RIP1 enhances baculovirus transgene expression. Parental A549 cells were transfected with or without 50 nM of siRNA against the RIP1 gene or non-targeting siCtrl as a negative control. Twenty-four hours post-transfection (hpt), the cells were transduced with vAcE virus. The EGFP fluorescence images were taken at 48 hpt. (B) Stable knockdown of RIP1 enhances baculovirus transgene expression. The green fluorescence images were taken at 48 hpt. (C) The mRNA expression level of RIP1 was detected by RT-PCR at 48 hpt. GAPDH mRNA expression levels served as an internal control. The band intensities in the 2% agarose gel were quantified by ImageJ software, and the silencing percent (Si%) was measured over the intensities observed under shCtrl conditions. (D) The knockdown efficiency of RIP1 was checked by qRT-PCR 0, 3, and 6 hpt. RIP1 mRNA expression levels were normalized by GAPDH expression to determine relative amounts. For (B)–(D), parental A549 or A549 cells stably expressing shRNA for RIP1 (A549-shRIP1) and non-target shRNA (A549-shCtrl) were transduced with vAcE at an MOI of 50.
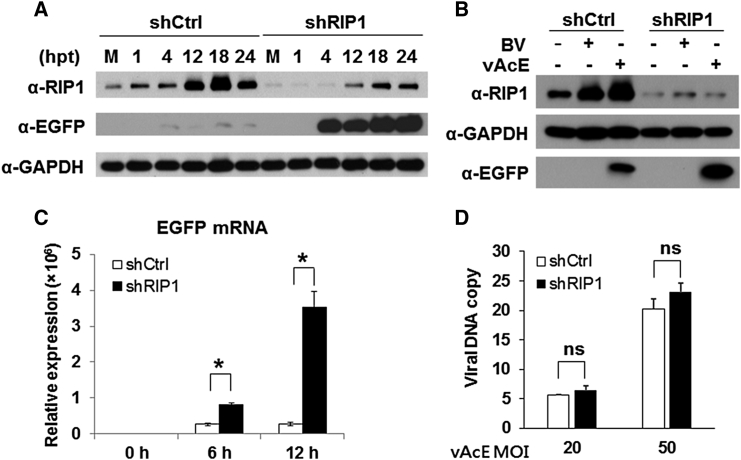
Next, we traced the RIP1 and EGFP protein levels in shCtrl or shRIP1 cells transduced with vAcE for various time points from 0–24 hr post-transduction (hpt) by western blotting. We found that RIP1 expression levels increased as early as 1 hpt and became more abundant as a function of time in the shCtrl cells but remained at low levels in shRIP1 cells, suggesting that baculovirus transduction modulates RIP1 protein expression in general. In contrast, EGFP protein derived from vAcE was significantly downregulated in shCtrl cells compared with shRIP1 cells, indicating that RIP1 protein inhibits baculovirus transgene expression. GAPDH protein served as an internal loading control in the western blot analyses (Figure 4A). We also observed increased RIP1 protein levels in shCtrl cells transduced either with recombinant vAcE or wild-type baculovirus (BV) compared with no baculovirus transduction at 18 hpt (Figure 4B).
Figure 4.
RIP1 Knockdown Enhances Baculovirus Transgene Expression without Affecting Viral Entry in an A549 Cell Line
(A) The protein expression levels of RIP1 and EGFP in cells transduced with vAcE at various time points, as indicated, were detected by western blotting. GAPDH served as an endogenous internal loading control in the experiment. (B) Western blot analyses of RIP1 in cells transduced with vAcE or wild-type BV at 18 hpt. (C) Transgene expression (mRNA levels) of EGFP was quantified in cells transduced with vAcE by qRT-PCR 0, 6, and 12 hpt. (D) Total cellular DNA was extracted from A549-shRIP1 and A549-shCtrl cells transduced with vAcE 2 hpt, and viral DNA copies were quantified by qPCR. Knockdown of RIP1 did not alter viral entry efficiency. All experiments were performed three times independently, and the data shown are of one representative transduction experiment. Asterisks indicate statistically significant differences compared with the control (*p < 0.05); ns, no significant difference.
In another set of experiments, we also observed that the mRNA expression level of EGFP, which was transduced into cells by vAcE, was significantly higher in shRIP1 cells compared with shCtrl cells (Figure 4C). To determine whether this baculovirus transgene enhancement was due to increased viral entry efficiency, we transduced shCtrl or shRIP1 cells with different concentrations of vAcE (MOI = 20 and 50) for 2 hr and then quantified the viral DNA by qPCR from the total cellular DNA. We did not observe statistical differences in the amount of viral DNA, suggesting that both shCtrl and shRIP1 cells were transduced with similar amounts of viral particles (Figure 4D). Overall, these results suggest that RIP1 does not affect viral entry but does restrict baculovirus transgene expression.
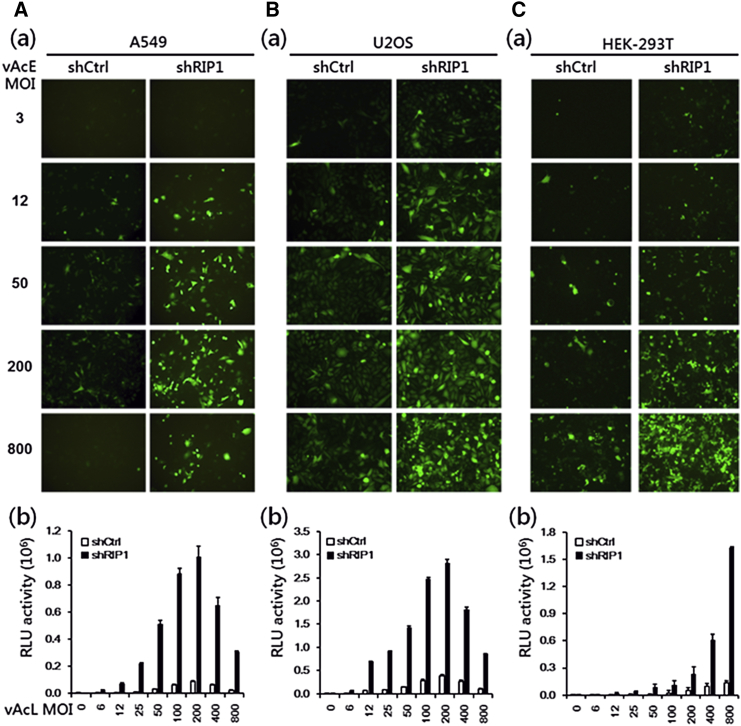
RIP1 Knockdown Elevates Dose-Dependent Baculovirus Transgene Expression in Mammalian Cells
It has been reported that baculovirus-mediated expression of reporter genes is relatively different among various mammalian cell lines.10, 24 Therefore, to determine whether baculovirus transgene enhancement under RIP1 knockdown is A549 cell line-specific or could be applicable to other cell lines, we examined the baculovirus dose-dependent effect in U2OS (human osteosarcoma) and HEK293T cell lines. To this end, we first established shRIP1-stable or shCtrl-stable U2OS and HEK293T cell lines and then transduced them with different concentrations of vAcE for 48 hr. As shown in Figure 5, the FI and the numbers of EGFP-positive cells increased according to viral dose in shRIP1 cells compared with shCtrl cells, with only minor differences among the cell lines tested. Apart from our visual observations, we quantified transgene expression levels by transducing cells with another recombinant baculovirus vAcL expressing CMV-promoter-driven firefly luciferase. Luciferase expression was significantly higher in shRIP1 cells compared with shCtrl cells in a dose-dependent fashion, and the expression pattern was similarly higher in A549 and U2OS cells compared with HEK293T-shRIP1 cells (Figure 5B). Maximum luciferase expression in shRIP1 cells was attained at MOI = 200 but dropped at a higher viral dose in both the A549 and U2OS cell lines. In contrast, HEK293T-shRIP1 cells showed gradual enhancement of luciferase expression with increasing the viral dose up to a maximum at MOI = 800 (Figure 5). Transduction efficiencies varied among these cell lines, possibly because the culture conditions differed and the activation efficiencies of RIP1 between cell lines were different. These data suggest that RIP1 knockdown-mediated baculovirus transgene enhancement is not restricted to A549 cells but that the activation efficiency of various mammalian cell lines may be different. EGFP and luciferase expression was driven by the CMV promoter in the vAcE and vAcL viruses, respectively. We confirmed that our findings are not attributable to the CMV promoter alone because transduction of vAcmC (SV40 promoter-driven mCherry) and vAceE25 (an EF-1α promoter with CMV enhancer-driven EGFP, kindly provided by Dr. Y. C. Hu) showed increased dose-dependent mCherry and EGFP expression in A549-shRIP1 compared with A549-shCtrl cells (Figures S1 and S2).
