Abstract
Background
In the search for specific phenotypes of chronic obstructive pulmonary disease (COPD) computed tomography (CT) derived Parametric Response Mapping (PRM) has been introduced. This study evaluates the association between PRM and currently available biomarkers of disease severity in COPD.
Methods
Smokers with and without COPD were characterized based on questionnaires, pulmonary function tests, body plethysmography, and low-dose chest CT scanning. PRM was used to calculate the amount of emphysema (PRMEmph) and non-emphysematous air trapping (i.e. functional small airway disease, PRMfSAD). PRM was first compared with other biomarkers for emphysema (Perc15) and air trapping (E/I-ratioMLD). Consequently, linear regression models were utilized to study associations of PRM measurements with clinical parameters.
Results
166 participants were included with a mean ± SD age of 50.5 ± 17.7 years. Both PRMEmph and PRMfSAD were more strongly correlated with lung function parameters as compared to Perc15 and E/I-ratioMLD. PRMEmph and PRMfSAD were higher in COPD participants than non-COPD participants (14.0% vs. 1.1%, and 31.6% vs. 8.2%, respectively, both p < 0.001) and increased with increasing GOLD stage (all p < 0.001). Multivariate analysis showed that PRMfSAD was mainly associated with total lung capacity (TLC) (β = −7.90, p < 0.001), alveolar volume (VA) (β = 7.79, p < 0.001), and residual volume (β = 6.78, p < 0.001), whilst PRMEmph was primarily associated with Kco (β = 8.95, p < 0.001), VA (β = −6.21, p < 0.001), and TLC (β = 6.20, p < 0.001).
Conclusions
PRM strongly associates with the presence and severity of COPD. PRM therefore appears to be a valuable tool in differentiating COPD phenotypes.
Keywords: Computed tomography, Parametric response mapping, Copd, Phenotypes, Emphysema, Small airway disease
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation of the airways and lung parenchyma, inevitably leading to (partly irreversible) pulmonary changes. Due to smoking and biomass fuel exposure, the morbidity and mortality of patients inflicted with this disease continues to increase world-wide [1]. COPD has a complex pathophysiology not fully captured by lung function tests only. As such there is a clear clinical need for diagnostic techniques and biomarkers that accurately characterize COPD in addition to lung function tests [2,3], that are able to identify specific subtypes, i.e. phenotypes, with unique prognostic and therapeutic information [4,5].
Several techniques have been proposed to discriminate different radiological COPD phenotypes, based on computed tomography (CT). For example, emphysema can be determined on CT by measuring the extent of low attenuation areas providing an objective method of emphysema assessment [6]. On an expiratory CT scan, air trapping can be quantified by measuring the ratio of the mean low attenuation at expiration to the mean low attenuation at inspiration [7]. Although these CT-biomarkers are associated with several clinical parameters for COPD severity [8], generally they each capture mainly one component of this heterogeneous disease. Additionally, current available techniques for measuring small airway disease are unable to distinguish air trapping as a result of small airway disease when there is evidence of emphysema. Hence, there is a need of a more complete and accurate quantitative CT tool.
In search of a more robust CT-biomarker for COPD quantification, a novel technique has been introduced called Parametric Response Mapping (PRM) [9]. PRM spatially aligns paired inspiratory and expiratory CT scans allowing, for the first time, differentiation of emphysematous from non-emphysematous air trapping (i.e. functional small airway disease) within the lung parenchyma.
Recently, it has been shown that PRM adds value to currently-known CT-measurements in diagnosing COPD [10]. It is able to detect changes over time [11], and is associated with age and smoking status [12]. Preliminary results have been published on the association of PRM with clinical COPD parameters such as forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) and it has been shown that PRM is associated with lung function decline [9,13]. However, the association of PRM with established markers of COPD disease severity, such as lung function parameters, diffusion capacity, BODE index and exacerbation frequency, remains to be determined in a well-characterized cohort, which is an essential step in the validation process of biomarkers [8].
The aim of this study was to evaluate the new imaging biomarker PRM with clinical and functional COPD parameters across the entire COPD spectrum.
2. Methods
2.1. Participants
This retrospective post-hoc study included participants that took part in a multicenter cross-sectional study located at the University Medical Center Utrecht (UMCU) and the University Medical Center Groningen (UMCG). The primary aim of this study was to evaluate acute and chronic inflammatory responses induced by smoking [14]. Included participants were: a group of young (age 18–40 years) smokers with 0–10 packyears and normal lung function, ex or current smokers with more than 20 packyears (age 40–75 years) and normal lung function, and COPD patients with GOLD stages varying from 1 to 4. Participants were extensively characterized, based on questionnaires, pulmonary function tests, body plethysmography, low-dose chest CT scanning, body mass index (BMI), and six minute walking distance (6MWD). Exclusion criteria were the presence of α-1-antitrypsin deficiency, an acute (pulmonary) infection, a prior history of inflammatory lung diseases other than COPD, treatment with antibiotics or corticosteroids within 8 weeks prior to inclusion, a recent diagnosis of cancer or participation in another study. The study was approved by the medical ethics committees of the UMCU and the UMCG (project approval number NL23437.042.08). The study is registered at clinicaltrial.gov with registration numbers NCT00807469 and NCT00850863. Written informed consent was obtained for all participants.
