Abstract
Background & objectives:
Although the need for a combination of antiepileptic drugs (AEDs) in the treatment of epilepsy is well justified, but an associated increase in adverse effects (AEs) lends a restriction to polytherapy. The aim of this study was to evaluate AEs and drug load (prescribed daily dose/defined daily doses) of AEDs in patients with epilepsy (PWE).
Methods:
Consecutive PWEs attending Epilepsy clinic in a tertiary care hospital in New Delhi, India, were enrolled in the study. Demographic variables, such as age, gender, diagnosis, age at onset of seizures, frequency of seizures, use of all AEDs and adverse event profile (AEP) score were noted. Routine laboratory tests including lipid profile, fasting blood glucose, haematological parameters and liver and kidney function tests were done.
Results:
A total of 697 consecutive patients were included in this study. Of them, 64.4 per cent were male; mean age was 29.6 ± 10.6 yr. Generalized seizures and focal seizures were recorded in n=386 (55.4%) and n=311 (44.6%), respectively. Monotherapy and polytherapy with two and greater than or equal to three AEDs were prescribed in 264 (37.9%), 243 (34.9%) and 190 (27.2%) patients, respectively. The average AED load, duration of treatment as well as AEP score were found to be significantly higher in combination of greater than or equal to three AEDs as compared to both monotherapy and combination of two AEDs, whereas no significant difference was observed between monotherapy and combination of two AEDs. Patients on monotherapy were in good control of seizures as compared to polytherapy. There was no significant change in biochemical parameters between the groups.
Interpretation & conclusions:
Polytherapy with combination of greater than or equal to three AEDs was associated with higher AEs and lower seizure control as compared to both monotherapy and combination of two AEDs. AEs did not correlate with AED load, seizure type, gender and age of the patients but were associated with both numbers of AEDs as well as seizure frequency in PWE.
Keywords: Adverse event profile score, antiepileptic drug load, antiepileptic drugs, epilepsy, monotherapy, polytherapy, seizures
Epilepsy affects an estimated 50 million people worldwide1,2, and the overall prevalence rate of epilepsy in India has been reported to be varied from 3.0-11.9/1000 population3. Despite that several antiepileptic drugs (AEDs) being introduced in the past two decades, many patients do not respond to a single AED. Several studies have been conducted indicating the superiority of combination of AEDs over monotherapy4,5. However, the treatment is initiated with monotherapy, and subsequently, other AEDs are added on if seizures are not adequately controlled6.
Conventional AEDs as monotherapy are commonly prescribed in developing countries with limited resources7,8. Though most prefer phenytoin (PHT) as initial monotherapy followed by carbamazepine (CBZ) and sodium valproate (VPA) based on the seizure type, this behaviour is highly variable not only in different countries but also among primary and tertiary care hospital set ups within a country9. Local socio-economic, cultural and ethnic factors, genetic profile of an individual and availability of drugs also affect the treatment regimens10. Moreover, baseline differences in patients’ demographic, seizure frequency and severity limit the direct comparison of individual trials11.
The eminence of life in patients with epilepsy (PWE) is compromised because of sequelae of seizures as well as the adverse effects (AEs) of medication12. Prescription of AEDs requires consideration of both drug efficacy and possible AEs in each patient. The relation between neurological AEs and epilepsy is not clearly understood, and it is difficult to know whether AEs are a consequence of epilepsy, a reaction to epilepsy or an AE of treatment13. Further, the presence of various neurological AEs may heighten sensitivity to general symptoms. Therefore, any measure of adverse events must have the ability to discriminate successfully between symptoms caused by AED use and those that are directly related to epilepsy or mood.
It is thus necessary to monitor and balance the goal of seizure control against adverse treatment effects. Different strategies are required for different scenarios and different patients. There are limited studies7,8 reporting the association of AEs with treatment regimens as well as seizure outcome simultaneously. This study was aimed to assess adverse event profile (AEP) as a measure of AEs occurring with AEDs and to explore the relation between AEs, epilepsy, AED load and number of AEDs prescribed. The primary objectives were to determine the AEs (AEP score) and drug load [prescribed daily dose (PDD)/defined daily dose (DDD)] of monotherapy, combination of two AEDs and greater than or equal to three AEDs in PWE. The secondary objectives were to determine the effectiveness of monotherapy and polytherapy of AEDs as well as factors responsible for AEs in PWE.
