Abstract
Injury is the leading cause of death and disability in childhood. Injured children are at high risk for developing alterations in stress response systems and post-traumatic stress symptoms (PTSS) that may compromise long-term physical and psychological health. In a prospective, observational cohort study, we examined individual differences in, and correlates of, stress-reactivity of the hypothalamic-pituitary-adrenal axis (HPA; salivary cortisol) and autonomic nervous system (ANS; salivary alpha amylase, sAA) following pediatric injury. Participants were 8-15 years of age and hospitalized for traumatic brain injury (TBI; n=55; M age =13.9 yrs; 40 males) or extracranial injury (EI; n=29; M age 12.3 yrs, 20 males) following vehicular accidents. Six months post-injury, saliva was collected before and after the Trier Social Stress Test and later assayed for cortisol and sAA. Relative to a healthy non-injured comparison group (n=33; M age =12.5 yrs, 16 males), injured children (ages 8 to 12 years), but not adolescents (ages 13 to 15 yrs), had higher cortisol levels; regardless of age, injured participants showed dampened cortisol reactivity to social evaluative threat. Compared to participants with EI, children with TBI had elevated cortisol and adolescents had elevated sAA. With respect to PTSS, individual differences in sAA were negatively correlated with avoidance in the TBI group and positively correlated with emotional numbing within the EI group. Importantly, psychological and neurobiological sequelae were weakly related to injury severity. Given the high prevalence of pediatric injury, these sequelae affect many children and represent a significant public health concern. Consequenty, surveillance of post-traumatic sequelae should include the full spectrum of injury severity. Monitoring the activity, reactivity, and regulation of biological systems sensitive to environmental insults may advance our understanding of individual differences in sequelae and adaptation following traumatic pediatric injury.
Keywords: hypothalamic pituitary adrenal, autonomic nervous system, cortisol, salivary alpha amylase, injury, post-traumatic stress
1. Introduction
Stressful experiences in childhood may adversely influence psychosocial adjustment and health outcomes later in life (McEwen et al., 2015). Physical injury is a major source of acute and chronic stress. Injury, the leading cause of mortality and morbidity in the pediatric age range, represents a serious public health concern. Over 135,000 children under 16 years of age are hospitalized for injuries annually in the United States (Centers for Disease Control and Prevention, 2016). Between 25–57% of injured children develop significant post-traumatic stress symptoms (PTSS) (Schreier et al., 2005). Like other forms of childhood adversity, injuries occur more often in children from disadvantaged backgrounds (Laflamme et al., 2010). Traumatic injuries may have unrecognized long-term neurobiological consequences that contribute to the constellation of findings associated with exposure to other forms of childhood adversity (Gunnar and Vazquez, 2001; Trickett et al., 2014). Despite the frequent occurrence of traumatic injury during childhood and adolescence, little is known about the impact of different types of injury sustained at different developmental stages on stress response systems and the subsequent development of PTSS.
The main components of the psychobiology of the stress response involve the reactivity and regulation of the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) (e.g., Chrousos & Gold, 1992). Reactivity of stress response systems refers to activation and engagement of resources in response to a stressor while regulation refers to return to baseline levels of activity following stressor cessation. The sympathetic branch of the ANS reacts quickly (within minutes) to threat or challenge and optimizes fight or flight responses through release of catecholamines into the general circulation. By contrast, activation of the HPA axis involves a slower to respond (20–30 mins) cascade of endocrine signals resulting in the eventual release of cortisol into the blood from the adrenal glands. The release of cortisol, a potent glucocorticoid, mobilizes energy stores. Chronic exposure to adversity has the potential to alter the set-point or threshold of HPA activity and reactivity, resulting in either over- or underactivity (Yehuda et al., 2010). Although acute stress reactions may be adaptive, the cumulative effects of chronic activation of these environmentally sensitive systems contribute to poor physical and psychological health, including internalizing disorders (Lupien et al., 2009), immune dysfunction, hypertension, insulin resistance (McEwen, 1998; Pacella et al., 2013), and hypopitutitarism (Reifschneider et al., 2015).
Traumatic injury is a risk factor for chronic negative physical and/or psychological health sequelae (Ewing-Cobbs et al., 2014), including post-traumatic stress disorder (PTSD) or significant subclinical PTSS (Zatzick et al., 2010). PTSS include intrusive thoughts, avoidance, negative changes in mood and cognitions, and alteration in arousal and reactivity that persist more than one month (American Psychiatric Association, 2013). Numerous factors predict PTSS after unintentional traumatic injury, including perceived life threat, acute distress and elevated heart rate, preinjury or comorbid psychological problems, maladaptive appraisals, avoidant coping style, and parental distress (Price et al., 2016).
In addition to the life challenges associated with physical injury and hospitalization, TBI has potential to directly influence variation in the psychobiology of the stress response through changes in the volume and integrity of the amygdala (Juranek et al., 2012) and hippocampus (Wilde et al., 2007), key limbic system structures regulating the HPA axis. Alteration in these structures likely contributes to hypopituitarism, growth hormone deficiency and pubertal disturbances that can evolve or persist years after injury and are reported in nearly 30% of children with moderate to severe TBI (Reifschneider et al., 2015). Endocrine dysfunction, particularly precocious puberty and hypothyroidism, has been noted in youth during the first year after mild to moderate TBI (Kaulfers et al., 2010). Based on alteration in neural structures regulating the HPA and the high rate of neuroendocrine dysfunction following pediatric TBI, brain injury is likely to be associated with more specific involvement of the HPA axis than bodily-skeletal injury.
Given the role that individual differences in biological sensitivity play in moderating the effects of early adversity on later developmental outcomes, and the high prevalence of injury during childhood (CDC, 2016), it is surprising that no studies have examined the effects of injury on HPA and ANS reactivity and regulation to specific stressors. A large volume of literature shows that youth exposed to various forms of adversity (Koss et al., 2016; Trickett et al., 2014) and youth with internalizing and externalizing disorders show blunted or attenuated cortisol reactivity (Bae et al., 2015). Recent technical advances have enabled minimally invasive measurement of a surrogate marker of ANS arousal, alpha-amylase (sAA), in saliva (Granger et al., 2007). Youth experiencing chronic stress (e.g., maltreatment) have lower sAA levels (Gordis et al., 2008) while youth with social anxiety (Payne et al., 2014) and externalizing disorders (Bae et al., 2015) had higher levels than comparison youth. Elevated diurnal sAA levels have also been associated with PTSD (Keeshin et al., 2015).
Several pediatric and adult injury studies examined the relation between acute elevation in basal or diurnal cortisol or catecholamine levels and later development of PTSS following injuries sustained primarily in vehicle accidents that required hospital admission. Patients with concussion symptoms, including loss of consciousness for > 15 minutes, or moderate to severe TBI were excluded. In youth samples, PTSS assessed 6 weeks after injury were predicted by acute 12-hour urinary cortisol, but not urinary epinephrine, norepinephrine, or dopamine levels (Delahanty and Nugent, 2006; Pervanidou et al., 2007). In a longitudinal study, Pervanidou and colleagues found that elevated evening levels of salivary or serum cortisol at both acute and 1-month intervals predicted PTSD 6 months after injury. Although cortisol levels tended to normalize over time, basal plasma noradrenaline was elevated at 1 month and increased further at 6 months after the injury (Pervanidou et al., 2007). Similarly, in adults, PTSS 4 to 6 months after injury were predicted by higher norepinephrine and lower cortisol obtained from 12-hour overnight urinary samples (Gandubert et al., 2016) and low morning and elevated afternoon salivary cortisol sampled 2 days after hospitalization (McFarlane et al., 2011). Evidence to date suggests that alteration in stress responsive systems occurs within the first several days following injury exposure and predicts PTSS during the first year.
