Abstract
Emergence of extensively drug-resistant tuberculosis (XDR-TB) has significantly threatened to jeopardize global efforts to control TB, especially in HIV endemic regions. XDR-TB is mainly an iatrogenically created issue, and understanding the epidemiological and risk factors associated with it is of paramount importance in curbing this menace. Emergence of this deadly phenomenon can be prevented by prompt diagnosis and effective treatment with second-line drugs in rifampicin-resistant TB (RR-TB) as well as multidrug-resistant TB (MDR-TB) patients. Optimal treatment of RR-TB, MDR-TB and XDR-TB cases alone will not suffice to reduce the global burden. The TB control programmes need to prioritize on policies focusing on the effective as well as rational use of first-line drugs in every newly diagnosed drug susceptible TB patients so as to prevent the emergence of drug resistance.
Keywords: Diagnosis, epidemiology, extensively drug-resistant tuberculosis, HIV, India, multidrug-resistant tuberculosis-pregnancy, second-line drugs, XDR-TB
Introduction
Drug-resistant tuberculosis (DR-TB) has been reported in the era of early introduction of anti-tubercular chemotherapy, but recently, extensively drug-resistant TB (XDR-TB) is posing a great threat to global control of TB. The concept of XDR-TB was first introduced at the Centers for Disease Control and Prevention (CDC) in March 20051. Shortly thereafter, the data on resistance to second line drugs (SLDs) were reported during an epidemic outbreak at KwaZulu-Natal, South Africa, resulting in alarming mortality among TB cases co-infected with HIV in February 20062. The global emergence of XDR-TB has raised the concern that the current existence of mostly drug susceptible TB will be replaced with a complicated form of TB with limited treatment strategies. The emergence of XDR-TB can be prevented by effective management with SLDs in rifampicin-resistant TB (RR-TB) as well as multidrug-resistant TB (MDR-TB) cases. This review aims to focus on the comprehensive management strategies for patients suffering from XDR-TB.
Definition
XDR-TB was defined as those TB cases with documented resistance to isoniazid (H) and rifampicin (R) and at least three of the six main classes of SLDs [aminoglycosides, polypeptides, fluoroquinolones (FQs), thioamides, cycloserines (Cs) and para-aminosalicylic acid]3. However, the WHO revised the case definition of XDR-TB as ‘TB with resistance to at least H and R as well as further resistance to any FQs [ofloxacin (Ofx), levofloxacin (Lfx) or moxifloxacin (Mfx)] and second-line injecTable drugs (SLIDs) [kanamycin (Km), amikacin (Amk) or capreomycin (Cm)] at an emergency meeting of the Global XDR-TB Task Force. This definition was considered in view of difficulty in testing some SLDs and less treatability of some forms of drug resistance as compared to others4. Further, two new terminologies pre-XDR-TB and extremely drug-resistant TB (XXDR-TB) have been introduced recently based on SLDs resistance patterns. Pre-XDR-TB was defined as a subset of MDR-TB cases that are resistant to either FQ or SLID but not to both, thereby not fulfilling the criteria of XDR-TB. Another term XXDR-TB also known as totally drug-resistant TB (TDR-TB) was proposed for cases resistant to all available first-line drugs and second-line drugs (SLDs)5. It was further recommended that these results should not be utilized solely to guide treatment as there is still lack of adequate reproducibility and reliability of drug susceptibility test (DST) for the remaining SLDs as well as standardized methodology for testing6,7. There could also be a discrepancy as in vitro DST results might depict resistance for a particular strain of Mycobacterium tuberculosis although there might not be actually any resistance in vivo and the prognostic relevance of these results without an internationally accepted and standardized DST would remain unclear.
Epidemiology
Global
Worldwide, XDR-TB constitutes 9.7 per cent [95% confidence interval (CI): 7.4-12%] of MDR-TB patients as reported by the WHO in 20157. Prevalence of XDR-TB cases from different countries of the world is summarized in Table I8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. Fourteen countries reported ≥10 XDR-TB cases with the proportion of MDR-TB cases with XDR-TB. Among these countries, the cases were highest in four countries7- Belarus (29%), Georgia (15%), Latvia (19%) and Lithuania (25%). The estimation of prevalence of resistance to SLDs was first documented by CDC, by surveying the network of Supranational Reference Laboratories (SRLs) during 2000-200426. Of the 23 eligible laboratories, 14 (61%) produced drug susceptibility results from 17,690 isolates, which represented data from 48 countries. Of the 17,690 isolates, 3520 (19.9%) isolates met criteria for MDR-TB. Of the 3520 MDR-TB isolates, 347 (9.9%) isolates met criteria for XDR-TB. The resistance patterns for XDR-TB isolates included resistance to aminoglycosides, Cm and FQs - 90 (3.4%); aminoglycosides, FQs and thioamides - 102 (3.4%) and FQs, thioamides and para-aminosalicyclic acid (PAS) - 94 (3.8%)26. Further, 48.1 per cent of all XDR-TB isolates were resistant to all four first-line drugs, leading to the resistance of isolates to more than seven drugs. The proportion of XDR-TB among MDR-TB patients was 6.5 per cent when estimated for industrialized nations26. Fifty five (13.6%) and 200 (15.4%) MDR-TB patients met criteria for XDR-TB among patients from Russia and Eastern Europe as well as Republic of Korea, respectively. In a study from Hong Kong27, 30 (17%) MDR-TB isolates among re-treatment TB cases had resistance to more than three SLDs, fulfilling criteria for XDR-TB. A drug resistance survey conducted among 447 culture-positive new and re-treatment TB cases in Abkhazia, Republic of Georgia, revealed that 3 per cent had additional resistance to three SLDs classes, consistent with XDR-TB28. Clusters of XDR-TB cases have been reported in South Africa and Iran with high prevalence of HIV co-infection and mortality8,29. In the Fourth WHO/International Union against Tuberculosis and Lung Disease anti-TB drug resistance surveillance report30, 35 countries and two special administrative regions have contributed data on XDR-TB with variable quality assurance for laboratory testing. Of the 4012 MDR-TB cases, 301 (7%) were reported to be XDR-TB with low numbers of cases from Central and Western Europe, the Americas and in Asian countries30. The XDR-TB proportion among MDR-TB was variable from 0 per cent in 11 countries to 30 per cent in Japan because of relatively low MDR-TB burden representing a few absolute cases. A higher proportion was observed in the nine countries of the former Soviet Union with approximately 10 per cent of all MDR-TB cases as XDR-TB ranging from 4.0 per cent in Armenia to almost 24.0 per cent in Estonia because these proportions represented a much larger absolute number of cases30. A study was conducted in Lima, Peru, among 810 TB patients from 1999 to 2002 where SLDs resistance was performed on 651 patients and 48 (7.4%) patients who had XDR-TB15. Another study from South Africa reported 996 (5.6%) of 17,615 MDR isolates as XDR-TB between 2004 and October 200731. Proportions were variable across Provinces with KwaZulu-Natal reporting 14 per cent MDR cases as XDR-TB. About 5.4 per cent of MDR-TB cases were found to have XDR-TB as reported from 46 countries based on continuous surveillance or representative surveys of SLDs resistance with the prevalence of more than 10 per cent XDR-TB cases in eight countries and majority were located in Eastern Europe and Central Asia31. A cumulative total of 105 countries have confirmed at least one case of XDR-TB by 20147. The WHO in 2016 reported that 58 countries and territories were treating 7234 XDR-TB patients with SLDs worldwide with a maximum number of cases notified from four countries - India (2130), Ukraine (1206), the Russian Federation (1205) and South Africa (719)32. Seventy four countries reported 7579 XDR-TB cases by the end of 2015. Thirty six per cent were reported to have DST for both FQs and SLIDs among MDR-TB and RR-TB patients with lowest coverage in the WHO Western Pacific and South-East Asia regions as notified in 201532.
Table I.