2.2. Clinical characteristics
Demographic variables were obtained for all participants and included information about smoking history. 6MWD was determined according to ATS guidelines [15]. Participants had to walk at their own pace and could stop if necessary with the availability of the use of oxygen. The Medical Research Council (MRC) dyspnea score was determined for all participants. The St George’s Respiratory Questionnaire (SGRQ) was obtained from participants with COPD in accordance with the Medical School guidelines, without modification of the questionnaire [16]. Also, the amount of exacerbations and antibiotic or corticosteroid courses in the past year were recorded. The BODE-index was calculated based on FEV1, the 6MWD, BMI, and the MRC-dyspnea score [17].
2.3. Functional characteristics
Spirometry was performed according to the European Respiratory Society (ERS)/American Thoracic Society (ATS) guidelines [18]. Post-bronchodilator FEV1, FVC, vital capacity (VC), total lung capacity (TLC), and residual volume (RV) were recorded. All participants underwent whole body plethysmography to calculate alveolar volume (VA) and diffusion capacity of the lung for carbon monoxide (TLCO). After correction of TLCO for current hemoglobin level (TLCOc) the gas transfer corrected for lung volume (KCO) was calculated.
2.4. Image acquisition
All participants underwent inspiratory and expiratory low-dose multi detector volumetric thin-slice chest CT. For the inspiratory acquisition, participants weighing less than 50 kg were exposed to 30 mAs at 90 kVp, participants weighing between 50 and 80 kg were exposed to 30 mAs at 120 kVp, and participants weighing more than 80 kg were exposed to 30 mAs at 140 kVp. During the expiratory acquisition participants weighing less than 80 kg were exposed to 20 mAs at 90 kVp and participants weighing more than 80 kg were exposed to 20 mAs at 120 kVp.
2.5. CT quantification
Lung segmentation and image registration of paired CT scans were performed using Lung Density Analysis (Imbio, LLC, Minneapolis, MN), which is a FDA approved medical device in the US. After image processing, paired histograms of the inspiratory and expiratory CT scans were analyzed and voxels were classified based on their attenuation values. Voxels between −1000 Hounsfield Units (HU) and −950 HU in the inspiratory CT and between −1000 HU and −856 HU in the expiratory CT represented emphysema (PRMEmph). All voxels between −950 HU and −810 HU in the inspiratory CT and between −1000 HU and −857 HU in the expiratory CT represented non-emphysematous air trapping or functional small airway disease (PRMfSAD). All voxels between −950 HU and −810 HU in the inspiratory CT and between −856 and −500 HU in the expiratory CT represented normal lung tissue (PRMNorm), see Fig. 1. PRM-values were corrected for lung volume on CT to achieve relative lung volumes.
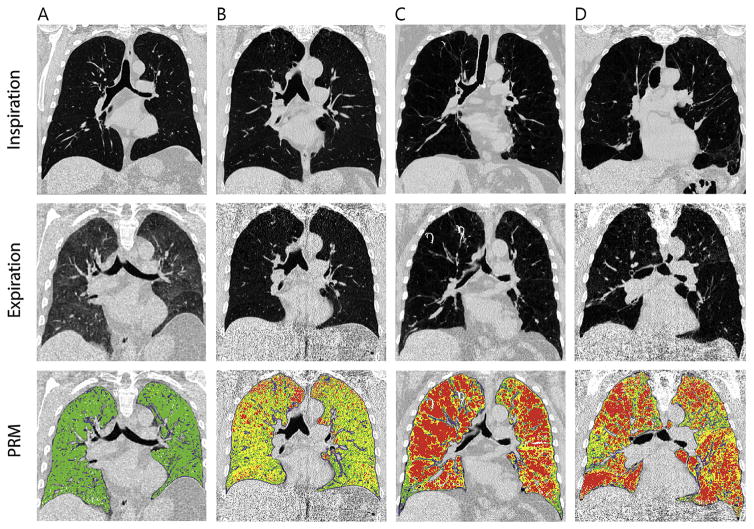
Fig. 1.
Parametric response mapping in four different patients. Normal lung tissue is denoted green (PRMNormal), functional small airway disease yellow (PRMfSAD), and emphysematous lung tissue red (PRMEmph). (A) 46-year old male with 25 pack years. He does not have COPD. The PRM image shows all green voxels, representing normal lung tissue. (B) 58-year old male with 40 pack years and COPD GOLD 3 (FEV1/FVC: 37%; FEV1% predicted: 41,2%). The PRM image shows that a large part of both lungs contain yellow voxels representing functional small airway disease. PRMfSAD was 49.9%. (C) 58-year old male with 31 pack years and COPD GOLD stage 4 (FEV1/FVC: 24%; FEV1% predicted: 20.1%). According to the red voxels of the PRM-image, his lung function impairment is mainly the result of emphysema. PRMEmph was 49.3%. (D) 70-year old male with 35 pack-years and COPD GOLD stage 3 (FEV1/FVC: 23%; FEV1% predicted: 32.2%). According to the PRM-image, his lung function impairment is due to both small airway disease and emphysema. PRMEmph was 38.5% and PRMfSAD was 32.1%. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
PRM was compared with previously reported CT-biomarkers for emphysema (Perc15) and air trapping (expiratory to inspiratory ratio of mean lung density, E/I-ratioMLD). Perc15 represents the HU value of which below 15% of the voxels on inspiratory CT are distributed. The lower Perc15, the more emphysema is present. E/I-ratioMLD is a method of dividing the mean lung density derived from the expiratory scan by the mean lung density derived from the inspiratory scan. E/I-ratioMLD is expressed in a percentage. The higher this percentage, the more air trapping is present.