Present Study
The primary aim of the study was to characterize salivary cortisol and sAA stress-related reactivity and regulation in children and adolescents sustaining traumatic injury in comparison to a healthy non-injury control group. Within the injury group we expected that youth with TBI, in comparison to those experiencing EI, would show distinct salivary cortisol and sAA stress reactivity and regulation. A secondary aim examined the degree to which, within the injury groups, individual differences in salivary cortisol and sAA correlated with self-reported subclinical levels of PTSS.
2. Methods
2.1. Participants
2.1.1. Recruitment
Youth ages 8–15 injured in vehicular accidents and treated in the Emergency Department or Level 1 Pediatric Trauma Center at Children’s Memorial Hermann Hospital/University of Texas Health Science Center at Houston for either a TBI or EI were screened for enrollment. Of 190 children injured by 12-16-15 who met study inclusion criteria, 104 were enrolled and eligible for the 6-month follow-up. Nineteen children dropped out between baseline and 6 month visits, one child was excluded, and one was removed by the investigators due to receiving hormone injections, resulting in a sample of 84 (M age =13.9 yrs, 40 males) injured youth. Participants with TBI (n=55, 40 males) and EI (n=29, 20 males) met the following inclusion criteria: 1) injured in a vehicle accident between ages 8 and 15 years, 2) proficiency in English or Spanish, 3) residing within a 125 mile catchment radius, 4) no prior history of major neuropsychiatric disorder (intellectual deficiency or low functioning autism spectrum disorder) that would complicate assessment of the impact of injury on behavioral outcomes, 5) no Type 1 or 2 diabetes, other metabolic or endocrine disorder, or systemic health problems (e.g., hypertension), 6) no prior medically attended TBI, and 7) no habitual use of steroids, tobacco, or alcohol. The latter four criteria were assessed during screening using a brief parent interview.
Children with EI were included to account for possible preinjury characteristics, such as risk-taking behavior, that may influence outcomes, to examine the stresses of injury and hospitalization, and to allow comparison of the effects of brain injury above and beyond that of bodily injury. A healthy comparison group was recruited from the community (n = 33, M age 12.5 years; 16 males) and met criteria 2–7. Informed written consent was obtained from each child’s guardian according to Institutional Review Board guidelines; written assent was obtained from all participants.
2.1.2. Injury characteristics
Demographic and injury characteristics of the TBI, EI, and healthy comparison participants and injury variables are provided in Table 1. The external cause of injury in a vehicle accident was selected to meet the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) criterion A for a PTSD diagnosis that specifies exposure to a potentially life-threatening situation. For TBI participants, severity of brain injury was rated using the lowest post-resuscitation Glasgow Coma Scale (GCS) score, which evaluates eye opening, motor, and verbal responses to stimulation (Teasdale and Jennett, 1974). Mild, moderate, and severe TBI were indicated by total scores of 13–15, 9–12, and 3–8, respectively. GCS scores were abstracted from the medical record by trained assistants. Severity of bodily injury was based on the Abbreviated Injury Scale (AIS), which classifies injury to specified anatomical regions on a scale from 1 to 6 (minor to life-threatening). We used the Injury Severity Score (ISS), which incorporates the highest AIS scores from three anatomical regions and ranges from 0 to 75 (Baker et al., 1974). For TBI participants, the modified ISS score excluding injuries to the head was also used (Mayer et al., 1980). Injury severity scores were obtained from the hospital trauma registry. In children with mild or moderate TBI, skeletal or body AIS scores were limited to ≤ 2 to minimize any confounding influence of severe extracranial injury on accurate assessment of GCS scores. Participants in the EI group also had no evidence of blunt head trauma or concussion symptoms.
Table 1.
Demographic and injury information for traumatic brain injury, extracranial injury, and healthy comparison groups
| Group |
|
|||
|---|---|---|---|---|
| Traumatic Brain Injury (n=55) |
Extracranial Injury (n=29) |
Healthy Comparison (n=33) |
Statistic (p) | |
| Demographic Variables | ||||
|
| ||||
| Age M (SD) | 12.9 (2.3) | 12.3 (2.3) | 12.5 (2.7) | F (2, 114) = .604, p = .548 |
| Sex % Male | 67 | 69 | 48 | Χ2(2, N=117) = 3.81 (.149) |
| Race (n) | Χ2(4, N=117) = 8.08 (.089) | |||
| Caucasian | 42 | 22 | 18 | |
| African American | 9 | 7 | 10 | |
| Other/Multiracial | 4 | 0 | 5 | |
| Ethnicity (n) | ||||
| Hispanic | 27 | 15 | 13 | Χ2(6, N=117) = 8.42 (.209) |
| Maternal education (n) | Χ2(4, N=117) = 12.98 (.011) | |||
| ≤ High School | 24 | 16 | 7 | |
| ≤ College | 17 | 8 | 21 | |
| Graduate Degree | 14 | 5 | 5 | |
| Preinjury Diagnoses (n) | ||||
| ADHD | 8 | 4 | 1 | |
| Anxiety | 1 | 1 | 0 | |
| Depression | 0 | 1 | 0 | |
|
| ||||
| Injury Variables | ||||
|
| ||||
| Cause of Injury (n) | Χ2(2, N=84) = 1.52 (.468) | |||
| Fall from Moving Vehicle | 13 | 4 | ||
| tor Vehicle Crash | 22 | 15 | ||
| Struck by Motor Vehicle | 20 | 10 | ||
| Injury Severity Score (ISS) M (SD) | 17 (11.6) | 9.8 (6.5) | t(82) = 3.17 (.002) | |
| Modified ISS M (SD) | 7.3 (9.3) | 9.4 (6.6) | t(82) = −1.11 (.269) | |
| Treatment Intensity (n) | Χ2(2, N=84) = 18.03 (<.001) | |||
| Released from ED | 12 | 9 | ||
| Admitted to hospital | 12 | 17 | ||
| Admitted to PICU | 31 | 3 | ||
| Length of Stay (days) M (SD) | 7.3 (6.4) | 4.9 (4.2) | t(61) = 1.518 (.134) | |
| Surgery n (%) | 17 | 17 | Χ2(1, N=84) = 6.05 (.014) | |
| Glasgow Coma Score (n) | ||||
| 3–8 | 20 | -- | ||
| 9–12 | 6 | -- | ||
| 13–15 | 29 | -- | ||
2.2. Procedures
2.2.1. Preinjury questionnaire and interview
To assess functioning of the child and family just prior to the injury, the primary caregiver completed questionnaires and interview within 2 weeks of the injury. This is standard procedure in studies of pediatric injury (Max et al., 2015). Parents of healthy children described the child and family’s current level of functioning at the time of enrollment. Parents completed the Child Behavior Checklist (CBCL) (Achenbach, 1991). The internalizing and externalizing T-scores normed for age and sex were examined to characterize preinjury behavioral problems. Exposure to adversity was assessed by trained interviewers based on 6 categories defined by Biederman and colleagues (Biederman et al., 2002). The following common indicators were assessed: 1) severe marital discord defined as divorce or separation, 2) low social status defined as levels IV or V on the Hollingshead Index; 3) large family size defined as three or more children living in the child’s primary home; 4) history of investigation by protective service agency regarding this child, 5) parental criminal conviction, and 6) treatment of parental mental health problems. Items were scored yes/no and summed to yield a total score.
2.2.2. Six month follow-up questionnaires
Children and their parents independently rated changes associated with puberty, including growth in height, body hair, and skin changes using the Petersen Pubertal Development Scale (PDS, Petersen et al., 1998). Sex-specific changes included breast development and menstruation for females and deepening of voice and facial hair for males. Each item was then coded on a 5-point scale similar to Tanner staging (Shirtcliff and Essex, 2008); ratings were averaged to yield a score ranging from 1 (pre-pubertal) to 5 (post-pubertal). If ratings of parents and children differed by more than 1 point, they were asked to discuss and come to consensus. The consensus rating was used in analyses.