Figure 5.
RIP1 Knockdown Elevates Dose-Dependent Baculovirus Transgene Expression in Mammalian Cells
(A–C) Transgene (reporter gene) expression levels of stably-expressing shRNA for RIP1 (shRIP1) or for non-target shCtrl of A549 (A), U2OS (B), and HEK293T (C) cells transduced with vAcE and vAcL at various MOIs as indicated for 48 hr. Shown is a reporter assay of EGFP expression (a) and luciferase expression (b) driven by a CMV promoter carried on the recombinant baculoviruses vAcE and vAcL. All experiments were performed three times independently, and the data shown are of one representative transduction experiment.
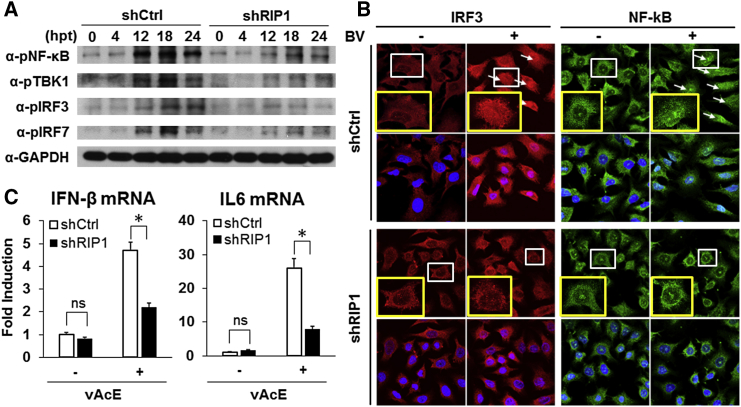
Silencing of RIP1 Blocks Activation of TBK1/IRF3/IRF7/NF-κB Proteins in Baculovirus-Induced Immune Signaling
It has been well documented that RIP1 is involved in several signaling pathways ranging from TLRs, DNA damage sensors, and intracellular double-stranded RNA (dsRNA) sensors15, 26, 27 and, furthermore, that baculovirus transduction induces the host innate immune responses through TLR-dependent and -independent signaling pathways.16, 28 To understand the molecular mechanism of how RIP1 knockdown enhances baculovirus transgene expression, we examined the activation of RIP1 downstream proteins—TBK1/IRF3/IRF7/nuclear factor κB (NF-κB)—in response to baculovirus transduction. Upon stimulation, these proteins become phosphorylated, transmit a signal to downstream proteins, or translocate to the nucleus to induce the expression of cytokine or IFN genes29, 30 that could affect baculovirus transgene expression.17
To examine the activation of RIP1 downstream factors, we transduced A549-shRIP1 and A549-shCtrl cells with vAcE for various time points, and the cell lysates were subjected to western blotting with phospho-specific antibodies. None of the four TBK1/IRF3/IRF7/NF-κB proteins in A549-shCtrl cells were activated up to 4 hpt, but they were strongly activated at 12 hpt and 18 hpt in response to baculovirus transduction (Figure 6A). Activation diminished to some extent at 24 hpt in the case of TBK1/IRF7/NF-κB proteins, but IRF3 remained active up to 24 hpt. In contrast, activation of these four proteins in A549-shRIP1 cells was almost abolished up to 24 hpt (Figure 6A). To test the inhibition of nuclear translocation of the phosphorylated transcription factors (IRF3/NF-κB) in shRIP1 cells, we performed an immunofluorescence assay of A549-shCtrl and A549-shRIP1 cells transduced with or without baculovirus (vAcE). Nuclear translocations of IRF3 and NF-κB proteins were clearly observed in A549-shCtrl cells, but, in contrast, nuclear translocation was significantly impeded in A549-shRIP1 cells, as in the case of A549-shCtrl cells transduced with baculovirus (Figure 6B). Furthermore, we also found that expression of IL-6 and IFN-β genes (downstream genes of NF-kB and IRF3) was dramatically downregulated in A549-shRIP1 compared with A549-shCtrl cells transduced with baculovirus (Figure 6C). These results indicate that RIP1 is an essential mediator of baculovirus-induced immune responses through the TBK1/IRF3/IRF7/NF-κB proteins.
Figure 6.
RIP1 Knockdown Inhibits Activation of Downstream Signaling Mediators
(A–C) A549-shRIP1 or A549-shCtrl cells were transduced with wild-type BV in (A) and (B) or with vAcE in (C) at an MOI of 50. (A) The cell lysates were collected at various time points post-transduction and subjected to western blot analyses with phospho-TBK1, phospho-IRF3/7, and phospho-NF-κB antibodies to detect their activation. (B) Cells were immunostained with phospho-IRF3 antibodies and phospho-NF-κB 24 hpt, and images were taken by laser confocal microscopy (LSM510). White arrows indicate nuclear translocation of IRF3 and NF-κB, respectively, in A549-shCtrl cells. The single cells (white lined) are enlarged (yellow lined) under every condition for better resolution. (C) Relative mRNA expression levels of IFN-β and IL-6 genes quantified by qRT-PCR at 3 hpt. The mRNA expression levels of IFN-β and IL-6 genes were normalized by GAPDH mRNA expression to determine relative amounts. All experiments were performed three times independently, and the data shown are of one representative transduction experiment. Asterisks indicate statistically significant differences compared with the control (*p < 0.05).