Characteristics of important studies from the world showing prevalence and outcome of extensively drug-resistant tuberculosis (XDR-TB)
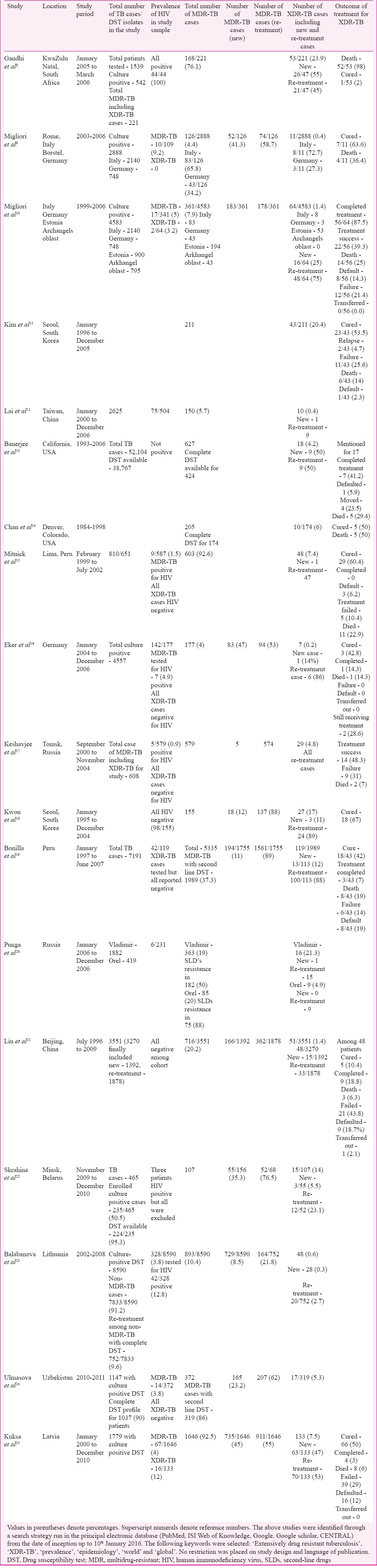
India
According to the data reported on XDR-TB from India, it varied from 0.3 to 60 per cent of MDR-TB as shown in Table II33,34,36,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59. Sub-national community survey from India in 2006 in the State of Gujarat reported XDR-TB in 3.2 per cent (95% CI, 1.2-6.6%) of 219 MDR-TB patients. All XDR-TB cases were previously treated cases. It was also reported that the high prevalence 24 per cent of pre-XDR-TB with only Ofx resistance among MDR-TB isolates39. Another study conducted by Tuberculosis Research Centre (TRC) [now National Institute of Research in Tuberculosis (NIRT)], Chennai, reported 10 cases of XDR-TB and 12 cases of pre-XDR-TB from two cohorts of 104 MDR-TB cases48. Among those XDR-TB patients, one was diagnosed pre-treatment, six had initial susceptibility to Km as well as Ofx and three with only initial Ofx resistance. In a study at Lucknow, XDR-TB was found in 8.5 per cent of MDR-TB cases, and 42.3 per cent of MDR-TB cases were pre-XDR-TB60. In analysis of studies involving 10 tertiary care centres in nine cities in India 598 isolates of XDR-TB were reported61. Another study conducted at a tertiary care TB hospital in New Delhi49, observed that 45 (20.17%) isolates were XDR-TB on analyzing sputum cultures of 223 diagnosed MDR-TB patients with maximum resistance to four drug combinations namely Km, ethionamide (Eto), Ofx and PAS (5.82%), followed by Km and Ofx (3.13%) and Ofx, Km and Eto (1.79%); 1.34 per cent isolates showed pan-resistance and other combinations in the remaining isolates. Majority of the XDR-TB isolates showed resistance to three or more drugs combination pattern, highlighting need for urgent and timely sensitivity report for SLDs to help clinicians start proper regimens to treat MDR-TB patients to prevent occurrence of XDR-TB49. A study conducted from a tertiary care hospital at Delhi reported 24 (44.4%) isolates proven positive for Mycobacterium tuberculosis among 54 HIV-positive patients. Among the 24 isolates, 12 (50%) qualified for MDR-TB, of which XDR-TB resistance was observed in 4 (33.3%) patients. All four XDR-TB patients expired within 2.6 months of diagnosis35. Another study on drug resistance survey in five Tibetan settlements in India reported that 14.5 per cent of 307 patients had MDR-TB62. A cross-sectional survey conducted among adults and children anti-retroviral therapy (ART)-centre attendees in Mumbai revealed that drug resistance was diagnosed in 68 (34%) of 202 culture-positive cases. Of these 68 cases, 38 per cent were MDR-TB, 21 per cent were pre-XDR (MDR-TB plus resistance to either an FQ or SLID), 6 per cent XDR-TB and 2 per cent XXDR-TB (XDR-TB plus resistance to any group IV/V drug)55. A study conducted at four tertiary care hospitals in Delhi reported prevalence of XDR-TB among 483 MDR-TB patients as 3.7 per cent. According to the latest WHO report, 8976 cases of MDR-TB as well as RR-TB were tested for resistance to SLDs with 3048 cases being XDR-TB as confirmed by laboratory testing in India32. Further studies are required at community level from different regions of India to estimate actual incidence and prevalence rate of XDR-TB to establish early diagnosis of drug resistance and implementation of appropriate treatment strategy by TB control programme.
Table II.
Characteristics of important studies from India showing prevalence and outcome of extensively drug-resistant tuberculosis (XDR-TB)

Factors responsible for XDR-TB
Drug resistance in M. tuberculosis is due to the mutations in the genome. Mismanagement of MDR-TB cases primarily results in this form of TB due to the following reasons: faulty or improper treatment, non-adherence to treatment guidelines, inappropriate, incomplete or erratic use of SLDs and use of poor-quality SLDs. A study from South Korea reported that factors responsible for XDR-TB were total duration of prior SLDs intake, inaccessibility to approach reliable laboratory for DST against first-line drugs as well as SLDs, lack of experience, poor training and skills to manage DR-TB and factors linked to poor control practices63. The lack of measures to ensure adherence and monitoring of treatment with prescribed regimens, especially in private sectors, was considered to be a major risk factor for XDR-TB63. The two strongest risk factors for XDR-TB are failure of regimens used for treatment of MDR-TB including SLIDs as well as FQ and close contact with an individual with documented XDR-TB64. It was observed in a study at Lithuania and Estonia that younger age group, male gender, and known contact with an MDR-TB were associated with increased risk of primary infection with XDR-TB and MDR-TB strains while defaults and failures in the past triggered XDR-TB development65. A three-year prospective study from Russia reported that risk of developing resistance to Cm and of XDR-TB was higher among MDR-TB patients who were treated with regimens containing less than three effective drugs (9.4 vs. 0%) as compared to those who were treated with regimens containing more than three effective drugs (8.6 vs. 0.8%)66. A study conducted at Delhi predicted factors that were associated significantly with XDR-TB such as family history of TB, low socio-economic status, comorbid illness and previous intake of SLIDs51.
Diagnosis
A clinical diagnosis of XDR-TB is not possible. However, the strongest risk factors to suspect XDR-TB include failure of an MDR-TB treatment regimen and close contact with an individual with documented XDR-TB or an individual, for whom treatment with a regimen including SLDs has failed64. A detailed evaluation of history of previous exposure to anti-tubercular treatment (ATT) including first-line drugs as well as SLDs is essential. XDR-TB cases constitute two major subgroups: (i) MDR-TB at diagnosis (not exposed to SLDs) and (ii) MDR-TB therapy failure (exposed to SLDs)67. The classification is important as it is a major variable for XDR-TB therapy response as XDR-TB patients belonging to the later group are difficult to treat as compared to former one. The MDR-TB patients are treated under programmatic management of drug-resistant tuberculosis (PMDT) by Revised National Tuberculosis Control Programme (RNTCP) of India with Category IV (CAT IV) which is a standardized six drug regimens (Eto, Cs, Lfx, Km, ethambutol-E and pyrazinamide-Z) taken daily for 24-27 months68. This standardized regimen contains anti-tubercular drugs that are grouped on the basis of efficacy, experience of use and drug class as recommended by the WHO64 (Table III). The non-responders and failures of CAT IV are offered SLDs DST (FQs and aminoglycosides).
Table III.
Grouping of anti-tuberculosis drugs
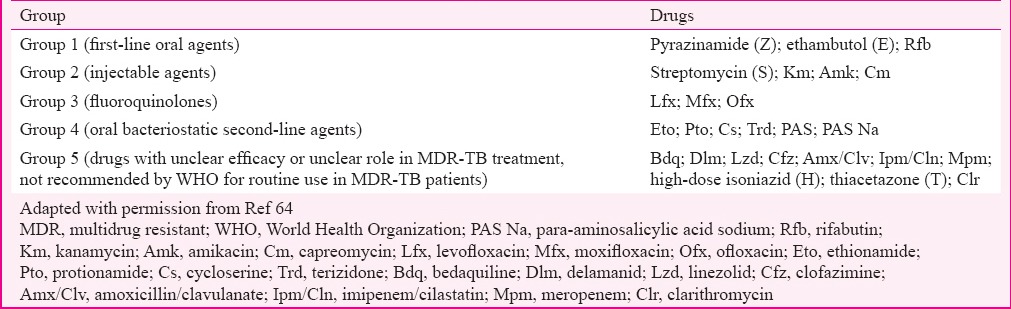
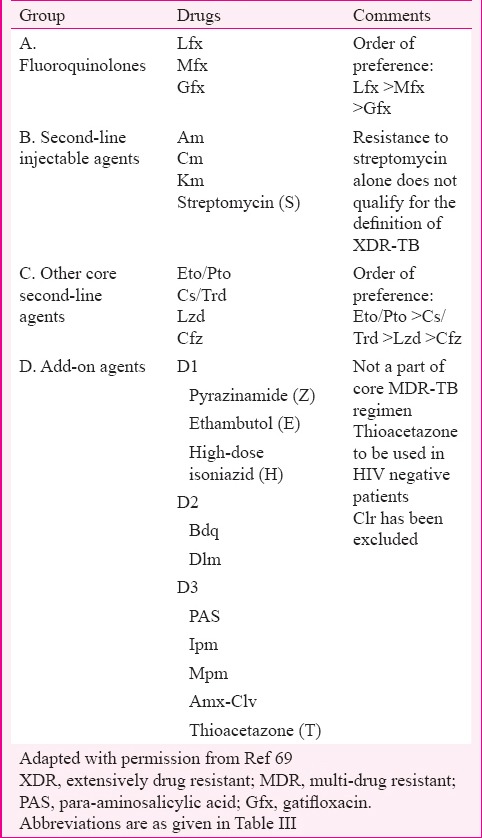
A patient of MDR-TB who is on second-line ATT or CAT IV and has sixth month follow up culture positive is said to be ‘XDR-TB suspect’ and the culture isolate is to be sent for DST for SLDs. In addition, any CAT IV patient showing reversion, i.e. two serial consecutive positive cultures after culture conversion, would also be suspected for treatment failure and the culture isolate of the second positive culture would be sent for second-line DST (mainly Km and FQ)68. WHO has recently re-grouped anti-tubercular drugs used in the design of conventional MDR-TB as well as XDR-TB treatment regimens based on the current evidence on their effectiveness and safety69 as mentioned in Table IV. Clofazimine (Cfz) and linezolid (Lzd) are now recommended as core SLDs in the MDR-TB regimen according to recent classification, while PAS is considered an add-on agent. Clarithromycin (Clr) and other macrolides are excluded from the current classification of drugs for the treatment of MDR-TB69. The only way to diagnose XDR-TB currently is by DST to first-line drugs and SLDs which may take up to 16 weeks if done by conventional method.