The Child PTSD Symptom Scale (Foa et al., 2001) is a validated self-report scale yielding a total post-traumatic stress score created from summing 17 items. Based on factor analysis and the 4 factor model of PTSD, we divided items into re-experiencing, active avoidance, emotional numbing, and arousal factors (Kassam-Adams et al., 2010). An impairment rating assessed impact on daily activities.
Child Behavior Checklist internalizing and externalizing T-scores normed for age and sex were examined to characterize behavioral problems at follow-up.
2.2.3. Psychosocial stress task and determination of salivary analytes
All participants completed The Trier Social Stress Test for Children (TSST-C) (Buske-Kirschbaum et al., 1997), an established laboratory procedure involving public speaking (social evaluative stress) and mental subtraction (cognitive stress) producing time-linked changes in stress responses that may be indexed by salivary cortisol and sAA. Reactivity of cortisol and sAA to the TSST-C and modifications has been validated in ages 7–25 (Yim et al., 2015).
Prior to beginning the TSST-C, participants acclimated to the office and completed a neuropsychological test battery for approximately 1.5 hours and did not eat, drink (except water), or exercise. Participants completed questionnaires and rested in a quiet room during pre- and post-stressor time periods. After a 3 minute preparation period, the child was videotaped giving a speech about themselves to a committee of doctors and told to try to perform better than other children. The speech continued for 6 minutes with no positive feedback with prompts to continue if the child paused for 10 seconds. Each child then engaged in serial mental subtraction for 4 minutes beginning at 1027, with difficulty titrated to age and ability level (if age < 13, subtract by 5, if older or making no errors, subtract by 13). Following errors, they were corrected and instructed to start over. At the end of the procedure, they were debriefed.
Following Granger and colleagues (2012), saliva samples were collected using 1 × 4 CM polyolefin swabs placed under the participants tongue for 2 mins at 20 minutes before and right before the TSST-C. Post-stressor samples were collected immediately after (sAA only), as well as 20 and 40 (cortisol only) minutes after to capture the distinct profiles of the analytes. Samples were frozen immediately at −20˚ C prior, and transported frozen to the Institute for Interdisciplinary salivary bioscience for analyses. Samples were assayed in duplicate for cortisol using a commercially available enzyme immunoassay assay (Salimetrics, LLC, Carlsbad, CA) without modification to the manufacturers recommended protocol. The test volume was 25 ul, range of calibrators from .01 to 3.0 ug/dL, and lower limit of sensitivity was .007 ug/dL. Samples were assayed for sAA using a commercially available (Salimetrics LLC, Carlsbad, CA) enzyme reaction kit. Determinations are expressed in ug/dl for cortisol and U/mL for sAA. Intra- and inter-assay coefficients of variation were, on average, less than 10% and 15% respectively. Raw value distributions for cortisol and sAA values was examined and at each time interval and extreme values (3 standard deviations above the mean) were winsorized. Following reassignment of 7 cortisol and 10 sAA values, distributions were reexamined and were positively skewed. AUC values from all 4 collection times during the TSST-C were calculated with respect to ground (AUCg) to assess the total secretion and with respect to increase (AUCi) to assess change in relation to the stressor (Khoury et al., 2015).
2.3. Statistical approach
After examination of variable distributions, comparability of demographic and psychosocial variables for the TBI, EI, and healthy comparison groups was examined using ANOVA or chi square. Psychosocial variables were examined to determine if the groups had comparable life adversity as well as internalizing and externalizing behaviors prior to study enrollment. Bivariate relations of age, sex, and pubertal development with cortisol and sAA AUCg&i were examined to assess whether the demographic variables should be considered as covariates.
Generalized linear models with a negative binomial distribution and log link function examined the effects of group, saliva collection time during the TSST-C, age, and their interactions on salivary analyte values. Advantages of this approach include assessment of appropriateness of the log transformation and evaluation of model fit. Age at 6 the month follow-up was treated as a categorical variable (child ages 8–12; adolescent ages 13–16). Orthogonal planned comparisons examined the impact of 1) both injury groups versus the healthy group and 2) the TBI versus the EI group on cortisol and sAA levels across the 4 collection intervals. Findings from main effects of group and age and their interactions are described in terms of impact on analyte levels; analyses of the time effect refer to reactivity and regulation. Post-hoc analyses of cortisol reactivity (time 3-time/2) and regulation (time 4–time 3) used the Bonferroni-Holm correction with a critical value of .025.
To address the second aim, Spearman partial correlation coefficients controlling for age examined the relation of cortisol and sAA AUCg&i with injury variables and subscale scores from the Child PTSD Scale.
3. Results
3.1 Preliminary analyses
Demographic and injury variables were compared across the TBI, EI, and healthy groups; Table 1 contains the descriptive and inferential statistics. The groups did not differ on age, sex, race, or ethnicity. Maternal education was higher in the healthy comparison group than in the injury groups. The type of vehicle accident was similar across TBI and EI groups; the majority of patients were injured in vehicle or vehicle-pedestrian collisions. As expected, the ISS score was significantly higher in the TBI than EI group. On the modified ISS scale excluding the head, the groups did not differ, suggesting similar severity of extracranial injury. For the TBI group, 84% sustained external injuries and approximately 30% sustained injury to the extremities or chest. For the EI group, 76% sustained an extremity injury while 52% had internal injuries involving the abdomen or chest. The length of hospital stay did not differ across injury groups. The TBI group included a broad range of severity. The lowest post-resuscitation GCS score of 3–8 indicated that 36% of the sample sustained a severe TBI. The majority of children sustained a mild or complicated-mild TBI as indicated by GCS scores from 13–15.
Regarding medications taken at follow-up, 9 youth (8 in TBI group) used 12 medications. Medications were classified as potentially influencing salivary analytes through altering subjective experience (stimulants=3, anticonvulsants=1, antidepressants=1, pain=3) or altering salivary composition or volume (decongestant=3, antiviral=1, alpha adrenergic receptor agonist=1) (Granger et al., 2009).
3.2 Potential covariates
Psychosocial and behavioral functioning just prior to enrollment in the study were examined to determine if levels were similar in the injury and comparison groups. Psychosocial adversity ratings and CBCL internalizing and externalizing T-scores did not differ across age or groups, suggesting comparable child and adolescent adjustment and preinjury trauma exposures. Prior to injury, ADHD was the most common psychological health diagnosis.
TSST-C time of administration did not differ across groups. The average time of the first sampling was 13:26 (SD=1:19). The mean (SD) minutes elapsing between the first and subsequent samples was 20.1 (1.2), 39.6 (1.3), 60.1 (1.4), and 80.1 (1.4). We also assessed whether age, pubertal stage, and sex were related to cortisol and sAA AUC values and should be included in the multivariable models. Age at the time of evaluation was significantly correlated with the AUCg&i measures for cortisol, rs(115)=.43 &.39, ps < .001, but not with sAA, rs(114)= .05 & -.05, ns. Pubertal status at the 6-month follow-up was highly correlated with age, r(115)=.77, p< .001, and did not differ across groups. Partialling out the influence of age, pubertal status was not significantly correlated with either sAA or cortisol AUCg&i. Sex was not significantly related to AUCg&i of either analyte. To maintain parallel analyses for cortisol and sAA, age was included as an independent variable in all analyses.