Material & Methods
In this cross-sectional observational study, patients were recruited from a consecutive sample of eligible, consenting PWE attending the epilepsy clinic in Outpatient Department (OPD) of Neurology, All India Institute of Medical Sciences, New Delhi, India, from October 2010 to September 2013. The retrospective duration of AED exposure was identified from patients’ case records, and then the patients were prospectively followed up for the study of AEs, prescription pattern and effectiveness. The retrospective AED exposure varied for each patient, and the prospective follow up was for at least two consecutive visits. Patients were followed during their routine OPD visits which were at three or six months depending on the patient requirement and logistics. The duration of follow up varied from 6 to 12 months. The study was approved by the Institutional Ethics Subcommittee (IESC/T-245/2010), and all participants provided a written informed consent.
Patients of age ≥18 yr with an established diagnosis of epilepsy were included for evaluation. These patients were on sTable doses of AEDs for at least three months. Choice of medication was not influenced by the study protocol and was essentially a clinical decision. Polytherapy was given to patients who did not respond to monotherapy and refractory cases as well. AED doses were adjusted by the treating physician as per clinical circumstances with special attention to efficacy for the seizure type/syndrome and tolerability.
Patients were prescribed the following AEDs: phenobarbital (PB), PHT, CBZ, clobazam (CLB), clonazepam (CLZ), VPA, levetiracetam (LEV), lamotrigine (LTG), oxcarbazepine (OXC), topiramate (TPM), zonisamide (ZNS), lacosamide (LCM) or any combination of these. All the enrolled patients were broadly divided into three groups: (i) monotherapy with either AED; (ii) combination of two AEDs; and (iii) combination of greater than or equal to three AEDs.
Baseline demographic data including gender, age, age at onset of seizures, type(s) and frequency of seizures, epilepsy characteristics according to the International League Against Epilepsy (ILAE)14, details of current AED regimens (including dosages) and concomitant medications were collected at the time of enrolment. Number of seizures were ascertained from seizure diaries maintained by all patients. All study participants were regularly monitored by the treating physician.
Antiepileptic drugs (AED) load [prescribed daily dose (PDD)/defined daily dose (DDD)]: AED load for each patient was calculated as a sum of the ratio of PDD/DDD for each AED included in the treatment regimen, where DDD corresponded to the assumed average maintenance daily dose of a drug for its main indication15. AED load reflects the load of the dose of the AEDs on individual PWE. It was calculated to evaluate whether the polytherapy resulted in reduced dose of another drug or increased overall doses of the AEDs. It is a validated method to calculate the load of AEDs on individual PWE16.
Effectiveness in terms of seizure frequency: The effectiveness of AEDs in terms of seizure frequency was classified into four categories based on the occurrence of seizure since last change in treatment regimen17. Category 1: seizure free, with no seizure in the past 12 months (after starting treatment) and remained so throughout follow up; Category 2 included patients with at least one seizure in six months. Patients in Category 3 had at least one seizure in a month. Patients exhibiting Category 4 never became seizure free or had at least one seizure in a week.
Adverse event profile (AEP): AEs were identified using the 21-item AEP questionnaire18. Briefly, the frequency of occurrence of each AE in the previous four weeks was rated on a four-point digital scale (4=frequent, 3=sometimes, 2=rarely and 1=no occurrence). In the present analysis, range of AEP scores varied from a minimum of 21 to a maximum of 84, with high scores indicating more frequent symptom reporting. The AEP scoring method is reliable, valid and has been widely used in epilepsy research16,19,20.
Routine biochemical testing: The blood samples from the patients were collected between 0800 and 1000 h, following a 12 h fast. The biochemical parameters including fasting blood glucose, kidney function tests (KFT) (urea, creatinine and uric acid), liver function tests (LFT) [aspartate transaminase (AST) and alanine transaminase (ALT)], total bilirubin and alkaline phosphatase), lipid profile (LP) (total cholesterol, triglycerides, low-density lipids, high-density lipids and very low density lipids), electrolytes (sodium, potassium, calcium and phosphate), haematological parameters (HPs) [haemoglobin, platelets count, white blood cell (WBC) count and erythrocyte sedimentation rate (ESR)] were estimated as part of routine clinical practice on the day after the first visit of the patient.