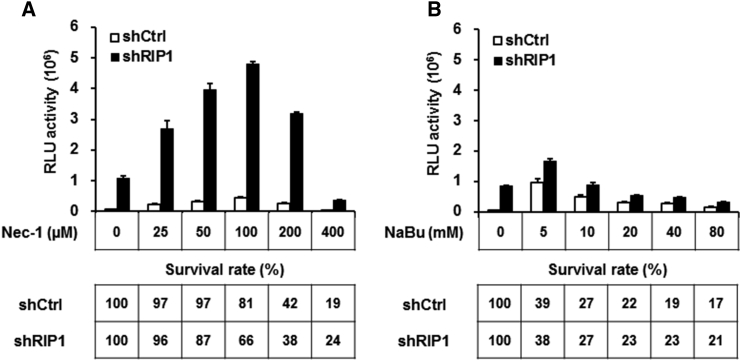
Necrostatin-1 Promotes Baculovirus Transgene Expression in RIP1 Knockdown Cells
Necrostatin-1 (Nec-1), a small-molecule inhibitor of RIP1 kinase, has been demonstrated to affect various RIP1-mediated cellular processes.31 We examined the effect of Nec-1 on RIP1-mediated baculovirus transgene inhibition. Nec-1 was added to A549-shCtrl and A549-shRIP1 cells at various concentrations, and, 24 hr later, cells were transduced with vAcL (MOI = 50) for 48 hr. As shown in Figure 7A, luciferase activities increased greatly, reaching a maximum at 100 μM, and then declined with further dosages of Nec-1 in A549-shRIP1 cells, whereas a milder effect was observed for A549-shCtrl cells compared with untreated controls (0 μM). This result suggests that inhibiting the function of residual RIP1 by Nec-1 in RIP1 knockdown cells enhances baculovirus transgene expression. Next, we examined the effect of sodium butyrate (NaBu, a histone deacetylase inhibitor), which is commonly used to enhance baculovirus transgene expression under similar experimental conditions.32 Compared with the untreated control (0 mM), NaBu (5 mM) had a significant effect on luciferase activity in RIP1 knockdown cells, but its effect gradually declined with increasing dosages. We did not observe significant transgene activation at a lower concentration of NaBu (2.5 mM) (Figure S3) in A549 cells. NaBu is more effective than Nec-1 in stimulating baculovirus-mediated gene expression, but Nec-1 is strikingly more potent than NaBu in RIP1 knockdown cells, yielding a significant enhancement in baculovirus-mediated luciferase activity (Figure 7B). These results indicate that Nec-1 treatment promotes baculovirus transgene expression in RIP1 knockdown cells and imply that this strategy could be very useful in enhancing baculovirus-mediated transgene expression in mammalian cells.
Figure 7.
Necrostatin-1 Promotes Baculovirus Transgene Expression in RIP1 Knockdown Cells
(A and B) A549-shRIP1 or A549-shCtrl cells were cultured in medium containing progressively increasing concentrations of Necrostatin-1 (Nec-1) (A) or the HDAC inhibitor sodium butyrate (NaBu) (B), as indicated, for 24 hr and then transduced with vAcL at MOI = 50. Luciferase activities at 48 hpt were measured by a luminometer. Percentages of viable cells were measured by incubating with 10% v/v AlamarBlue for 1 hr, and the AlamarBlue intensity in each well was measured with a fluorescence reader. All experiments were performed three times independently, and the data shown are of one representative transduction experiment.
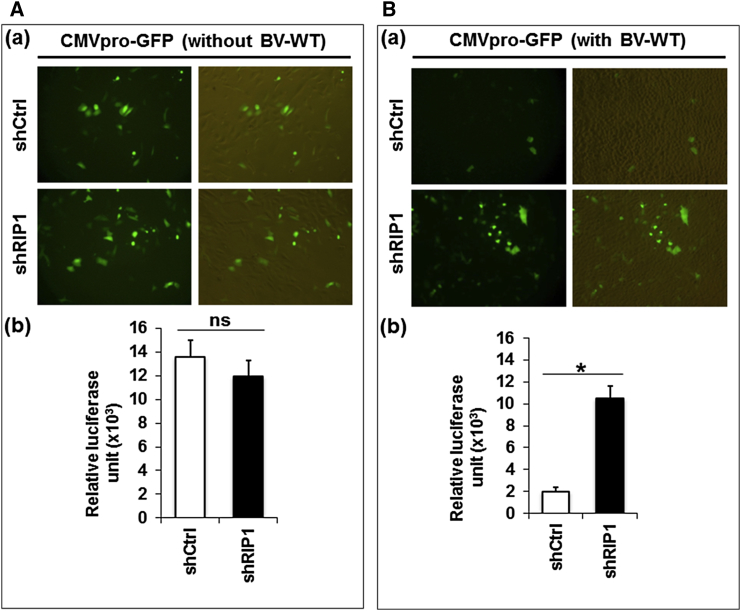
Baculovirus Transduction, but Not Plasmid DNA Transfection, Results in RIP-1-Mediated Inhibition of Marker Gene Expression
Our data suggest that baculovirus-induced immune responses attenuate transgene expression. In line with our findings, a recent report18 also demonstrated that immune responses triggered by baculovirus are responsible for baculovirus transgene inhibition in MEFs. We wondered whether inhibition of baculovirus-mediated foreign gene expression is triggered by baculovirus transduction or due to the introduction of foreign recombinant DNA carried by the infecting baculovirus. To answer this question, we first used recombinant plasmid DNA carrying the EGFP gene under a CMV promoter (pABcE) to transfect A549-shCtrl and A549-shRIP1 cells. 5 hpt, cells were transduced with or without wild-type baculovirus. After incubation for a further 24 hr, EGFP fluorescence was assayed, and we found it to be significantly reduced in A549-shCtrl cells transduced with wild-type baculovirus compared with mock transduction. In contrast, no significant differences in EGFP fluorescence were observed in A549-shRIP1 cells transduced with or without baculovirus, suggesting that RIP1 was not induced by plasmid transfection to restrict foreign gene expression (Figure 8A). We also confirmed this finding with another recombinant plasmid DNA (pABcL) carrying the firefly luciferase gene. After transfection of A549-shCtrl and A549-shRIP1 cells with plasmid pABcL, we observed similar luciferase activity in these two cells without baculovirus transduction. However, luciferase activity was significantly reduced in A549-shCtrl cells, but not in A549-shRIP1 cells, upon baculovirus transduction (Figure 8B). Collectively, these results demonstrate that RIP1 was induced by baculovirus transduction, but not by plasmid transfection, to restrict foreign gene expression.