3.3 The effect of age at injury and type of injury on TSST-C
3.3.1. Reactivity and regulation of cortisol and sAA following brain or bodily injury
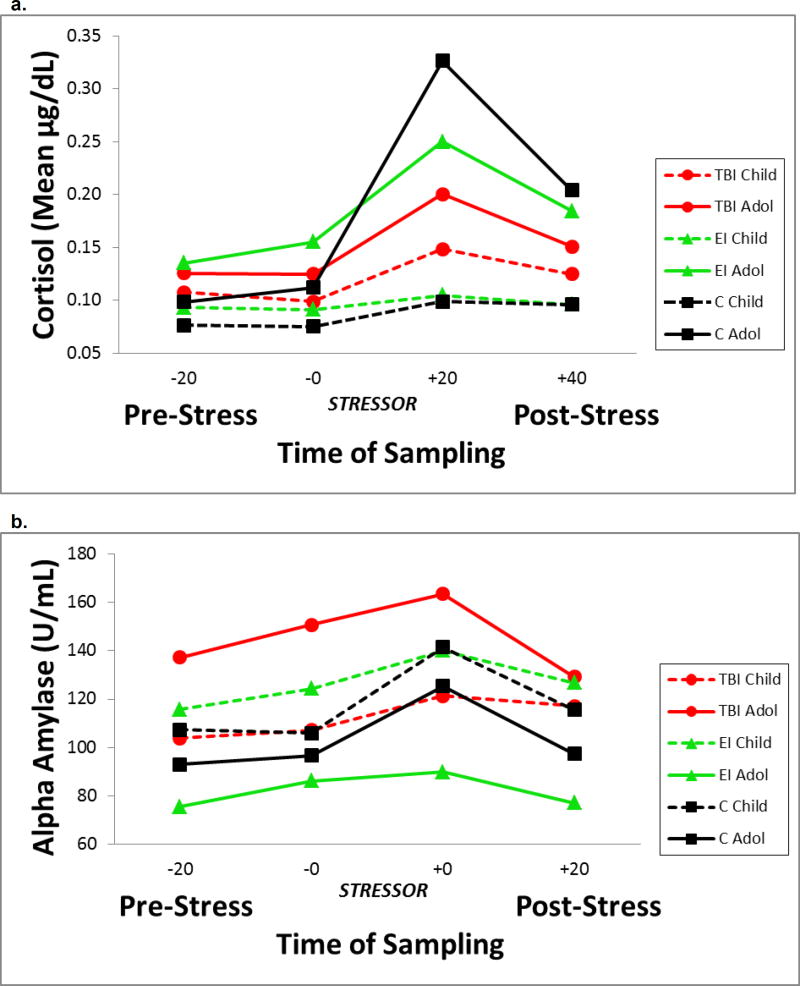
The effects of group, time of saliva sampling on the TSST-C, age, and their interactions were modeled separately for cortisol and sAA. The two pre-stressor samples did not differ within groups and were averaged to yield the mean pre-stressor level for each analyte. The age × group × time interaction was nonsignificant and was trimmed from each model. Table 2 contains the tests of statistical significance for main effects and two-way interactions from the trimmed models for each analyte. Figure 1 a & b show the average values for cortisol and sAA in response to the TSST-C, for children and adolescents by group and time of sampling. Transformed cortisol and sAA values were used in all analyses, but raw data (ug/dl and U/mL) are presented in the text and tables for ease of interpretation.
Table 2.
Statistics for Generalized Linear Models Examining Effects of Group, Age, and Time of Sampling of Salivary Analytes During the Trier Social Stress Test-Child
| Cortisol | Alpha Amylase | ||||
|---|---|---|---|---|---|
|
|
|||||
| Source | df | Chi Square | p | Chi Square | p |
| Main Effects | |||||
| Planned Group Comparisons | |||||
| Injury vs. Healthy | 1 | 1.43 | .231 | 0.09 | .770 |
| TBI vs. EI | 1 | 0.02 | .902 | 1.80 | .180 |
| Time | 3 | 33.55 | <.001 | 16.42 | .001 |
| Age | 1 | 29.03 | <.001 | 0.83 | .362 |
| Interaction Effects | |||||
| Age × Group | |||||
| Injury vs. Healthy | 1 | 5.07 | .024 | 0.02 | .897 |
| TBI vs. EI | 1 | 4.73 | .030 | 4.36 | .037 |
| Age × Time | 3 | 12.20 | .007 | 3.54 | .316 |
| Group × Time | 6 | 9.51 | .153 | 3.86 | .700 |
Note: Significant findings indicated in bold.
Figure 1.
Salivary cortisol (1a) and alpha amylase (1b) values during the Trier Social Stress Test-Child version for children and adolescents with traumatic brain injury, extracranial injury and healthy participants
a. Significant group by age interaction for injured vs healthy groups. Cortisol values were higher in injured children, particularly those with TBI, than in heathy children. Values did not differ for adolescents. The injured groups showed a significantly attenuated cortisol response to the stressor compared to the healthy group, suggesting dysregulation of the HPA stress response.
b. sAA levels and reactivity did not vary in injured versus healthy participants. Compared to the EI group, sAA levels did not differ in children but were significantly higher in adolescents with TBI.
For salivary cortisol, planned group comparisons revealed a significant group × age effect for the injury vs. healthy contrast. Collapsing across time of sampling, injured children had higher cortisol levels than the healthy children; however, cortisol levels did not differ by group for the adolescents. The simple main effects indicated that comparison children differed from the injured children, Χ2 (1)=8.81, p=.003, but the control adolescents did not differ from injured adolescents. The group × time interaction was nonsignificant, suggesting that changes in overall cortisol levels were similar across the 4 samples in each group. Post-hoc analyses of cortisol reactivity (time 3-time 2) and regulation (time 4-time 3) used the Bonferroni-Holm correction with a critical value of .025. The healthy comparison group showed significantly greater reactivity than the injured groups, Χ2 (1)=5.86, p=.015; no differences in post-stressor regulation of cortisol were obtained. The age × time interaction was significant; as expected, adolescents showed significantly greater increase in cortisol in response to the stressor than children.
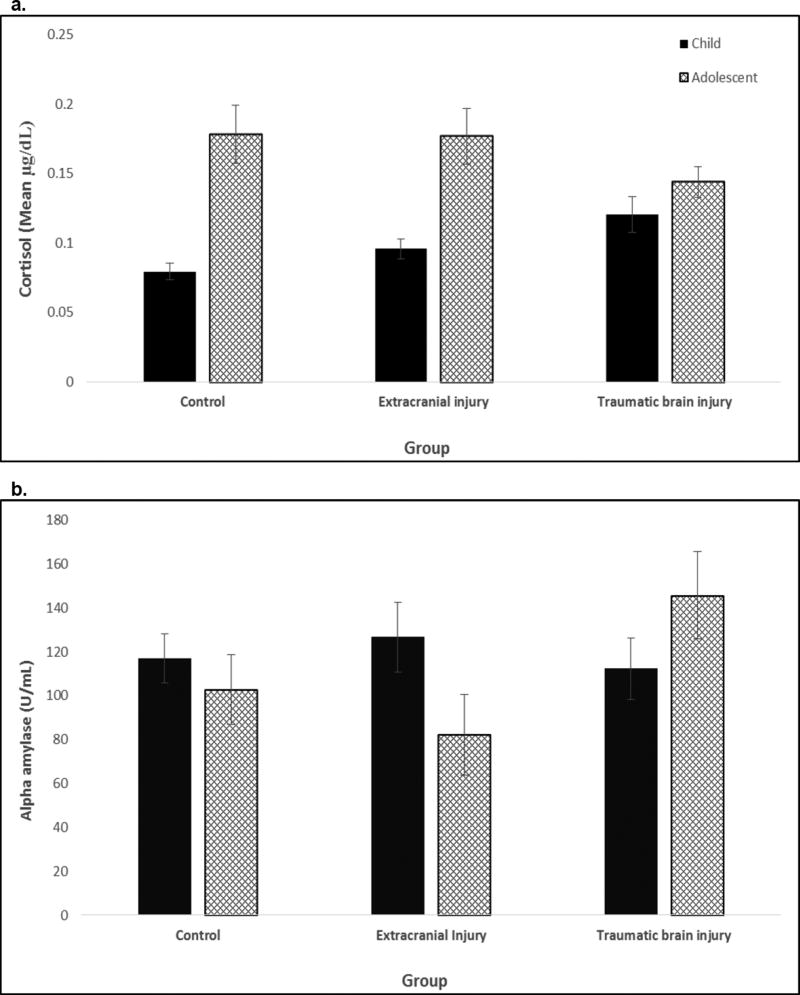
For the planned comparison of TBI and EI groups, a significant age × group interaction for cortisol was also obtained. Collapsing across time of sampling, children with TBI tended to have higher values than those with EI, Χ2 (1)=3.04, p = .081; no differences were noted for adolescents. Comparing cortisol within each group by age, cortisol was elevated in adolescents with EI relative to children with EI, Χ2 (1)= 19.62 p < .001. However, within the TBI group, there was no significant elevation in cortisol in adolescents relative to children. Figure 2a shows the cortisol age by group interaction; children with TBI had elevated levels while adolescents with TBI had attenuated cortisol levels. The TBI and EI groups showed similar overall patterns of reactivity and regulation.