Table IV.
Revised grouping of anti-tuberculosis drugs
All MDR-TB patients should preferably be tested for XDR-TB in high-resource settings. The detection of cases also depends on the availability of SLDs DST. According to the WHO, in areas with limited second-line DST, patients should be tested at the very least if suspected to have risk factors for XDR-TB. Further, in areas where no capacity for second-line DST exists, this investigation has to be done from a capable SRL. Individuals presumed to have XDR-TB should have at least DST to H, R, the three SLIDs (Km, Amk and Cm) and FQs used in the country64. The routine testing of DST for SLDs is not feasible on account of its difficulty, cost and poor reliability. Even in well-developed countries, despite adequate facilities available for performing DST for SLDs, interpretation of the results requires careful analysis by trained staff because of lack of standardized concentrations for each drug and wide variation in definitions of resistance. DST of drugs such as groups 4 and 5 drugs (Table III) has poor reproducibility and reliability64. Therefore, precautions must be taken while adding these drugs in individual regimens based on DST. Urgent interventions are required for XDR-TB patients with concomitant HIV-infection carrying high risk of mortality that include assessment for the risk of SLDs resistance and initiation of an empiric XDR regimen if probability of infection is high while awaiting for conventional phenotypic DST results64. More rapid tests such as radiometric methods such as BACTEC 460 and MGIT 960, should be used70. Some of the newer liquid and agar techniques can determine the presence of XDR-TB within 14 days. Other more rapid systems are being tested for identification of drug resistance e.g. colorimetric assays and broth-based assays for rapid detection of M. tuberculosis and for resistance to H and R. Genotypic methods for detecting specific genetic mutations and responsible for certain types of drug resistance are available for only MDR-TB e.g. Genotype MTBDR assay, Genotype MTBDR plus assay, INNO-LiPA assay15,71. The second-line line probe assay (LPA) for FQs and aminoglycosides known as GenoType MTBDRsl assay has the potential to be used as a rule-in test for XDR-TB where capacity for the same is available. In a study, sensitivity and specificity of the Genotype MTBDRsl test was found to be 87 and 96 per cent, respectively for FQs; 100 and 100 per cent for Am; 77 and 100 per cent, respectively for Km; 80 and 98 per cent respectively, for Cm and 57 and 92 per cent respectively, for E72. Two versions (version 1 and 2) of MTBDRsl assay have been introduced by the WHO in 201673. MTBDRsl version 1 detects M. tuberculosis complex (MTBC) and resistance to FQs (mutation in gyrA gene), SLID (mutation in rrs gene) and E (mutation in embB gene) in smear-positive specimens as well as culture isolates. The MTBDRsl version 2 detects MTBC and resistance to FQs (mutation in gyrA and gyrB gene) as well as SLID (mutation in rrs gene and eis promoter region) excluding E. The additional advantage is that it can be applied in smear-negative specimens also. The assay displays a sensitivity and specificity of 85.1 and 98.2 per cent to detect FQ resistance when performed directly (using smear-positive sputum samples) and 83.1 and 97.7 per cent, respectively, when performed indirectly (using culture isolates)74. MTBDRsl exhibited sensitivities and specificities of 76.9 and 99.5 per cent for the SLID class when performed indirectly (using isolates) and 94.4 and 98.2 per cent, respectively, when performed using smear-positive sputum samples (directly).
The sensitivity and specificity of MTBDRsl for both aminoglycosides and FQs were 100 and 100 per cent and for E were 91.7 and 100 per cent in a study conducted at Lucknow75. Another study conducted at Mumbai revealed sensitivity and specificity of MTBDRsl for aminoglycosides (100 and 100%), FQs (91.3 and 95.5%) and E (56.2 and 81%)76. The targeted resistance was detected in less time as compared to phenotypic DST. However, due to low negative predictive value to FQ (88%) and E (43.21%), the assay results must be interpreted in coordination with the phenotypic DST. The WHO Expert Group has concluded that Genotype MTBDRsl LPA may be preferred over phenotypic culture-based DST, to detect resistance to SLDs in both adults as well as children. However, this recommendation requires high-quality evidence for validation as this assay has less sensitivity for detection of SLDs resistance to reliably rule out XDR-TB. In addition, the cross-resistance between various SLDs makes this test less reliable to identify the individual drugs to be used for treatment64. There is high correlation of resistance-conferring mutations to FQs between LPA and conventional phenotypic resistance, especially for Ofx and Lfx as compared to Mfx and Gfx. This proves that inclusion of Mfx or Gfx in a regimen should be best guided by phenotypic DST results. These limitations make Genotype MTBDRsl LPA still inferior to conventional phenotypic DST and cannot replace the latter. The second-line LPA for FQs and SLIDs has the potential to be used as a rule-in test for XDR-TB where capacity for same is available, especially in high prevalence settings64.
The RNTCP of India has expanded access to DST of baseline SLDs, especially for Km and Lfx to all MDR-TB cases at the initiation of treatment, and the results would be received after six to eight weeks77. This will help in appropriate modifications of the treatment regimens and improvement of treatment outcomes if additional resistance (including pre-XDR-TB and XDR-TB cases) is detected early. Currently, 25 laboratories has been certified by RNTCP for SLDs DST that includes five national reference laboratories, 13 intermediate reference laboratories, five medical colleges, one non-governmental organization and one private-SRL77. Baseline SLD for MDR-TB patients has been rolled out across the country by linking States and Union Territories to the certified laboratories. The National Committee of Operational Research has approved a study for the validation of LPA for detecting resistance to FQs, injecTable aminoglycosides (Km, Amk) and cyclic peptides (Cm) in Programme Setting in India using MTBDRsl assay77. This assay has to be implemented all over the centres in India to achieve rapid detection of XDR-TB from high burden of MDR-TB patients in the near future.
Treatment
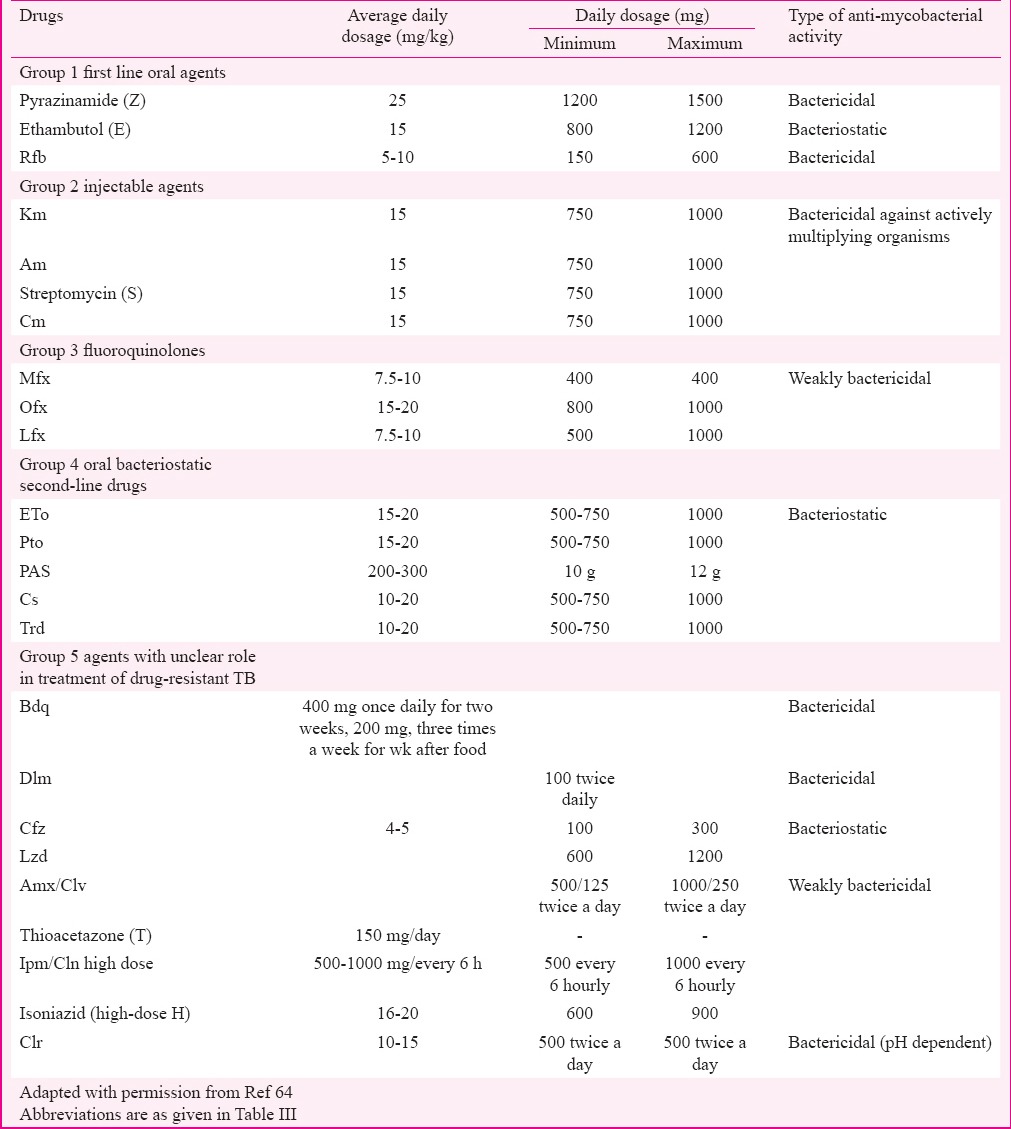
Treatment of XDR-TB is difficult and expensive. Less powerful agents (second-line and group V agents) are required for treatment because R and H cannot be used and drugs have to be used for a longer duration as well. The treatment can be an individualized regimen or a standardized one, depending on the previous drugs taken by the patient (by constructing a drug-o-gram), degree of resistance in the community and the financial status of the patient. A drug is considered to be effective if the baseline DST shows susceptibility to that drug; however, if DST results are not available, a drug may still considered to be effective if not previously taken for at least one month15. At least five drugs with proven susceptibility or which are most likely to be effective should be used in constructing a regimen64 (Table V). The first drug should be selected from group 1 in addition to Z. Another drug to be selected from group 2 is an injecTable agent, to which the strain is susceptible and its use can be extended to 12 months or more based on culture conversion. It is recommended to use an injecTable agent that has never been used before or consider designing the regimen without an injecTable agent in case of reported resistance to all injecTable agents. If one of the injecTable agents is considered effective but toxicity is a limiting factor, then alternative route of inhalation through a nebulizer should be considered but the use of this route is still questionable due to lack of evidence64. Further, drug from group 3 such as higher generation FQ (Mfx or Gfx) can be added. Use of all group 4 drugs that have not been used or less exposed in a previous regimen may be effective. Two or more group 5 drugs [such as bedaquiline (Bdq)] can be added. Bdq (TMC 207) is a newly approved drug by the WHO for the treatment of MDR-TB and XDR-TB, which belongs to the diarylquinoline class of antibiotics78. This drug has high bactericidal activity against drug resistance proved on the basis of a multicentric phase II trial. It was observed that patients on Bdq achieved significantly higher rates of sputum culture conversion at six months (79 vs. 58%) and cure rate at 2.5 yr (58 vs. 32%)79.