2.6. Statistical analysis
After normality was checked with Q-Q plots, mean (standard deviation, SD) and median (inter-quartile range, IQR) values were calculated as appropriate. PRM-values were compared with Perc15 and E/I-ratioMLD by calculating Spearman correlation coefficients between CT-metrics and FEV1% predicted, FEV1/FVC, RV/TLC, and KCO. Consequently, PRM-values were compared between groups using Student’s t-tests and one-way ANOVA for normally distributed values and with Mann-Whitney U tests and Kruskal Wallis tests for non-normally distributed data. Univariate linear regression analyses were performed to evaluate the association between PRM values as dependent variables and clinical variables. Standardized regression coefficients were obtained by standardizing the independent parameters (z-scores) and subsequently inserting these into the regression analysis. R squared values were used to express the percentages of variance explained. Multivariate regression analysis included parameters from the univariate analysis with a p-value below 0.2. Missing values (at worst 10%) were imputed with the multiple imputation method (20 iterations). Subsequent analyses were pooled over 20 imputations. Multi-collinearity among the variables was evaluated by using Pearson correlation coefficients and was considered acceptable when lower than 0.8. A significance level of <0.05 was set with Bonferroni correction for multiple testing.
3. Results
3.1. Participants
Of the 195 participants initially included in the study 6 participants withdrew their informed consent and 23 participants could not be evaluated using PRM because of CT quality issues or because expiratory images were lacking. This resulted in 166 participants eligible for analysis. 53 data points were missing, with a maximum of 4 per variable (2.4%). Mean ± SD age was 50.5 ± 17.7 years, 50 (30.1%) participants being female, 116 (69.9%) male. Baseline characteristics are shown in Table 1. Based on the GOLD criteria 95 (57.2%) participants were classified as having COPD (32 participants were classified as having COPD GOLD 1, 23 GOLD 2, 27 GOLD 3, and 13 GOLD 4, respectively).
Table 1.
Baseline characteristics.
| Parameter | Young smokers without COPD (n = 47) | Older smokers without COPD (n = 24) | GOLD 1 (n = 32) | GOLD 2 (n = 23) | GOLD 3 (n = 27) | GOLD 4 (n = 13) |
|---|---|---|---|---|---|---|
| Age, years | 25.5 ± 7.7 | 55.3 ± 9.6 | 62.6 ± 8.0 | 63.8 ± 6.1 | 61.1 ± 6.3 | 56.2 ± 10.1 |
| Male sex, n(%) | 28 (59.6%) | 19 (79.2%) | 26 (81.3%) | 19 (82.6%) | 17 (63.0%) | 7 (53.8%) |
| BMI, kg/m2 | 23.4 ± 2.8 | 26.1 ± 3.7 | 26.8 ± 4.9 | 26.0 ± 3.6 | 23.9 ± 4.0 | 26.0 ± 7.5 |
| Packyears | 4.0 ± 5.3 | 29.0 ± 12.3 | 40.1 ± 17.9 | 33.8 ± 9.3 | 41.9 ± 18.0 | 35.5 ± 20.6 |
| Current smokers, n(%) | 40 (85.1%) | 22 (91.7%) | 20 (62.5%) | 14 (60.9%) | 13 (48.1%) | 3 (23.1%) |
| FEV1% predicted, % | 108.1 ± 8.8 | 111.9 ± 14.3 | 95.8 ± 10.3 | 63.9 ± 9.4 | 40.8 ± 5.6 | 23.5 ± 4.0 |
| FEV1/FVC, % | 84.8 ± 6.1 | 79.1 ± 6.1 | 63.2 ± 6.3 | 49.9 ± 9.1 | 36.9 ± 5.9 | 29.2 ± 5.6 |
| FEV1/VC, % | 85.1 ± 6.9 | 77.7 ± 5.4 | 60.1 ± 5.9 | 46.7 ± 8.3 | 34.2 ± 5.4 | 23.5 ± 3.8 |
| TLC, L | 7.1 ± 1.2 | 7.2 ± 1.1 | 8.0 ± 1.5 | 7.7 ± 1.4 | 7.7 ± 1.4 | 8.4 ± 1.4 |
