Abstract
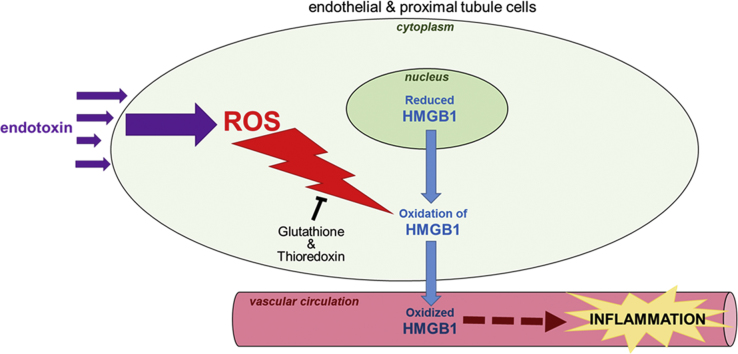
During sepsis, the alarmin HMGB1 is released from tissues and promotes systemic inflammation that results in multi-organ damage, with the kidney particularly susceptible to injury. The severity of inflammation and pro-damage signaling mediated by HMGB1 appears to be dependent on the alarmin's redox state. Therefore, we examined HMGB1 redox in kidney cells during sepsis. Using intravital microscopy, CellROX labeling of kidneys in live mice indicated increased ROS generation in the kidney perivascular endothelium and tubules during lipopolysaccharide (LPS)-induced sepsis. Subsequent CellROX and MitoSOX labeling of LPS-stressed endothelial and kidney proximal tubule cells demonstrated increased ROS generation in these cells as sepsis worsens. Consequently, HMGB1 oxidation increased in the cytoplasm of kidney cells during its translocation from the nucleus to the circulation, with the degree of oxidation dependent on the severity of sepsis, as measured in in vivo mouse samples using a thiol assay and mass spectrometry (LC-MS/MS). The greater the oxidation of HMGB1, the greater the ability of the alarmin to stimulate pro-inflammatory cyto-/chemokine release (measured by Luminex Multiplex) and alter mitochondrial ATP generation (Luminescent ATP Detection Assay). Administration of glutathione and thioredoxin inhibitors to cell cultures enhanced HMGB1 oxidation during sepsis in endothelial and proximal tubule cells, respectively. In conclusion, as sepsis worsens, ROS generation and HMGB1 oxidation increases in kidney cells, which enhances HMGB1's pro-inflammatory signaling. Conversely, the glutathione and thioredoxin systems work to maintain the protein in its reduced state.
Abbreviations: CBP, CREB-binding protein; DAMPs, damage-associated molecular patterns; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HK2, Human Kidney Proximal Tubule cells - type 2; HMGB1, high mobility group box protein 1; HUVEC, human umbilical vein endothelial cells; IL-1β, interleukin 1 beta; IL-17, interleukin 17; LPS, lipopolysaccharide; MIP-1α, macrophage inflammatory protein type one alpha; MIP-1β, macrophage inflammatory protein type one beta; MIP-2, macrophage inflammatory protein type two; NADPH, nicotinamide adenine dinucleotide phosphate; PAMPs, pathogen-associated molecular pattern; ROS, reactive oxygen species; TNFα, tumor necrosis factor alpha
Keywords: Redox, Oxidative stress, HMGB1, Sepsis, Cytokines
Graphical abstract
Highlights
-
•
Endotoxins (LPS) increase cellular oxidative stress during sepsis.
-
•
During its translocation, HMGB1 gets oxidized in the cytoplasm.
-
•
Thioredoxin and glutathione keep HMGB1 in a reduced redox state during sepsis.
-
•
HMGB1 oxidation enhances its stimulation of inflammatory cyto-/chemokine release.
1. Introduction
Sepsis is a major cause of death in hospitalized patients [1]. During sepsis, enhanced oxidative stress and stimulation of pro-inflammatory cyto-/chemokines promote tissue damage, which leads to kidney and other organ failure [2], [3], [4]. During the early onset of endotoxemia, pathogen microbial elements referred to as pathogen-associated molecule patterns (PAMPs) (including lipopolysaccharide - LPS) activate immune, endothelial and epithelial cells by stimulating toll-like receptors (TLRs) [5], [6], [7]. Activation of TLRs induces the rapid release of early pro-damage signals, including damage-associated molecular patterns (DAMPs) that are referred to as alarmins, into the circulation [8], [9]. Once released into the circulation, these alarmin molecules stimulate the release of pro-inflammatory factors and widen damage systemically.
A major alarmin that is released from tissues within the first 24 h of endotoxemia is High Mobility Group Box 1 protein (HMGB1). HMGB1 is typically found in the nucleus of a variety cells (including immune, endothelial and epithelial cells) where it is bound to DNA. After activation of TLR on these cells [8], acetylation of HMGB1 triggers its translocation from the nucleus to the circulation [10]. Once in the circulation, HMGB1 interacts with a variety of target cell receptors including RAGE, TLR2, and TLR4 [11], [12], [13], [14], [15], [16], [17] and stimulates release of pro-inflammatory cyto-/chemokines [14], [15], [17], [18], [19], [20]. As sepsis worsens, the pro-damage effects of HMGB1 worsens causing progressive tissue and organ damage [21].