Table V.
Doses of anti-tubercular drugs used in multidrug-resistant and extensively drug-resistant tuberculosis
Bdq has been given approval for use along with the background regimen under conditional access through the RNTCP PMDT programme77. It is planned to introduce this drug in six referral sites initially to establish its safety profile among Indian patients for improving outcome amongst MDR-TB and XDR-TB patients. Delamanid (Dlm) (OPC 67683) and pretomanid (Ptm) (PA-824) belong to the nitroimidazole class of antibiotics, currently undergoing phase II and phase III clinical trials78. A single cohort study reported that patients receiving Dlm for two months had a higher rate of two-month sputum-culture conversion than patients receiving placebo [7 of 16 (44%) vs. 1 of 10 (10%), P=0.10]80. Rates of sustained culture conversion and successful treatment outcomes were higher, whereas mortality was lower among patients treated for six months or more than among those treated for two months or less81. Other newer drugs such as sutezolid (Szd) (PNU-100480) and SQ109 are currently undergoing phase II clinical trials82. Benzothiazinones (BTZ043) are the most recently introduced drugs that are undergoing pre-clinical development phases83. High-dose H should be considered if there is documentation of low-level resistance or absence of the katG gene. Localized disease should be considered for adjuvant surgery if possible. Various measures should be undertaken while treating MDR-TB and XDR-TB patients for better outcomes such as rigorous respiratory infection control, hospitalization in case of poor general condition of the patient or coexistence of major comorbidities, provision of shelter if the social condition of the patient prevents proper home care, management of HIV co-infection, comprehensive monitoring and full social support to ensure adherence, full access to palliative and end-of-life care services and focus on patient-centred approach64.
The MDR-TB patients diagnosed with baseline FQ and injecTable aminoglycosides resistance are treated with modified CAT IV regimen, whereas the same resistance patterns among non-responders and failures of CAT IV are treated with CAT V regimen for up to 24-30 months daily regimen68,77. The standardized regimen for Cat V consists of seven drugs, with two reserve/substitute drugs. The intensive phase (IP) (6-12 months) consists of seven drugs - Cm, PAS, Mfx, high-dose H, Cfz, Lzd and amoxyclav whereas continuation phase (CP) (18 months) consists of six drugs – PAS, Mfx, high-dose H, Cfz, Lzd and amoxyclav. The dosages of the drugs would vary as per the weight bands of the patient. All drugs are to be given on a daily basis under supervision of a directly observed treatment (DOT) provider. Injection of Cm is given six days/week. The details of the drugs and dosages are summarized in Table V. The reserve/substitute drugs i.e. Clr and thiacetazone (T) could be included in the regimen of XDR-TB patients preferably in place of PAS if the latter was used previously during their CAT IV treatment of MDR-TB. The other indications for using these reserve drugs are inability to tolerate one or more of the drugs included in standardized regimen and resistance to Cm at baseline. The switching from IP to CP is advised only after achievement of culture conversion. The IP can be extended from six months up to a maximum of 12 months in case of delay in culture conversion68. The PMDT site committee could decide on administering Cm injection intermittently (three times/week) for the months 7-12 in case of extension. This strategy has been widely adopted in various States of India since its implementation in 201268. A total of 392 XDR-TB cases were initiated on second-line standardized treatment under PMDT in 201384. Subsequently, 939 XDR-TB cases including Ofx-resistant cases were detected among 2184 MDR-TB cases from January to September 201485. Of the 939 XDR-TB cases, 879 (93.6%) were treated with standardized treatment. According to the current report, 2130 XDR-TB cases were registered and initiated on treatment in 2015 with maximum cases belonging to Maharashtra (899), Gujarat (288) and Delhi (166)77. The WHO has recently introduced standardized as well as individualized regimens in managing MDR and XDR-TB patients based on the recent classification69 as shown in Table IV. A regimen with at least five effective TB medicines should be prescribed in the IP, including Z and four core SLDs - (group A: 1 drug, group B: 1 drug, and group C: at least two drugs). If the regimen is considered ineffective especially in case of XDR-TB where resistance to both groups A and B drugs is reported, additional drugs from groups D2 or D3 are introduced to make it more effective with total of five drugs. The two recent drugs Bdq and Dlm now categorized under group D2 within the add-on agents used to treat MDR-TB and XDR-TB. WHO has also recommended to treat a subset of MDR-TB patients with shorter regimen of 9-12 months instead of a conventional regimen with no previous history of SLDs intake and in whom sensitivity to FQs and SLID detected at baseline or highly suspected69. These regimens need to be implemented all over the country under PMDT strategy in coming years with better hope in improving outcome.
Monitoring of treatment
No randomized controlled trials have been conducted on XDR-TB, regarding the appropriate duration of treatment. Therefore, it is largely based on consensus68. Baseline evaluation should include complete blood count with platelets, thyroid function test, liver function test, kidney function test, serum electrolytes, audiometry, vision tests, electrocardiography, blood sugar, pregnancy test in women of reproductive age group and also psychosocial counselling. All patients need to be offered HIV testing after pre-test counselling, unless known to be HIV positive. Assessment with chest X-ray and evaluation for surgery will also be done. While on follow up, culture should be done monthly during IP after three months of starting treatment and quarterly during CP. In case of extension of IP due to delay in culture conversion, the follow up schedule may be modified accordingly68,77.
There are various adverse effects such as nephrotoxicity (Cm), electrolyte wasting (Cm), hypothyroidism (PAS, Eto), hepatotoxicity (PAS), ototoxicity (injecTable aminoglycoside), psychiatric disturbances (Cs/Tzd) and QT prolongation (Bdq, Dlm) which are difficult to monitor and may be life-threatening, therefore requiring intensive monitoring. A significant part of monitoring treatment is assessment and management of toxicity or adverse drug reactions occurring due to second-line anti-tubercular drugs64. The ability to monitor patients for adverse effects daily is one of the major advantages of directly observed therapy (DOT) over self-administration of drug-resistant TB treatment. WHO has recently introduced concept of active TB safety drug monitoring and management (aDSM) to detect, manage and report suspected or confirmed drug toxicities. It involves active and systematic clinical and laboratory assessment of patients on treatment with newer anti-TB drugs, novel MDR-TB regimens or XDR-TB regimens7,32,69. It is envisaged that aDSM will become an integral component of the PMDT apart from spontaneous or voluntary reporting and targeted reporting.