| RV, L | 1.6 ± 0.5 | 2.3 ± 0.5 | 2.9 ± 0.8 | 3.4 ± 0.8 | 4.1 ± 0.9 | 5.3 ± 1.2 |
| RV/TLC, % | 23.3 ± 6.6 | 31.6 ± 5.9 | 36.2 ± 6.8 | 43.7 ± 7.3 | 53.1 ± 7.5 | 63.1 ± 6.1 |
| TLCOc, mmol/min/kPa | 10.6 ± 2.3 | 8.9 ± 1.6 | 7.3 ± 2.4 | 6.1 ± 2.0 | 3.9 ± 1.7 | 3.5 ± 2.2 |
| VA, L | 6.4 ± 1.1 | 6.3 ± 1.0 | 6.8 ± 1.1 | 5.9 ± 1.1 | 5.4 ± 1.0 | 4.7 ± 0.7 |
| KCO, mmol/min/kPa/L | 1.7 ± 0.3 | 1.4 ± 0.2 | 1.1 ± 0.3 | 1.0 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.3 |
| 6MWD, m | – | – | 531 ± 80 | 540 ± 85 | 450 ± 89 | 329 ± 117 |
| MRC dyspnea scale | 0.6 ± 0.5 | 0.7 ± 0.6 | 1.3 ± 0.7 | 1.8 ± 0.9 | 2.5 ± 1.3 | 3.9 ± 1.0 |
| BODE index | – | – | 0.1 ± 0.4 | 0.8 ± 1.0 | 3.1 ± 1.5 | 6.0 ± 1.7 |
| SGRQ score | – | – | 21.0 ± 12.2 | 30.4 ± 14.2 | 38.9 ± 13.3 | 49.7 ± 15.0 |
| No. of exacerbations | – | – | 0.6 ± 1.9 | 0.4 ± 0.8 | 1.3 ± 2.1 | 1.5 ± 1.6 |
| No. of antibiotic courses | 0.2 ± 0.6 | 0.1 ± 0.4 | 0.2 ± 0.4 | 0.7 ± 1.0 | 1.5 ± 2.0 | 1.7 ± 1.4 |
| No. of prednisolone courses | 0 | 0 ± 0.2 | 0.2 ± 0.5 | 0.4 ± 0.8 | 1.4 ± 2.2 | 1.8 ± 1.4 |
| PRMNormal, % | 77.0 (66.8–80.9) | 72.7 (67.8–78.1) | 51.4 (45.4–59.1) | 42.8 (33.1–46.9) | 30.2 (22.7–34.1) | 18.7 (12.3–29.8) |
| PRMEmph, % | 0.9 (0.1–1.1) | 1.4 (0.3–1.5) | 3.7 (1.9–7.8) | 7.4 (4.0–14.3) | 14.7 (10.1–30.2) | 26.2 (17.8–46.4) |
| PRMfSAD, % | 4.1 (1.3–7.4) | 9.8 (4.6–14.1) | 25.4 (19.1–31.2) | 31.2 (25.8–37.4) | 35.1 (31.8–41.7) | 37.1 (31.0–37.5) |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; VC: vital capacity; TLC: total lung capacity; RV: residual volume; TLCOc: pulmonary diffusion capacity for carbon monoxide, corrected for hemoglobin count; VA: alveolar volume; KCO: transfer coefficient for carbon monoxide; BMI: body mass index; 6MWD: six minute walking distance; MRC: Medical Research Council; SGRQ: St. George’s respiratory questionnaire; PRMNormal: extend of normal lung tissue; PRMEmph: extend of emphysema; PRMfSAD: extend of small airway disease.
3.2. Comparison with other CT-metrics
PRM-values were compared with Perc15 and E/I-ratioMLD by evaluating correlation coefficients with clinical variables. This showed that both PRMEmph and PRMfSAD were more strongly correlated with FEV1% predicted, FEV1/FVC, FEV1/VC, RV/TLC, and KCO, than Perc15 and E/I-ratioMLD. Complete results with correlation coefficients are shown in Table 2.
Table 2.
Spearman’s Rho correlation coefficients between CT-metrics (PRMEmph, PRMfSAD, Perc15, and E/I-ratioMLD) and clinical parameters. All correlation coefficients were statistically significant (all p < 0.001).
| FEV1% predicted | FEV1/FVC | FEV1/VC | RV/TLC | KCO | |
|---|---|---|---|---|---|
| PRMEmph | −0.763 | −0.858 | −0.862 | 0.770 | −0.797 |
| PRMfSAD | −0.724 | −0.819 | −0.827 | 0.779 | −0.752 |
| Perc15 | 0.572 | 0.636 | 0.632 | −0.503 | 0.523 |
| E/I-ratioMLD | −0.645 | −0.723 | −0.730 | 0.736 | −0.677 |
PRMEmph: extent of emphysema as determined by parametric response mapping; extent of small airway disease as determined by parametric response mapping; Perc15: emphysema score as 15th percentile of attenuation distribution curve on inspiratory scan; E/I-ratioMLD: expiration to inspiration ratio of mean lung density.