Figure 2.
Age by group interactions for cortisol (2a) and alpha amylase (2b) values for children and adolescents in the healthy control, extracranial injury, and traumatic brain injury groups
a. Cortisol values were higher in injured children than in healthy children; adolescent values did not differ by group. Cortisol tended to be higher in children with TBI than EI.
b. sAA values did not differ in injured and healthy participants; however, sAA was significantly higher in adolescents with TBI than EI and did not differ in children.
For sAA, the planned comparisons of the injury group vs the healthy group revealed nonsignificant group and group × age effects. The main effect of time was significant; as expected, sAA changed in response to the stressor.
The comparison of sAA across TBI and EI groups revealed a significant group × age interaction. sAA was higher for the TBI than EI group at older, Χ2 (1)=4.24, p=.039, but not younger ages, Χ2 (1)=0.45, ns. Neither reactivity nor regulation varied across the TBI and EI groups. Figure 2b shows sAA levels by age and group collapsing across time of sampling.
3.3.2. Relation of salivary analytes, injury severity, and PTSS
Table 3 provides the Spearman correlation coefficients for cortisol and sAA AUC with age, sex, pre-enrollment psychosocial, pubertal status, follow-up psychosocial, and injury severity. Cortisol AUCg&i were significantly correlated with age and pubertal status. Cortisol and sAA AUCg&i were not significantly associated with psychosocial adversity or pre-injury or post-injury parent ratings of internalizing and externalizing behavior problems. Regarding injury variables, the GCS score was not significantly correlated with cortisol or sAA AUCg or AUCi. The ISS score was not related to cortisol AUCg or to AUCi for either group. However, ISS correlated significantly with sAA AUCg for the combined injury groups, r(82)=.286, p=.008; this relation was accounted for by the correlation with the TBI group, r(53)=.331, p = .014, but not for the EI group. Modified ISS scores were not significantly related to AUC for either group.
Table 3.
Relation of demographic, psychosocial, and injury variables with area under the curve cortisol and alpha amylase values
| Cortisol | Alpha amylase | |||
|---|---|---|---|---|
|
|
||||
| AUC ground | AUC increase | AUC ground | AUC increase | |
| Demographic (n=117) | ||||
| Age | .427** | .386** | .045 | −.046 |
| Sex | −.087 | .084 | .027 | .108 |
| Pre-Enrollment Psychosocial (n=117) | ||||
| Psychosocial Adversity | −.044 | −.025 | .093 | .020 |
| CBCL Internalizing | −.008 | .028 | −.007 | −.034 |
| CBCL Externalizing | −.044 | −.071 | .048 | .000 |
| 6 Month | ||||
| Pubertal Development | .365** | .335** | -.014 | −.034 |
| CBCL Internalizing | −.035 | −.102 | .113 | .025 |
| CBCL Externalizing | .007 | −.059 | .164 | −.014 |
| Injury Variables (n=84) | ||||
| ISS | .125 | .103 | .286** | .067 |
| MISS | .109 | .019 | .170 | .030 |
| GCS - Admission | −.153 | −.182 | −.131 | −.033 |
p < 0.01
We examined relations of PTSS scores with injury severity and AUC. None of the injury variables was significantly related to PTSS scores. Table 4 contains the correlation coefficients for the cortisol and sAA AUCg&i with factor and impairment scores from the self-report Child PTSD Scale for the TBI and EI groups. Cortisol AUCg and the AUCi from both analytes were unrelated to symptom factor or impairment scores. Emotional numbing was positively correlated with sAA AUCg only for the EI group; Fischer’s r to z transformation indicated that the relation between numbing and group did not differ across the TBI and EI groups. sAA AUCg was significantly negatively correlated with active avoidance scores for the TBI group; higher sAA was associated with less avoidance of reminders of the injury. In the EI group, the coefficient was positive although nonsignificant. Fischer’s r to z transformation indicated that the relation between avoidance scores and sAA AUCg differed significantly between the TBI and EI groups, z= −2.28, p = .02. To explore this association, we examined whether memory of being injured was related to sAA AUCg and avoidance scores in the TBI group. From interviews completed 6 weeks after the injury; the reported memory of the accident was categorized as complete (n=9), partial (n=21), or none (n=26). The presence or absence of memory for the injury event was not related to the active avoidance score. Neither cortisol nor sAA AUCg or AUCi was significantly related with CBCL internalizing or externalizing scores at follow-up.
Table 4.
Mean Children’s PTSD Scale Scores and Partial Correlation with Cortisol and Alpha Amylase Area Under the Curve Values for Traumatic Brain (TBI) and Extracranial Injury (EI) Groups
| Children’s PTSD Scale Score M (SD) |
Cortisol (r) | Alpha Amylase (r) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC ground | AUC increase | AUC ground | AUC increase | |||||||
| TBI | EI | TBI | EI | TBI | EI | TBI | EI | TBI | EI | |
| Re-experiencing | 2.36 (3.32) | 2.31 (2.42) | −0.108 | −0.047 | −0.055 | 0.164 | −0.082 | 0.041 | −0.083 | −0.212 |
| Active Avoidance | 1.22 (1.82) | 1.03 (1.57) | 0.018 | −0.083 | 0.018 | 0.069 | −0.319* | 0.212† | −0.145 | 0.219 |
| Emotional Numbing | 2.16 (2.95) | 1.41 (2.15) | −0.047 | −0.146 | −0.056 | −0.157 | 0.045 | 0.398* | −0.161 | 0.184 |
| Arousal | 3.05 (3.08) | 3.62 (3.58) | −0.015 | −0.017 | −0.011 | 0.104 | −0.092 | 0.196 | −0.249 | −0.072 |
| Impairment | 1.35 (1.96) | 0.90 (1.70) | −0.081 | 0.238 | −0.059 | 0.274 | 0.075 | 0.301 | −0.199 | 0.015 |
| Total Score | 8.80 (9.24) | 8.38 (8.12) | −0.078 | −0.085 | −0.050 | 0.065 | −0.149 | 0.318 | −0.231 | −0.044 |
p < .05, age partialled.
Fisher r to z transformation, TBI vs. EI p <.05
4. Discussion
In this study, the nature of trauma and age at injury were linked to individual differences in youth’s subsequent HPA axis activity and regulation, as well as to ANS arousal. Compared to a healthy non-injured group, injured children (8 to 12 years), but not adolescents (13 to 15 years), had elevated cortisol, and injured participants (regardless of age) showed dampened cortisol reactivity to social evaluative threat. In contrast, sAA level and reactivity did not vary in injured versus healthy youth. Injury type also influenced analyte levels. Compared to participants with EI, children with TBI had elevated cortisol and adolescents had elevated sAA. With respect to PTSS, individual differences in sAA AUCg were negatively correlated with avoidance after TBI and positively correlated with emotional numbing after EI. This is the first study (to the best of our knowledge) to systematically evaluate the psychobiology of the stress response to social evaluative threat during the chronic stage of recovery from nonintentional pediatric trauma. The findings suggest that monitoring the activity, reactivity, and regulation of environmentally sensitive biological systems such as the HPA axis and the ANS may advance our understanding of individual differences in long-term adaptation following pediatric injury.
