Abstract
Chronic stress produces differential dendritic remodeling of pyramidal neurons in medial prefrontal cortex of male and female rats. In males, this dendritic remodeling is reversible. However, the timeline of recovery, as well as the potential for reversibility in females, is unknown. Here, we examined dendritic recovery of pyramidal neurons in layer II–II of prelimbic cortex in male and female rats following chronic restraint stress (3 h/day for 10 days). Dendritic morphology and spine density were analyzed immediately following the cessation of stress, or following a 7 or 10 day recovery period. Chronic stress produced apical dendritic retraction in males, which was coupled with a decrease in the density of stubby spine on apical dendrites. Further, following a 10-day recovery period, the morphology of neurons from stressed rats resembled that of unstressed rats. Male rats given a 7 day recovery period had apical dendritic outgrowth compared to unstressed rats. Immediately after cessation of stress, females showed only minimal dendritic remodeling. The morphology of neurons in stressed females resembled those of unstressed rats following only 7 days of recovery, at which time there was also a significant increase in stubby spine density. Males and females also showed different changes in baseline corticosterone concentrations during recovery. These findings not only indicate that dendritic remodeling in prelimbic cortex following chronic stress is different between males and females, but also suggest chronic stress induces differential hypothalamic-pituitary-adrenal axis dysregulation in males and females. These differences may have important implications for responses to subsequent stressors.
Keywords: prefrontal cortex, sex differences, corticosterone, dendritic morphology, spine density
INTRODUCTION
Stress can disrupt a variety of cognitive and emotional behaviors (Holmes and Wellman, 2009), and can also precipitate or exacerbate several psychological disorders, including depression, posttraumatic stress disorder, and schizophrenia (Harder et al., 1980, Brown and Harris, 1989). Alterations in the structure and function of medial prefrontal cortex (mPFC) may be a key factor in the pathophysiology of many of these disorders (Harder et al., 1980, Weinberger et al., 1986, Milad et al., 2009), and mPFC modulates several behaviors that are disrupted by stress, including working memory (Hains et al., 2009, Mika et al., 2012), attentional-set shifting (Liston et al., 2006), fear conditioning (Conrad et al., 1999, Farrell et al., 2010), and the retrieval of extinction memory (Miracle et al., 2006, Wilber et al., 2011).
Further, there is evidence that the number of self-reported stressful life events is positively correlated with risk for depression (Risch et al., 2009) and PTSD (Lian et al., 2014), and alterations in prefrontal cortex volume are observed following repeated stressors, even in non-patient populations (Papagni et al., 2011). Thus, incomplete or aberrant recovery from stress may leave some individuals vulnerable to the deleterious effects of subsequent stressors.
The morphology of neurons in mPFC seems to be especially sensitive to the effects of stress. Indeed, both acute and chronic stress profoundly alter the morphology of pyramidal neurons in the prelimbic (PL) region of the rodent mPFC. PL in rodents is structurally and functionally homologous to dorsal lateral PFC in humans (Uylings et al., 2003, Seamans et al., 2008), a region implicated in the cognitive deficits associated with stress-related psychopathologies (Sheline et al., 2010). In males, acute (Izquierdo et al., 2006), mild (Brown et al., 2005), and chronic stress (Cook and Wellman, 2004, Radley et al., 2004, Liu and Aghajanian, 2008) produce apical dendritic retraction in PL. In the case of chronic stress, this retraction is coupled with a decrease in spine density (Radley et al., 2006, Radley et al., 2008), whereas an increase in spine density is found following acute stress (Nava et al., 2015). These changes in dendritic morphology likely have important implications for neuronal function in PL, and therefore may contribute to stress-induced behavioral alterations.
Although corticolimbic morphology can undergo rapid and robust changes in response to stress, these changes are reversible in males. For example, chronic-stress induced dendritic remodeling in PL of males is reversible, with dendritic length resembling that of unstressed rats by 21 days after the cessation of stress (Radley et al., 2005, Bloss et al., 2010). There is also evidence that a shorter length of recovery time may be sufficient. For example, hippocampal neurons of male rats also undergo dendritic retraction following chronic stress, but following 10 days of recovery post-stress, this retraction is ameliorated (Conrad et al., 1999). Therefore, chronic stress-induced changes in mPFC neurons may be reversible in a shorter time than has previously been shown.
The prevalence of stress-linked disorders differs between men and women, with women being twice as likely to develop major depression and posttraumatic stress disorder as men (Solomon and Herman, 2009). Given this difference in susceptibility, it is unsurprising that stress can have divergent effects on behaviors modulated by mPFC, as well as dendritic morphology in males and females. For example, while chronic stress disrupts temporal order recognition memory in males, females show no deficits (Wei et al., 2014). Additionally, whereas acquisition of conditioned fear is enhanced in male rats following chronic stress (Conrad et al., 1999, Farrell et al., 2010), there is evidence that females show impairment in fear acquisition following stress (Baran et al., 2009). Further, in contrast to the dendritic retraction following chronic stress observed in males, females show apical dendritic outgrowth in PL (Garrett and Wellman, 2009). The potential for reversibility in females has yet to be examined. Therefore, to further characterize the process of dendritic recovery in mPFC, we assessed dendritic morphology in PL of male and female rats immediately following chronic restraint stress, as well as after 7 and 10 days of post-stress “recovery.” We focused on PL in the present study, as the majority of studies examining stress effects on dendritic morphology have focused on this region of mPFC, where chronic stress-induced dendritic retraction has been robustly demonstrated in males (e.g., Cook and Wellman, 2004, Radley et al., 2005, Garrett and Wellman, 2009).
EXPERIMENTAL PROCEDURES
Subjects and Stressors
Male and female Sprague Dawley rats (approximately 68 days of age at start; Harlan, Indianapolis, IN; N = 73) were group-housed (3 per cage) in standard laboratory cages (48 cm × 20 cm × 26 cm), with ambient temperature 23–25 °C, free access to food and water, and a 12:12 light/dark cycle (lights on at 0800 h). Rats were either left unstressed or subjected to chronic restraint stress for 10 days, and were given a recovery period of 0, 7, or 10 days, resulting in 8 groups (Fig. 1A): unstressed males (n = 11) and females (n = 12), 0d Rec males (n = 8) and females (n = 8), 7d Rec males (n = 7) and females (n = 9), and 10d Rec males (n = 9) and females (n = 9). All rats were weighed daily throughout the stress procedure. Immediately after weighing, unstressed rats were returned to their home cages and left undisturbed for 3 hours in a separate room. Stressed rats were placed in semi-cylindrical restrainers (6.35 cm diameter × 15.24 cm length, modified so the tail piece locks into place; Braintree Scientific) for 3 hours in their home cages, a manipulation that produces significant increases in plasma corticosterone levels (Cook and Wellman, 2004). All rats within a cage were assigned to the same experimental group, and all stressed rats underwent restraint simultaneously. Rats were left undisturbed during the recovery period. All procedures were conducted between 8:00 am and 8:00 pm (i.e., during the light phase), were in accordance with NIH Guidelines, and were approved by the Bloomington Animal Care and Use Committee.
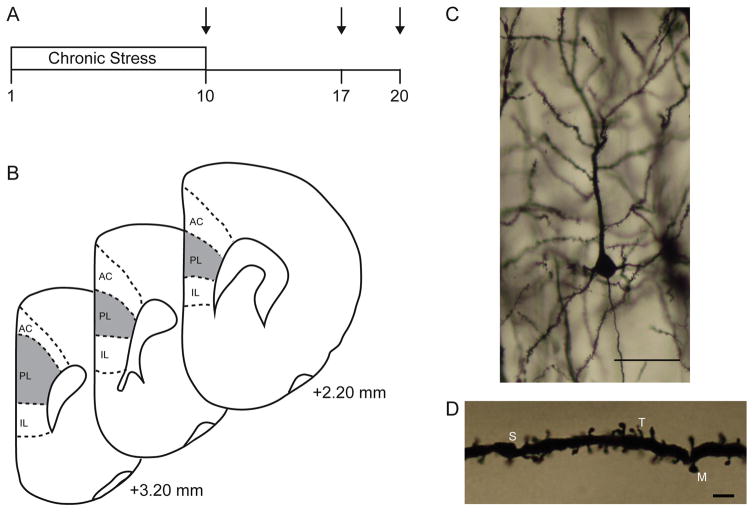
FIG. 1.
(A) Schematic depiction of experimental timeline for assessing dendritic morphology in chronically stressed males and females during a post-stress recovery period. Arrow represents tissue collection. (B) Schematic diagram of coronal sections through rat prefrontal cortex. The portions of prelimbic cortex from which neurons were sampled is shown (shaded areas). Coordinates indicate position relative to bregma (Paxinos and Watson, 1998). (C) Digital micrograph of Golgi-stained neuron in layer II–III of mPFC. Scale bar = 50 μm. (D) Digital micrograph demonstrating different spine types on a pyramidal neuron in layer II–III of mPFC. M, mushroom; S, stubby; T, thin. Scale bar = 5 μm.