Figure 8.
Baculovirus Transduction, but Not Plasmid DNA Transfection, Results in RIP-1-Mediated Inhibition of Marker Gene Expression
(A and B) Recombinant plasmids containing the CMV promoter-driven reporter EGFP gene (a) or luciferase (b) were transfected into A549-shRIP1 or A549-shCtrl cells for 5 hr and then cells were transduced without (A) or with (B) wild-type baculovirus (BV) at MOI = 50 for 24 hr. The green fluorescence images (a) and luciferase activities (b) indicate that baculovirus transduction inhibits foreign gene expression. All experiments were performed three times independently, and the data shown are of one representative transduction experiment. Asterisks indicate statistically significant differences compared with the control (*p < 0.05).
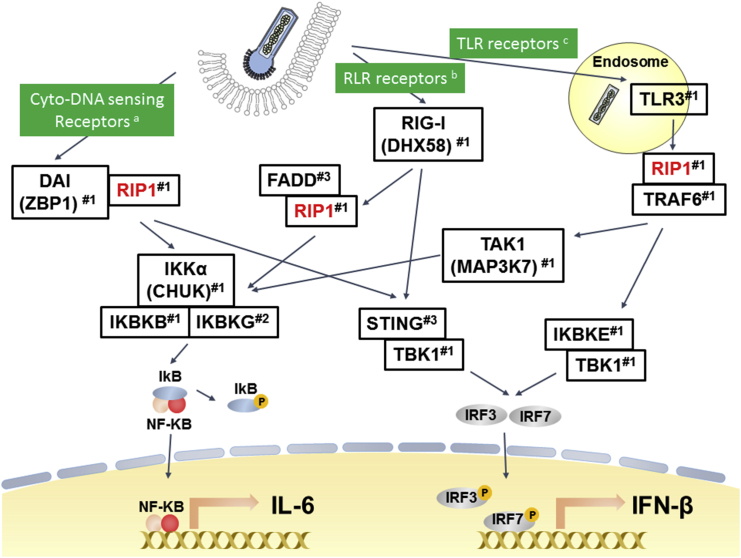
In summary (Figure 9), our findings reveal that knockdown of RIP1, a common mediator upstream of TBK1 in TLR-, RIG-I-, and cytosolic DNA-sensing receptor signaling pathways,26, 29, 30, 33, 34, 35 inhibits the downstream signaling cascade, which then subsequently blocks expression of IFNs and cytokines. Thus, inhibition of RIP1-mediated immune responses leads to enhancement of baculovirus transgene expression.
Figure 9.
Involvement of RIP1 in the Major Antiviral Innate Immune Signaling Pathways Affecting Baculovirus Transgene Expression
According to the KEGG pathway database and several reports,26, 27, 30 RIP1 is upstream of TBK1 and a common mediator of the TLR-, RIG-I-, and cytosolic DNA-sensing receptor signaling pathways. It is not known whether RIP1 is involved in the NOD signaling pathway. Knockdown of NOD signaling pathway genes also increases baculovirus transgene expression (this study). In previous studies, the innate immune response-related cytosolic DNA-sensing receptor,28 RLR,18 and TLR316 were responsive to baculovirus transduction in mammalian cells. RIP1 also plays roles in regulation of the cytosolic DNA-sensing51, RLR,52 and TLR353 pathways. Silencing of RIP1 blocks the activation of downstream mediators (TBK1 and IRF3/7) and affects the expression of interferons and cytokines (IFN-β and IL-6 in this study), which consequently enhances baculovirus transgene expression. Furthermore, silencing of each component (Table 1; #1) in these pathways also enhanced baculoviral transgene expression, demonstrating the importance of these receptors in viral transgene regulation. Moreover, silencing of RIP1 did not have severe effects on cell viability, making it a promising candidate in applications of baculovirus transgene expression. #1, target genes, listed in Table 1; #2, target genes, not listed in Table 1; #3, innate immunity genes without available shRNA clones in the screening; a, see Abe and Matsuura28 and Ma and He51; b, see Ono et al.18 and Barber52; c, see Chen et al.16 and Hiscott53.
Discussion
Baculovirus transgene expression technology has been broadly applied in mammalian cells. Although baculovirus is capable of transducing many cell lines, transgene expression levels are adversely affected in general or even inhibited by host immune responses. To improve baculovirus-mediated transgene expression, comprehensive screening of host immune responses and study of the mechanisms governing these restrictions are crucial, not only to improve gene expression but also to provide novel insights into the interactions between viruses and non-host cells. In this study, we used a comprehensive lentivirus-based shRNA library to search for most (if not all) genes that are specific for innate immune response sensors, adaptors, and early downstream signaling involved in four antiviral pathways. We identified numerous genes from these signaling pathways that negatively regulate baculovirus transgene expression. Among the silenced genes, RIP1, a common mediator in three antiviral pathways (TLR, RIG-I, and cytosolic DNA sensing), was identified as a strong negative regulator of baculovirus transgene expression. Our data indicate that RIP1 knockdown greatly inhibits the baculovirus-induced immune response and, consequently, enhances transgene expression.
It has previously been demonstrated that virus-induced pro-inflammatory cytokines and type I IFNs may inhibit transgene expression while also inducing cytotoxicity in target cells and tissues.36, 37 Recently, it was shown that TLR- and RIG-I-mediated signaling components are activated in response to baculovirus and that the triggered immune response hinders transgene expression.17, 18 Knockdown of antiviral immune signaling mediators is believed to be beneficial for viral growth in a cell type-dependent and/or virus-specific manner. For example, silencing of IRF7 in Madin Darby canine kidney (MDCK) cells contributed to high-yield production of influenza virus,22 but knockdown of IRF7 did not show any beneficial role for baculovirus transgene expression in our study or in MEF cells.18 As expected, direct inhibition of NF-κB did not increase baculovirus transgene expression because NF-κB has been shown to be a major activator of the CMV promoter.38 Furthermore, silencing of the NOD1 and NOD2 receptors and genes, such as ERBB2IP and SUGT1, which all function in the NOD signaling pathway, enhanced baculovirus transgene expression, suggesting that the NOD pathway could also be involved in baculovirus-mediated gene expression. Intriguingly, silencing of IL-1β and TLR3 enhanced baculovirus transgene expression better than RIP1 knockdown, but cell viability was greatly affected under IL-1β- and TLR3-silenced conditions.