4.1 Psychobiology of stress after traumatic injury
Stress reactivity is heterogeneous and complexly determined. In children and adolescents, reactivity profiles and analyte levels may vary depending on several developmental, demographic, and trauma-related factors, including age at the time of trauma, trauma type and chronicity, and time since trauma (De Bellis and Zisk, 2014). In the present study, age interacted with injury exposure to produce atypical and divergent patterns of cortisol reactivity. Prior to their injury, children and adolescents had relatively low psychosocial adversity. The attenuated cortisol in adolescents then appears to reflect their age at trauma exposure and assessment rather than any pre-existing psychosocial exposure or post-injury internalizing or externalizing psychological comorbidity. Our findings converge with those from youth exposed to other types of trauma and various forms of adversity that reported elevated cortisol reactivity and/or level in children and attenuated cortisol reactivity in trauma-exposed adolescents (Gunnar and Vazquez, 2001; Trickett et al., 2014). In studies examining cortisol patterns in traumatized adolescents and adults, the influence of age at onset and age at assessment should be considered in conjunction with vulnerability factors to explain variability in cortisol reactivity.
Puberty is increasingly viewed as a critical developmental period influencing stress reactivity (Gunnar and Vazquez, 2001). Pubertal status did not account for additional variability in cortisol reactivity above that of chronological age in our sample. However, pubertal status, but not age, predicted cortisol reactivity in a community sample in whom the effects of age and pubertal development were dissociated (Doom et al., 2015). Future studies should further examine the relation of age and pubertal status to see if the findings generalize beyond healthy youth to clinical samples with variable adversity and injury exposures.
In addition to age at trauma exposure, time since trauma and trauma type may explain variability in patterns of cortisol reactivity and/or level of cortisol secretion. Time since trauma was similar across our sample and averaged 6 months, which is generally considered “recent trauma” in studies of exposure to childhood adversities. Consequently, the pattern of elevated cortisol level in children and attenuated level and reactivity in adolescents may be related primarily to age at injury rather than to time since injury in cases of single incident physical injury. Although there is variability across studies, cross-sectional studies examining diurnal cortisol in school-aged children and adolescents exposed to a range of adversities have reported initial cortisol hypersecretion (Carrion et al., 2002; Trickett et al., 2010) that may be followed by hypocortisolism (Trickett et al., 2014). However, it is unclear whether basal cortisol levels change sequentially or whether the change is related to other moderator variables. In longitudinal studies, stress reactivity in youth experiencing physical or sexual abuse at 9–12 years of age was blunted when assessed several years after trauma. Interestingly, when assessed at age 18, the percentage of youth with blunted reactivity decreased and there were no longer differences in maltreated and comparison youth, suggesting that the HPA axis may normalize or recalibrate in some youth years after stress exposure (Peckins et al., 2015).
Does brain injury influence stress response systems above and beyond changes associated with bodily injury? Studies of traumatic stress in children sustaining physical injuries usually exclude patients with loss of consciousness or moderate to severe TBI, limiting understanding of the impact of bodily versus brain injury on stress biomarkers. Due to the high incidence of hypopituitarism after TBI, we expected the TBI group to show greater reduction in cortisol levels than the EI group. Contrary to expectation, overall cortisol level and reactivity assessed 6 months after injury did not differ significantly in the TBI and EI groups. Due to the lack of longitudinal studies, it is unknown whether pediatric injury is associated with progressive attenuation or normalization of cortisol level and reactivity.
The hypothesis that sAA values would be elevated in both injury groups was partially supported given the elevation in adolescents with TBI compared to those with EI. Individual differences in sAA levels have been related to both behavioral problem profiles and environmental stressors following a psychosocial stress challenge. Elevated sAA predicted increased trait anxiety in children (Allwood et al., 2011b) and has been noted in adults with a history of childhood adversity (Kuras et al., 2017). Dysregulation of the ANS may contribute to the increased internalizing problems including anxiety and depression noted following pediatric injury (Max et al., 2015).
Although age at injury and type of injury were related to stress system reactivity, cortisol AUC was not related to severity of brain or bodily injury. Our findings converge with prior studies emphasizing that neuroendocrine abnormalities are not strongly related to severity of TBI and may emerge during chronic stages of recovery (Reifschneider et al., 2015). There is a paucity of literature on long-term neuroendocrine changes after physical injury in youth. Our finding of similar HPA axis dysregulation in injured participants with brain and bodily injury raises the question whether long-term neuroendocrine screening should be recommended for youth with EI as well as TBI.
Regarding sAA, AUC values were not related to ISS scores in patients with bodily injury. Although sAA AUCg was significantly correlated with ISS scores in participants with TBI, it was unrelated to GCS scores and to the modified ISS score excluding the head. The source of this inconsistency is unclear; studies examining other stress system biomarkers following pediatric injury have also not shown consistent relations with indices of injury severity. Our findings are similar to those of Pervanidou and colleagues, who found that ISS scores were not related to salivary or serum cortisol or to plasma concentrations of noradrenaline obtained shortly following the accident (Pervanidou et al., 2007). Assessment of the child’s initial physiological reactivity and appraisal of potential harm may show stronger relations with stress biomarkers than objective measures of injury severity (Price et al., 2016).
4.2 Post-traumatic Stress
Across ages and different trauma exposures, time since exposure is a core variable moderating the relation of PTSS to stress markers. In maltreatment samples, diurnal salivary cortisol levels were positively related to PTSS when trauma occurred within a year of sampling and negatively correlated when trauma was more distal (Weems and Carrion, 2007). Nonintentional injury studies examining multiple analytes obtained acutely have generally found positive correlation of 12-hour urinary cortisol, norepinephrine and/or epinephrine concentrations with later developing PTSS in children and adults (Delahanty et al., 2005; Gandubert et al., 2016). In particular, the divergence of plasma noradrenaline and cortisol level across the first 6-months in youth who developed PTSD points to alteration across interacting HPA and ANS stress response systems (Pervanidou et al., 2007). Pervanidou et al. (2007) hypothesized that PTSD may be related to initial cortisol dysregulation that does not inhibit the catecholaminergic response in limbic structures including the locus coeruleus and amygdala. Similarly, we found that PTSS assessed 6 months after injury were related only to sAA AUCg and not to cortisol AUC. Taken together, these findings provide support for the hypothesis that PTSS following trauma reflects evolving autonomic system dysregulation (Hendrickson and Raskind, 2016), which has also been implicated following brain injury (Williamson et al., 2013).
There appears to be some specificity in the relation of salivary markers with PTSS clusters following physical trauma exposures. Active avoidance and emotional numbing, but not hyperarousal or re-experiencing symptoms, were related to sAA AUC values in the present study and to hair cortisol values in adults with mild TBI (Pacella et al., 2017). In relation to avoidance and numbing PTSS clusters in adults, functional imaging studies reported negative correlation with activation in dorsomedial prefrontal cortex (Frewen et al., 2012) and rostral anterior and subcallosal cingulate regions, in conjunction with positive correlation with superior temporal cortex activation (Hopper et al., 2007). These findings are consistent with an emotion dysregulation model positing post-traumatic alteration in pathway integrity and network connectivity of structures involved in top-down inhibition of limbic structures that regulate emotional reactivity (Yehuda et al., 2015). High levels of noradrenergic signaling may contribute to emotion dysregulation through inhibition of prefrontal regulatory systems, resulting in increased activity and reactivity in the amygdala (Hendrickson and Raskind, 2016).