During sepsis, excessive ROS is generated by various cells and multiple sources, including enhanced activity of NAPDH oxidase and dehydrogenase/xanthine oxidase enzymes [22], [23], [24], [25], [26], [27], [28], [29], altered mitochondrial bioenergetics [30], fatty acid oxidation (peroxisomal metabolism) and impaired peroxisomal catalase activity [31]. Enhanced levels of ROS (superoxide, hydrogen peroxide, hydroxyl, etc.) promote the lapse into septic shock by promoting a multitude of redox reactions throughout tissues that subsequently alters protein and enzymatic function, modulates changes in microcirculatory hemodynamics, and stimulates vascular structural changes. HMGB1 is a target of such redox reaction molecules [32], [33]. HMGB1 contains two redox sensitive cysteine moieties (at positions C23 and C45) in its 215 amino acid structure whose redox states greatly impact HMGB1 function [34]. When these cysteine residues are in reduced thiol form, the protein's pro-damage signaling is minimal [35], [36]. However, as oxidative stress worsens and these thiols are oxidized to form a disulfide bond, HMGB1's function shifts to promote severe inflammation.
In the present study, we examined the redox state of HMGB1 during sepsis, the cellular compartment where HMGB1 redox is effected, the cellular antioxidants that keep HMGB1 reduced, and the effect that HMGB1 redox has on the proteins ability to stimulate cyto-/chemokine release. Since the kidneys are particularly vulnerable to sepsis, we examined these phenomena in kidneys cells.
2. Materials and methods
2.1. Animals
The animal study protocol was in accordance with the NIH's Guide for the Care and Use of Laboratory Animals, the American Veterinary Medical Association (AVMA) guidelines and approved by the Institutional Animal Care and Use Committee. C57BL male mice aged >16 weeks were used in all experiments. Details of animal usage are described in the Supplementary methods online.
2.2. Cell cultures
The endothelial cell line Ea.hy926, which are human umbilical vein endothelial cells (HUVEC), and human kidney proximal tubule (HK-2) cells (ATCC, Chicago, IL) were used in experiments. Details of cell cultures are described in the Supplementary methods online.
2.3. Experimental protocol and analysis
Details of experimental protocols and analysis (including LPS treatment, ROS measurement in the circulation, nuclear/cytoplasmic fractionation, HMGB1 isolation, thiol measurement, LC-MS/MS analysis, glutathione/thioredoxin analysis, cyto-/chemokine analysis, ATP measurement and statistical analysis) are described in the Supplementary methods online.
3. Results and discussion
3.1. ROS generation
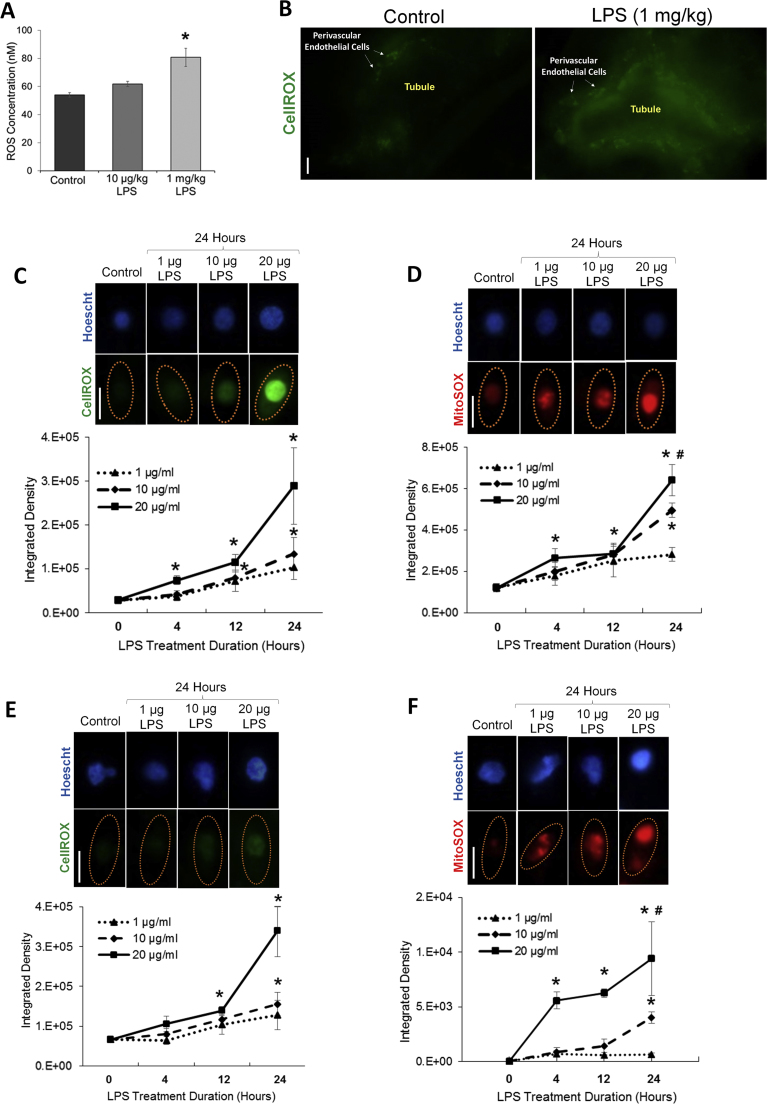
We first examined total ROS levels in the circulation of animals 24 h after LPS-induced sepsis. In these experiments, two different doses of LPS were administered intraperitoneally to separate sets of animals, a low LPS dose (10 µg/kg) and a high LPS (1 mg/kg) dose. The low dose increased ROS in the circulation only slightly in animals, although the increase was not statistically significantly (Fig. 1a). In contrast, the high LPS dose resulted in a significant increase in circulating ROS within 24 h after LPS delivery.