3.3. Functional characteristics and PRM
PRMNorm was significantly lower in COPD participants than non-COPD participants (38.0% vs. 71.8%, p < 0.001) and decreased with increasing GOLD stage (p < 0.001). PRMEmph and PRMfSAD were both higher in COPD participants as compared to non-COPD participants (14.0% vs. 1.1%, and 31.6% vs. 8.2%, respectively, both p < 0.001) and were higher with increasing GOLD stage (both p < 0.001). Results of PRM values classified by GOLD stage are shown in Fig. 2. Results of post-hoc tests are shown in Tables 1–5 of the Supplementary material.
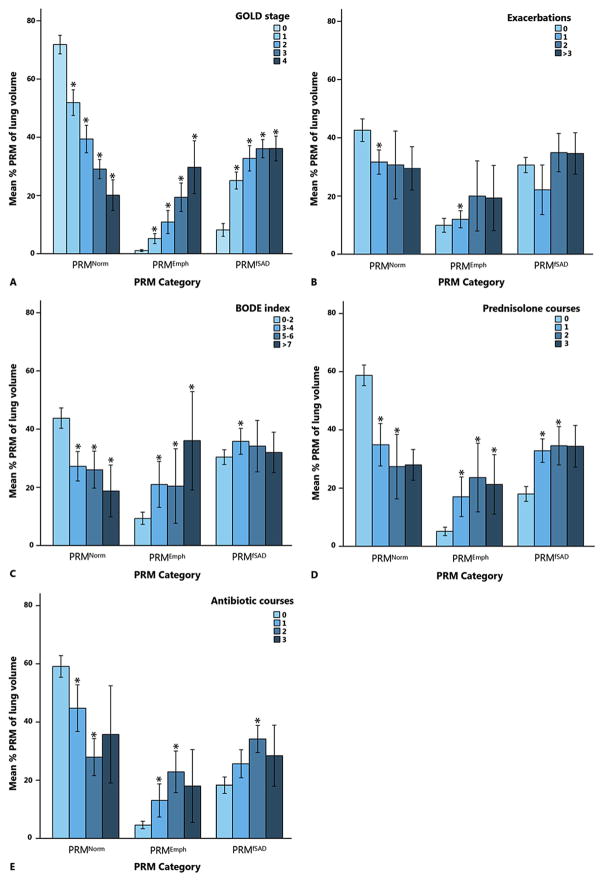
Fig. 2.
Values of PRM categories classified by (A) GOLD stage, (B) the number of exacerbations in the past year, (C) BODE index, (D) the number of prednisolone courses in the past year, and (E) the number of antibiotic courses in the past year. GOLD stage 0 = no COPD.
PRM: parametric response mapping; GOLD: global initiative for Chronic Obstructive Lung Disease; BODE: body mass index, airflow obstruction, dyspnea, and exercise; PRMNormal: extent of normal lung tissue; PRMEmph: extent of emphysema; PRMfSAD: extent of small airway disease; *: significantly different from GOLD stage 0 in (A), 0 exacerbations in (B), BODE index 0–2 in (C), 0 prednisolone courses in (D), and 0 antibiotic courses in (E).
Univariate analysis showed that higher PRMfSAD and PRMEmph values were associated with lower lung function (i.e. FEV1% predicted and FEV1/FVC). Furthermore, an absolute decrease in KCO with one mmol/min/kPa/L resulted in an increase in PRMfSAD and PRMEmph of 23.3% and 16.6%, respectively. Complete results of the univariate analyses coefficients can be found in Table 3.
Table 3.
Univariate and multivariate results with PRM measurements as dependent variables and clinical parameters as independent variables.
| PRMfSAD | PRMEmph | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
|
|
|
|
|||||
| Effect size (95% CI) | P-value | Effect size (95%CI) | P-value | Effect size (95%CI) | P-value | Effect size (95%CI) | P-value | |
| Age, years | 0.6 (0.5–0.7) | <0.001 | 0.21 (0.10–0.33) | <0.001 | 0.3 (0.2–0.4) | <0.001 | – | – |
| BMI, kg/m2 | 0.6 (0.03–1.1) | 0.04 | – | – | −0.4 (−0.9–−0.01) | 0.04 | −0.27 (−0.49–−0.04) | 0.02 |
| Packyears, years | 0.4 (0.3–0.5) | <0.001 | – | – | 0.2 (0.1–0.2) | 0.001 | −0.07 (−0.13–−0.01) | 0.02 |
| FEV1% predicted | −0.3 (−0.4–−0.3) | <0.001 | −0.21 (−0.30–−0.13) | <0.001 | −0.3 (−0.3–−0.2) | <0.001 | −0.10 (−0.16–−0.01) | <0.001 |
| FEV1/FVC, % | −0.6 (−0.6–−0.5) | <0.001 | – | – | −0.4 (−0.5–−0.4) | <0.001 | – | – |
| FEV1/VC, % | −0.004 (−0.01–−0.004) | <0.001 | – | – | −0.003 (−0.004–−0.003) | <0.001 | – | – |
| TLC, L | 1.8 (0.2–3.4) | 0.03 | −7.90 (−11.21–−4.58) | <0.001 | 2.8 (1.6–4.0) | <0.001 | 6.20 (4.09–8.31) | <0.001 |
| RV, L | 7.5 (6.1–8.9) | <0.001 | 6.78 (3.48–10.07) | <0.001 | 6.8 (5.9–7.7) | <0.001 | −1.94 (−3.99–0.10) | 0.06 |
| RV/TLC, % | 0.8 (0.7–0.9) | <0.001 | – | – | 0.6 (0.5–0.7) | <0.001 | – | – |
| VA, L | −2.2 (−4.2–−0.1) | 0.04 | 7.79 (4.62–10.97) | <0.001 | −2.6 (−4.0–−1.3) | <0.001 | −6.21 (−8.25–−5.93) | <0.001 |
| KCO, mmol/min/kPa/L | −23.3 (−27.2–−19.4) | <0.001 | −5.84 (−11.01–−0.66) | 0.03 | −16.6 (−19.2–−13.9) | <0.001 | −8.95 (−11.98–−5.93) | <0.001 |
| TLCOc, mmol/min/kPa | −3.0 (−3.6–−2.5) | <0.001 | – | – | −2.2 (−2.6–−1.8) | <0.001 | – | – |
| MRC dyspnea scale | 6.1 (4.6–7.6) | <0.001 | – | – | 6.0 (4.9–7.0) | <0.001 | – | – |
| 6MWD, m | −0.04 (−0.1–−0.02) | <0.001 | – | – | −0.06 (−0.1–−0.04) | <0.001 | – | – |