Based on prior studies reporting positive correlation of PTSS in the first year after injury with diurnal salivary cortisol (Weems and Carrion, 2007) and urinary cortisol and norepinephrine levels (Delahanty et al., 2005), the negative correlation between avoidance and sAA in our TBI sample was unexpected. Some children with TBI who show high ANS arousal and low avoidance may not avoid thinking about the reminders or consequences of their injury due to impaired recall of the event related to post-traumatic or retrograde amnesia. There is debate whether patients who lose consciousness develop the full complement of PTSS since impaired memory of the traumatic event may reduce the likelihood of developing re-experiencing and avoidance symptoms. Consistent with this hypothesis, a large multi-site study reported reduced risk of developing PTSD symptoms in adults with moderate to severe TBI compared to patients with physical injury and no TBI (Zatzick et al., 2010). Even though patients with loss of consciousness may not remember the injury event, they are still exposed to physiological and psychological trauma. In the present study, avoidance symptoms were not significantly related to whether participants with TBI had full, partial, or no memory of the injury event. Other factors besides memory may account for the association of higher sAA and less avoidance of reminders of the injury. Additional research will need to ascertain whether the relation of low avoidance symptoms with elevated ANS system priming is adaptive or detrimental and whether it varies with other injury characteristics and psychological health outcomes.
In the chronic stage of recovery from EI, children with higher sAA were more likely to show emotional numbing. Similarly, Weems et al. (Weems et al., 2003) identified a directional relationship in which early hyperarousal predicted later developing emotional numbing, but not vice versa. Following pediatric injury, effortful avoidance and dysphoria symptoms, which overlap with emotional numbing, have been more strongly related than other symptom clusters to impairment in functional outcomes, include//ng general health and days missed from school (Kassam-Adams et al., 2010). Both emotional numbing and arousal levels are correlated with exposure to violence and to aggression and delinquent behaviors (Allwood et al., 2011a). As such, they may be risk factors for the development of subsequent behavioral disturbances.
4.3 Limitations, future directions and concluding comments
The sample targeted children hospitalized following vehicle-related injuries. The findings should be extended to children with other external causes of injury and those discharged from the emergency department. Injury severity measures showed no to weak associations with analyte levels and stress scores. The individual’s reaction to the injury may be more salient than objective measures of injury severity for the occurrence of PTSS. Whether measures of cognitive attribution would show stronger relations than PTSS scores with cortisol or sAA AUC values, or whether similar relations would be apparent in patients injured by mechanisms other than vehicular accidents and requires additional investigation. The sample size was relatively small and likely contributed to insufficient power to identify contributions of pubertal status and sex to individual differences in cortisol and sAA activity and regulation. Future studies should evaluate coordination across stress response systems as well as the contribution of changes to inflammatory and gonadal systems to understanding the impact of injury sustained at different neurodevelopmental stages.
Given the high incidence of pediatric injury and public health burden, it is critically important to understand links between neurobiological changes and long-term physical and psychological health outcomes. Brain versus body injury and age at injury were major determinants of individual differences in HPA axis and ANS activity and regulation. The weak relation of injury severity with the development of PTSS and dysregulation of both HPA and ANS stress axes suggests that surveillance and intervention efforts should target the entire spectrum of injury severity in youth hospitalized following injury to the brain or body. Improved understanding of relations between neurobiological and psychological responses following injury is critically important to guide development of interventions in children and adolescents sustaining injuries. Targeted strategies should be developed to assess efficacy of behavioral and/or pharmacological therapies to reduce PTSS, normalize stress system reactivity, and improve health related quality of life following pediatric injury (Abelson et al., 2014).
Highlights.
HPA and ANS stress systems were dysregulated after nonintentional pediatric injury
HPA and ANS activity and regulation varied with age at injury and type of injury
Cortisol reactivity to social stress was attenuated after brain or body injury
After brain injury, cortisol elevated in children, sAA elevated in adolescents
sAA, but not salivary cortisol, predicted specific post-traumatic stress symptoms
Acknowledgments
This work was funded in part by National Institutes of Health R01 NS046308. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: In the interest of full disclosure, DAG is founder and Chief Scientific and Strategy Advisor at Salimetrics LLC and Salivabio LLC. These relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine.
Drs. Ewing-Cobbs, Prasad, Cox, and Swank and Mr. Duque have no disclosures or conflicts of interest.
References
- 1.Abelson JL, Erickson TM, Mayer SE, Crocker J, Briggs H, Lopez-Duran NL, Liberzon I. Brief cognitive intervention can modulate neuroendocrine stress responses to the Trier Social Stress Test: buffering effects of a compassionate goal orientation. Psychoneuroendocrinology. 2014;44:60–70. doi: 10.1016/j.psyneuen.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achenbach T. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont College of Medicine; Burlington, VT: 1991. [Google Scholar]
- 3.Allwood MA, Bell DJ, Horan J. Posttrauma numbing of fear, detachment, and arousal predict delinquent behaviors in early adolescence. J. Clin. Child Adol. Psychol. 2011a;40:659–667. doi: 10.1080/15374416.2011.597081. [DOI] [PubMed] [Google Scholar]
- 4.Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biol. Psychol. 2011b;88:57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth. American Psychiatric Publishing; Washington, D.C.: 2013. [Google Scholar]
- 6.Bae YJ, Stadelmann S, Klein AM, Jaeger S, Hiemisch A, Kiess W, Ceglarek U, Gaudl A, Schaab M, von Klitzing K, Thiery J, Kratzsch J, Dohnert M. The hyporeactivity of salivary cortisol at stress test (TSST-C) in children with internalizing or externalizing disorders is contrastively associated with alpha-amylase. J Psychiatr. Res. 2015;71:78–88. doi: 10.1016/j.jpsychires.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: a method of describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 8.Biederman J, Faraone SV, Monuteaux MC. Differential effect of environmental adversity by gender: Rutter’s index of adversity in a group of boys and girls with and without ADHD. Am. J. Psychiatr. 2002;159:1556–1562. doi: 10.1176/appi.ajp.159.9.1556. [DOI] [PubMed] [Google Scholar]
- 9.Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom. Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol. Psychiatr. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, N.C.f.I.P.a.C. Web-based injury statistics query and reporting system (WISQARS) 2016 [Google Scholar]
- 12.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc. Psychiatr Clin. N. Am. 2014;23:185–222. doi: 10.1016/j.chc.2014.01.002. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delahanty DL, Nugent NR. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Ann. N. Y. Acad. Sci. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- 14.Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102–111. doi: 10.1016/j.psyneuen.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewing-Cobbs L, Bloom DR, Prasad MR, Waugh JK, Cox CS, Jr, Swank PR. Assessing recovery and disability after physical trauma: the Pediatric Injury Functional Outcome Scale. J. Pediatr. Psychol. 2014;39:653–665. doi: 10.1093/jpepsy/jsu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foa EB, Johnson KM, Feeny NC, Treadwell KRH. The child PTSD symptom scale: A preliminary examination of its psychometric properties. J. Clin Child Psychol. 2001;30:376–384. doi: 10.1207/S15374424JCCP3003_9. [DOI] [PubMed] [Google Scholar]