Statistical analysis: Data were analyzed using Statistical Package for the Social Sciences for Windows, Version 16 (SPSS Inc., Chicago, IL, USA) and presented as number (percentage) or median (range) as appropriate. Data were analyzed initially by descriptive statistics. Comparison between groups was done by one-way analysis of variance for continuous variables and Wilcoxon signed rank test for discrete variables. Differences in frequency of utilization of individual AEDs between groups were compared using Pearson's Chi-square test. Multivariate linear regression was used to assess the contribution of demographic, disease-related and AED-related variables to AEP scores.
Results
A total of 697 consecutive patients were enrolled in the study and total 12 AEDs, including both older and newer, were prescribed in different treatment regimens with a maximum of five AEDs in a patient. Demographic description of the enrolled patients in each category is provided in Table I. Distribution of patients in each category did not differ significantly with 37.9, 34.9 and 27.2 per cent of patients in monotherapy, combination of two AEDs and combination of greater than or equal to three AEDs, respectively. Although mean age of patients did not differ significantly between each category, age at onset of seizures was significantly lower in patients receiving polytherapy [combination of two (P<0.05) and greater than or equal to three AEDs (P<0.001)] as compared to monotherapy. Prescription of monotherapy was significantly (P<0.01) higher in patients with generalized seizures as compared to focal seizures. In patients with generalized seizures, prescription of monotherapy was significantly higher than that of polytherapy (P<0.01).
Table I.
Demographic characteristics of patients receiving monotherapy and polytherapy with antiepileptic drugs (AEDs) (n=697)
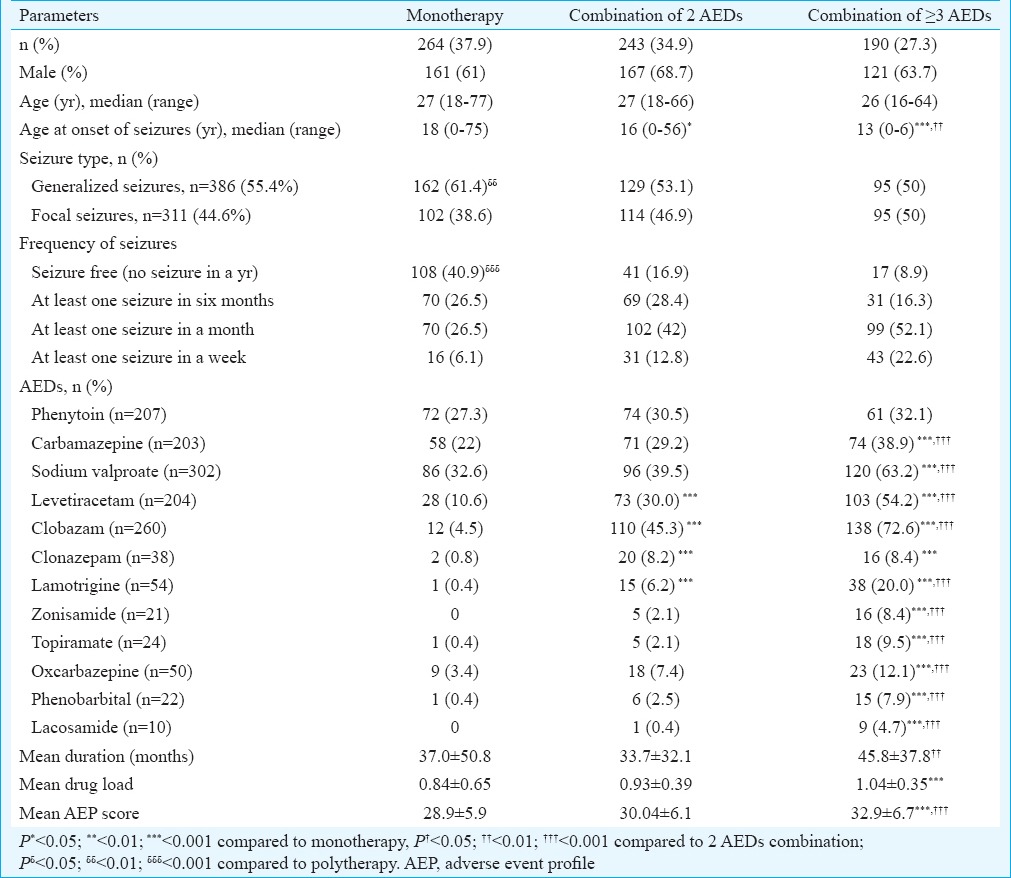
Effectiveness in terms of seizure frequency: Significantly more patients were seizure free with monotherapy (40.9%) as compared to polytherapy (25.8%) in category 1 (no seizure in a year). No significant difference was found between groups for patients in category 2 (at least one seizure in six months) of seizure outcome. There were significantly higher number of patients in category 3 (at least one seizure in a month) and category 4 (at least one seizure in a week) on combination of two and greater than or equal to three AEDs in comparison to those on monotherapy. Combination of greater than or equal to three AEDs did not cause any significant change in seizure frequency as compared to one and two AEDs (Table I).