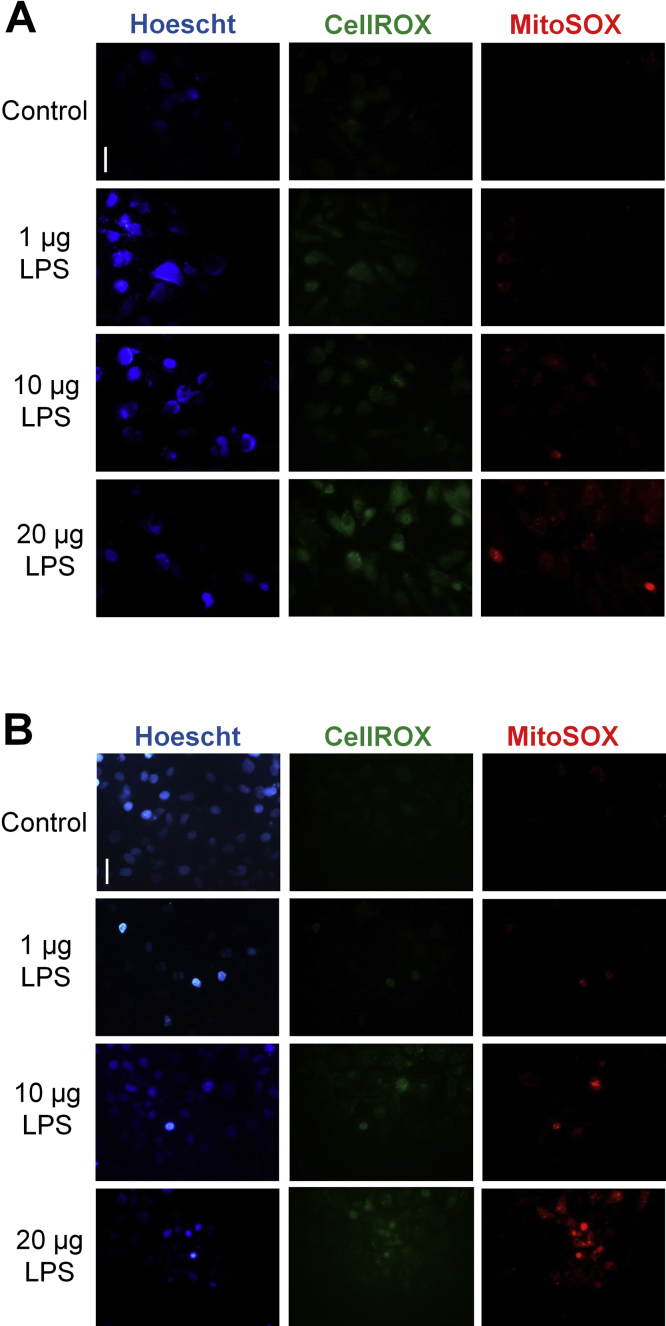
Fig. 1.
Oxidative stress is increased in the circulation and in the kidneys during sepsis. (A) Levels of ROS in the circulation of mice 24 h after administration of low (10 µg/kg) or high (1 mg/kg) LPS dose. (B) ROS generation (as indicated by the green fluorescence of CellROX staining) in exposed kidneys of live animals 24 h after LPS (1 mg/kg) delivery. CellROX staining of kidneys was visualized by real-time intravital microscopy. The perivasculature is labeled surrounding the indicated tubules. (C) Levels of intracellular ROS in HUVEC over a 24-h period during treatment with three different doses of LPS (1 µg/ml, 10 µg/ml, and 20 µg/ml). Images in panel C demonstrate an increase of intracellular ROS at 24 h (as indicated by the increase in green fluorescence of CellROX; nuclei are labeled blue with Hoescht; cells are outlined in yellow in images). The line graph displays subsequent ROS quantification. (D) Levels of mitochondrial generated ROS in HUVEC over a 24-h period during treatment with three different doses of LPS (1 µg/ml, 10 µg/ml, and 20 µg/ml). Images in panel D demonstrate an increase of mitochondrial ROS at 24 h (as indicated by the increase in red fluorescence of MitoSOX; nuclei are labeled blue with Hoescht; cells are outlined in yellow in images). Subsequent quantification is given in the line graph. (E) Levels of intracellular ROS in HK-2 cells over a 24-h period during treatment with three different doses of LPS (1 µg/ml, 10 µg/ml, and 20 µg/ml). Images in panel E demonstrate an increase of intracellular ROS at 24 h (as indicated by the increase in green fluorescence of CellROX; nuclei are labeled blue with Hoescht; cells are outlined in yellow in images). The line graph displays subsequent ROS quantification. (F) Levels of mitochondrial generated ROS in HK-2 cells over a 24-h period during treatment with three different doses of LPS (1 µg/ml, 10 µg/ml, and 20 µg/ml). Images in panel F demonstrate an increase of mitochondrial ROS at 24 h (as indicated by the increase in red fluorescence of MitoSOX; nuclei are labeled blue with Hoescht; cells are outlined in yellow in images). Subsequent quantification is given in the line graph. Magnification = 600x. Line bar represents 25 µm. *p ≤ 0.05 vs. control; #p ≤ 0.05 vs. 24-h (1 µg/ml LPS). n = 5–9 different cell cultures per experimental group for graphs C-F (see Supplemental Methods online for details concerning the amount of cells analyzed in each cell culture sample). Slight corrections in brightness and contrast were made to images in Fig. 1 only for clarity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Since the kidney is especially susceptible to oxidative damage, we examined ROS generation in kidneys during sepsis. To localize kidney ROS generation, we intrarenally perfused the membrane permeable ROS labeling dye CellROX into septic mice 24 h after LPS (1 mg/kg) delivery and imaged exposed kidneys in live animals using real-time intravital microscopy. CellROX reacts with oxidative molecules, such as superoxide and hydroxyl, which are generated from NADPH oxidase, xanthine oxidase and mitochondrial respiration. CellROX staining demonstrated enhanced ROS generation in the kidney perivascular endothelium and tubules (Fig. 1b) during sepsis.