Estrous Phase Characterization
On the day of perfusion, vaginal lavages were performed and exfoliate cytology was examined immediately under light microscopy. Estrous phase was determined based on the morphology of cells present (Garrett and Wellman, 2009). Due to the small number of rats in proestrus (n = 3) and estrus (n = 2) compared to diestrus (n = 33), we did not analyze our data relative to estrous phase.
Corticosterone EIA
Immediately prior to perfusion, blood was collected via cardiac puncture and allowed to clot at room temperature for 30 minutes followed by centrifugation at 13,000 rpm for 5 minutes to obtain serum. Corticosterone was measured via a commercially available EIA kit (Enzo Life Sciences, Plymouth Meeting, PA) that shows low crossreactivity with other major steroid hormones. Samples were diluted (1:20) with assay buffer and run in duplicates according to instructions provided by the manufacturer. The sensitivity of the assay was 27 pg/mL, and intra-assay variation was 1.77% and 2.62% for each plate.
Histology and Dendritic Analysis
Brains were processed using a modification of Glaser and van der Loos’ Golgi stain, as described previously (Glaser and Van der Loos, 1981, Martin and Wellman, 2011). On the final day of either stress or recovery, rats were deeply anesthetized with urethane and transcardially perfused with saline. To verify the stress manipulation, adrenal glands were removed and weighed. Brains were removed and immersed in Golgi-Cox solution for 14 days and then moved to 30% sucrose in saline (Gibb and Kolb, 1998). Brains were sectioned at 200 μm on a vibratome (Campden Instruments, MA752). Sections were mounted, alkalinized, developed in Dektol (Kodak), fixed in Ilford rapid fixer, dehydrated in a graded series of ethanols, cleared in xylenes, and coverslipped (Wellman, 2016).
Pyramidal neurons in layer II–III of prelimbic cortex were reconstructed (Fig. 1B). Prelimbic cortex is readily identified by its position on the medial wall of the rostral cortex, and its location dorsal to infralimbic cortex, which is markedly thinner and has fewer, less well-defined layers (Zilles and Wree, 1995), and ventral to anterior cingulate cortex, which is thicker than PL and located adjacent to the inflection of the forceps minor, which results in a distinct bend to the apical trunk of its layer II–III neurons. Within PL, layer II–III is readily identifiable in Golgi-stained material based on its characteristic cytoarchitecture. Its position is immediately lateral to the relatively cell-poor layer I (which also contains the distal dendritic tufts of layer II–III pyramid cells) and medial to layer V. In mPFC, this boundary is pronounced because of the greater cell-packing density and smaller somata of pyramidal cells in layer II–III relative to layer V (Cajal, 1995, Zilles and Wree, 1995). Pyramidal neurons were defined by a distinct, single apical dendritic tree extending from the apex of the soma toward the pial surface of the cortex, two or more basilar dendritic trees extending from the base of the soma, and the presence of dendritic spines (Fig. 1C). Neurons selected for reconstruction did not have truncated apical branches, had at least one nontruncated basilar tree, and were unobscured by neighboring neurons and glia, with dendrites that were easily discriminable by focusing through the depth of the tissue. In 4 sections evenly spaced through the rostral-caudal extent of PL, all pyramidal neurons meeting these criteria were identified (mean = 24 ± 6 per animal). Eight neurons per animal (four from each hemisphere) were then randomly selected from all of those identified and reconstructed at a final magnification of 600x. Morphology of apical and basilar arbors was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida, MBF Biosciences) with the experimenter blind to condition. For basilar dendrites, only non-truncated basilar trees were drawn, and per-tree analyses were performed.
Spines were also counted on eight neurons per animal. For each neuron, a segment averaging 39.37 ± 0.35 μm in length was drawn from one terminal apical and one terminal basilar dendrite. Whenever possible, spines were assessed on previously reconstructed neurons. We examined distal branches because stress and corticosterone administration induce dendritic remodeling in these branches (Wellman, 2001, Cook and Wellman, 2004, Liu and Aghajanian, 2008). Spines were classified as stubby, thin, or mushroom, based on standard morphological criteria (Fig. 1D; Peters and Kaiserman-Abramof, 1970).
Statistical analyses
Chronic stressors such as immobilization and restraint attenuate normal weight gain in males (Marti et al., 1994, Cook and Wellman, 2004) and increase relative adrenal weight (Heiderstadt et al., 2000). Therefore, to verify the stress manipulation, weight gain and adrenal weight were compared using two-way ANOVAs (stress x sex). To examine possible differences in lasting basal hypothalamic-pituitary-adrenal (HPA) axis activity as a result of chronic stress, mean serum corticosterone was compared using a two-way ANOVA (stress x sex).
To assess changes in the amount and distribution of dendritic material, a three-dimensional version of a Sholl analysis (Sholl, 1956) was used, in which the total length of dendrites between an overlay of concentric spheres of 10 μm radius centered on the soma was assessed. To simplify analyses, the lengths of apical and basilar arbors were divided into thirds (proximal, middle, and distal) based on the cumulative lengths of unstressed males and females. These data were compared across groups using three-way repeated measures ANOVAs (distance from soma x stress x sex). Total spine density and densities of each spine type were also compared using two-way ANOVAs (stress x sex). We also estimated the total number of spines, and the total number of each spine type, on terminal branches by multiplying spine density by terminal branch length. These data were compared using two-way ANOVAs (stress x sex). For all analyses, measures were averaged across neurons within each animal; thus, the unit of analysis for statistics is the individual animal. Planned comparisons were done when appropriate, and consisted of F tests done within the context of the overall ANOVA (Hayes, 1994, Maxwell and Delaney, 2004), comparing unstressed rats to stressed rats given 0, 7, or 10 days to recover and comparing each recovery group to the 0 day recovery rats.
Finally, to examine potential relationships between CORT and dendritic morphology, Pearson correlations were calculated between apical or basilar branch length (divided into proximal-middle-distal portions) and serum CORT. Fisher r to z transformations were used to compare r-coefficients between unstressed rats and stressed rats with varying recovery periods.
RESULTS
Verification of Stress Manipulation
Body Weight
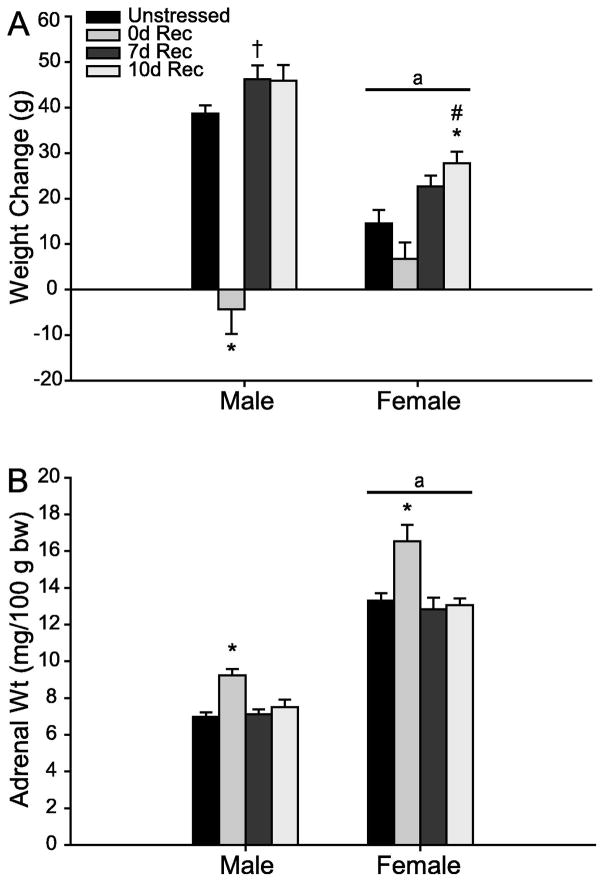
Unsurprisingly, weight gain differed between males and females (Fig. 2A; main effect of sex, F1,65 = 36.32, p < 0.05). Further, chronic stress and subsequent recovery significantly altered weight gain in males and females (main effect of stress, F3,65 = 47.21, p < 0.05), although this effect was different in males and females (sex x stress interaction, F3,65 = 13.05, p < 0.05). Planned comparisons revealed that 0d Rec males showed significant weight attenuation compared to unstressed males (F1,17 = 72.89, p < 0.001). Further, 7d Rec males gained significantly more weight than unstressed males (F1,18 = 5.05, p = 0.04). A similar trend approached significance in 10d Rec males (F1,16 = 4.08, p = 0.06). Further, both 7d and 10d Rec males gained significantly more weight than 0d Rec males (7d Rec, F1,15 = 71.04, p < 0.001; 10d Rec, F1,13 = 56.99, p < 0.001), although these two groups did not differ from each other (F1,14 = 0.01, ns).
FIG. 2.
(A) Mean weight change in unstressed versus stressed male and female rats receiving a 0d, 7d, or 10d recovery. Stress attenuated weight gain in 0d Rec male and female rats. (B) Mean adrenal-weight-to-body- weight ratios (adrenal weight/100 g body weight) in unstressed versus stressed male and female rats receiving a 0d, 7d, or 10d recovery. Stress increased relative adrenal weight in 0d Rec male and female rats. Error bars represent SEM. * p < 0.05 compared to same-sex controls; † p < 0.10 compared to same-sex controls; # p < 0.05 compared to same-sex 0d Rec rats; a p < 0.05 compared to male rats.