| SGRQ score | 0.1 (−0.01–0.2) | 0.26 | – | – | 0.4 (0.3–0.5) | <0.001 | – | – |
Legend: PRMfSAD: extend of small airway disease; PRMEmph: extend of emphysema; PRMPD: extend of parenchymal disease; CI: confidence interval; BMI: body mass index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; TLC: total lung capacity; RV: residual volume; VA: alveolar volume; MRC: Medical Research Council. Parameters with a p-value of less than 0.2 in the univariate analysis were included in the multivariate analysis, unless there was multicollinearity present. Adjusted R2 of the models was 0.69 for PRMfSAD and 0.75 for PRMEmph.
3.4. Clinical characteristics and PRM
Clinical characteristics stratified by quintiles of PRMfSAD and PRMEmph are found in Tables 6 and 7 of the Supplementary material. Age, packyears, and 6MWD were significantly associated with higher PRMfSAD and PRMEmph measurements (all p ≤ 0.001). A higher PRMEmph was furthermore associated with a higher SGRQ score (β = 0.4, p < 0.001), whilst PRMfSAD was not. PRMEmph was higher with an increase in the following categorical parameters: the number of exacerbations (p = 0.003), BODE index (p < 0.001), MRC scale (p < 0.001), the number of prednisolone (p < 0.001), and antibiotic courses (p < 0.001). PRMfSAD was higher with an increasing MRC scale (p < 0.001) and with the number of prednisolone (p < 0.001) and antibiotic courses (p < 0.001). Results for PRMEmph and PRMfSAD remained statistically significant after post-hoc testing with Bonferroni corrections. Results of PRM values classified by BODE index, number of exacerbations, number of prednisolone courses, and number of antibiotic courses, are shown in Fig. 2.
3.5. Multivariate analysis
Multicollinearity was present between FEV1/FVC and FEV1/VC, RV/TLC, FEV1% predicted, and KCO. Therefore, parameters with the highest standardized regression coefficients and R squared values in univariate analysis were included in the multivariate analysis. Results of the final linear multivariate models with PRM measurements as dependent variables are shown in Table 3. PRMfSAD was primarily associated with total lung capacity (TLC) (β = −7.90, p < 0.001), alveolar volume (VA) (β = 7.79, p < 0.001), and residual volume (β = 6.78, p < 0.001). PRMEmph was primarily associated with Kco (β = 8.95, p < 0.001), VA (β = −6.21, p < 0.001), and TLC (β = 6.20, p < 0.001). Adjusted R2 for the final models was 0.69 for PRMfSAD and 0.75 for PRMEmph.
4. Discussion
We showed that PRM biomarkers of small airway disease and emphysema increased with each GOLD stage and were well associated with clinically important parameters assessing COPD morbidity such as packyears, FEV1% predicted, FEV1/FVC, BMI, 6MWD, and SGRQ score. The strong associations of PRM with multiple functional and clinical parameters suggest that PRM could provide important information on disease phenotype and severity [2].
Data on the added value of PRM to other known CT-derived biomarkers for diagnosing COPD has been provided [10], but no comparisons were made including other clinical parameters for COPD severity. Therefore, we compared the correlation between PRM as well as Perc15 (emphysema) and E/I-ratioMLD (air trapping) with clinical parameters. The results demonstrated that both PRMEmph and PRMfSAD were more strongly correlated with parameters for disease severity such as FEV1% predicted and KCO, as compared to Perc15 and E/I-ratioMLD. Hence, we acknowledged PRM as a technique superior to Perc15 and E/I-ratioMLD and provided data on its associations with clinical and functional parameters.