Our experiments show that several antiviral immune pathways can be activated in response to baculovirus. Therefore, blocking one particular pathway may not be a good strategy to achieve efficient transgene expression. We found that blocking a common mediator, such as RIP1, which functions in at least three signaling pathways, enhanced transgene expression more effectively without affecting cell viability. RIP1 is an important component of many immune signaling pathways. It regulates mitogen-activated protein kinase (MAPK) and NF-kB activation as well as cell death. The intermediate domain of RIP1 regulates cell fate between necroptosis and RIP1-kinase-dependent apoptosis.35 In the absence of RIP1, TLR3-mediated signals activating NF-κB were abolished, suggesting that TLR3-induced NF-κB activation is dependent on RIP kinases.26
We found that silencing of RIP1 not only enhanced baculovirus transgene expression in A549, U2OS, and HEK293T cells (Figure 5) but also in Vero-E6 and Chinese hamster ovary (CHO) cells (data not shown), suggesting that it could be a potential restriction factor in many cell types. Cells deficient in antiviral immune genes would be expected to exhibit increased baculovirus transgene expression. Therefore, it is puzzling why our baculovirus-transduced HEK239T cells, which are deficient in cGAS/STING proteins,39 showed lower transgene expression levels at lower MOIs (Figure 5) compared with A549 and U2OS cells. Thus, we anticipate that other antiviral factor(s) in addition to RIP1 might potentially restrict baculovirus transgene expression in HEK293T cells.
RIP1 knockdown led to inhibition of phosphorylation levels and nuclear translocation of downstream proteins (IRF3 and NF-κB) in signaling pathways, consequently reducing the expression of effector genes such as IFN-β and IL-6. Our observations are consistent with earlier studies showing the antiviral role of RIP1 in viral infections. In more recent studies, RIP1 has been found to associate with RIG-I in the presence of dsRNA and also play an important role in the type I IFN pathway.29 Murine cytomegalovirus M45 protein inhibits NF-κB and P38 MAPK activation through RIP1 interaction to block pro-inflammatory and innate immune signaling pathways.33, 34 Another report has also shown that the Tax protein of human T cell lymphotropic virus (HTLV) disrupts IRF7 function by binding to RIP1 and TRIF to escape from cellular host defense responses.29
It has been well documented that, upon induction by viral infections, the host immune response inhibits transgene expression.40, 41, 42 Baculovirus DNA may stimulate TLR3, RIG-I, and STING during transduction.43, 44 In our study, RIP1 knockdown may have blocked TLR3 signaling and also influenced the translocation of NF-κB and IRF3/7 into the nucleus (Figure 6), which, in turn, decreased the expression of IL16 and IFN-β and resulted in suppression of the intrinsic innate immune response. Previous studies have also shown that viral DNA can stimulate the type I IFN and NF-κB-dependent pathways.43, 44 Our results further show that, as well as playing a role in this mechanism, RIP1 may also affect RIG-I and STING signaling.
Similar to adeno-associated virus40 and herpes simplex virus (HSV-1)43, 44 viral vectors, our study and an earlier report18 reveal that baculovirus also induces type I IFN and multiple cytokines. IFNs released from infected host cells, in turn, activate IFN receptor (IFNR) and downstream STAT1 transcription factors to induce expression of antiviral genes. It has been demonstrated that type I IFN/STAT1 signaling is critical for transcriptional silencing of HSV viral amplicon-encoded transgenes both in vivo and in vitro.44 Some reports have also shown that type 1 IFN signaling leads to activation of promyelocytic leukemia (PML) gene expression and the formation of PML nuclear bodies (PML-NBs, also known as ND10). PML-NBs have been shown to associate with viral DNA of simian virus 40, HSV, and cytomegalovirus in nuclei and to recruit the histone acetylase (HDAC) complex to silence viral gene expression during natural infection by these viruses.45, 46 In our previous studies, we found that the baculovirus transactivator IE2 associates with PML protein in the NBs of baculovirus-transduced mammalian cells.47, 48. Taken together, these observations lead us to speculate that low amounts of IFN and cytokines in baculovirus-transduced RIP1 knockdown cells may reduce PML-NB formation, resulting in enhancement of baculovirus transgene expression.
In summary, by using a high-throughput methodology to screen high numbers of host immune defense genes, we identified a series of target genes that function as negative regulators against baculovirus transgene expression. We found that RIP1 is one of the most significant factors inhibiting baculovirus transgene expression without resulting in extensive cell death. We also compared the effects of a RIP1-specific inhibitor (Nec-1)49 with a commonly used HDAC inhibitor (NaBu) on the enhancement of baculovirus transgene expression. Interestingly, Nec-1 significantly boosted baculovirus-mediated transgene expression in RIP1-silenced cells and was much more potent in its effects than NaBu. Gene expression of IFNs and cytokines can be downregulated by RIP1 silencing, and we found that RIP1 knockdown enhanced baculovirus transgene expression but did not affect viral entry into mammalian cells. Furthermore, we also discovered an intriguing phenomenon in that baculovirus transduction, but not recombinant DNA plasmid transfection, triggered host responses and, in turn, inhibited foreign gene expression. Overall, our study has not only analyzed virus-host interactions through host immune-responsive genes, but it has also elucidated that the combination of RIP1 silencing plus Nec-1 treatment could be a very useful system to stimulate high-level baculovirus transgene expression in many mammalian cells.
Materials and Methods
Cells and Viruses
Insect cells (Sf21) were grown at 26°C in TC100 insect medium containing 10% (v/v) fetal bovine serum (FBS) (Gibco). A549 cells were grown in Ham’s F12K medium (Gibco), U2OS cells were grown in McCoy’s medium (Gibco), and 293T cells were grow in DMEM (Gibco). Each medium was supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% (v/v) FBS at 37°C in an air/CO2 (19:1) incubator. vAcE, vAcL, vAc-mC, and vAceE are recombinant baculoviruses expressing GFP, luciferase, mCherry, and EGFP, respectively, by the CMV, SV40, and CMV-enhancer/EF-1α promoters.23, 25 Briefly, the EGFP coding region was amplified by PCR from pEGFP-C1 and inserted into pTriEx-3 (Novagen) to generate pAcE. The luciferase coding region was amplified by PCR from pTRE-luc and inserted into pTriEx-3 to generate pAcL. Both EGFP and luciferase were driven by the CMV immediate-early enhancer and promoters. PCR-amplified mCherry was inserted into pTriEx-3 under the control of the SV40 promoter to generate pAcmC. The pAcE, pAcL, and pAcmC plasmids were co-transfected with a linearized viral DNA of AcMNPV (vAcRP23.Laz, Pharmingen) into Sf21 cells using Cellfectin (Life Technologies). The resulting recombinant baculoviruses (vAcE, vAcL, and vAcmC) were isolated through endpoint dilutions.