Chronic stress did not significantly attenuate weight gain in females (unstressed vs 0d Rec, F1,18 = 2.71, ns). However, increased weight gain in 7d Rec females tended toward significance compared to unstressed females (F1,19 = 4.11, p = 0.06), and was significantly greater compared to 0d Rec (F1,15 = 14.07, p = 0.002). Ten day recovery females gained significantly more weight than both unstressed (F1,19 = 10.59, p = 0.004) and 0d Rec (F1,15 = 23.74, p < 0.001) females. Females in the 7d and 10d rec groups did not differ from each other (F1,16 = 2.20, ns).
Adrenal Weight
In agreement with previous work (Konkle et al., 2003), females had a higher adrenal-to-body weight ratio (Fig. 2B; main effect of sex, F1,65 = 366.26, p < 0.05). Further, chronic stress, as well as the presence of a recovery period, significantly altered adrenal weight in both males and females (main effect of stress, F3,65 = 16.82, p < 0.05). Planned comparisons revealed that chronic stress increased adrenal weight relative to body weight in both 0d Rec males (F1,17 = 30.34, p < 0.001) and 0d Rec females (F1,18 = 13.38, p = 0.002), while 7d and 10d Rec males and females did not differ from same-sex controls (all Fs ≤ 1.38, all ns). On the other hand, 7d and 10d Rec males and females had significantly lower relative adrenal weights relative compared to stressed males and females that had no recovery period (all Fs ≥ 10.71, all ps ≤ 0.006). Adrenal weights did not differ between 7day and 10d males (F1,14 = 1.07, ns) or females (F1,16 = 0.03, ns).
Corticosterone Analyses
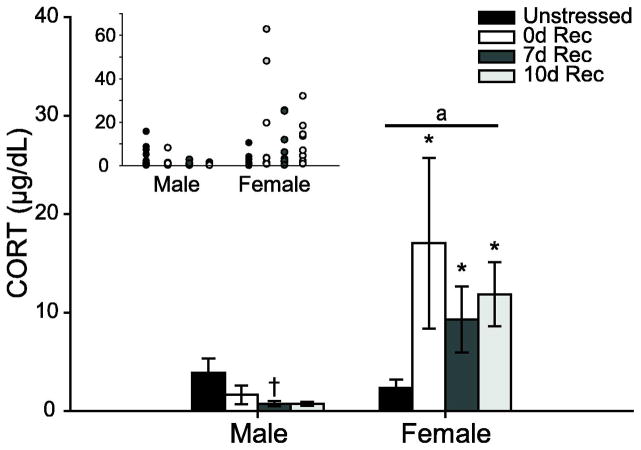
Sex Differences in Baseline Corticosterone Following Chronic Stress
Baseline corticosterone levels differed between males and females (Fig. 3; main effect of sex, F1,65 = 13.14, p < 0.05). Although there was no main effect of stress (F3,65 = 1.33, ns), there was a significant sex by stress interaction (F3,65 = 2.71, p = 0.05). Planned comparisons revealed that at all recovery time points, stressed females had higher serum corticosterone concentrations than unstressed females (0d Rec, F1,18 = 4.32, p = 0.05; 7d Rec, F1,19 = 5.24, p = 0.03; 10d Rec, F1,19 = 10.28, p = 0.005), while 0d, 7d, and 10d Rec females did not differ from each other (all Fs ≤ 0.76, all ns). Female 7d and 10d Rec groups had significantly higher corticosterone than males at the same recovery time points (7d, F1,16 = 6.49, p = 0.02; 10d, F1,14 = 8.92, p = 0.01). There were no significant differences in baseline corticosterone levels between unstressed males and stressed males at any recovery point, although there was a trend for decreased corticosterone in the 7d Rec group (0 days, F1,17 = 1.34, ns; 7 Days, F1,18 = 3.53, P = 0.08; 10 days, F1,16 = 2.78, ns).
FIG. 3.
Mean serum CORT at perfusion in unstressed versus stress male and female rats receiving a 0d, 7d, or 10d recovery. While 7d Rec male rats tended to have suppressed baseline CORT, stressed females had elevated CORT at all times post-stress. Error bars represent SEM. Inset: Scatterplot of individual datapoints. Stressed female rats have a high degree of individual variability. * p < 0.05 compared to same-sex controls; † p < 0.10 compared to same-sex controls; a p < 0.05 compared to male rats.
Dendritic Morphology Analyses
Apical Dendritic Reorganization Following Chronic Stress
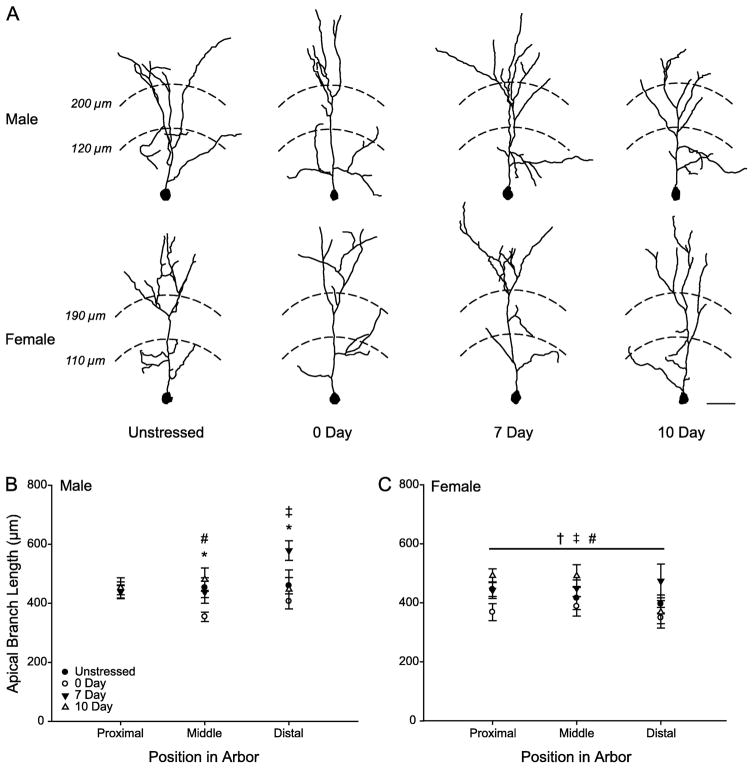
Stress significantly altered apical dendritic length (F3,65 = 6.25, p < 0.05). Although there was no main effect of sex (F1,65 = 2.58, ns) or distance from soma (F1,65 = 0.03, ns) and no interaction between sex and stress (F3,65 = 0.13, ns), there were significant interactions between distance from the soma and stress (F3,65 = 2.56, p < 0.05), as well as distance from the soma and sex (F2,130 = 3.37, p < 0.05). The three-way interaction between distance from the soma, sex, and stress was nonsignificant (F6,130 = 0.65, ns), and therefore, two-way ANOVAs (stress x distance from soma) were used to examine the effects of stress on apical branch length separately in males and females.
In males, stress tended to alter apical branch length (F3,31 = 2.73, p = 0.06). While there was no main effect of distance from the soma (F2,62 = 1.80, ns), apical dendritic length varied across stress conditions as a function of distance from the soma (Fig. 4B; distance from soma x stress interaction, F6,62 = 2.37, p < 0.05). Planned comparisons revealed no significant differences proximal to the cell body (all Fs ≤ 0, all ns). In contrast, dendritic length in the middle third of the apical arbor was significantly reduced in 0d Rec males compared to unstressed males (F1,17 = 5.50, p = 0.03). Further, a trend towards increased dendritic length in 7d Rec males compared to 0d Rec males approached significance (F1,15 = 3.86, p = 0.07) and reached significance in 10d Rec males (F1,13 = 9.75, p = 0.008). Surprisingly, in the distal portion of the apical arbor, branch length in 7d Rec males was significantly greater than that of either unstressed males (F1,18 = 7.66, p = 0.01) or 0d Rec males (F1,15 = 16.05, p = 0.001), and this difference approached significance compared to 10d Rec males (F1,14 = 3.64, p = 0.08). All other planned comparisons were nonsignificant (all Fs ≤ 0.84, all ns).
Fig. 4.
(A) Computer-assisted reconstruction of apical arbors of Golgi-stained neurons in layer II–III of PL in unstressed, 0d, 7d, and 10d Rec male and female rats. Neurons are at or near the mean for each group. Scale bar = 50 μm. (B) Mean length of apical branches in unstressed, 0d, 7d, and 10d Rec male rats with 10-μm concentric circles summed across the proximal, middle, and distal third of the arbor. 0d Rec male rats have decreased dendritic length in the middle third of the arbor, while 7d Rec male rats have increased dendritic length in the distal third. (C) Mean length of apical branches in unstressed, 0d, 7d, and 10d Rec females with 10-μm concentric circles summed across the proximal, middle, and distal third of the arbor. Stressed females have no changes in dendritic length across the apical arbor at any time post-stress. * p < 0.05 Unstressed vs 0d Rec males; † p < 0.10 Unstressed vs 0d Rec females; ‡ p < 0.05 0d Rec vs 7d Rec of same sex; # p < 0.05 0d Rec vs 10d Rec of same sex.