Distribution of antiepileptic drugs (AEDs): All the AEDs were distributed similarly amongst all the treatment regimens except ZNS and LCM which were prescribed only as part of polytherapy not monotherapy. Percentage of use of PHT did not differ significantly amongst different treatment regimens. Amongst monotherapy, VPA was most frequently prescribed followed by CBZ, PHT and LEV. Prescription of CBZ was significantly higher in combination of greater than or equal to three AEDs. Frequency of use of VPA, LEV, CLB, CLZ, LTG, ZNS, TPM, OXC, PHB and LCM was significantly (P<0.001) higher in polytherapy.
In terms of percentage prescribing pattern, LCM was more frequently prescribed followed by ZNS>TPM>PHB>LTG>CLB>LEV>CLZ>VPA>CBZ> PHT in combination of greater than or equal to three AEDs suggesting that the newer AEDs were mostly prescribed in polytherapy, whereas older AEDs were almost uniformly dispersed amongst monotherapy and polytherapy regimens.
Antiepileptic drugs (AED) load and duration: Individual AED load was expressed as a ratio of PDD/DDD. The average AED load increased significantly from 0.84±0.65 in monotherapy to 0.93±0.39 for two AEDs and 1.04±0.35 for greater than or equal to three AEDs (P<0.001) (Table I). Amongst different AEDs, drug load of CBZ, VPA and LEV was found to be significantly higher in polytherapy as compared to monotherapy. The drug load of CLB and TPM was higher in combination of greater than or equal to three AEDs as compared to two AEDs and monotherapy. The ratio of PDD/DDD for PHT, CLZ, CBZ, LTG, ZNS, OXC and PHB did not vary significantly between the groups (Fig. 1).
Fig. 1.
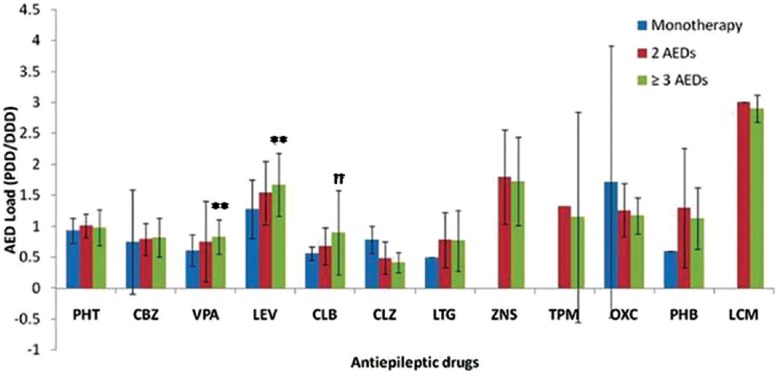
Drug load (prescribed daily dose/defined daily dose, PDD/ DDD) of different antiepileptic drugs (AEDs) separately in patients on monotherapy (n=264), combination of two antiepileptic drugs (n=243) and combination of greater than or equal to three antiepileptic drugs (n=190). **P<0.01 compared to monotherapy; ††P<0.01 compared to 2 AEDs combination. PHT, Phenytoin; CBZ, carbamazepine; VPA, sodium valproate; LEV, levetiracetam; CLB, clobazam; CLZ, clonazepam; LTG, lamotrigine; ZNS, zonisamide; TPM, topiramate; OXC, oxcarbazepine; PB, phenobarbital; LCM, lacosamide.