Due to the potential of kidney endothelial and tubule cells to produce ROS, we further examined ROS generation in endothelial (HUVEC) and HK-2 cells during sepsis. We initially examined the time course of ROS generation in these cells. In experiments, cultured cells were separately treated with three different doses of LPS; a low (1 µg/ml), medium (10 µg/ml) or high (20 µg/ml) LPS dose to mimic mild-to-severe sepsis, respectively. All doses increased ROS in both endothelial and proximal tubule cells, although the highest dose promoted a drastically greater generation of ROS (Fig. 1c,e; Supplemental Fig. 1). In these experiments, cellular ROS was again detected in live cells by CellROX. Since mitochondria are major producers of ROS, particularly superoxide, we also measured mitochondrial specific ROS generation using the dye MitoSOX. Similar to CellROX, MitoSOX is also membrane permeable, with both dyes fluorescing once they are oxidized and bound to cellular DNA. Comparable to experiments with CellROX, mitochondrial ROS generation was also significantly elevated within 24 h of treatment with LPS in endothelial and proximal tubule cells, with the higher LPS doses prompting greater mitochondrial ROS generation in both cell types (Fig. 1d,f; Supplemental Fig. 1).
3.2. HMGB1 redox
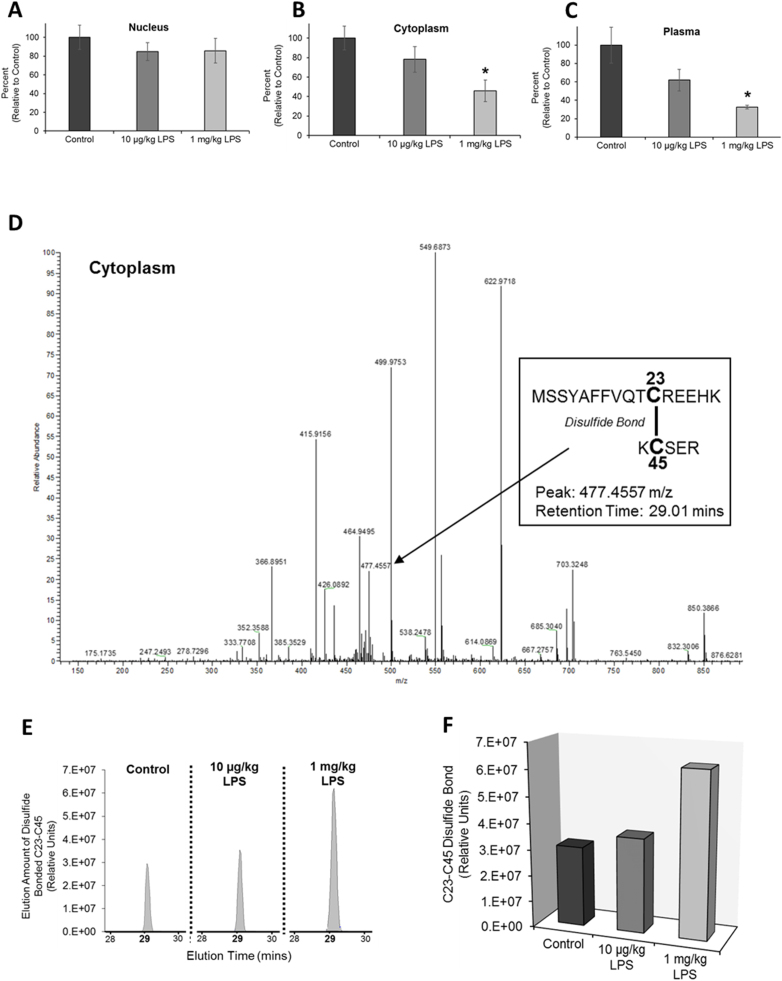
We have previously shown that during LPS-induced stress, HMGB1 undergoes nuclear-to-cytoplasmic translocation in kidney cells and is then released into the circulation [37]. Therefore, we next examined the redox state, and more specifically the thiol content of HMGB1, during the protein's LPS-induced translocation and release. In these experiments, HMGB1 thiol content was measured in HMGB1 isolated from nuclear and cytoplasmic kidney fractionates and from the plasma after LPS administration. These experiments used whole kidney fractionates and did not distinguish between specific cell type differences in HMGB1 redox (i.e. endothelial versus epithelial cells). When animals were treated with a low dose of LPS (10 µg/kg) to mimic mild sepsis, HMGB1 demonstrated increased oxidation in the cytoplasm and circulation, as indicated by decreased thiol content. However, the increase was statistically insignificant (Fig. 2a-c). Conversely, HMGB1 demonstrated significant oxidation in the cytoplasmic compartment and remained oxidized in the circulation during high LPS dose treatment (Fig. 2a-c). To confirm oxidation of HMGB1 in the cytoplasm of kidney cells, we also subjected these samples to mass spectrometry analysis. Liquid chromatography in tandem with mass spectrometry (LC-MS/MS) confirmed oxidation of HMGB1 in the cytoplasm during LPS-induced stress. LC-MS/MS identified the oxidation of cysteine residues at positions 23 and 45 (C34 and C45) and subsequent formation of a disulfide bond between these two residues during LPS treatment, with enhanced disulfide bond formation as LPS-induced stress was increased (Fig. 2d-f). The difference in HMGB1 thiol content during low versus high LPS-induced stress presumably indicates a heterozygous mixture of HMGB1 whose cysteines are still in reduced thiol form along with cysteines that have formed a disulfide bond. This was similarly observed in experiments conducted by Venereau at. al., in which supernatants of THP-1 monocytes treated with LPS contained both all-thiol and disulfide bonded HMGB1 [35].