Apical dendritic reorganization was also seen in females (Fig. 4C; main effect of stress, F3,34 = 3.7, p < 0.05). This effect did not vary across the dendritic arbor (main effect of distance from soma, F2,68 = 1.66, ns; distance from soma x stress interaction, F6,68 = 1.08, ns). Therefore, follow-up comparisons were performed on total apical branch length, collapsed across the arbor. A small decrease in apical branch length in 0d Rec females compared to unstressed females approached significance (F1,18 = 3.66, p = 0.07). Further, 7d Rec and 10d Rec females had greater branch length than 0d Rec females (7d, F1,15 = 7.50, p = 0.02; 10d, F1,15 = 7.14, p = 0.02). Apical branch length was not different in 7d and 10d Rec relative to unstressed females (all Fs ≤ 0.77, ns).
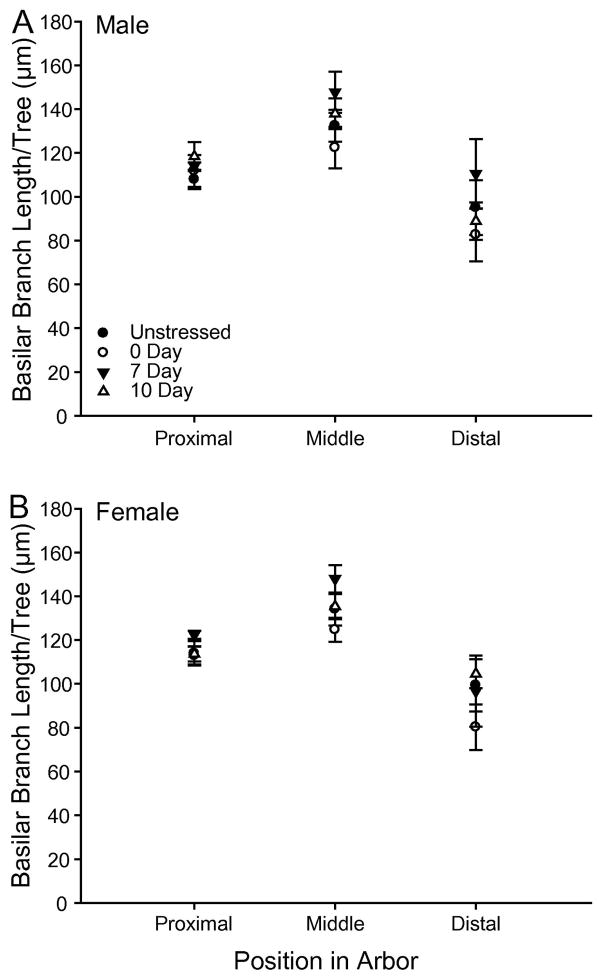
Consistent with prior work (e.g., Cook and Wellman, 2004, Brown et al., 2005, Izquierdo et al., 2006), basilar dendritic length varied by distance from soma (F2,130 = 2.37, p < 0.05). However, there was no main effect of stress (F3,65 = 2.14, ns) or sex (F1,65 = 0.11, ns) on basilar branch length (Fig. 5), and no stress by sex interaction (F3,65 = 0.94, ns).
Fig. 5.
(A) Mean length of basilar branches per tree in unstressed, 0d, 7d, and 10d Rec male rats with 10-μm concentric circles summed across the proximal, middle, and distal third of the arbor. (B) Mean length of basilar branches per tree in unstressed, 0d, 7d, and 10d Rec female rats with 10-μm concentric circles summed across the proximal, middle, and distal third of the arbor. Overall basilar branch length did not vary across stress condition or sex. Error bars represent SEM.
Relationships between Baseline Corticosterone and Apical Dendritic Length
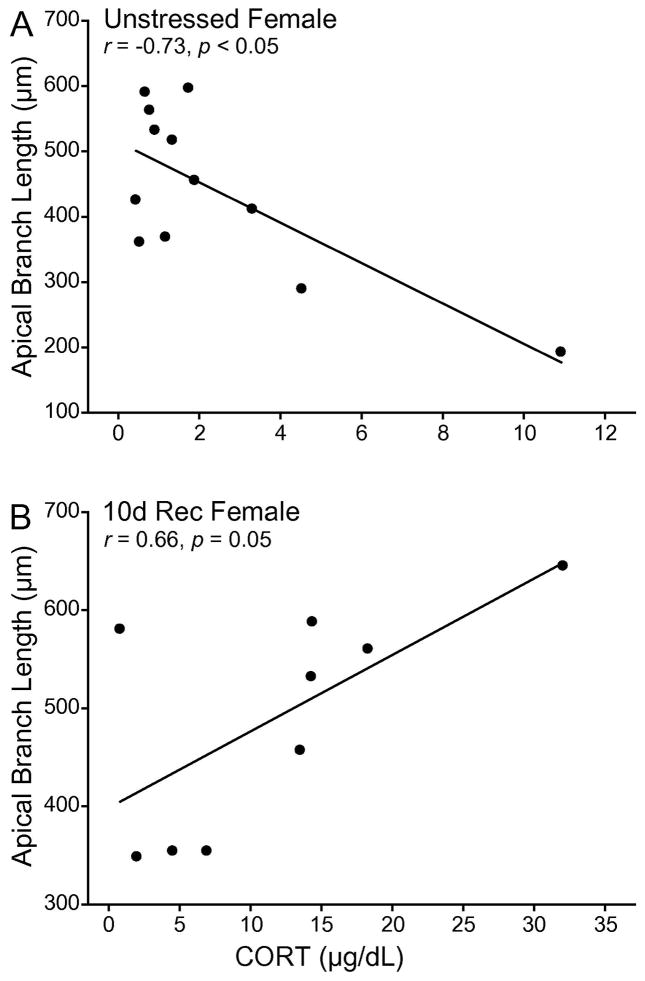
In unstressed females, corticosterone strongly and negatively correlated with total apical branch length (Fig. 6A; r = −0.69, p < 0.05), which was likely due specifically to a significant correlation between branch length and corticosterone in the middle portion of the apical arbor (r = −0.73, p < 0.05). This correlation was not present in 0d Rec females (r = −0.47, ns) and 7d Rec females (r = 0.25, ns). The association was reversed in 10d Rec females, with branch length correlating positively with corticosterone concentration (Fig. 6B; r = 0.66, p = 0.05), and this correlation was significantly different than that of unstressed females (z = −3.26, p < 0.05). No other correlations reached significance.
Fig. 6.
(A) Linear regression for average PL apical branch length in unstressed female rats vs. serum CORT. (B) Linear regression for average PL apical branch length in 10d Rec female rats vs. serum CORT. Apical branch length is negatively correlated with serum CORT un unstressed females. This correlation is reversed in 10d Rec female rats.
Spine Analyses
Apical Spine Density
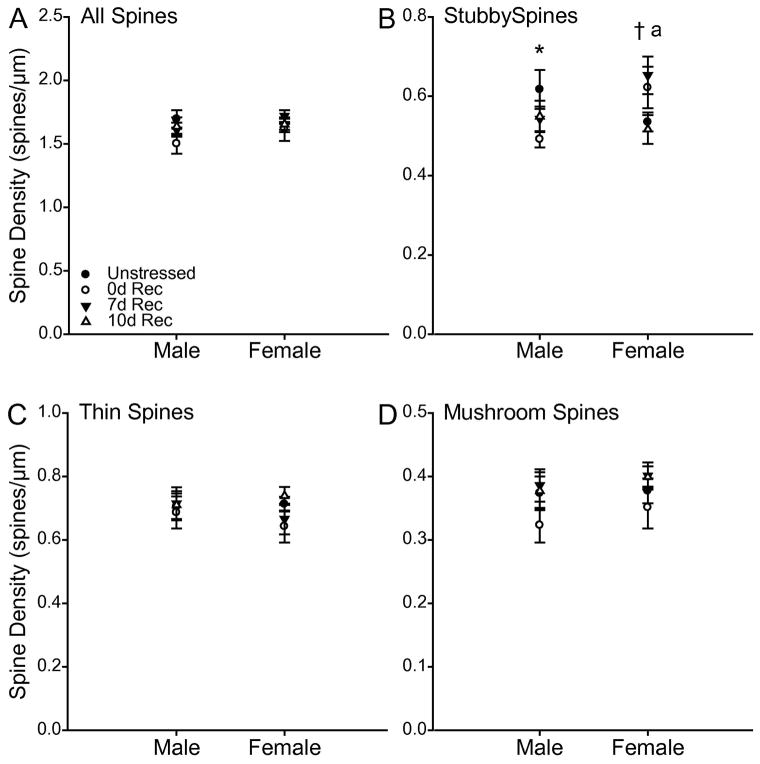
Total apical spine density, as well as the densities of apical thin and mushroom spines were unaffected by stress (Fig 7; all Fs ≤ 1.87, all ns), did not differ between males and females (all Fs ≤ 0.96, all ns), and there were no significant interactions (all Fs ≤ 1.25, all ns). In contrast, despite no main effects of stress (F3,65 = 0.93, ns) or sex (F1, 65 = 1.25, ns) on stubby spine density, stress altered stubby spine density differentially in males and females (Fig. 7B; for sex x stress interaction, F3,65 = 3.72, p < 0.05). Planned comparisons revealed that whereas 0d Rec males had a decrease in stubby spine density relative to unstressed males (F1,17 = 4.31, p = 0.05), 7d Rec females showed an increase in stubby spine density relative to unstressed females. (F1,19 = 5.77, p = 0.03). This pattern of changes resulted in a significant sex difference in stubby spine density immediately following chronic stress (F1,14 = 5.38, p = 0.04), whereby males had a significantly lower stubby spine density compared to females. A similar, although nonsignificant, trend was found at 7 days post-stress (F1,16 = 3.75, p = 0.07). No other planned comparisons reached significance (all Fs ≤ 2.40, all ns).