The duration of treatment (months) also varied significantly and was found to be longer in patients on combination of greater than or equal to three AEDs as compared to two AEDs (P<0.01) but not compared to monotherapy. No significant difference was observed between monotherapy and combination of two AEDs with respect to the duration of treatment (Table I).
Adverse event profile (AEP) questionnaire: The average AEP score increased significantly in combination of greater than or equal to three AEDs as compared to both monotherapy and combination of two AEDs (P<0.001). However, the AEP score did not differ significantly amongst monotherapy and combination of two AEDs (Table I).
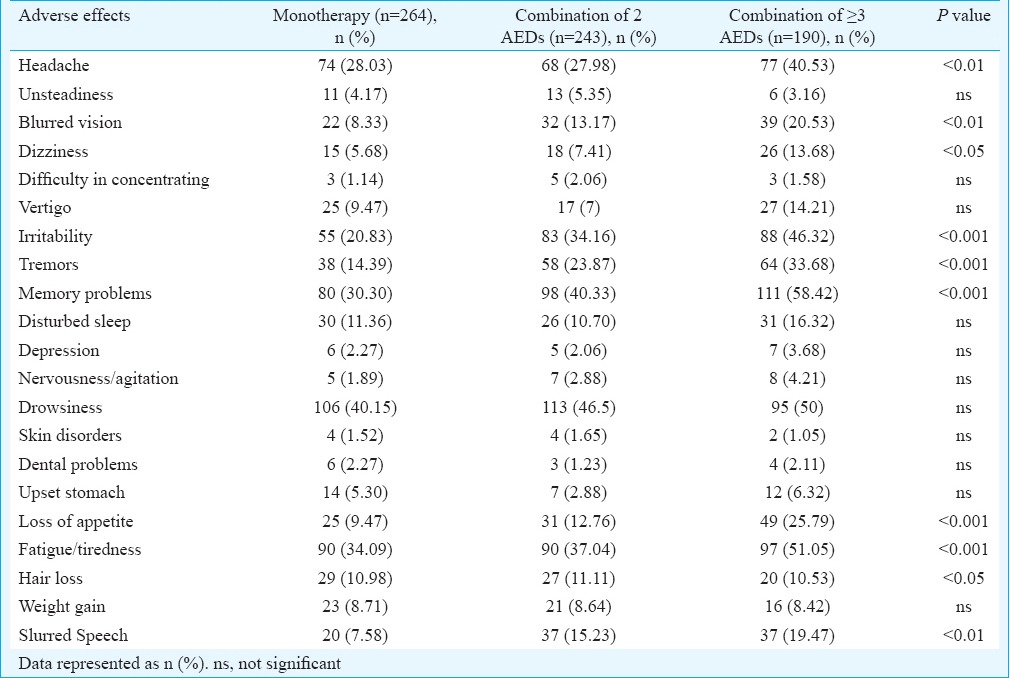
Frequency of AEs reported as occurring always/frequently (rating 4) or sometimes (rating 3) in the AEP questionnaire is illustrated in Table II. AEs were divided into two categories: neurological and systemic disorders. All neurological AEs were found to be significantly more in patients on combination of greater than or equal to three AEDs in comparison to monotherapy as well as two AEDs except unsteadiness, difficulty in concentrating, vertigo, disturbed sleep, depression, nervousness/agitation and drowsiness which did not differ between combination of two and greater than or equal to three AEDs. Systemic adverse effects like loss of appetite, fatigue/tiredness, hair loss and slurred speech were found to differ significantly among different treatment regimens.
Table II.
Percentage of patients reporting individual adverse effects as identified by the adverse event profile questionnaire receiving monotherapy and polytherapy of antiepileptic drugs (AEDs) (n=697)
Factors responsible for adverse effects in patients with epilepsy: Multivariate regression analysis revealed that factors such as age, gender, age at onset of seizures, type of seizures and AED load did not have any correlation with occurrence of AEs. However, a number of prescribed AEDs significantly affected the occurrence of AEs in PWE (P=0.001). There was no correlation between AEP scores and AED load amongst groups in patients on monotherapy (r=0.016; P=0.81) as well as polytherapy (two AEDs: r=0.023, P=0.71 and greater than or equal to three AEDs: r=0.36; P=0.62) (Fig. 2).