Fig. 2.
Sepsis-induced oxidative stress leads to oxidation of HMGB1. HMGB1 thiol content in the (A) nuclear and (B) cytoplasmic compartments of kidney cells and in the (C) plasma 24 h after treatment with either low (10 µg/kg) or high (1 mg/kg) LPS dose. *p ≤ 0.05 vs. control; n = 5. (D-F) LC-MS/MS analysis was conducted on cytoplasmic fractions to confirm HMGB1 oxidation in the cytoplasm. (D) LC-MS/MS spectra trace of kidney cytoplasmic samples show two HMGB1 peptides with a retention time of 29.01 mins that are connected by a disulfide bond between C23 and C45 (peptide sequences are indicated in the figure). The disulfide bonded peptides appear as a peak at 477 m/z (identified as disulfide bond between C23 and C45 by MassMatrix software analysis). The absence of the thiol marker iodoacetamide and sulfonate marker dimedone further indicates these two cysteines are disulfide bonded. (E) Sample comparison of the elution traces (and subsequent area assignments) of disulfide bonded peptides with a peak of 477 m/z and a retention time of 29.01 mins. Samples compared included HMGB1 isolated from cytoplasmic fractions of mice whole kidneys 24 h after mice were treated with low and high LPS dose. (F) Quantification of the elution trace area assignments representative of the amount of HMGB1 C23-C45 disulfide bonded in each sample. Statistical significance is not given in panel F because multiple (n = 5) individual samples were pooled together and analyzed collectively for each of the three sample conditions.
Our results indicate HMGB1 becomes oxidized in the cytoplasm of kidney cells before its release into the circulation. This finding suggests increased intracellular generated ROS from sources such as NADPH oxidase and dysfunctional mitochondria oxidize HMGB1 in the cytoplasm during its nuclear-to-cytoplasmic translocation prior to release into the plasma. Furthermore, similar to the study by Venereau et al. utilizing monocytes, HMGB1 in the nucleus of kidney cells remains in reduced thiol form.
3.3. HMGB1 reduction by thioredoxin and glutathione
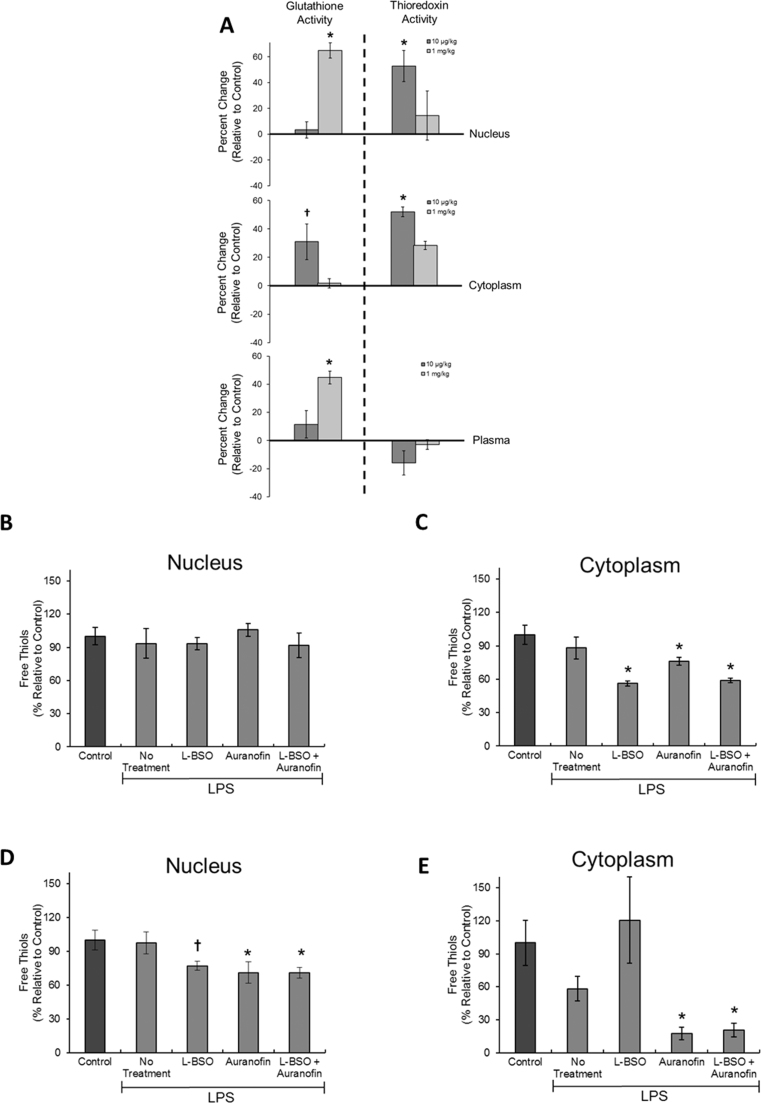
The thioredoxin and glutathione reduction pathways are two parallel, interdependent enzymatic systems present in all eukaryotic cells [29]. While both pathways are composed of multiple enzymes, including critical reductases, the thioredoxin and glutathione enzymes themselves are key enzymes in their respective systems [38]. Both systems play critical roles in the maintenance of intra-/extracellular redox balance [30] by maintaining proteins in their reduced state [29], [39], [40]. Thioredoxin and glutathione have both been shown to interact and reduce isolated purified HMGB1 [32], [33]. Therefore, we questioned if these systems maintain reduction of HMGB1 during sepsis. We first examined total thioredoxin and glutathione activity in