High-Throughput shRNA Screening
Human alveolar basal epithelial A549 cells were plated in 96-well plates (5 × 103 cells/well) 24 hr prior to transduction. Lentiviruses capable of expressing shRNA against individual human gene transcripts were provided by the National RNAi Core Facility (Institute of Molecular Biology, Academia Sinica; http://rnai.genmed.sinica.edu.tw/index/). Cells were transduced with lentiviruses at an MOI of 10 in the presence of Polybrene (hexadimethrine bromide, 8 μg/mL) by spin transduction (1,170 × g, 15 min, 37°C) and incubated at 37°C for 24 hr. The cells were incubated with fresh F12K medium containing puromycin (2 μg/mL). GFP fluorescence activity and cell viability were measured 48 hr after baculovirus transduction. Cell viability was measured using an AlamarBlue assay (AlamarBlue cell viability assay, Life Technologies). The shRNA target genes selected from the KEGG pathway database (http://www.genome.jp/kegg/pathway.html) are related to human antiviral response pathways, including TLRs, RLRs, NLRs, and cytosolic DNA-sensing pathways. Every selected gene was probed with five lentiviruses carrying shRNA targeting to different target sites of the individual expressed transcript.
Generation of Stably Gene-Silenced A549 Cell Lines
A549 cells were transduced with vesicular stomatitis virus G protein (VSV-G)-pseudotyped shRNA lentivirus (self-inactivating viral vector [SIN]) according to the manufacturer’s protocol (Institute of Molecular Biology, Academia Sinica; http://rnai.genmed.sinica.edu.tw/webContent/web/protocols/wicket:pageMapName/wicket-0). Forty-eight hours after addition of virus, transduced cells were selected for stable clones by adding 2 μg/mL puromycin to growth medium for 2 weeks, and then surviving cells were collected for further applications.
RNA Extraction and qRT-PCR Analysis
Total RNA and total cellular DNA were extracted at various time points with TRIzol reagent (Invitrogen) and a DNeasy blood and tissue kit. Total RNA was extracted from A549 cells using RNAmini (QIAGEN) and reverse-transcribed using SuperScript III (Life Technologies). The quantity and quality of RNA were checked using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). The amounts of each transcript were measured by qPCR using specific primers (Table 2).
Table 2.
List of Primers Used for qRT-PCR and qPCR
| Primer Name | Sequence (5′–3′) |
|---|---|
| EGFP-qFw | TATATCATGGCCGACAAGCA |
| EGFP-qRv | TGTTCTGCTGGTAGTGGTCG |
| GAPDH-qFw | CCCCACACACATGCACTTACC |
| GAPDH-qRv | CCTAGTCCCAGGGCTTTGATT |
| GP41-Fw | CGTAGTGGTAGTAATCGCCGC |
| GP41-Rv | AGTCGAGTCGCGTCGCTTT |
| IFNb-qFw | TGCCATTCAAGTGATGCTCCA |
| IFNb-qRv | TCCAGAAGCTCTGTCTGCTGATGTA |
| IL6-qFW | GTCAGGGGTGGTTATTGCAT |
| IL6-qRv | AGTGAGGAACAAGCCAGAGC |
| RIP1-qFw | GGCATTGAAGAAAAATTTAGGC |
| RIP1-qRv | TCACAACTGCATTTTCGTTTG |
Genomic DNA Extraction and qPCR Analysis
A549 cells were incubated with vAcE baculovirus for 2 hr and then washed with PBS three times and PBS with 0.1% trypsin once to remove free virus particles from cells. For viral entry efficiency, the total cellular DNA was extracted by a High Pure PCR Template Preparation Kit (Roche), and viral DNA copy number was determined by qPCR using gp41-specific primers50 (Table 2).
Western Blotting
Stable RIP1 knockdown clones of A549 cells (4 × 105) in a 60-mm dish were transduced with vAcE baculovirus at an MOI of 50 for various times as indicated. Cells were washed with ice-cold PBS and suspended in 0.2 mL lysis buffer (89901, Thermo Fisher Scientific) containing a protease inhibitor cocktail (B14001, Biotool). Cell lysates were sonicated (XL-2020, Misonix) and quantified by Bradford assay (23236, Thermo Fisher Scientific). Proteins (25 μg) were subjected to SDS-PAGE and transferred to nitrocellulose (NC) membranes (NBA085C001EA, PerkinElmer). After washing, the membranes were blocked with 5% skim milk in Tris-Tween buffer saline (TTBS) and incubated with primary antibodies (1:1,000 dilution) at 4°C overnight. The primary antibodies were specific for RIP1 (610459, BD Transduction Laboratories), phospho (Ser536) in nuclear factor kappa-light-chain-enhancer of activated B cells (pNF-κB; Ser536) (3033, Cell Signaling Technology), IRF3 (ab50772, Abcam), IRF7 (ab62505, Abcam), or GAPDH (GTX100118, GeneTex). After washing, the membranes were incubated with goat anti-mouse (115-035-003, Jackson ImmunoResearch) (for RIP1 and IRF3) or goat anti-rabbit (111-035-003, Jackson ImmunoResearch) (for pNF-κB and IRF7) secondary antibodies (1:5,000 dilution) conjugated with peroxidase at room temperature for 1 hr. The immune complexes were visualized with a Western Bright enhanced chemiluminescence (ECL) kit (K12045-D50, Advansta) and detected by X-ray films.
Immunofluorescence Assay
Eight-well Lab-Tek II chamber slides (Nunc) were seeded with RIP1 knockdown stable clones of A549 cells (4 × 104/well), and the cells were transduced with vAcE baculovirus at an MOI of 50 for various times as indicated. 24 hpt, the cells were washed three times with Dulbecco’s PBS (DPBS; Invitrogen) and fixed with 4% paraformaldehyde for 10 min. Cells were washed three times with DPBS buffer and then permeabilized by incubation with −20°C 100% acetone for 10 min. After three 5-min washes in DPBS, the cells on the slides were blocked with blocking buffer (10% FBS in DPBS) for 1 hr and then incubated with the appropriate primary antibodies (1:150 dilution in blocking buffer) overnight at 4°C. Primary antibodies were pNF-κB (Ser536) (3033, Cell Signaling Technology), IRF3 (ab50772, Abcam), or IRF7 (ab62505, Abcam). Cells were then washed three times with washing buffer (0.1% Tween 20 in DPBS, DPBS-T) and incubated for 1 hr in the dark with 1:200 dilutions of Alexa Fluor 405 goat anti-mouse immunoglobulin G (IgG) for IRF3 or Alexa Fluor 555 goat anti-rabbit IgG for pNF-κB and IRF3 (Invitrogen). Cells were then stained with Hoechst 33342 (Invitrogen) for 15 min before being washed three times in washing buffer and sealed with aqueous mounting medium (HIS002B, Serotec). Fluorescent images were visualized with a Zeiss laser confocal microscope (LSM510).
Cell Viability Assay
To determine relative cell metabolic activity, 10% v/v AlamarBlue was added to the cells and incubated for 1 hr. Reductions in AlamarBlue concentrations were measured with a fluorescence reader (EnSpire, PerkinElmer) at an excitation wavelength of 560 nm and an emission wavelength of 590 nm.
Plasmid Transfection
Transfections were performed using Lipofectamine 2000 reagent (Invitrogen). 1 × 104 A549 cells were plated onto individual wells of a 24-well plate. A549 cells were transfected with 1000 ng of the reporter plasmid with the gfp gene driven by the CMV promoter. The transfection reagent was replaced by F12K medium after 5 hr.