Outcomes of treatment
There is a scarcity of data on the treatment outcomes of patients with XDR-TB, and the existing data so far are highly variable. Further, the influence of HIV status and fate of patients with treatment failure remain unknown. The WHO has reported data regarding global treatment outcome for 4236 XDR-TB patients representing 47 countries by the end of 2013 (successful treatment - 28%, death - 27%, failure - 21% and default - 23%)32. The Russian Federation reported highest number of XDR-TB patients for whom outcomes were reported and also mortality was highest (>40%) in India and South Africa32. In a study conducted in Peru between 1999 and 2002, more than 60 per cent of XDR-TB patients were cured after receiving treatment either at home or in community-based settings with appropriate regimen15. In a retrospective cohort study of 608 patients with MDR-TB in Russia between 2000 and 2004, 29 (4.8%) patients had XDR-TB. In this study, 48.3 per cent of XDR-TB had treatment cure or completion17. The largest outbreak of XDR-TB occurred in the Tugela Ferry of KwaZulu-Natal region in South Africa in an HIV-positive population, characterized by a high mortality rate8. There was 99 per cent mortality. This was a nosocomial outbreak in an area of high HIV-endemicity, not necessarily that the XDR-TB aspect was driven by HIV. Therefore, HIV-positive patients are not inherently at higher risk of XDR-TB; the main driver is the drug resistance pattern among the surrounding population. Studies conducted in South Africa found no significant difference in mortality rates between HIV-positive patients on highly active ART (HAART) and HIV-negative ones86,87. The important risk factors responsible for poor outcomes were prior anti-TB therapy, FQ resistance or prior FQ use, Cm resistance, extensive radiological lesions including cavitation, HIV seropositivity, other immunocompromised states, low body mass index, hypoalbuminaemia, older age, male sex, low haematocrit and early default during one year of treatment88. The major risk factor for resistance to SLDs was previous intake as well as incorrect prescription of these drugs89. Early availability of the second-line DST results (within 31 days of treatment initiation) was found to be associated with a better outcome of XDR-TB treatment compared with those where DST results were available after 31 days of treatment19. Treatment success was highest if at least six drugs were used in the IP90. A meta-analysis by Jacobson et al91 assessed treatment outcomes for XDR-TB and factors associated with favourable responses. It was observed that the use of later generation FQs for the treatment of XDR-TB significantly improved treatment outcomes despite documented resistance to that drug. Patients with XDR-TB infected by a strain susceptible to other first-line drugs, to a later generation FQ and to other SLIDs, such as Cm, might carry a prognosis almost similar to that of MDR-TB.
There is limited evidence regarding treatment outcome of XDR-TB from India. According to the WHO Global Report (2016), 2130 XDR-TB cases were prescribed SLD under PMDT of RNTCP with treatment success rate of 37 per cent in 201332. There is a need to introduce new classes of anti-TB drugs that can be used for treating XDR-TB. Lzd is one of the drugs, which has been utilized in managing XDR-TB cases. A systematic review and meta-analysis included individual data analysis of 121 patients from 12 uncontrolled studies focusing on efficacy, safety and tolerability of Lzd-containing regimens. The treatment success was achieved in 81.8 per cent of the cases92. Dlm, Bdq and PA-824 are other new anti-TB agents with potential evidence to enhance the cure rate of XDR-TB in the near future. A study conducted in South Africa has shown that independent predictors of probability of culture conversion were no history of MDR-TB and use of Cfz93. A systematic review by Gopal et al94 suggested that Cfz-containing regimens could be beneficial in the treatment of XDR-TB with drug-resistant strains and who had limited alternative treatment options. Another study reported that successful treatment eradicated XDR-TB strains despite the low treatment success rate among patients with XDR-T95. Worldwide, 70 countries had started regimens containing Bdq and 39 countries had used Dlm by the end of 2015 to improve treatment outcome32. Majority of the patients treated with Bdq were reported by the Russian Federation and South Africa32.
Role of surgery
Surgery has an important role in the treatment of XDR-TB cases due to an inability to achieve complete cure with the available ATT regimens. Patients especially with localized disease can undergo surgery as an adjunct to anti-TB therapy. It should not be considered as the last resort but during early anti-TB therapy considering risk-benefit ratio. Anti-TB therapy should be initiated at least for two months before surgery and continued for 12-24 months thereafter. It is generally preferred in those cases with localized disease, persistent sputum positivity and resistance to large number of drugs64. Most common operative procedure in patients with pulmonary disease is resection surgery9. Stringent infection control measures should be taken before, during and after the surgery since infectious substances and aerosols are generated in large quantities during surgery and during mechanical ventilation. It was observed that overall cure rate was substantially higher (81 vs. 56%) when surgery was more frequently and aggressively applied in cases of resistant pulmonary TB96. A study was conducted at Georgia97 among 75 patients comprising 51 MDR-TB and 24 XDR-TB who underwent adjunctive thoracic surgery - lobectomy (54%), segmentectomy (35%) and pneumonectomy (11%). Of the 72 patients with evaluable outcomes, 59 (82%) had favourable outcomes including 90 per cent of MDR and 67 per cent of XDR-TB patients. Postoperative complications were observed in 7 (9%) patients without any mortality. This study also predicted various factors that were associated with poor treatment outcomes such as bilateral disease, XDR pattern, previous usage of increasing effective drugs, positive pre-operative sputum culture and major post-operative surgical complication97.
HIV and XDR-TB
The management strategy of HIV-positive XDR-TB patients is identical to that of HIV-naïve XDR-TB patients. However, mortality in HIV-positive XDR-TB patients without the use of ART is extremely high8,55. Adverse events are observed more common in such patients because of various pharmacokinetics as well as pharmacodynamics interactions between ART and anti-TB therapy, diverse pharmacogenomics properties and occurrence of immune reconstitution inflammatory syndrome (IRIS). Therefore, intense monitoring is essential for both response to therapy and adverse events. Thiacetazone (T) is not recommended for HIV-positive patients68. ART was found to improve survival in patients with DR-TB, and initiation of ART in relation to TB treatment should be similar to patients with drug susceptible TB. Multiple clinical trials of new regimens are on-going and preliminary data from the Bdq Clinical Access Programme in South Africa98 suggest improved short-term outcomes when Bdq±Lzd and/or Cfz was given to patients with XDR and pre-XDR TB including patients with HIV co-infection. However, the risk of QT prolongation and ART drug interactions related to Bdq are to be considered98.
XDR-TB in paediatric patients
Children with DR-TB generally acquire primary resistance from an index case with DR-TB. The diagnostic criteria and indications for treatment need to be specified as there is a lack of adequate sputum specimens when requested from young children. Alternative technique such as sputum induction with nebulized hypertonic saline may facilitate collection of specimens. Gastric lavage is another commonly used procedure for collecting specimens when nebulization is unsuccessful. It is especially employed for conventional culture with DST and GeneXpert MTB/RIF but not used for smear microscopy in isolation because of the low yield. DST should be used to guide therapy in paediatric cases wherever possible as they are often culture negative with paucibacillary TB. Management of culture-negative children should include clinical evidence of active TB disease, history of contact with a documented case of DR-TB including previous treatment as well as exposure to anti-TB drugs and the results of DST64. The evidence for use of SLDs for extended periods in children is limited. The regimen should be designed carefully considering risk-benefit ratio. The current evidence has shown that paediatric cases with DR-TB have generally tolerated SLDs especially FQs, Eto, PAS and Cs well without experiencing any significant adverse events99. In general, anti-TB drugs should be given according to body weight64 as mentioned in Table VI. Monthly monitoring of body weight is essential for paediatric cases as indicated for adjustment of doses of ATT according to the change in weight bands. All drugs should be prescribed at the higher recommended dosage ranges, except E which should be given at 15 mg/kg as compared to 25 mg/kg in adults64. It is more difficult to monitor for optic neuritis in children.
Table VI.
Paediatric dosing of second-line anti-tuberculosis drugs
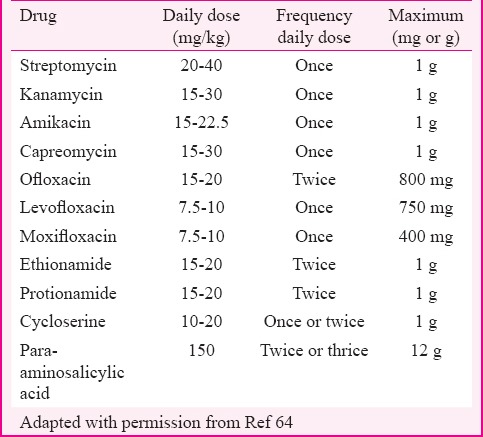
Response monitoring to treatment is difficult in children who are not culture-positive initially. Chest radiography is also considered inferior to monitoring of weight gain for the assessment of treatment response64. Children under three years of age who are contacts of patients with MDR-TB or XDR-TB can be given chemoprophylaxis to prevent high risk of disease progression. The proposed recommendation is to use two or three oral drugs for a minimum of nine months based on susceptibility pattern of the index case of DR-TB100.
XDR-TB in pregnancy
All female patients of reproductive age group should undergo screening for pregnancy upon baseline evaluation before initiation of treatment for active DR-TB. It is not considered to be a contraindication for treatment as active DR-TB imposes greater risks to the lives of both mother and foetus64. Birth control is strongly recommended for all non-pregnant women receiving therapy for DR-TB because of frequent and severe toxicity profile of SLDs affecting both mother and foetus64. The risks and benefits of treatment should be carefully considered along with aggressive counselling to achieve smear conversion as early as possible to protect the health of the mother and foetus, both before and after birth. The treatment should be initiated preferably in the second trimester because of higher incidence of teratogenicity in the first trimester or sooner if condition of patient is critical. The therapy should be initiated with at least three or four oral drugs with documented susceptibility and then reinforced with an injecTable agent and possibly other drugs immediately postpartum64. InjecTable agents, especially aminoglycosides, should be avoided in the regimens of pregnant patients because of increased risk of foetal ototoxicity. However, Cm is to be prescribed if an injecTable agent is definitely required with less risk of ototoxicity as compared to others64. Eto should also be avoided in pregnancy as it can aggravate the risk of symptoms such as nausea as well as vomiting and also teratogenic effects with controversial evidence.