PRMEmph and PRMfSAD were both well associated with clinical parameters such as pulmonary function tests, body plethysmography and quality of life questionnaires. PRMEmph increased with higher BODE index and was found to be associated with the number of exacerbations, prednisolone courses, and antibiotic courses in the past year. Both PRMEmph and PRMfSAD allowed, next to identification and severity of disease, differentiation of clinical heterogeneity in COPD characterized by fSAD and emphysema. This is an important feature of PRM as varying phenotypes may have different life course outcomes and therefore can be used to characterize patients and institute personalize treatment in the future.
An increase in PRMfSAD and PRMEmph was associated with a decrease in FEV1% predicted, which is based on airflow limitation due to central and peripheral airway changes, but the strong association of PRMEmph with reduced lung diffusion highlights its ability to detect gas exchange abnormalities. Our results confirm previously shown associations of FEV1, FEV1/FVC, RV, and CT-derived air trapping [19,20]. However, the advantage of PRM is the ability to differentiate between emphysematous and non-emphysematous air trapping allowing a more realistic estimate of small airway disease.
Results on PRMEmph are in accordance with previous results on the association between CT-derived emphysema, FEV1/FVC, and reduced lung diffusion [20]. Namely, increasing PRMEmph values were mainly associated with a decrease in VA and TLCOc, and an increase in TLC. Therefore, PRMEmph could possibly identify a more generalized airflow obstruction where emphysema plays a more dominant role.
When evaluating a new biomarker, three criteria have to be met to appropriately validate a surrogate end-point: the presence of the imaging biomarker has to be closely coupled or linked to the presence of the target disease; the technique has to be accurate, reproducible, and feasible over time; and changes in the imaging biomarker must be closely related to changes in the true endpoint [21,22]. Our study is a first step in testing and validating the CT based PRM technique for COPD evaluation. We provided crucial information by analyzing a well-characterized cohort of (ex) smokers without COPD and COPD patients across all GOLD stages. We showed that PRM is associated with a variety of well-established parameters of COPD severity, which is an important step in the evaluation of this biomarker. Future studies should focus on reproducibility of PRM and longitudinal evaluation for PRM in the context of COPD progression and therapeutic response assessment.
With the current interest in different phenotypes of COPD, many CT-biomarkers have been suggested, but are often difficult to interpret or replicate in a clinical setting [23,24]. For example, the expiratory to inspiratory mean lung density (E/I-ratioMLD) can be used to capture air trapping on CT, but it is not able to distinguish between air trapping as a result from emphysema or air trapping as a result from small airway disease. With the introduction of PRM, several radiological aspects of COPD could be captured in one overview providing quantitative information on both fSAD and emphysema. Because of the extensive information and the simplicity of the technique, we therefore consider PRM to be a promising CT-biomarker.
Some limitations need to be addressed. First, we were not able to confirm our results histologically. However, histologically defining non-emphysematous air trapping is challenging and obtaining histology in patients with mild COPD might not be feasible. Second, further validation with longitudinal data is needed in order to use PRM for evaluation of changes over time. Third, the technique of PRM may introduce errors due to misregistration. However, this issue has been addressed before and it has been shown that even misalignment up to 5 mm had very little effect on the relative volumes of PRM [11]. In addition, we quantified emphysema and fSAD on low-dose images. Low dose imaging causes a shift in the measurements towards higher emphysema and fSAD percentages, although the magnitude is not fully known. In an association study like ours this problem is limited, but when defining emphysema and fSAD presence based on fixed cut-off values, dose (noise) needs to be taken into account. Last, in this study we evaluated the association between PRM and clinical parameters in subjects with COPD. A history of asthma was an exclusion criterium for COPD in our study. However, we did not measure bronchodilator reversibility, bronchial hyper-responsiveness, or blood and sputum eosinophilia in all subjects. Therefore, we were not able to depict COPD patients with asthmatic features who might be diagnosed with asthma-COPD overlap syndrome (ACOS). It is important to recognize this COPD-ACOS subtype as these patients are more likely to have frequent exacerbations and worse quality of life. Further research is needed to evaluate PRM in this specific subgroup.
In conclusion, PRM provides CT-quantification analysis in COPD patients, with differential levels for more extensive disease. Considering the ability to quantify multiple lung abnormalities associated with the different phenotypes of COPD, this technique could be valuable in the diagnosis, differentiation, and management of this disease.
Supplementary Material
Abbreviations
- 6MWD
six minute walking distance
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- E/I-ratioMLD
expiration to inspiration ratio of mean lung density
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- KCO
gas transfer corrected for lung volume
- MRC
Medical Research Council
- Perc15
emphysema score as 15th percentile of attenuation distribution curve on inspiratory scan
- PRM
parametric response mapping
- RV
residual volume
- SGRQ
St George’s Respiratory Questionnaire
- TLC
total lung capacity
- TLCO
diffusion capacity of the lung for carbon monoxide
- VA
alveolar volume
- VC
vital capacity
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.rmed.2016.11.021.