the cellular compartments of kidney cells and in the circulation 24 h after low and high LPS dose administration. Despite a negligible change in circulating thioredoxin levels 24 h after LPS administration, the change in intracellular and extracellular levels of thioredoxin and glutathione were dependent on the severity of endotoxin stress. Specifically, our results demonstrated thioredoxin activity was initially increased in the nucleus and cytoplasm during mild sepsis, but as sepsis became more severe, thioredoxin activity decreased in these compartments (Fig. 3a). In contrast, glutathione activity increased significantly in both the nucleus and circulation as LPS-induced sepsis worsened (Fig. 3a). Interestingly, cytoplasmic glutathione levels drastically declined as sepsis worsened, suggesting the simultaneous increase of circulating glutathione was a result of its enhanced release from the cytoplasm into the circulation.
Fig. 3.
Glutathione and thioredoxin maintain HMGB1 in reduced form during sepsis. (A) Glutathione and thioredoxin activity in the nuclear and cytoplasmic compartments of fractionated whole kidneys and in the plasma 24 h after mice were treated with low (10 µg/kg) or high (1 mg/kg) LPS dose. Values in the graphs are represented by percent change relative to control. n = 4–5 (except thioredoxin plasma and cytoplasm treated with 10 µg/kg of LPS, which had an n = 3). Thiol content of HMGB1 localized in the (B) nucleus and (C) cytoplasm of HUVEC 24 h after treatment with LPS (10 µg/ml) and specific inhibitors of glutathione (L-BSO) or thioredoxin (Auranofin). *p ≤ 0.05 vs. control. n = 4–5. Thiol content of HMGB1 localized in the (D) nucleus and (E) cytoplasm of HK-2 cells 24 h after treatment with LPS (10 µg/ml) and L-BSO or Auranofin. *p ≤ 0.05 vs. control; †p≤0.10 vs. control. n = 4–5.
We next examined the ability of these antioxidant systems to maintain reduced HMGB1 within kidney cells during LPS-induced stress. These experiments were conducted on HUVEC and HK-2 cell cultures. A relatively low dose of LPS was implemented in these experiments (10 µg/ml) as to not overwhelm the antioxidant capacity of the thioredoxin and glutathione systems. In endothelial cells, while inhibition of glutathione and thioredoxin had no effect on HMGB1 redox in the nucleus, conversely, inhibition of either antioxidant enhanced HMGB1 oxidation in the cytoplasm (Fig. 3b-c). In tubular epithelial cells, the thioredoxin inhibitor auranofin increased HMGB1 oxidation in the nucleus and cytoplasm, while the glutathione inhibitor L-BSO only had an influence on HMGB1 redox in the nucleus (Fig. 3d-e). These findings indicate glutathione and thioredoxin maintain HMGB1 in its reduced form in kidney cells during sepsis, with cell-type and cellular-compartment dependent differences in their specific effects on HMGB1 redox.