Breastfeeding
Women with active DR-TB breastfeeding their infants should receive a full course of anti-TB treatment timely to prevent transmission of tubercle bacilli through milk. It is also recommended to provide infant formula feeding option as an alternative to breastfeeding. If the mother is sputum smear-positive, her family members should take care of the infant until sputum smear conversion occurs. If this is not feasible, both the mother and infant should be kept in well-ventilated areas or outdoors. Another option is to use of surgical mask or an N-95 respirator by mother until she becomes sputum smear-negative64.
Contraception
Birth control is advised to all non-pregnant sexually active women receiving therapy for DR-TB. The oral contraceptives can be used safely except if the patient is taking rifamycin-containing regimens and also experiencing vomiting immediately or within two hours leading to decreased absorption as well as efficacy of the anti-TB treatment64. The latter issue can be managed by asking patients to take their contraceptives during vomiting-free periods with anti-TB treatment. Patients should use a barrier method of contraception in addition after tolerating contraceptive pills for at least one month. Condoms can be a reasonable solution, especially for those patients who are uncomforTable in taking additional pills. It has additional advantage of protection against sexually transmitted disease, but it is less effective as compared to contraceptive pills when not used correctly64.
WHO response strategies for XDR-TB
WHO convened a Global Task Force on XDR-TB in October 2006 to combat the threat of XDR-TB. The proposed objectives were strengthening of basic activities to control TB and HIV, prevention of further emergence of MDR-TB and XDR-TB and speeding up of treatment of such cases, thereby increasing coverage101. The revised plan decided to extend the treatment to cover 1.6 million MDR-TB and XDR-TB cases by 2015 instead of 800,000 MDR-TB cases32. Treatment success rates among patients with MDR and XDR-TB still remained consistently and unacceptably low since the initiation of global monitoring by WHO. According to the WHO recommendations, all patients with MDR-TB should undergo testing for SLDs to determine if they have XDR-TB7,32. The End TB Strategy32 emphasizes on early diagnosis of TB and universal DST for rapidly and accurately detecting TB and drug resistance. Rapid expansion of laboratory services and programmatic management are required to achieve universal accessibility of services for MDR and XDR-TB. The delivery of treatment should be patient-centred all over the countries with preference to provision of ambulatory services over hospitalization for severe cases. Newer regimens containing safer, affordable and more effective medicines with shorter duration and ease of administration are required to improve treatment outcomes. Other strategies, such as improvement in quality of life, enabling adherence to treatment, management of adverse events, comprehensive palliative and end-of-life care and psycho-social support, should also be implemented. Adequate resources for detection and treatment and building capacity among health care workers are essential to provide high-quality care.
Control of XDR-TB
The primary aim in the control of XDR-TB is to prevent emergence of drug resistance by most cost-effective way of treatment under Directly Observed Treatment and Short-course (DOTS) chemotherapy strategy. Proper management of MDR-TB cases including judicious introduction of SLDs in the regimen remains the major principle to prevent emergence and transmission of XDR-TB cases since MDR-TB and XDR-TB cases respond poorly to short-course chemotherapy. Although newer drugs for TB are coming up in the near future such as Ptm, Szd, SQ109 and benzothiazinones apart from recently introduced Bdq and Dlm, the key to success depends on appropriate diagnosis and effective treatment of XDR-TB patients. There is a need for a continuous and periodic drug resistance surveillance that will guide in deciding type of chemotherapy for treatment and also evaluation of longitudinal trends of progress in TB control by the current and past chemotherapy programmes. The guidelines of national programmes need to be updated periodically based on levels of resistance, training of professionals in private sector, strengthening of existing National Tuberculosis Control Programme and ensuring compliance enhancing measures such as provision of free/subsidized anti-TB drugs, supervised treatment and health education.
Conclusion
Overall, the emergence of XDR-TB reminds that global TB control requires a sustained commitment by scientific, political and financial authorities. One of the first priorities is to effectively diagnose XDR-TB in clinical practice by increasing the laboratory capacity worldwide. All the reference laboratories in each country should perform high quality conventional DST for all the SLDs to diagnose XDR-TB effectively. Molecular tests to rapidly identify H and R resistance together with DST for SLDs will contribute in rapid diagnosis, but there is a requirement of large investments in training personnel and facilities to make it feasible in resource-limited countries. New research in the areas involving application of molecular biology in the field of epidemiology could help in better understanding of the mechanisms leading to drug resistance, development of newer diagnostic tools and effective drugs to control DR-TB. The effective management of XDR-TB depends on judicious prescription of SLDs to reduce morbidity and mortality and transmission based on the current scenario. Optimal treatment of RR-TB, MDR-TB and XDR-TB cases alone will not control the global burden. The TB control programmes should emphasize on policies focusing on the effective use of first-line drugs in every new patient so as to prevent the emergence of MDR-TB, XDR-TB and XXDR-TB or TDR-TB.
Footnotes
Conflicts of Interest: None.
References
- 1.Shah NS, Wright A, Drobniewski F. Extreme drug resistance in tuberculosis (XDR-TB): Global survey of supranational reference laboratories for Mycobacterium tuberculosis with resistance to second-line drugs. Int J Tuberc Lung Dis. 2005;9:S77. [Google Scholar]
- 2.Moll A, Gandhi NR, Pawinski R, Andrews J, Zeller K, Sturm AW, et al. Identification of a multidrug-resistant tuberculosis cluster as a cause of death among HIV co-infected patients in rural South Africa. 13th Conference on Retroviruses and Opportunistic Infections; 7 February 2006; Denver, Colorado, United States of America. 2006 [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs - worldwide 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–5. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Notice to readers: Revised definition of extensively drug-resistant tuberculosis. MMWR Morb Mortal Wkly Rep. 2006;55:1176. [Google Scholar]
- 5.Migliori GB, Loddenkemper R, Blasi F, Raviglione MC. 125 years after Robert Koch's discovery of the tubercle bacillus: The new XDR-TB threat. Is “science” enough to tackle the epidemic? Eur Respir J. 2007;29:423–7. doi: 10.1183/09031936.00001307. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Emergency update 2008. WHO/HTM/TB/2008.402. Geneva: WHO; 2008. [Google Scholar]
- 7.World Health Organization. Global tuberculosis report 2015, 20th ed WHO/HTM/TB/2015.22. Geneva: WHO; 2015. [Google Scholar]
- 8.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 9.Migliori GB, Ortmann J, Girardi E, Besozzi G, Lange C, Cirillo DM, et al. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg Infect Dis. 2007;13:780–2. doi: 10.3201/eid1305.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliori GB, Besozzi G, Girardi E, Kliiman K, Lange C, Toungoussova OS, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–6. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 11.Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–5. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 12.Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, et al. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect Dis. 2008;47:e57–63. doi: 10.1086/591702. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee R, Allen J, Westenhouse J, Oh P, Elms W, Desmond E, et al. Extensively drug-resistant tuberculosis in California 1993-2006. Clin Infect Dis. 2008;47:450–7. doi: 10.1086/590009. [DOI] [PubMed] [Google Scholar]
- 14.Chan ED, Strand MJ, Iseman MD. Treatment outcomes in extensively resistant tuberculosis. N Engl J Med. 2008;359:657–9. doi: 10.1056/NEJMc0706556. [DOI] [PubMed] [Google Scholar]
- 15.Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eker B, Ortmann J, Migliori GB, Sotgiu G, Muetterlein R, Centis R, et al. Multidrug-and extensively drug-resistant tuberculosis, Germany. Emerg Infect Dis. 2008;14:1700–6. doi: 10.3201/eid1411.080729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: A retrospective cohort study. Lancet. 2008;372:1403–9. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 18.Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 19.Bonilla CA, Crossa A, Jave HO, Mitnick CD, Jamanca RB, Herrera C, et al. Management of extensively drug-resistant tuberculosis in Peru: Cure is possible. PLoS One. 2008;3:e2957. doi: 10.1371/journal.pone.0002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punga VV, Jakubowiak WM, Danilova ID, Somova TR, Volchenkov GV, Kazionnyy BY, et al. Prevalence of extensively drug-resistant tuberculosis in Vladimir and Orel regions, Russia. Int J Tuberc Lung Dis. 2009;13:1309–12. [PubMed] [Google Scholar]