Fig. 7.
(A) Total apical spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. (B) Apical stubby spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. Chronic stress decreased stubby spine density in 0d Rec male rats, but increase spine density in 7d Rec females. (C) Apical thin spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. (D) Apical mushroom spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. Error bars represent SEM. * p < 0.05 Unstressed vs 0d Rec males; † p < 0.05 Unstressed vs 7d Rec females; a p < 0.05 0d Rec females vs 0d Rec males.
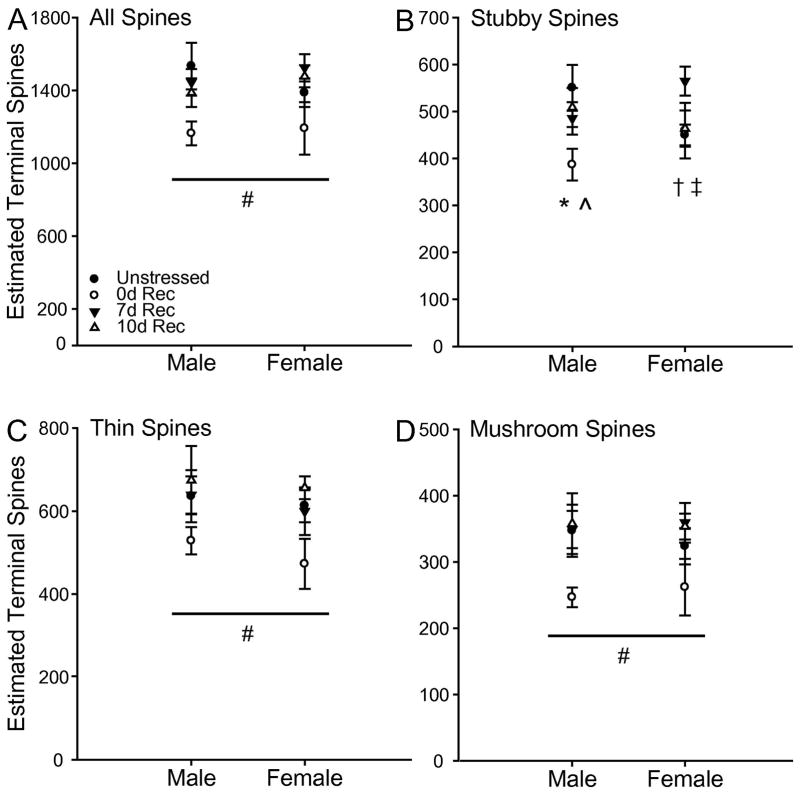
Estimated Apical Terminal Spines
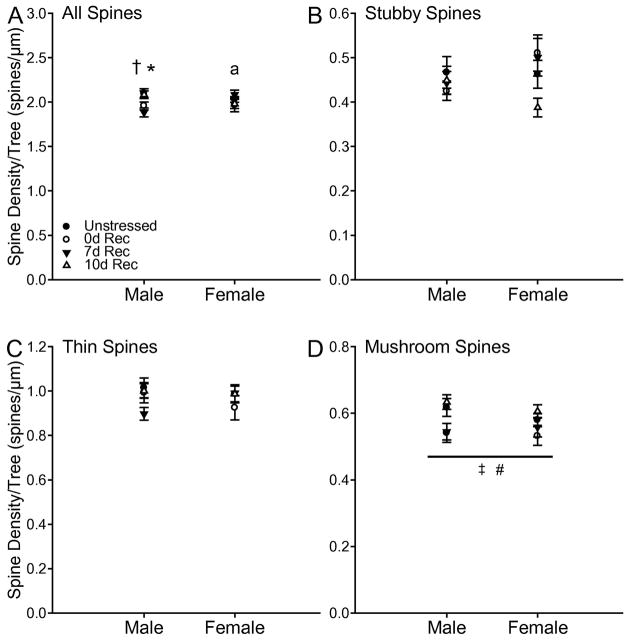
Estimation of terminal spines revealed a different pattern of stress-induced changes on apical dendritic spines. Stress altered the estimated number of total (F3,65 = 4.20, p < 0.05), thin (F3, 65 = 3.23, p < 0.05), and mushroom (F3,65 = 3.92, p < 0.05) spines. These effects did not vary by sex (All Fs < 0.82, all ns), and there were no significant stress by sex interactions (All Fs < 0.53, all ns). Planned comparisons revealed 0d Rec rats had fewer estimated total spines than unstressed (F1,37 = 6.58, p = 0.01), 7d Rec (F1,32 = 11.37, p = 0.002), and 10d Rec rats (F1,30 = 9.62, p = 0.004). A similar pattern was found for estimated thin (unstressed, F1,37 = 5.73, p = 0.02; 7d Rec, F1,32 = 5.73, p = 0.02; 10d Rec, F1,30 = 10.35, p = 0.003) and mushroom (unstressed, F1,37 = 5.86, p = 0.02; 7d Rec, F1,32 = 11.426, p = 0.002; 10d Rec, F1,30 = 10.63, p = 0.003) spines. For apical stubby spines, although there was no main effect of stress (F3,65 = 2.25, ns) or sex (F1,65 = .003, ns), there was a significant stress by sex interaction (F3,65 = 2.69, p = 0.05). Planned comparisons revealed a decrease in estimated apical stubby spines in 0d Rec males compared to unstressed (F1,17 = 6.46, p = 0.02) and 10d Rec males (F1,13 = 5.31, p = 0.04), a pattern that approached significance compared to 7d Rec males (F1,15 = 4.12, p = 0.06). In contrast, 7d Rec female rats had an increased estimated number of stubby spines compared to unstressed females (F1,19 = 9.63, p = 0.006), which tended also to be the case compared to 10d Rec female rats (F1,16 = 4.18, p = 0.06). No other comparisons reached significance (all Fs < 3.70, all ns).
Basilar Spine Density
Significant changes in basilar spines were also observed (Fig 9). While there was no main effect of stress or sex on total basilar spine density, there was a significant stress by sex interaction (F3,65 = 3.54, p < 0.05). Planned comparisons revealed that the 0d Rec male group tended to have a decrease in spine density (F1,17 = 3.75, p = 0.07). This decrease was significant in 7d Rec males (F1,18 = 8.28, p = 0.01). In contrast, no comparisons reached significance in females (all Fs ≤ 0.99, all ns), although the decrease in overall spine density in 7d Rec males resulted in a significant sex difference at this time point (F1,16 = 7.95, p = 0.01).
Fig. 9.
(A) Total basilar spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. Chronic stress decreased basilar spine density in 0d and 7d Rec male rats. (B) Basilar stubby spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. (C) Basilar thin spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. (D) Basilar mushroom spine density in unstressed, 0d, 7d, and 10d Rec male and female rats. Chronic stress decrease mushroom spine density in 0d and 7d Rec rats. Error bars represent SEM. * p < 0.05 Unstressed vs 7d Rec males; † p < 0.10 Unstressed vs 0d Rec males; a p < 0.05 7d Rec females vs 7d Rec males; ‡ p < 0.05 Unstressed vs 0d Rec; # p < 0.10 Unstressed vs 7d Rec.
For densities of stubby and thin spines, there were no main effects of stress (stubby, F3,65 = 1.05, ns; thin, F3,65 = 0.93, ns) or sex (stubby, F1,65 = 0.69, ns; thin, F1,65 = 0.02, ns), and no significant interactions (stubby, F 3,65 = 1.81, ns; thin, F3,65 = 1.37, ns). In contrast, stress significantly altered the density of mushroom spines (F3,65 = 4.55, p < 0.05), and this effect did not differ between males and females (main effect of sex, F1,65 = 0.73, ns; stress x sex interaction, F 3,65 = 0.44, ns). Planned comparisons revealed that 0d Rec rats had significantly decreased density of mushroom spines (F1,37 = 5.94, p = 0.02), a pattern that approached significance in 7d Rec rats (F1,37 = 3.52, p = 0.07). Further, 10d Rec rats had significantly greater density of mushroom spines compared to either 0d Rec (F1,30 = 11.39, p = 0.002) or 7d Rec (F1,30 = 7.52, p = 0.01) rats. No other comparisons reached significance (all Fs ≤ 0.74, all ns).