Footnotes
Conflicts of interest
Dr. C.J. Galbán and Dr. B.D. Ross report grants from NIH, during the conduct of the study. Dr. C.J. Galbán and Dr. B.D. Ross both have a patent Voxel-based analysis of registered medical images acquired from multiple phases with royalties paid to University of Michigan from Imbio, LLC. Dr. B.D. Ross is founder and shareholder of Imbio, LLC. Dr. Postma reports grants from Top Institute Pharma, during the conduct of the study; grants and other from GSK, other from Boehringer Ingelheim, other from TEVA, other from Takeda, grants and other from Chiesi, grants from Genetech, grants from Roch, grants and other from Astra Zeneca, outside the submitted work. Dr. M. van den Berge reports grans from TEVA, Chiesi, GlaxoSmithKline and Top Institute Pharma. Drs. E. Pompe, Dr. P.A. de Jong, Dr. F.A.A. Mohamed Hoesein, Prof. Dr. J.W.J. Lammers, Prof. Dr. L. Koenderman, and Dr. N.H.T. ten Hacken report grants from Top Institute Pharma (including Nycomed and GlaxoSmithKline), during the conduct of the study.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, Decramer M, Wedzicha JA, et al. An official american thoracic society/european respiratory society statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):e4–e27. doi: 10.1164/rccm.201501-0044ST. [DOI] [PubMed] [Google Scholar]
- 3.Cho MH, Castaldi PJ, Hersh CP, et al. A genome-wide association study of emphysema and airway quantitative imaging phenotypes. Am J Respir Crit Care Med. 2015;192(5):559–569. doi: 10.1164/rccm.201501-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes. The future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch DA, Austin JHM, Hogg JC, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner society. Radiology. 2015;277(1):192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka S, Kurihara Y, Yagihashi K, Nakajima Y. Quantitative assessment of peripheral airway obstruction on paired expiratory/inspiratory thin-section computed tomography in chronic obstructive pulmonary disease with emphysema. J Comput Assist Tomogr. 2007;31:384–389. doi: 10.1097/01.rct.0000243457.00437.10. [DOI] [PubMed] [Google Scholar]
- 8.Nakano Y, Van Tho N, Yamada H, Osawa M, Nagao T. Radiological approach to asthma and COPD-the role of computed tomography. Allergol Int. 2009;58:323–331. doi: 10.2332/allergolint.09-RAI-0124. [DOI] [PubMed] [Google Scholar]
- 9.Galban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pompe E, van Rikxoort EM, Schmidt M, et al. Parametric response mapping adds value to current computed tomography biomarkers in diagnosing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1084–1086. doi: 10.1164/rccm.201411-2105LE. [DOI] [PubMed] [Google Scholar]
- 11.Boes JL, Hoff BA, Bule M, et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS) Acad Radiol. 2015;22(2):186–194. doi: 10.1016/j.acra.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudewijn IM, Postma DS, Telenga ED, et al. Effects of ageing and smoking on pulmonary computed tomography scans using parametric response mapping. Eur Respir J. 2015;46(4):1193–1196. doi: 10.1183/09031936.00009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt SP, Soler X, Wang X, et al. Association between functional small airways disease and FEV1 decline in COPD. Am J Respir Crit Care Med. 2016;15(5):498–505. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo Tam Loi AT, Hoonhorst SJM, Franciosi L, et al. Acute and chronic inflammatory responses induced by smoking in individuals susceptible and non-susceptible to development of COPD: from specific disease phenotyping towards novel therapy. Protocol of a cross-sectional study. BMJ Open. 2013;3:e002178. doi: 10.1136/bmjopen-2012-002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crapo RO, Casaburi R, Coates AL, et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 17.Celli BR, Cote CG, Martin J, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen OF, Brackel HJL, Bogaard JM, Kerrebijn KF. Wave-speed-determined flow limitation at peak flow in normal and asthmatic subjects. J Appl Physiol. 1997;83:1721–1732. doi: 10.1152/jappl.1997.83.5.1721. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed Hoesein FAA, de Jong PA, Lammers J-WJ, et al. Contribution of CT quantified emphysema, air trapping and airway wall thickness on pulmonary function in male smokers with and without COPD. COPD. 2014;11:503–509. doi: 10.3109/15412555.2014.933952. [DOI] [PubMed] [Google Scholar]
- 21.Brody AS, Tiddens HAWM, Castile RG, et al. Computed tomography in the evaluation of cystic fibrosis lung disease. Am J Respir Crit Care Med. 2005;172:1246–1252. doi: 10.1164/rccm.200503-401PP. [DOI] [PubMed] [Google Scholar]
- 22.Boes JL, Bule M, Hoff BA, et al. The impact of sources of variability on Parametric Response Mapping of lung CT scans. Tomography. 2015;1(1):69–77. doi: 10.18383/j.tom.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby M, Pike D, Coxson HO, Mccormack DG. COPD: do imaging measurements of emphysema and airway disease explain symptoms and exercise capacity? Radiology. 2015;277(3):872–880. doi: 10.1148/radiol.2015150037. [DOI] [PubMed] [Google Scholar]
- 24.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1:129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.