- 21.Liu CH, Li L, Chen Z, Wang Q, Hu YL, Zhu B, et al. Characteristics and treatment outcomes of patients with MDR and XDR tuberculosis in a TB referral hospital in Beijing: A 13-year experience. PLoS One. 2011;6:e19399. doi: 10.1371/journal.pone.0019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skrahina A, Hurevich H, Zalutskaya A, Sahalchyk E, Astrauko A, van Gemert W, et al. Alarming levels of drug-resistant tuberculosis in Belarus: Results of a survey in Minsk. Eur Respir J. 2012;39:1425–31. doi: 10.1183/09031936.00145411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balabanova Y, Radiulyte B, Davidaviciene E, Hooper R, Ignatyeva O, Nikolayevskyy V, et al. Risk factors for drug-resistant tuberculosis patients in Lithuania 2002-2008. Eur Respir J. 2012;39:1266–9. doi: 10.1183/09031936.00133911. [DOI] [PubMed] [Google Scholar]
- 24.Ulmasova DJ, Uzakova G, Tillyashayhov MN, Turaev L, van Gemert W, Hoffmann H, et al. Multidrug-resistant tuberculosis in Uzbekistan: Results of a nationwide survey, 2010 to 2011. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.42.20609. pii:20609. [DOI] [PubMed] [Google Scholar]
- 25.Kuksa L, Riekstina V, Leimane V, Ozere I, Skenders G, Van den Bergh R, et al. Multi- and extensively drug-resistant tuberculosis in Latvia: Trends, characteristics and treatment outcomes. Public Health Action. 2014;4(Suppl 2):S47–53. doi: 10.5588/pha.14.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kam KM, Yip CW. Surveillance of Mycobacterium tuberculosis susceptibility to second-line drugs in Hong Kong 1995-2002, after the implementation of DOTS-plus. Int J Tuberc Lung Dis. 2004;8:760–6. [PubMed] [Google Scholar]
- 28.Pardini M, Iona E, Varaine F, Karakozian H, Arzumanian H, Brunori L, et al. Mycobacterium tuberculosis drug resistance, Abkhazia. Emerg Infect Dis. 2005;11:501–3. doi: 10.3201/eid1103.040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masjedi MR, Farnia P, Sorooch S, Pooramiri MV, Mansoori SD, Zarifi AZ, et al. Extensively drug-resistant tuberculosis:2 years of surveillance in Iran. Clin Infect Dis. 2006;43:841–7. doi: 10.1086/507542. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO/IUALTD Global Project on Anti-Tuberculosis Drug Resistance Surveillance (2002-2007) 4th Global Report WHO/HTM/TB/2008.394. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 31.World Health Organization. Multidrug and Extensively Drug-resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. WHO/HTM/TB/2010.3. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 32.World Health Organization. Global tuberculosis report 2016. WHO/HTM/TB/2016.13. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 33.Jain S, Rodrigues C, Mehta A, Udwadia ZF. High prevalence of XDR-TB from a tertiary care hospital in India. Proceedings of the American Thoracic Society International Conference, May 2007 San Francisco, USA. 2007 Abstract A510. [Google Scholar]
- 34.Mondal R, Jain A. Extensively drug-resistant Mycobacterium tuberculosis India. Emerg Infect Dis. 2007;13:1429–31. doi: 10.3201/eid1309.070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Sankar MM, Gopinath K. High rate of extensively drug-resistant tuberculosis in Indian AIDS patients. AIDS. 2007;21:2345–7. doi: 10.1097/QAD.0b013e3282f125c9. [DOI] [PubMed] [Google Scholar]
- 36.Jose L, Mundayoor M, Ajaykumar R. Annual Report of the Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram. Thiruvananthapuram: RGCB; 2007. p. 220. [Google Scholar]
- 37.Rodrigues C. XDR TB-perspectives from a referral tertiary care hospital in Mumbai. In: Sood OP, Sharma SK, editors. Challenges of MDR/XDR tuberculosis in India. Proceedings of Round Table Conference Series. Vol. 22. New Delhi, India: Ranbaxy Science Foundation; 2008. pp. 39–42. [Google Scholar]
- 38.Michael JS, Shalini BE, Mathews MS. Drug resistant tuberculosis – An experience of southern states of India. In: Sood OP, Sharma SK, editors. Challenges of MDR/XDR tuberculosis in India. Proceedings of Round Table Conference Series. Vol. 22. New Delhi, India: Ranbaxy Science Foundation; 2008. pp. 35–8. [Google Scholar]
- 39.Ramachandran R, Nalini S, Chandrasekar V, Dave PV, Sanghvi AS, Wares F, et al. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis. 2009;13:1154–60. [PubMed] [Google Scholar]
- 40.Dhingra VK, Malik S, Hanif M, Arora VK. XDR tuberculosis: A report from the New Delhi tuberculosis centre, India. J Coll Physicians Surg Pak. 2009;19:133–5. [PubMed] [Google Scholar]
- 41.Sharma SK, George N, Kadhiravan T, Saha PK, Mishra HK, Hanif M. Prevalence of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A retrospective hospital-based study. Indian J Med Res. 2009;130:392–5. [PubMed] [Google Scholar]
- 42.Datta BS, Hassan G, Kadri SM, Qureshi W, Kamili MA, Singh H, et al. Multidrug-resistant and extensively drug resistant tuberculosis in Kashmir, India. J Infect Dev Ctries. 2009;4:19–23. doi: 10.3855/jidc.669. [DOI] [PubMed] [Google Scholar]
- 43.Rajasekaran S, Chandrasekar C, Mahilmaran A, Kanakaraj K, Karthikeyan DS, Suriakumar J. HIV coinfection among multidrug resistant and extensively drug resistant tuberculosis patients – A trend. J Indian Med Assoc. 2009;107:281–2. 284-6. [PubMed] [Google Scholar]
- 44.Paramasivan CN, Rehman F, Wares F, Sundar Mohan N, Sundar S, Devi S, et al. First- and second-line drug resistance patterns among previously treated tuberculosis patients in India. Int J Tuberc Lung Dis. 2010;14:243–6. [PubMed] [Google Scholar]
- 45.Balaji V, Daley P, Anand AA, Sudarsanam T, Michael JS, Sahni RD, et al. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One. 2010;5:e9527. doi: 10.1371/journal.pone.0009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakraborty N, De C, Bhattacharyya S, Mukherjee A, Santra S, Banerjee D, et al. Drug susceptibility profile of Mycobacterium tuberculosis isolated from HIV infected and uninfected pulmonary tuberculosis patients in Eastern India. Trans R Soc Trop Med Hyg. 2010;104:195–201. doi: 10.1016/j.trstmh.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Khanna A, Raj VS, Tarai B, Sood R, Pareek PK, Upadhyay DJ, et al. Emergence and molecular characterization of extensively drug-resistant Mycobacterium tuberculosis clinical isolates from the Delhi Region in India. Antimicrob Agents Chemother. 2010;54:4789–93. doi: 10.1128/AAC.00661-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas A, Ramachandran R, Rehaman F, Jaggarajamma K, Santha T, Selvakumar N, et al. Management of multi drug resistance tuberculosis in the field: Tuberculosis Research Centre experience. Indian J Tuberc. 2007;54:117–24. [PubMed] [Google Scholar]
- 49.Myneedu VP, Visalakshi P, Verma AK, Behera D, Bhalla M. Prevalence of XDR TB cases – A retrospective study from a tertiary care TB hospital. Indian J Tuberc. 2011;58:54–9. [PubMed] [Google Scholar]
- 50.James P, Gupta R, Christopher DJ, Thankagunam B, Veeraraghavan B. MDR- and XDR-TB among suspected drug-resistant TB patients in a tertiary care hospital in India. Clin Respir J. 2011;5:19–25. doi: 10.1111/j.1752-699X.2009.00184.x. [DOI] [PubMed] [Google Scholar]
- 51.Porwal C, Kaushik A, Makkar N, Banavaliker JN, Hanif M, Singla R, et al. Incidence and risk factors for extensively drug-resistant tuberculosis in Delhi region. PLoS One. 2013;8:e55299. doi: 10.1371/journal.pone.0055299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dholakia YN, Shah DP. Clinical profile and treatment outcomes of drug-resistant tuberculosis before directly observed treatment strategy plus: Lessons for the program. Lung India. 2013;30:316–20. doi: 10.4103/0970-2113.120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakshmipathy D, Ramasubban G, Lily Kulandai LT, Sridhar R, Narahari MH, Meenakshi N. Extensively drug resistant tuberculosis (XDR-TB) by phenotypic drug susceptibility using BACTEC Micro MGIT culture system – A pilot study in hospital based population in Chennai, India. Int J Curr Microbiol Appl Sci. 2014;3:129–35. [Google Scholar]
- 54.Udwadia ZF, Moharil G. Multidrug-resistant-tuberculosis treatment in the Indian private sector: Results from a tertiary referral private hospital in Mumbai. Lung India. 2014;31:336–41. doi: 10.4103/0970-2113.142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isaakidis P, Das M, Kumar AM, Peskett C, Khetarpal M, Bamne A, et al. Alarming levels of drug-resistant tuberculosis in HIV-infected patients in metropolitan Mumbai, India. PLoS One. 2014;9:e110461. doi: 10.1371/journal.pone.0110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao P, Chawla K, Shenoy VP, Mukhopadhyay C, Brahmavar V, Kamath A, et al. Study of drug resistance in pulmonary tuberculosis cases in South Coastal Karnataka. J Epidemiol Glob Health. 2015;5:275–81. doi: 10.1016/j.jegh.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalal A, Pawaskar A, Das M, Desai R, Prabhudesai P, Chhajed P, et al. Resistance patterns among multidrug-resistant tuberculosis patients in greater metropolitan Mumbai: Trends over time. PLoS One. 2015;10:e0116798. doi: 10.1371/journal.pone.0116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee P, Karmakar PR, Basu R, Lahiri SK. Sociodemographic and clinical profile of multi drug resistant tuberculosis patients: A study at drug resistant tuberculosis centers of Kolkata. IOSR J Dent Med Sci. 2015;14:52–8. [Google Scholar]