Estimated Basilar Terminal Spines
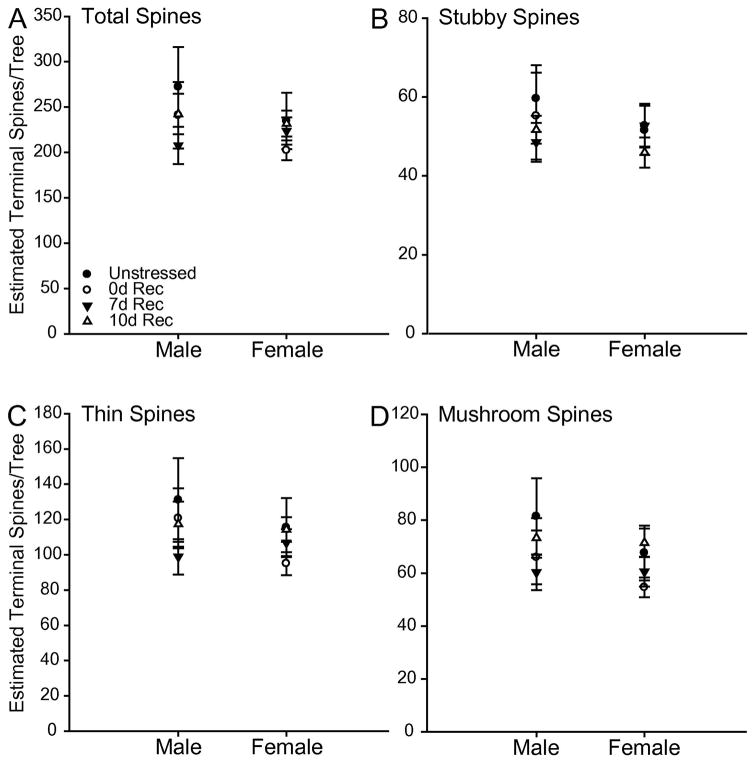
The estimated total number of basilar spines did not differ by stress condition (F3,65 = 0.74, ns) or sex (F1,65 = 0.71, ns), and there was no stress by sex interaction (F3,65 = 0.40, ns). This was also the case for the estimated number of stubby (main effect of stress, F3,65 = 0.42, ns; main effect of sex, F1,65 = 0.39, ns; stress x sex, F3,65 = 0.33, ns), thin (main effect of stress, F3,65 = 0.75, ns; main effect of sex, F1,65 = 0.69, ns; stress x sex, F3,65 = 0.44, ns), and mushroom (main effect of stress, F3,65 = 1.32, ns; main effect of sex, F1,65 = 0.97, ns; stress x sex, F3,65 = 0.28, ns) spines.
DISCUSSION
The purpose of the current study was to characterize dendritic recovery following chronic stress in males and females, and to determine if changes in dendritic morphology are associated with alterations in basal corticosterone levels. The three major findings from this study are: 1) males and females showed different patterns of apical dendritic retraction immediately following the cessation of chronic restraint stress; this retraction was reversed by 7 days post-stress in females and 10 days poststress in males; 2) males, but not females, showed dendritic outgrowth at 7 days post-stress; and 3) chronic stress had differential long-lasting effects on baseline HPA axis activity in males and females.
Dynamic Dendritic Reorganization during Stress Recovery in Male Rats
Consistent with previous work (Cook and Wellman, 2004, Radley et al., 2004, Liu and Aghajanian, 2008, Garrett and Wellman, 2009), chronic restraint stress attenuated weight gain and increased relative adrenal weight, suggesting that the manipulation was stressful. Further, relative adrenal weight and body weight gain were normalized by 7 days post-stress. Also consistent with previous studies (e.g., Cook and Wellman, 2004, Garrett and Wellman, 2009), chronic stress induced apical dendritic retraction in the medial prefrontal cortex of male rats. However, apical dendritic length resembled that of unstressed rats by 10 days post-stress. This agrees with previous work demonstrating that dendritic retraction in hippocampal area CA3 resulting from 21 days of restraint stress was reversed by 10 days post-stress (Conrad et al., 1999). Further, our findings expand on Radley and colleagues’ (2005) finding that the apical dendritic retraction induced by chronic restraint stress (6 hours/day for 21 days) was fully reversed by 21 days post-stress. Our results indicate that this reversal can happen quite quickly. However, note that in the present study, daily restraint was only 3 hours in duration and occurred for only 10 days. Thus, it is possible that a longer, more intense stressor may result in a different time course of recovery than that found here. In addition, a recent study found that acute stress produced apical dendritic retraction 24 hours and 14 days later, but not at 7 days post-stress (Nava et al., 2015). Therefore, although apical dendritic length resembled that of unstressed rats by 10 days post-stress here, it is possible that the dynamic dendritic remodeling that we have shown continues between 10 and 21 days post-stress, and further alterations in apical dendritic organization could be found at later time points.
Perhaps the most surprising finding from the current study is the apical dendritic outgrowth in male rats 7 days post-stress, followed by a return to lengths similar to those seen in unstressed rats by 10 days post-stress. This initial outgrowth and subsequent pruning is reminiscent of the pattern of dendritic remodeling in injury models following unilateral lesions (reviewed in Macias, 2008). For example, following unilateral motor cortex lesions, pyramidal neurons in layer V showed increased dendritic arborization for the first two weeks post-lesion, followed by partial pruning (Jones and Schallert, 1992). Importantly, changes in dendritic morphology correlated with functional return of the affected limb (Jones and Schallert, 1992, 1994), suggesting that branching patterns, branch distribution, and overall shape of the dendritic arbor can determine the functional properties of neurons. Indeed, this structure-function relationship has been demonstrated in several models (Rall et al., 1992, Mainen and Sejnowski, 1996, Koch and Segev, 2000, Lu et al., 2001, Grudt and Perl, 2002). Thus, although outside the scope of the current study, it is possible that the dendritic reorganization in male rats here has important functional consequences.
One potential functional consequence of the dendritic reorganization shown here is altered HPA axis regulation. Activation of prelimbic cortex has a net inhibitory effect on stress-evoked HPA axis activation via projections to the fusiform and dorsomedial subdivisions of the bed nucleus of the stria terminalis, which in turn send inhibitory projections to the periventricular nucleus of the hypothalamus (PVN; Radley et al., 2009). Interestingly, Ostrander and colleagues (2006) showed that 7 days post-chronic variable stress, males showed HPA axis hypoactivation in response to a novel acute stressor, which was associated with decreased c-fos expression in the PVN. Therefore, the outgrowth of apical dendrites in PL at 7 days post-stress in males could increase the excitability of these cells (Wilber et al., 2011), thereby inhibiting HPA axis activity. This is consistent with the findings described above (Ostrander et al., 2006, Ostrander et al., 2009), as well as the slight reduction in baseline corticosterone seen in the current study. Alternatively, there is evidence that chronic stress-induced dendritic retraction in hippocampus produces more excitable neurons (Kole et al., 2004). Therefore, increased dendritic length could decrease the excitability of neurons due to a decrease in membrane resistance. Thus, future studies should assess the functional consequences of the dendritic reorganization within PL during the recovery period following chronic stress.
Dendritic Reorganization of Prelimbic Cortex in Female Rats
In contrast to the dynamic dendritic reorganization in PL of male rats, females showed only marginally significant dendritic retraction of apical dendrites in PL. This finding is in contrast to the apical dendritic outgrowth in mPFC of female rats we previously demonstrated (Garrett and Wellman, 2009). Methodological differences between the two studies may account for this discrepancy. First, in the present study, we restricted our analysis to PL, whereas the Garrett and Wellman study examined both anterior cingulate cortex and PL. Stress can have opposing effects on various subregions of prefrontal cortex, at least in males (e.g., Liston et al., 2006). Therefore, it is possible that the increase in apical dendritic length found in the previous study could be primarily driven by changes in anterior cingulate, rather than PL. Further, we used a 10 day chronic restraint paradigm here, as opposed to the 7 day stressor used in the previous study. Stressors of different lengths and intensities can have varying effects on dendritic morphology in other brain regions (Vyas et al., 2002, McLaughlin et al., 2007, Maroun et al., 2013, Grillo et al., 2015). Thus, perhaps in females, shorter-term chronic stressors produce dendritic growth, whereas longer-term chronic stressors produce dendritic retraction. Finally, different criteria for selecting neurons were used in this study. Whereas the previous study limited selection to neurons on which all basilar trees were nontruncated and unobstructed, we selected from neurons that had at least one basilar tree that met these criteria, and thus were likely reconstructing neurons from a subpopulation of larger pyramidal cells.