Fig. 2.
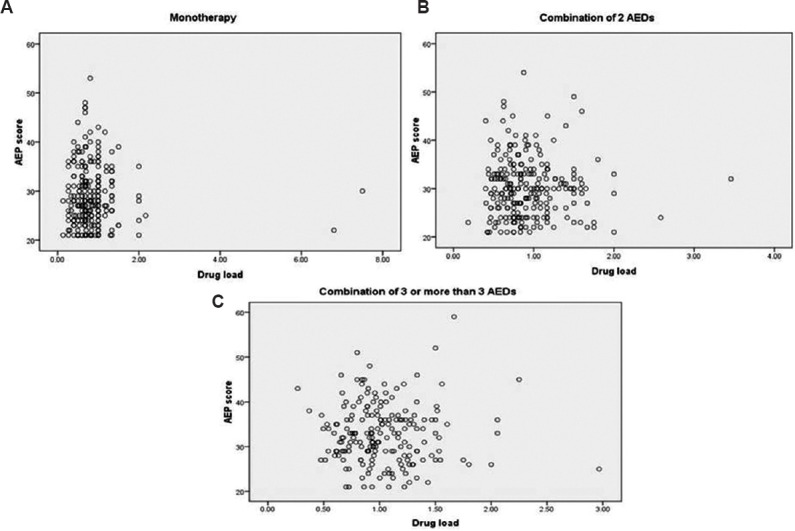
Relationship between adverse event profile (AEP) scores and antiepileptic drug load (drug load) in the patients (n=697). (A) Patients on monotherapy (r=0.023, P=0.71, n=264); (B) combination of two antiepileptic drugs (r=0.016, P=0.81, n=243); (C) combination of greater than or equal to three antiepileptic drugs (r=0.36, P=0.620, n=190).
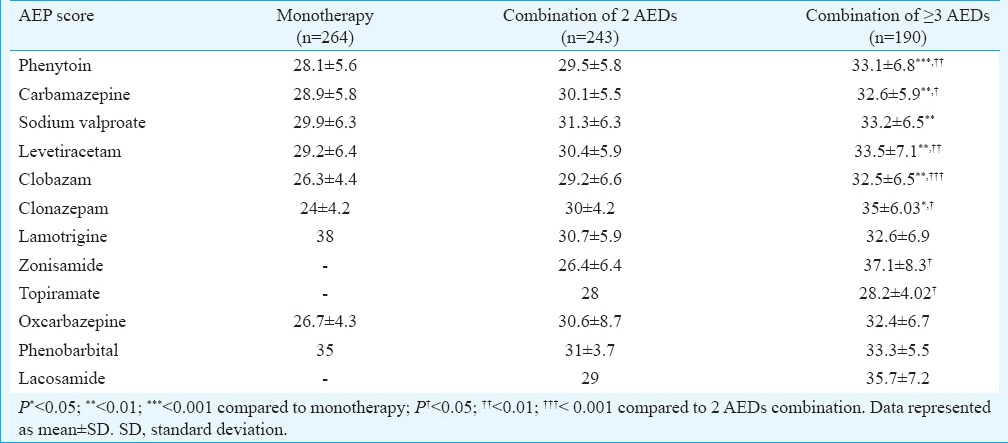
Relationship of AEP score with different AED regimens: The AEP score varied significantly amongst different treatment regimens (Table III). Combination of greater than or equal to three AEDs resulted in significantly higher AEP score in patients on PHT, CBZ, LEV, CLB and CLZ as compared to monotherapy as well as two AEDs. No such difference was observed amongst patients on TPM, LTG and ZNS as compared to their respective monotherapies. There was no significant difference in AEP score of different treatment regimens amongst patients on LTG, OXC, PHB and LCM.
Table III.