- 59.Udwadia ZF, Mullerpattan JB, Shah KD, Rodrigues CS. Possible impact of the standardized Category IV regimen on multidrug-resistant tuberculosis patients in Mumbai. Lung India. 2016;33:253–6. doi: 10.4103/0970-2113.180800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain A, Dixit P, Prasad R. Pre-XDR & XDR in MDR and ofloxacin and kanamycin resistance in non-MDR Mycobacterium tuberculosis isolates. Tuberculosis (Edinb) 2012;92:404–6. doi: 10.1016/j.tube.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Michael JS, John TJ. Extensively drug-resistant tuberculosis in India: A review. Indian J Med Res. 2012;136:599–604. [PMC free article] [PubMed] [Google Scholar]
- 62.Salvo F, Dorjee K, Dierberg K, Cronin W, Sadutshang TD, Migliori GB, et al. Survey of tuberculosis drug resistance among Tibetan refugees in India. Int J Tuberc Lung Dis. 2014;18:655–62. doi: 10.5588/ijtld.13.0516. [DOI] [PubMed] [Google Scholar]
- 63.Jeon CY, Hwang SH, Min JH, Prevots DR, Goldfeder LC, Lee H, et al. Extensively drug-resistant tuberculosis in South Korea: Risk factors and treatment outcomes among patients at a tertiary referral hospital. Clin Infect Dis. 2008;46:42–9. doi: 10.1086/524017. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2014.11. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 65.Ignatyeva O, Balabanova Y, Nikolayevskyy V, Koshkarova E, Radiulyte B, Davidaviciene E, et al. Resistance profile and risk factors of drug resistant tuberculosis in the Baltic countries. Tuberculosis (Edinb) 2015;95:581–8. doi: 10.1016/j.tube.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Smith SE, Ershova J, Vlasova N, Nikishova E, Tarasova I, Eliseev P, et al. Risk factors for acquisition of drug resistance during multidrug-resistant tuberculosis treatment, Arkhangelsk Oblast, Russia 2005-2010. Emerg Infect Dis. 2015;21:1002–11. doi: 10.3201/eid2106.141907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karkhanis VS, Joshi JM. Is XDR-TB a sub-group of MDR-TB?Need to reorganize alphabets again! Indian J Tuberc. 2012;59:187–9. [PubMed] [Google Scholar]
- 68.Revised National Tuberculosis Control Programme. Guidelines on Programmatic Management of Drug-resistant Tuberculosis (PMDT) in India. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; 2012. [Google Scholar]
- 69.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. WHO/HTM/TB/2016.04. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 70.Palomino JC. Nonconventional and new methods in the diagnosis of tuberculosis: Feasibility and applicability in the field. Eur Respir J. 2005;26:339–50. doi: 10.1183/09031936.05.00050305. [DOI] [PubMed] [Google Scholar]
- 71.Furin J. The clinical management of drug-resistant tuberculosis. Curr Opin Pulm Med. 2007;13:212–7. doi: 10.1097/MCP.0b013e3280f3c0b2. [DOI] [PubMed] [Google Scholar]
- 72.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:1683–9. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. The use of molecular line probe assay for the detection of resistance to second-line anti-tuberculosis drugs, policy guidance. WHO/HTM/TB/2016.07. Geneva: WHO; 2016. [Google Scholar]
- 74.Theron G, Peter J, Richardson M, Barnard M, Donegan S, Warren R, et al. The diagnostic accuracy of the GenoType® MTBDRsl assay for the detection of resistance to second-line anti-tuberculosis drugs. Cochrane Database Syst Rev. 2014;10:CD010705. doi: 10.1002/14651858.CD010705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh AK, Maurya AK, Kant S, Umrao J, Kushwaha RAS, Nag VL, et al. Rapid detection of drug resistance and mutational patterns of extensively drug-resistant strains by a novel GenoType® MTBDRsl assay. J Postgrad Med. 2013;59:179–85. doi: 10.4103/0022-3859.118034. [DOI] [PubMed] [Google Scholar]
- 76.Ajbani K, Nikam C, Kazi M, Gray C, Boehme C, Balan K, et al. Evaluation of genotype MTBDRsl assay to detect drug resistance associated with fluoroquinolones, aminoglycosides and ethambutol on clinical sediments. PLoS One. 2012;7:e49433. doi: 10.1371/journal.pone.0049433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.TB India 2016; Revised National Tuberculosis Control Programme: Annual Status Report. New Delhi, India: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; 2016. [Google Scholar]
- 78.Sloan DJ, Lewis JM. Management of multidrug-resistant TB: Novel treatments and their expansion to low resource settings. Trans R Soc Trop Med Hyg. 2016;110:163–72. doi: 10.1093/trstmh/trv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371:723–32. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 80.Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41:1393–400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta R, Geiter LJ, Wells CD, Gao M, Cirule A, Xiao H. Delamanid for extensively drug-resistant tuberculosis. N Engl J Med. 2015;373:291–2. doi: 10.1056/NEJMc1415332. [DOI] [PubMed] [Google Scholar]
- 82.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D, Zhu T, et al. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One. 2014;9:e94462. doi: 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinrich N, Dawson R, du Bois J, Narunsky K, Horwith G, Phipps AJ, et al. Early phase evaluation of SQ109 alone and in combination with rifampicin in pulmonary TB patients. J Antimicrob Chemother. 2015;70:1558–66. doi: 10.1093/jac/dku553. [DOI] [PubMed] [Google Scholar]
- 84.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–9. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.World Health Organization. Tuberculosis control in the South-East Asia region: Annual TB report 2015. Geneva: WHO; 2015. [accessed on July 19 2016]. Available from http://www.searo.who.int/tb/annual-tb-report-2015 . [Google Scholar]
- 86.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: A retrospective cohort study. Lancet. 2010;375:1798–807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 87.Kvasnovsky CL, Cegielski JP, Erasmus R, Siwisa NO, Thomas K, der Walt ML. Extensively drug-resistant TB in Eastern Cape, South Africa: High mortality in HIV-negative and HIV-positive patients. J Acquir Immune Defic Syndr. 2011;57:146–52. doi: 10.1097/QAI.0b013e31821190a3. [DOI] [PubMed] [Google Scholar]
- 88.Sotgiu G, Ferrara G, Matteelli A, Richardson MD, Centis R, Ruesch-Gerdes S, et al. Epidemiology and clinical management of XDR-TB: A systematic review by TBNET. Eur Respir J. 2009;33:871–81. doi: 10.1183/09031936.00168008. [DOI] [PubMed] [Google Scholar]
- 89.Migliori GB, Sotgiu G, D’Ambrosio L, Centis R, Lange C, Bothamley G, et al. TB and MDR/XDR-TB in European Union and European Economic Area countries: Managed or mismanaged? Eur Respir J. 2012;39:619–25. doi: 10.1183/09031936.00170411. [DOI] [PubMed] [Google Scholar]
- 90.Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, et al. Resistance to fluoroquinolones and second-line injecTable drugs: Impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–68. doi: 10.1183/09031936.00134712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: Systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sotgiu G, Centis R, D’Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: Systematic review and meta-analysis. Eur Respir J. 2012;40:1430–42. doi: 10.1183/09031936.00022912. [DOI] [PubMed] [Google Scholar]
- 93.Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: A cohort study. Lancet. 2014;383:1230–9. doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- 94.Gopal M, Padayatchi N, Metcalfe JZ, O’Donnell MR. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2013;17:1001–7. doi: 10.5588/ijtld.12.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blöndal K, Viiklepp P, Guðmundsson LJ, Altraja A. Predictors of recurrence of multidrug-resistant and extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:1228–33. doi: 10.5588/ijtld.12.0037. [DOI] [PubMed] [Google Scholar]
- 96.Pomerantz M, Madsen LA, Goble M, Iseman MD. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg. 1991;52:1108–11. doi: 10.1016/0003-4975(91)91289-8. [DOI] [PubMed] [Google Scholar]
- 97.Vashakidze S, Gogishvili S, Nikolaishvili K, Dzidzikashvili N, Tukvadze N, Blumberg HM, et al. Favorable outcomes for multidrug and extensively drug resistant tuberculosis patients undergoing surgery. Ann Thorac Surg. 2013;95:1892–8. doi: 10.1016/j.athoracsur.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meintjes G. Management of drug-resistant TB in patients with HIV co-infection. J Int AIDS Soc. 2014;17(4 Suppl 3):19508. doi: 10.7448/IAS.17.4.19508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukherjee JS, Joseph JK, Rich ML, Shin SS, Furin JJ, Seung KJ, et al. Clinical and programmatic considerations in the treatment of MDR-TB in children: A series of 16 patients from Lima, Peru. Int J Tuberc Lung Dis. 2003;7:637–44. [PubMed] [Google Scholar]
- 100.Zumla A, Abubakar I, Raviglione M, Hoelscher M, Ditiu L, McHugh TD, et al. Drug-resistant tuberculosis - current dilemmas, unanswered questions, challenges, and priority needs. J Infect Dis. 2012;205(Suppl 2):S228–40. doi: 10.1093/infdis/jir858. [DOI] [PubMed] [Google Scholar]
- 101.World Health Organization. The global MDR-TB and XDR-TB response plan 2007-2008. WHO/HTM/TB/2007.387. Geneva: WHO; 2007. [Google Scholar]