While females in this study did show some degree of apical dendritic retraction, it was small in magnitude compared that seen in males. Further, the pattern of retraction was different, with the retraction occurring more proximally in the arbor (see Fig 7). Thus, the effect of chronic stress on prelimbic cortex in males and females is different, with females seeming to be less affected by the deleterious effects of stress on dendritic morphology. This is in agreement with a growing body of literature that suggests that females may be more resilient to stress, in terms of both neuronal morphology and behavior. For example, following 21 days of restraint stress, CA3 pyramidal neurons in males undergo pronounced retraction of apical dendrites, whereas no changes are seen in the apical dendrites of female rats (Galea et al., 1997). Further, following chronic stress, males show behavioral deficits in a variety of spatial and non-spatial memory tasks including object placement (Beck and Luine, 2002), radial arm maze (Luine et al., 1994), Y-maze (Conrad et al., 1996), and object recognition (Beck and Luine, 1999). In contrast, females show either no change (Beck and Luine, 2002), or enhancements in several of these tasks (Bowman et al., 2001, Bowman et al., 2002, McLaughlin et al., 2005). To complicate this, however, in behavioral paradigms in which females initially outperform males (e.g., eyeblink conditioning), stress has the opposite effect. For example, exposure to brief restraint with concomitant tail shock facilitates eyeblink conditioning in males, but impairs conditioning in females (Wood and Shors, 1998). Thus, sex differences in the effects of stress on neuronal morphology and behavior seem to be stressor- and learning paradigm-dependent. Given this, it is possible that males and females are differentially sensitive to restraint stress, and therefore, the sex-specific dendritic reorganization that we observed may be a result of differences in perceived stressor intensity. Furthermore, given that all rats were group-housed and restraint occurred in the home cages alongside restrained cagemates, the sex-dependent stress effects found in the present study could be due to differential social buffering. Both possibilities deserve considerable attention in future studies.
Alterations in Apical and Basilar Spine Density
In addition to apical dendritic retraction immediately following chronic stress, neurons in PL of male rats also exhibited a decrease in the density of stubby spines. Several previous studies have shown decreases in total apical spine density in PL following chronic stress (Radley et al., 2006, Liu and Aghajanian, 2008, Hains et al., 2009), and at least one prior study has suggested that this loss of dendritic spines is driven primarily by a decrease in the density of larger spines (Radley et al., 2008). As larger spines are considered more stable (Rochefort and Konnerth, 2012), this suggests that chronic stress impedes spine stabilization. As we classified spines by morphology as opposed to size, it is difficult to draw direct comparisons between our results and these previous findings. Stubby spines are considered to be the least mature spine type on cortical pyramidal neurons, whereas thin and mushroom spines are thought to be more stable (Rochefort and Konnerth, 2012). Thus, a decrease in stubby spines without a concurrent increase in thin or mushroom spine density suggests a lack of spine stabilization following exposure to chronic stress, which is consistent with the notion that stress may decrease spine lability. This lack of concurrent increase in thin and mushroom spine densities combined with decreased dendritic length resulted in a net loss of spines, which likely has important implications for the function of these neurons immediately post-stress. Consistent with this notion, we previously found that stubby spine density in the IL region of mPFC correlates quite strongly with stress-induced extinction deficits (Moench et al., 2016).
To our knowledge, this is the first study examining changes in spine density of PL neurons in females following chronic stress. Immediately post-stress, decreases in the number but not density of thin and mushroom spines were seen in females, a dissociation likely due to subtle remodeling of apical dendrites. In contrast to the decreased density and estimated total number of stubby spines on apical terminal dendrites observed in male rats immediately following chronic stress, stressed female rats showed no change in density immediately following stress. Following a 7 day recovery period, both density and estimated total number of stubby spines had increased. This suggests that in the days following the cessation of stress, females, in contrast to males, may have an increase in spine lability in PL. As spines play an important role in the information processing of neurons, these lasting changes in spine density in the days following stress likely have important consequences for the overall functioning of PL, even several days after the cessation of stress, and again demonstrates sex differences in the lasting effects of stress in this region.
In agreement with previous findings from our lab and others, chronic stress did not alter the morphology of basilar dendritic arbors in PL of either males or females (Cook and Wellman, 2004, Radley et al., 2006, Garrett and Wellman, 2009). These previous findings have led to the general conclusion that, unlike apical dendrites, basilar dendrites in mPFC may be insensitive to the effects of stress. In contrast to this notion, however, here we found alteration in basilar spine density as a result of chronic stress. Specifically, neurons in PL of male rats had a decrease in overall spine density on basilar dendrites at 7 days post-stress, suggesting that changes in basilar spines may occur over a different timescale than that of apical spines. Further, both males and females exhibited a stress-induced decrease specifically in mushroom spines immediately after stress, which neared significance following 7 days of recovery. Thus, basilar dendrites may not be as impervious to stress as once thought. Further, there is some evidence that changes in basilar dendrite morphology and spine density may be hemisphere-specific and may vary by the time of day during which stress is administered (Perez-Cruz et al., 2009), indicating stress-induced changes in basilar dendrites may be more nuanced than those changes observed in apical dendrites. Indeed, this is consistent with the lack of significant alteration in estimates of total basilar spines, which suggests subtle changes in basilar dendritic length that countered the alterations in spine densities may have occurred. As pyramidal neurons segregate their inputs (Spratling, 2002), these changes in apical basilar dendritic spines suggest differential processing of input by each tree type in response to chronic stress.
Sex Differences in Stress-induced Alterations in Basal HPA Axis Activity
HPA axis dysregulation is a common feature of several stress-related psychological disorders. For example, hypercortisolism has long been implicated in depression and bipolar disorder (Carroll, 1982, Rush et al., 1996, Rybakowski and Twardowska, 1999, Maripuu et al., 2014), whereas hypocortisolism is more often associated with PTSD (Thaller et al., 1999, Yehuda et al., 2000, Kirschbaum et al., 2002, Rohleder et al., 2004). It has yet to be determined whether HPA dysregulation presents a risk factor for pathology or if it is part of the symptomatology of a given disorder. Given the numerous etiologies of stress-related disorders, both cases are likely. Here, we found that, whereas previously-stressed males tended to show attenuated baseline levels of serum corticosterone at 0 and 7 days post-stress, previously-stressed females had remarkably higher levels of corticosterone, even at 10 days post-stress. The trend toward corticosterone suppression in males agrees with previous work from Ostrander and colleagues (2006) who demonstrated that one week following chronic variable stress, male rats had attenuated adrenocorticotropic hormone and corticosterone levels following a heterotypic acute stressor, although they did not find significant basal differences in corticosterone at this time point. This difference could reflect the use of a different stress paradigm in the current study, but nonetheless suggests a possible trend in HPA axis hypoactivity in males following chronic stress.
Several mechanisms could underlie basal HPA axis hypoactivity in males. One possibility involves corticotropin releasing hormone (CRH) production and its relationship with HPA axis responsivity. During chronic stress, CRH production in the PVN increases. Sustained hypersecretion of CRH results in CRH receptor downregulation in the pituitary (Hauger et al., 1988, Aguilera et al., 2001). In the recovery period following the cessation of stress when CRH levels return to normal, receptor expression is still relatively low. Thus, attenuated CRH secretion, in combination with fewer pituitary CRH receptors, will lead to less adrenocorticotropic hormone (ACTH) production, and therefore, less corticosterone secretion. In support of this hypothesis, mice lacking CRH receptors lack the ability to mount a proper HPA axis response to stress (Smith et al., 1998). Therefore, the corticosterone suppression found here in males could represent a period of time in the recovery process prior to the normalization of pituitary CRH receptors.
Not only did females show starkly different corticosterone levels throughout the recovery period compared to males, there was also a high degree of variability in females that was not observed in males (see Fig. 3). Importantly, this variability was not due to the hormonal status of the females. One possible explanation for this variability could be the pulsatile nature of corticosterone release in females. Compared to males, females show larger and more frequent peaks of corticosterone throughout a 24 h period (Seale et al., 2004). As such, it is possible that some blood samples may have been collected at or near a peak of corticosterone secretion, while others were collected when corticosterone levels were relatively low. This seems unlikely, as unstressed females did not show the same variability as females in all three of the stressed groups. Instead, chronic stress resulted in HPA axis dysregulation that altered basal corticosterone levels in a subset of females. This suggests that, even within rodents, females may have a high degree of individual variation in recovery from stress Understanding this sex-dependent variation in stress responsivity could elucidate risk factors that may have profound effects on HPA axis adaptability, and thus responsivity to future stressors, and risk for stress-induced psychopathology.
Corticosterone Levels Correlate with Apical Dendritic Length in Females
Although the effects of stress did not vary across the apical arbor in females, dendritic length in the middle portion of the arbor correlated strongly with serum corticosterone levels. In unstressed females, higher concentrations of baseline corticosterone were associated with shorter apical dendrites. At ten days post-stress, however, this correlation was reversed, with shorter apical dendrites associated with lower baseline corticosterone levels. Interestingly, in stressed males allowed no recovery period, a negative correlation between corticosterone and apical branch length in the middle of arbor approached significance. Although the functional significance of the specificity of these associations to the middle portion of the arbor is presently unclear, there is evidence that even across the apical arbor, pyramidal neurons can receive inputs from several different regions. For example, in hippocampal CA1 pyramidal neurons, inputs from entorhinal cortex via the perforant path and thalamus synapse on distant dendritic tufts, whereas the more proximal branches receive inputs from the CA3 region via the Schaffer collaterals (Spruston, 2008). This raises the possibility that pyramidal neurons in layer II–III of prelimbic cortex could be receiving inputs onto the middle portion of the apical arbor that are specific to HPA axis function.