Adverse event profile (AEP) score of different antiepileptic drugs separately in patients on monotherapy (n=264), combination of 2 antiepileptic drugs (AEDs) (n=243) and combination of ≥3 AEDs (n=190)
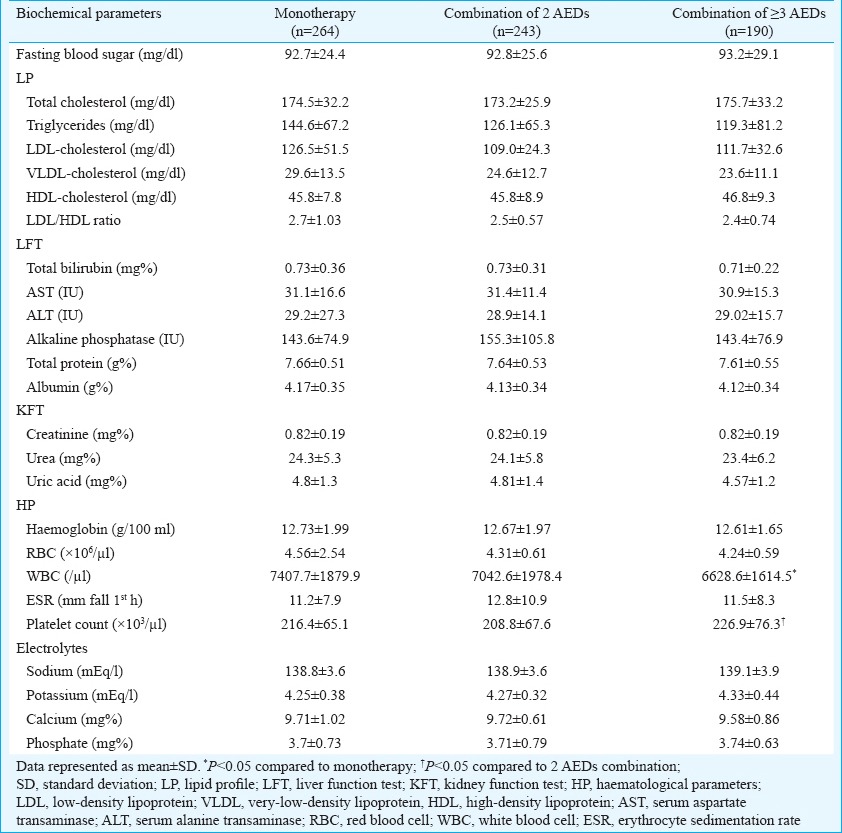
Relationship of biochemical parameters with different AED regimens: There were no significant changes in serum LP, LFT, KFT and electrolyte levels in patients on different AED regimens (Table IV). HPs also did not differ significantly between the regimens except WBCs and platelet count. Patients on combination of greater than or equal three AEDs had significantly higher WBC count as compared to monotherapy patients (P<0.05). Platelet count (P<0.05) was also significantly higher in patients on greater than or equal to three AEDs as compared to patients on combination of two AEDs. No such difference was observed between monotherapy and combination of two AEDs.
Table IV.
Biochemical parameters of patients on antiepileptic drugs (AEDs)
Discussion
Various treatment regimens are prescribed for control of seizures, and the AEs are major limiting factors in PWE11,19. The present study was aimed at analyzing the prescription pattern, AED load (PDD/DDD), effectiveness in terms of seizure frequency and AEs (AEP score) in patients treated with monotherapy and polytherapy of AEDs. Transitional polytherapy or long-term maintenance AED polytherapy in medically refractory epilepsy also contributed to a higher proportion of patients on polytherapy20.
The proportion of males on polytherapy was higher as compared to monotherapy and vice versa for females. This may be due to higher potential of AEDs for teratogenic side effects21. Prescription of polytherapy was found to be significantly higher in patients with an early age of onset of seizures as compared to monotherapy. The age at onset of seizures has a significant impact on determining the treatment, probability of reduction of seizures and cognitive side effects22. Early onset of seizures sometimes results in more complex seizures which in turn can lead to refractory epilepsy. The present results demonstrated higher frequency of use of monotherapy in patients with generalized seizures as compared to focal seizures despite the similar frequency of both types of seizures amongst the groups23. Focal seizures are known to have more cases of refractory epilepsy leading to use of more than two AEDs to control seizures24.