3.4. HMGB1 redox state impacts stimulation of cyto-/chemokine release and ATP generation
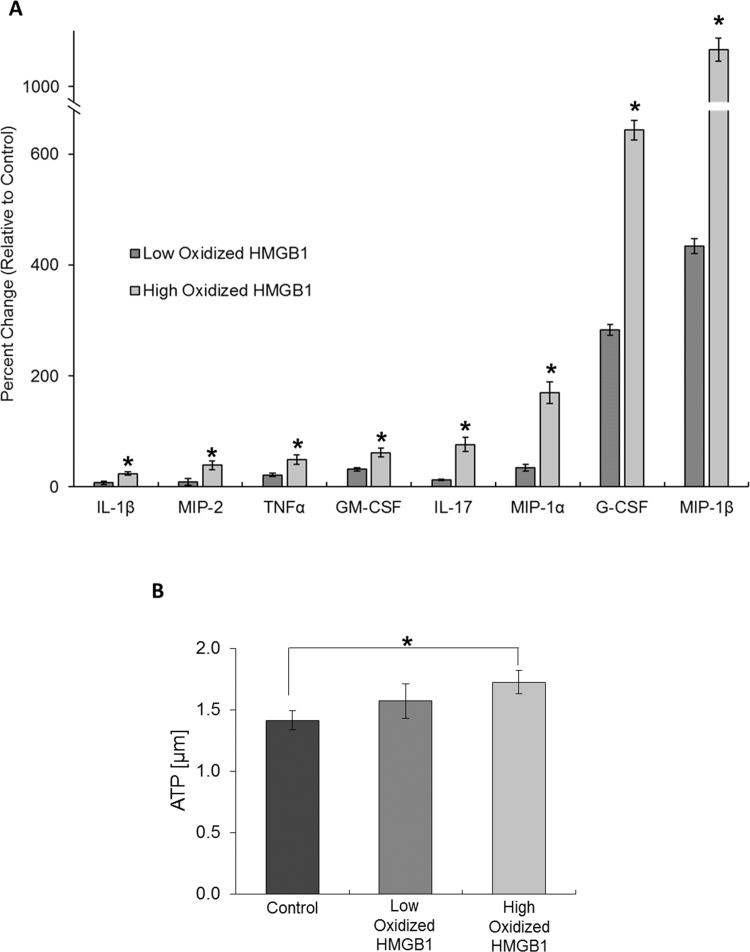
Once in the circulation, HMGB1 can stimulate the release of pro-inflammatory cyto-/chemokines from various cells and tissues [20], [35], [41], [42], [43], [44], [45], [46], [47]. However, the impact that the change in HMGB1 redox has on the protein's ability to promote cyto-/chemokine release during sepsis remains unclear. Therefore, we examined the specific cyto-/chemokines that are systemically released in response to HMGB1 that is oxidized to different extents due to differences in sepsis severity. For these experiments, HMGB1 was captured from the circulation of mice 24 h after treatment with either low (10 µg/kg) or high (1 mg/kg) LPS dose. HMGB1 was also captured from the circulation of control animals. The purified HMGB1 was then intravenously administered to healthy mice. After 6 h, cyto-/chemokine levels in the circulation was measured by Luminex Multiplex. As HMGB1 became more oxidized, the protein's ability to stimulate systemic release of pro-inflammatory cyto-/chemokines was enhanced, particularly regarding release of IL-1β, IL-17, TNFα, MIP-1α, MIP-1β, MIP-2, G-CSF and GM-CSF (Fig. 4a).
Fig. 4.
Oxidation of HMGB1 alters the alarmin's signaling function. (A) The increase of eight cytokines in the circulation of healthy mice 6 h after intravenous injection of low or high oxidized HMGB1. Values in the graph are percent change relative to control. *p ≤ 0.05 vs. control. n = 5–6. (B) Generation of ATP from mitochondria (isolated from whole kidneys) after 20 min treatment with low or high oxidized HMGB1. *p ≤ 0.05 vs. control. n = 5–6.
Sepsis is characterized by an increase in total cellular metabolism in order to provide the energy needed to sustain immune system activation [22]. However, the molecules that stimulate the hypermetabolic state are uncertain. When isolated mitochondria (purified from whole kidney homogenates) were treated with low and high oxidized HMGB1, mitochondrial ATP generation increased (Fig. 4b). As HMGB1 oxidization increased, its stimulatory effect on ATP generation was enhanced. This suggests HMGB1 and its oxidative state plays a role in stimulating a hypermetabolic state during sepsis.
4. Conclusions
During sepsis, oxidative stress increases in the kidney, which leads to oxidation of HMGB1 during its release from kidney cells. Oxidation of HMGB1 enhances its pro-inflammatory signaling. However, glutathione and thioredoxin work to maintain HMGB1 in a reduce state in kidney cells.
Funding
Studies were supported by AHA grant 12SDG9080006, ASN grant 010973-101, Westchester Community Foundation - Renal Clinical Fund, and a Seed Funding Grant award from the Biomedical and Health Sciences Research Council of the Touro College and University System (BBR).
Conflict of interest disclosures
All the authors declare no competing interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.08.001.
Appendix A. Supplementary material
Supplementary material
Fig. S1.
ROS generation in endothelial and proximal tubule cells during treatment with LPS. Levels of intracellular ROS in (A) HUVEC and (B) HK-2 cells 24 h after treatment with three different doses of LPS (1 µg/ml, 10 µg/ml, and 20 µg/ml). Intracellular ROS was detected by CellROX (green) and MitoSOX (red). Nuclei were labeled blue with Hoescht. Magnification = 400x. Line bar represents 25 µm. Slight corrections in brightness and contrast were made to images only for clarity.