- 18.Frewen PA, Dozois DJ, Neufeld RW, Lane RD, Densmore M, Stevens TK, Lanius RA. Emotional numbing in posttraumatic stress disorder: a functional magnetic resonance imaging study. J. Clin. Psychiatr. 2012;73:431–436. doi: 10.4088/JCP.10m06477. [DOI] [PubMed] [Google Scholar]
- 19.Gandubert C, Scali J, Ancelin ML, Carriere I, Dupuy AM, Bagnolini G, Ritchie K, Sebanne M, Martrille L, Baccino E, Hermes A, Attal J, Chaudieu I. Biological and psychological predictors of posttraumatic stress disorder onset and chronicity. A one-year prospective study. Neurobiol. Stress. 2016;3:61–67. doi: 10.1016/j.ynstr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm. Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of a risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp. Neurol. 2016;284:181–195. doi: 10.1016/j.expneurol.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma. Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 25.Juranek J, Johnson CP, Prasad MR, Kramer LA, Saunders A, Filipek PA, Swank PR, Cox CS, Jr, Ewing-Cobbs L. Mean diffusivity in the amygdala correlates with anxiety in pediatric TBI. Brain Imag. Behav. 2012;6:36–48. doi: 10.1007/s11682-011-9140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassam-Adams N, Marsac ML, Cirilli C. Posttraumatic stress disorder symptom structure in injured children: functional impairment and depression symptoms in a confirmatory factor analysis. J. Am. Acad. Child Adolesc. Psychiatr. 2010;49:616–625. doi: 10.1016/j.jaac.2010.02.011. 625.e611-614. [DOI] [PubMed] [Google Scholar]
- 27.Kaulfers AM, Backeljauw PF, Reifschneider K, Blum S, Michaud L, Weiss M, Rose SR. Endocrine dysfunction following traumatic brain injury in children. J. Pediatr. 2010;157:894–899. doi: 10.1016/j.jpeds.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Keeshin BR, Strawn JR, Out D, Granger DA, Putnam FW. Elevated salivary alpha amylase in adolescent sexual abuse survivors with posttraumatic stress disorder symptoms. J. Child Adolesc. Psychopharmacol. 2015;25:344–350. doi: 10.1089/cap.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury JE, Gonzalez A, Levitan RD, Pruessner JC, Chopra K, Basile VS, Masellis M, Goodwill A, Atkinson L. Summary cortisol reactivity indicators: Interrelations and meaning. Neurobiol. Stress. 2015;2:34–43. doi: 10.1016/j.ynstr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koss KJ, Mliner SB, Donzella B, Gunnar MR. Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology. 2016;66:31–38. doi: 10.1016/j.psyneuen.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuras YI, McInnis CM, Thoma MV, Chen X, Hanlin L, Gianferante D, Rohleder N. Increased alpha-amylase response to an acute psychosocial stress challenge in healthy adults with childhood adversity. Dev. Psychobiol. 2017;59:91–98. doi: 10.1002/dev.21470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laflamme L, Hasselberg M, Burrows S. 20 years of research on socioeconomic inequality and children’s unintentional injuries: understanding the cause-specific evidence at hand. Int. J. Pediatr. 2010 doi: 10.1155/2010/819687. Epub 2010 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 34.Max JE, Lopez A, Wilde EA, Bigler ED, Schachar RJ, Saunders A, Ewing-Cobbs L, Chapman SB, Yang TT, Levin HS. Anxiety disorders in children and adolescents in the second six months after traumatic brain injury. J. Pediatr. Rehabil. Med. 2015;8:345–355. doi: 10.3233/PRM-150352. [DOI] [PubMed] [Google Scholar]
- 35.Mayer T, Matlak ME, Johnson DG, Walker ML. The modified injury severity scale in pediatric multiple trauma patients. J. Pediatr. Surg. 1980;15:719–726. doi: 10.1016/s0022-3468(80)80271-5. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS, Gray J, Nasca C. Recognizing resilience: Learning from the effects of stress on the brain. Neurobiol. Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarlane AC, Barton CA, Yehuda R, Wittert G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36:720–727. doi: 10.1016/j.psyneuen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Pacella ML, Hruska B, Delahanty DL. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J. Anx. Disord. 2013;27:33–46. doi: 10.1016/j.janxdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Pacella ML, Hruska B, Steudte-Schmiedgen S, George RL, Delahanty DL. The utility of hair cortisol concentrations in the prediction of PTSD symptoms following traumatic physical injury. Soc. Sci. Med. 2017;175:228–234. doi: 10.1016/j.socscimed.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 41.Payne LA, Hibel LC, Granger DA, Tsao JC, Zeltzer LK. Relationship of salivary alpha amylase and cortisol to social anxiety in healthy children undergoing laboratory pain tasks. J. Child Adolesc. Behav. 2014;2 doi: 10.4172/jcalb.1000129. pii 1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peckins MK, Susman EJ, Negriff S, Noll J, Trickett PK. Cortisol profiles: A test for adaptive calibration of the stress response system in maltreated and nonmaltreated youth. Dev. Psychopathol. 2015;27:1461–1470. doi: 10.1017/S0954579415000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Papassotiriou I, Hindmarsh P, Bakoula C, Tsiantis J, Chrousos GP. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: Progressive divergence of noradrenaline and cortisol concentrations over time. Biol. Psychiatr. 2007;62:1095–1102. doi: 10.1016/j.biopsych.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Price J, Kassam-Adams N, Alderfer MA, Christofferson J, Kazak AE. Systematic review: A reevaluation and update of the integrative (trajectory) model of pediatric medical traumatic stress. J. Pediatr. Psychol. 2016;41:86–97. doi: 10.1093/jpepsy/jsv074. [DOI] [PubMed] [Google Scholar]
- 45.Reifschneider K, Auble BA, Rose SR. Update of endocrine dysfunction following pediatric traumatic brain injury. J. Clin. Med. 2015;4:1536–1560. doi: 10.3390/jcm4081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreier H, Ladakakos C, Morabito D, Chapman L, Knudson MM. Posttraumatic stress symptoms in children after mild to moderate pediatric trauma: a longitudinal examination of symptom prevalence, correlates, and parent-child symptom reporting. J. Trauma. 2005;58:353–363. doi: 10.1097/01.ta.0000152537.15672.b7. [DOI] [PubMed] [Google Scholar]
- 47.Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev. Psychobiol. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 49.Trickett PK, Gordis E, Peckins MK, Susman EJ. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment. 2014;19:27–37. doi: 10.1177/1077559513520466. [DOI] [PubMed] [Google Scholar]
- 50.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weems CF, Carrion VG. The association between PTSD symptoms and salivary cortisol in youth: The role of time since the trauma. J. Trauma. Stress. 2007;20:903–907. doi: 10.1002/jts.20251. [DOI] [PubMed] [Google Scholar]
- 52.Weems CF, Saltzman KM, Reiss AL, Carrion VG. A prospective test of the association between hyperarousal and emotional numbing in youth with a history of traumatic stress. J. Clin. Child Adolesc. Psychol. 2003;32:166–171. doi: 10.1207/S15374424JCCP3201_15. [DOI] [PubMed] [Google Scholar]
- 53.Wilde EA, Bigler ED, Hunter JV, Fearing MA, Scheibel RS, Newsome MR, Johnson JL, Bachevalier J, Levin HS. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Develop. Med. Child Neurol. 2007;49:294–299. doi: 10.1111/j.1469-8749.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 54.Williamson JB, Heilman KM, Porges EC, Lamb DG, Porges SW. A possible mechanism for PTSD symptoms in patients with traumatic brain injury: central autonomic network disruption. Front. Neuroengineer. 2013;6:13. doi: 10.3389/fneng.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology. 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
- 56.Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, Hobfoll SE, Koenen KC, Neylan TC, Hyman SE. Post-traumatic stress disorder. Nature reviews. Disease Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- 57.Yim IS, Quas JA, Rush EB, Granger DA, Skoluda N. Experimental manipulation of the Trier Social Stress Test-Modified (TSST-M) to vary arousal across development. Psychoneuroendocrinology. 2015;57:61–71. doi: 10.1016/j.psyneuen.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Zatzick DF, Rivara FP, Jurkovich GJ, Hoge CW, Wang J, Fan MY, Russo J, Trusz SG, Nathens A, Mackenzie EJ. Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Arch. Gen. Psychiatr. 2010;67:1291–1300. doi: 10.1001/archgenpsychiatry.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]