Luciferase Assay
Cells were washed twice with PBS and lysed by 100 μL cell culture lysis reagent (CCLR; 100 mM potassium phosphate [pH 7.8], 0.2% Triton X-100, and 2 mM β-mercaptoethanol). Cell lysates were centrifuged at 3,500 rpm for 30 min at 4°C, and 20 μL supernatant was mixed with 180 μL luciferase assay reagent (LAR; 25 mM phosphate buffer [pH 7.8], 4 mM EGTA, 15 mM MgSO4, 1 mM dithiothreitol, and 0.2 mM ATP) in a black 96-well microplate. The samples in each well were injected with 0.2 mM luciferin (Promega). Luciferase activity was measured by a luminometer (EnSpire, PerkinElmer) for each well. Afterward, the results were plotted as average luciferase activities from triplicate assays of three independent experiments.
Author Contributions
C.-H.W. and Y.-C.C. conceived and designed the experiments. C.-H.W., N.G.N., and L.-L.L. performed the experiments. C.-H.W., N.G.N., L.-L.L., S.-C.W., and Y.-C.C. analyzed the data and contributed reagents, materials, and analysis tools. C.-H.W., N.G.N., and Y.-C.C. wrote the paper.
Acknowledgments
We would like to thank the Academia Sinica RNAi Core Facility for shRNA-lentivirus experiments and the Academia Sinica IMB Imaging Core for help with confocal imaging. We would like to thank Dr. John O’Brien of the Academia Sinica IMB Editing Core for editing this manuscript. This research was funded by grants MOST 106-2321-B-001-012, MOST 106-3114-B-033-001, and MOST105-2321-B-001-040 from the Ministry of Science and Technology and NHRI-106A1-MRCO-0217171 and 022361 from Academia Sinica.
Footnotes
Supplemental Information includes three figures and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.07.002.
Supplemental Information
References
- 1.Chen C.Y., Lin C.Y., Chen G.Y., Hu Y.C. Baculovirus as a gene delivery vector: recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol. Adv. 2011;29:618–631. doi: 10.1016/j.biotechadv.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merrihew R.V., Clay W.C., Condreay J.P., Witherspoon S.M., Dallas W.S., Kost T.A. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J. Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y.C., Yao K., Wu T.Y. Baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev. Vaccines. 2008;7:363–371. doi: 10.1586/14760584.7.3.363. [DOI] [PubMed] [Google Scholar]
- 4.Cheshenko N., Krougliak N., Eisensmith R.C., Krougliak V.A. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846–854. doi: 10.1038/sj.gt.3301459. [DOI] [PubMed] [Google Scholar]
- 5.Liu F., Wu X., Li L., Liu Z., Wang Z. Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Protein Expr. Purif. 2013;90:104–116. doi: 10.1016/j.pep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy P. Genetically engineered particulate virus-like structures and their use as vaccine delivery systems. Intervirology. 1996;39:62–71. doi: 10.1159/000150476. [DOI] [PubMed] [Google Scholar]
- 7.Lykhova A.A., Kudryavets Y.I., Strokovska L.I., Bezdenezhnykh N.A., Semesiuk N.I., Adamenko I.N., Anopriyenko O.V., Vorontsova A.L. Suppression of proliferation, tumorigenicity and metastasis of lung cancer cells after their transduction by interferon-beta gene in baculovirus vector. Cytokine. 2015;71:318–326. doi: 10.1016/j.cyto.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Lin S.Y., Chung Y.C., Hu Y.C. Update on baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev. Vaccines. 2014;13:1501–1521. doi: 10.1586/14760584.2014.951637. [DOI] [PubMed] [Google Scholar]
- 9.Fornwald J.A., Lu Q., Wang D., Ames R.S. Gene expression in mammalian cells using BacMam, a modified baculovirus system. Methods Mol. Biol. 2007;388:95–114. doi: 10.1007/978-1-59745-457-5_5. [DOI] [PubMed] [Google Scholar]
- 10.Boyce F.M., Bucher N.L. Baculovirus-mediated gene transfer into mammalian cells. Proc. Natl. Acad. Sci. USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C.Y., Wu H.H., Chen C.P., Chern S.R., Hwang S.M., Huang S.F., Lo W.H., Chen G.Y., Hu Y.C. Biosafety assessment of human mesenchymal stem cells engineered by hybrid baculovirus vectors. Mol. Pharm. 2011;8:1505–1514. doi: 10.1021/mp100368d. [DOI] [PubMed] [Google Scholar]
- 12.Chuang C.K., Wong T.H., Hwang S.M., Chang Y.H., Chen G.Y., Chiu Y.C., Huang S.F., Hu Y.C. Baculovirus transduction of mesenchymal stem cells: in vitro responses and in vivo immune responses after cell transplantation. Mol. Ther. 2009;17:889–896. doi: 10.1038/mt.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe T., Kaname Y., Wen X., Tani H., Moriishi K., Uematsu S., Takeuchi O., Ishii K.J., Kawai T., Akira S., Matsuura Y. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J. Virol. 2009;83:7629–7640. doi: 10.1128/JVI.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan Y.K., Gack M.U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 2016;14:360–373. doi: 10.1038/nrmicro.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Festjens N., Vanden Berghe T., Cornelis S., Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 16.Chen G.Y., Shiah H.C., Su H.J., Chen C.Y., Chuang Y.J., Lo W.H., Huang J.L., Chuang C.K., Hwang S.M., Hu Y.C. Baculovirus transduction of mesenchymal stem cells triggers the toll-like receptor 3 pathway. J. Virol. 2009;83:10548–10556. doi: 10.1128/JVI.01250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H.P., Matsuura Y., Chen H.C., Chen Y.L., Chuang C.K., Abe T., Hwang S.M., Shiah H.C., Hu Y.C. Baculovirus transduction of chondrocytes elicits interferon-alpha/beta and suppresses transgene expression. J. Gene Med. 2009;11:302–312. doi: 10.1002/jgm.1299. [DOI] [PubMed] [Google Scholar]
- 18.Ono C., Ninomiya A., Yamamoto S., Abe T., Wen X., Fukuhara T., Sasai M., Yamamoto M., Saitoh T., Satoh T. Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells. J. Virol. 2014;88:2157–2167. doi: 10.1128/JVI.03055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J., Wang D., Gao R., Zhao B., Song J., Qi X., Zhang Y., Shi Y., Yang L., Zhu W. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499:500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 20.Han X., Li Z., Chen H., Wang H., Mei L., Wu S., Zhang T., Liu B., Lin X. Influenza virus A/Beijing/501/2009(H1N1) NS1 interacts with β-tubulin and induces disruption of the microtubule network and apoptosis on A549 cells. PLoS ONE. 2012;7:e48340. doi: 10.1371/journal.pone.0048340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronni T., Melén K., Malygin A., Julkunen I. Control of IFN-inducible MxA gene expression in human cells. J. Immunol. 1993;150:1715–1726. [PubMed] [Google Scholar]