Conclusions
The present study demonstrates sex differences in dendritic reorganization during recovery from chronic stress, as well as long-lasting changes in basal HPA axis activity. These results suggest that the long-term sequelae of stress—rather than its immediate effects—may be critical determinants of risk for stress-related psychological disorders. Further, given the increased risk for stress-related psychopathologies in women (Solomon and Herman, 2009), as well as the increased propensity to develop psychopathology following multiple life stressors (Risch et al., 2009, Lian et al., 2014), elucidating the mechanisms underlying sex differences in recovery patterns from chronic stress has important implications for developing targeted treatment options for these disorders.
Fig. 8.
(A) Estimated number of spines on apical terminal dendrites in unstressed 0d, 7d, and 10d Rec male and female rats. Chronic stress decreased the total number of terminal spines in both male and female 0d Rec rats compared to unstressed rats. (B) Estimated number of stubby spines on apical terminal dendrites in unstressed 0d, 7d, and 10d Rec male and female rats. In males, chronic stress decreased estimated stubby spine number in 0d Rec rats compared to both unstressed and 10d Rec rats, and this decrease approached significance compared to 7d Rec rats. (C) Estimated number of thin spines on apical terminal dendrites in unstressed 0d, 7d, and 10d Rec male and female rats. Chronic stress decreased the estimated number of thin spines in both male and female 0d Rec rats compared to unstressed rats. (D) Estimated number of mushroom spines on apical terminal dendrites in unstressed 0d, 7d, and 10d Rec male and female rats. Chronic stress decreased the estimated number of mushroom spines in both male and female 0d Rec rats compared to unstressed rats. # p < 0.05 0d Rec vs all other groups; * p < 0.05 0d Rec vs Unstressed and 10d Rec males; ^ p < 0.07 0d Rec vs 7d Rec males; † p < 0.05 Unstressed vs 7d Rec females; ‡ p < 0.07 7d Rec vs 10d Rec females.
Fig. 10.
(A) Estimated number of total basilar terminal spines in unstressed 0d, 7d, and 10d Rec male and female rats. (B) Estimated number of stubby basilar terminal spines in unstressed 0d, 7d, and 10d Rec male and female rats. (C) Estimated number of thin basilar terminal spines in unstressed 0d, 7d, and 10d Rec male and female rats. (D) Estimated number of mushroom basilar terminal spines in unstressed 0d, 7d, and 10d Rec male and female rats.
Highlights.
Male rats have a dynamic pattern of dendritic reorganization in prelimbic cortex in the days following chronic stress.
Chronic stress induces lasting increases in baseline corticosterone in female rats.
This divergent pattern of changes post-stress may leave males and females differentially sensitive to subsequent stressors.
Abbreviations
- 0d Rec
chronic stress plus no recovery period
- 10d Rec
chronic stress plus a 10 day recovery period
- 7d Rec
chronic stress plus a 7 day recovery period
- ACTH
adrenocorticotropic hormone
- CORT
corticosterone
- CRH
corticotropin releasing hormone
- HPA
hypothalamic-pituitary-adrenal
- mPFC
medial prefrontal cortex
- PL
prelimbic cortex
- PTSD
posttraumatic stress disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G, Rabadan-Diehl C, Nikodemova M. Regulation of pituitary corticotropin releasing hormone receptors. Peptides. 2001;22:769–774. doi: 10.1016/s0196-9781(01)00390-4. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:321–330. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: Role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive Effects of Stress and Aging on Structural Plasticity in the Prefrontal Cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression. In: Brown GW, Harris TO, editors. Life Events and Illness. Guilford; New York: 1989. pp. 49–93. [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Cajal S. Histology of the Nervous System. New York: Oxford University Press; 1995. [Google Scholar]
- Carroll BJ. The dexamethasone suppression test for melancholia. Br J Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesions of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol Learn Mem. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009:162. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy - application of a new, high clarity golgi-nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Risher M, Macht VA, Bumgardner AL, Hang A, Gabriel C, Mocaer E, Piroli GG, Fadel JR, Reagan LP. Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience. 2015;284:430–443. doi: 10.1016/j.neuroscience.2014.09.047. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol (Lond) 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MAT, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AFT. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DW, Strauss JS, Kokes RF, Ritzler BA, Gift TE. Life events and psychopathology severity among 1st psychiatric admissions. J Abnorm Psychol. 1980;89:165–180. doi: 10.1037//0021-843x.89.2.165. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Millan MA, Lorang M, Harwood JP, Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Hayes WL. Statistics. Fort Worth, TX: Harcourt Brace; 1994. [Google Scholar]
- Heiderstadt KM, McLaughiin RM, Wright DC, Walker SE, Gomez-Sanchez CE. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Laboratory Animals. 2000;34:20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Joksimovic L, Rohleder N, Wolf JM, Riegler S. Hypocortisolism and increased glucocorticoid sensitivity in Bosnian War PTSD victims. Psychosom Med. 2002;64:150–150. [Google Scholar]
- Koch C, Segev I. The role of single neurons in information processing. Nat Neurosci. 2000;3(Suppl):1171–1177. doi: 10.1038/81444. [DOI] [PubMed] [Google Scholar]
- Kole MHP, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Konkle ATM, Baker SL, Kentner AC, Barbagallo LSM, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Lian YL, Xiao J, Wang Q, Ning L, Guan SZ, Ge H, Li FY, Liu JW. The relationship between glucocorticoid receptor polymorphisms, stressful life events, social support, and post-traumatic stress disorder. Bmc Psychiatry. 2014;14:10. doi: 10.1186/s12888-014-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Inokuchi H, McLachlan EM, Li JS, Higashi H. Correlation between electrophysiology and morphology of three groups of neuron in the dorsal commissural nucleus of lumbosacral spinal cord of mature rats studied in vitro. J Comp Neurol. 2001;437:156–169. doi: 10.1002/cne.1276. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Macias M. Injury induced dendritic plasticity in the mature central nervous system. Acta Neurobiol Exp. 2008;68:334–346. doi: 10.55782/ane-2008-1699. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF. Relative Hypo-and Hypercortisolism Are Both Associated with Depression and Lower Quality of Life in Bipolar Disorder: A Cross-Sectional Study. PLoS One. 2014;9:12. doi: 10.1371/journal.pone.0098682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur J Neurosci. 2013;38:2611–2620. doi: 10.1111/ejn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti O, Marti J, Armario A. Effects of chronic stress on food-intake in rats - influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA Receptor Blockade Alters Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex. Cereb Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Lawrence Erlbaum Associates; 2004. [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, Talboom JS, Bimonte-Nelson HA, Sanabria F, Conrad CD. Chronic Stress Impairs Prefrontal Cortex-Dependent Response Inhibition and Spatial Working Memory. Behav Neurosci. 2012;126:605–619. doi: 10.1037/a0029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Nava N, Treccani G, Alabsi A, Kaastrup Mueller H, Elfving B, Popoli M, Wegener G, Nyengaard JR. Temporal Dynamics of Acute Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex and the Protective Effect of Desipramine. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv254. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Flak JN, Richtand NM, Herman JP. Chronic stress produces enduring decreases in novel stress-evoked c-fos mRNA expression in discrete brain regions of the rat. Stress. 2009;12:469–477. doi: 10.3109/10253890802641966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress. 2011;14:227–232. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perez-Cruz C, Simon M, Czeh B, Flugge G, Fuchs E. Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat prelimbic cortex: activity- and stress-induced changes. Eur J Neurosci. 2009;29:738–747. doi: 10.1111/j.1460-9568.2009.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. Small pryamidal neuron of rat cerebral cortex - perikayron, dendrites and spines. Am J Anat. 1970;127:321. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models to experimental data. Physiol Rev. 1992;72:S159–186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression A Meta-analysis. JAMA-J Am Med Assoc. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR, Jr, Weissenburger JE, Crowley GT, Khatami M, Vasavada N. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatr. 1996;57:470–484. doi: 10.4088/jcp.v57n1006. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. J Psychiatr Res. 1999;33:363–370. doi: 10.1016/s0022-3956(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol. 2004;16:516–524. doi: 10.1111/j.1365-2826.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: Insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. 1956:324–333. [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratling MW. Cortical region interactions and the functional role of apical dendrites. Behav Cogn Neurosci Rev. 2002;1:219–228. doi: 10.1177/1534582302001003003. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Thaller V, Vrkljan M, Hotujac L, Thakore J. The potential role of hypocortisolism in the pathophysiology of PTSD and psoriasis. Coll Antropol. 1999;23:611–619. [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatr. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiological dysfunction of dosolateral prefrontal cortex in schizophrenia.1. regional cerebral blood-flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Visualizing changes in neuronal dendritic morphology in response to stress and pharmacological challenge. Curr Protoc Neurosci. 2016;78:8.38.31–38.38.18. doi: 10.1002/cpns.18. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, Dolan S. Low cortisol and risk for PTSD in adult offspring of Holocaust survivors. Am J Psychiatry. 2000;157:1252–1259. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: Areal and laminal structure. In: Paxinos G, editor. The rat nervous system. San Diego, CA: Academic Press; 1995. pp. 649–685. [Google Scholar]