References
- 1.Angus D.C., Linde-Zwirble W.T., Lidicker J. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ghaly T., Rabadi M.M., Weber M. Hydrogel-embedded endothelial progenitor cells evade LPS and mitigate endotoxemia. Am. J. Physiol. Ren. Physiol. 2011;301:F802–F812. doi: 10.1152/ajprenal.00124.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aird W.C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 4.Vallet B., Wiel E. Endothelial cell dysfunction and coagulation. Crit. Care Med. 2001;29:S36–S41. doi: 10.1097/00003246-200107001-00015. [DOI] [PubMed] [Google Scholar]
- 5.Schroder N.W., Morath S., Alexander C. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 6.Lien E., Chow J.C., Hawkins L.D. A novel synthetic acyclic lipid A-like agonist activates cells via the lipopolysaccharide/toll-like receptor 4 signaling pathway. J. Biol. Chem. 2001;276:1873–1880. doi: 10.1074/jbc.M009040200. [DOI] [PubMed] [Google Scholar]
- 7.Cario E., Rosenberg I.M., Brandwein S.L. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 8.Rabadi M.M., Kuo M.C., Ghaly T. Interaction between uric acid and HMGB1 translocation and release from endothelial cells. Am. J. Physiol. Ren. Physiol. 2012;302:F730–F741. doi: 10.1152/ajprenal.00520.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zoelen M.A., Yang H., Florquin S. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaldi T., Talamo F., Scaffidi P. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G.Y., Tang J., Zheng P. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Mezayen R., El Gazzar M., Seeds M.C. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol. Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang R., Tang D., Schapiro N.E. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokkola R., Andersson A., Mullins G. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 15.Park J.S., Gamboni-Robertson F., He Q. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 16.Park J.S., Svetkauskaite D., He Q. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 17.Yu M., Wang H., Ding A. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 18.Park J.S., Arcaroli J., Yum H.K. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell Physiol. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 19.Yanai H., Ban T., Wang Z. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 20.Rabadi M.M., Ghaly T., Goligorksy M.S. HMGB1 in renal ischemic injury. Am. J. Physiol. Ren. Physiol. 2012;303:F873–F885. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratliff B.B., Rabadi M.M., Vasko R. Messengers without borders: mediators of systemic inflammatory response in AKI. J. Am. Soc. Nephrol. 2013;24:529–536. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 22.Pravda J. Metabolic theory of septic shock. World J. Crit. Care Med. 2014;3:45–54. doi: 10.5492/wjccm.v3.i2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson J.M. Reactive oxygen species in phagocytic leukocytes. Histochem. Cell Biol. 2008;130:281–297. doi: 10.1007/s00418-008-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F., Schuster D.P., Tyml K. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med. 2007;42:124–131. doi: 10.1016/j.freeradbiomed.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Li J.M., Shah A.M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 26.Barth E., Fischer G., Schneider E.M. Peaks of endogenous G-CSF serum concentrations are followed by an increase in respiratory burst activity of granulocytes in patients with septic shock. Cytokine. 2002;17:275–284. doi: 10.1006/cyto.2002.1010. [DOI] [PubMed] [Google Scholar]
- 27.Oldner A., Goiny M., Rudehill A. Tissue hypoxanthine reflects gut vulnerability in porcine endotoxin shock. Crit. Care Med. 1999;27:790–797. doi: 10.1097/00003246-199904000-00037. [DOI] [PubMed] [Google Scholar]
- 28.Lamarque D., Whittle B.J. Involvement of superoxide and xanthine oxidase in neutrophil-independent rat gastric damage induced by NO donors. Br. J. Pharmacol. 1995;116:1843–1848. doi: 10.1111/j.1476-5381.1995.tb16672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaymak C., Basar H., Sardas S. Reactive oxygen species (ROS) generation in sepsis. FABAD J. Pharm. Sci. 2011;36:41–47. [Google Scholar]
- 30.Duran-Bedolla J., Montes de Oca-Sandoval M.A., Saldana-Navor V. Sepsis, mitochondrial failure and multiple organ dysfunction. Clin. Investig. Med. 2014;37:E58–E69. doi: 10.25011/cim.v37i2.21087. [DOI] [PubMed] [Google Scholar]
- 31.Vasko R., Ratliff B.B., Bohr S. Endothelial peroxisomal dysfunction and impaired pexophagy promotes oxidative damage in lipopolysaccharide-induced acute kidney injury. Antioxid. Redox Signal. 2013;19:211–230. doi: 10.1089/ars.2012.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu D., Debnath P., Takayama Y. Redox properties of the A-domain of the HMGB1 protein. FEBS Lett. 2008;582:3973–3978. doi: 10.1016/j.febslet.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoppe G., Talcott K.E., Bhattacharya S.K. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp. Cell Res. 2006;312:3526–3538. doi: 10.1016/j.yexcr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Tang D., Kang R., Zeh H.J., 3rd High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011;14:1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venereau E., Casalgrandi M., Schiraldi M. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H., Lundback P., Ottosson L. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol. Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Rabadi M.M., Xavier S., Vasko R. High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 2015;87:95–108. doi: 10.1038/ki.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berndt C., Lillig C.H., Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 39.An N., Kang Y. Thioredoxin and hematologic malignancies. Adv. Cancer Res. 2014;122:245–279. doi: 10.1016/B978-0-12-420117-0.00007-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhou D., Shao L., Spitz D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markadieu N., San-Cristobal P., Nair A.V. A primary culture of distal convoluted tubules expressing functional thiazide-sensitive NaCl transport. Am. J. Physiol. Ren. Physiol. 2012;303:F886–F892. doi: 10.1152/ajprenal.00114.2012. [DOI] [PubMed] [Google Scholar]
- 42.Leelahavanichkul A., Huang Y., Hu X. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011;80:1198–1211. doi: 10.1038/ki.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulloa L., Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Mullins G.E., Sunden-Cullberg J., Johansson A.S. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand. J. Immunol. 2004;60:566–573. doi: 10.1111/j.0300-9475.2004.01518.x. [DOI] [PubMed] [Google Scholar]
- 45.Erlandsson Harris H., Andersson U. Mini-review: the nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Yang H., Czura C.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 47.Abraham E., Arcaroli J., Carmody A. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material