- 22.Hamamoto I., Takaku H., Tashiro M., Yamamoto N. High yield production of influenza virus in Madin Darby canine kidney (MDCK) cells with stable knockdown of IRF7. PLoS ONE. 2013;8:e59892. doi: 10.1371/journal.pone.0059892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tung H., Wei S.C., Lo H.R., Chao Y.C. Baculovirus IE2 Stimulates the Expression of Heat Shock Proteins in Insect and Mammalian Cells to Facilitate Its Proper Functioning. PLoS ONE. 2016;11:e0148578. doi: 10.1371/journal.pone.0148578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann C., Sandig V., Jennings G., Rudolph M., Schlag P., Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc. Natl. Acad. Sci. USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H.P., Ho Y.C., Hwang S.M., Sung L.Y., Shen H.C., Liu H.J., Hu Y.C. Variation of baculovirus-harbored transgene transcription among mesenchymal stem cell-derived progenitors leads to varied expression. Biotechnol. Bioeng. 2007;97:649–655. doi: 10.1002/bit.21261. [DOI] [PubMed] [Google Scholar]
- 26.Meylan E., Burns K., Hofmann K., Blancheteau V., Martinon F., Kelliher M., Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 27.Cusson-Hermance N., Khurana S., Lee T.H., Fitzgerald K.A., Kelliher M.A. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-kappaB activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 28.Abe T., Matsuura Y. Host innate immune responses induced by baculovirus in mammals. Curr. Gene Ther. 2010;10:226–231. doi: 10.2174/156652310791321279. [DOI] [PubMed] [Google Scholar]
- 29.Hyun J., Ramos J.C., Toomey N., Balachandran S., Lavorgna A., Harhaj E., Barber G.N. Oncogenic human T-cell lymphotropic virus type 1 tax suppression of primary innate immune signaling pathways. J. Virol. 2015;89:4880–4893. doi: 10.1128/JVI.02493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebsamen M., Heinz L.X., Meylan E., Michallet M.C., Schroder K., Hofmann K., Vazquez J., Benedict C.A., Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condreay J.P., Witherspoon S.M., Clay W.C., Kost T.A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Upton J.W., Kaiser W.J., Mocarski E.S. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mack C., Sickmann A., Lembo D., Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. USA. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Xia F., Hermance N., Mabb A., Simonson S., Morrissey S., Gandhi P., Munson M., Miyamoto S., Kelliher M.A. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol. Cell. Biol. 2011;31:2774–2786. doi: 10.1128/MCB.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung R.S., Qin L., Bromberg J.S. TNFalpha and IFNgamma induced by innate anti-adenoviral immune responses inhibit adenovirus-mediated transgene expression. Mol. Ther. 2001;3:757–767. doi: 10.1006/mthe.2001.0318. [DOI] [PubMed] [Google Scholar]
- 37.Olschowka J.A., Bowers W.J., Hurley S.D., Mastrangelo M.A., Federoff H.J. Helper-free HSV-1 amplicons elicit a markedly less robust innate immune response in the CNS. Mol. Ther. 2003;7:218–227. doi: 10.1016/s1525-0016(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 38.DeMeritt I.B., Milford L.E., Yurochko A.D. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J. Virol. 2004;78:4498–4507. doi: 10.1128/JVI.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M., Bertin T.K., Rogers G.L., Cela R.G., Zolotukhin I., Palmer D.J., Ng P., Herzog R.W., Lee B. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol. Ther. 2013;21:796–805. doi: 10.1038/mt.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kollarik M., Carr M.J., Ru F., Ring C.J., Hart V.J., Murdock P., Myers A.C., Muroi Y., Undem B.J. Transgene expression and effective gene silencing in vagal afferent neurons in vivo using recombinant adeno-associated virus vectors. J. Physiol. 2010;588:4303–4315. doi: 10.1113/jphysiol.2010.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki M., Cerullo V., Bertin T.K., Cela R., Clarke C., Guenther M., Brunetti-Pierri N., Lee B. MyD88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus. Hum. Gene Ther. 2010;21:325–336. doi: 10.1089/hum.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapgier A., Wynn R.F., Jouanguy E., Filipe-Santos O., Zhang S., Feinberg J., Hawkins K., Casanova J.L., Arkwright P.D. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J. Immunol. 2006;176:5078–5083. doi: 10.4049/jimmunol.176.8.5078. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki M., Chiocca E.A., Saeki Y. Early STAT1 activation after systemic delivery of HSV amplicon vectors suppresses transcription of the vector-encoded transgene. Mol. Ther. 2007;15:2017–2026. doi: 10.1038/sj.mt.6300273. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y., Song J., Lu J., Chen X. The histone deacetylase inhibitor sodium butyrate inhibits baculovirus-mediated transgene expression in Sf9 cells. J. Biotechnol. 2007;131:180–187. doi: 10.1016/j.jbiotec.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Chiang Y.W., Wu J.C., Wang K.C., Lai C.W., Chung Y.C., Hu Y.C. Efficient expression of histidine-tagged large hepatitis delta antigen in baculovirus-transduced baby hamster kidney cells. World J. Gastroenterol. 2006;12:1551–1557. doi: 10.3748/wjg.v12.i10.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C.Y., Chen H.Z., Chao Y.C. Maximizing baculovirus-mediated foreign proteins expression in mammalian cells. Curr. Gene Ther. 2010;10:232–241. doi: 10.2174/156652310791321215. [DOI] [PubMed] [Google Scholar]
- 48.Liu C.Y., Wang C.H., Hsiao W.K., Lo H.R., Wu C.P., Chao Y.C. RING and coiled-coil domains of baculovirus IE2 are critical in strong activation of the cytomegalovirus major immediate-early promoter in mammalian cells. J. Virol. 2009;83:3604–3616. doi: 10.1128/JVI.01778-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jie H., He Y., Huang X., Zhou Q., Han Y., Li X., Bai Y., Sun E. Necrostatin-1 enhances the resolution of inflammation by specifically inducing neutrophil apoptosis. Oncotarget. 2016;7:19367–19381. doi: 10.18632/oncotarget.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanarsdall A.L., Okano K., Rohrmann G.F. Characterization of the replication of a baculovirus mutant lacking the DNA polymerase gene. Virology. 2005;331:175–180. doi: 10.1016/j.virol.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y., He B. Recognition of herpes simplex viruses: toll-like receptors and beyond. J. Mol. Biol. 2014;426:1133–1147. doi: 10.1016/j.jmb.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber G.N. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr. Opin. Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiscott J. Another detour on the Toll road to the interferon antiviral response. Nat. Struct. Mol. Biol. 2004;11:1028–1030. doi: 10.1038/nsmb1104-1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.