A progressive decline in the likelihood of producing seizure freedom with successive AED regimens was demonstrated in the present study, most markedly from monotherapy to combination of greater than or equal to three AEDs. In other words, concomitant use of three AEDs provided no additional benefit over the simultaneous use of two AEDs. Although Poolos et al25 reported improved efficacy with two AEDs over monotherapy, but our study, with larger patient population, substantiated previous results26.
The AED load did not increase with an increase in the number of AEDs. In most instances, a period of transitional polytherapy was observed during the conversion from one AED to another resulting in an overwhelming increase in AED load. This process may also get complicated by drug interactions and complex AED pharmacokinetics20. The ratio of PDD/DDD was highest for LCM, either due to small sample size of patients on LCM or due to attempts at higher doses to attain seizure control.
The proportion of patients with AEs identified by the AEP (95.2%) was comparable to that found in earlier studies16,19. A significant difference in AEP score was seen between patients on monotherapy and polytherapy. Previous studies have reported an improved quality of life after conversion from polytherapy to monotherapy7,27. However, there are also findings of a lack of association between AE burden and number of AEDs16,28.
The AEP score of patients on different AEDs in various seizure outcome groups did not differ significantly except for CLB and PHB which showed significantly higher AEP score in patients with no seizure control as compared to other groups with improvement in terms of reduction in seizure frequency. This may be due to per se AEs of these AEDs. The lack of correlation between AEP score and seizure outcome verifies the correlation of AEP score with number of AEDs. Moreover, AEs were also affected by the toxicity burden imposed by overtreatment rather than disease manifestation29. Hesdorffer et al30 here proposed that the risk of sudden unexpected death in epilepsy depends on frequency of seizures rather than number of AEDs used.
The present study revealed the lack of relationship between AEs and AED load. Combination of two different AEDs, each at the respective DDD, is unlikely to produce the same effects as doubling the dose of an AED from one DDD to two DDDs. Moreover, evidences from various animal and clinical studies4,31 also show that not all AED combinations are the same and that at equal drug loads or equal therapeutic efficacy, some combinations are better tolerated than others.
Biochemical parameters did not differ significantly between the groups. Though WBC and platelet count differed significantly in patients on combination of greater than or equal to three AEDs as compared to other groups, the alterations were within the normal range and hence clinically insignificant. The biochemical changes taking place, when a patient is on a combination of drugs, vary with the number and type of drug, duration of treatment, duration of epilepsy and patient genotype32.
AEs can also be influenced by genetic profile, differences in duration of treatment, seizure type, age, gender, comorbidities and personality trait and mood states10,33. In the present study no correlation of AEs was observed with age, gender, age at onset of seizures, type of seizures and AED load.
Though the need for combination of AEDs in the treatment of epilepsy is well justified, the associated increase in AEs lends a restriction to polytherapy. The present results provided insight in the AEs, AED load, seizure frequency and biochemical alterations with a number of AEDs in PWE. The results suggested that the AEs in PWE were more associated with a number of AEDs rather than frequency of seizures. Hence, the search for newer more potent less toxic antiepileptics cannot be undermined specially for the drug refractory population.
Acknowledgement
This study was conducted under the Aegis of Center for Excellence, Department of Biotechnology and Adverse Drug Reaction Monitoring Centre, AIIMS, New Delhi, India. The authors thank the Council of Scientific and Industrial Research, New Delhi, for providing financial assistance (9/6(431)/2011, EMR-I) to the first author (RJ) for carrying out this research work. Authors acknowledge Shrimati M. Kalaivani from the department of Biostatistics for her support and guidance.
Footnotes
Conflicts of Interest: None.
References
- 1.Birbeck GL. Revising and refining the epilepsy classification system: Priorities from a developing world perspective. Epilepsia. 2012;53(Suppl 2):18–21. doi: 10.1111/j.1528-1167.2012.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radhakrishnan K. Challenges in the management of epilepsy in resource-poor countries. Nat Rev Neurol. 2009;5:323–30. doi: 10.1038/nrneurol.2009.53. [DOI] [PubMed] [Google Scholar]
- 3.Amudhan S, Gururaj G, Satishchandra P. Epilepsy in India I: Epidemiology and public health. Ann Indian Acad Neurol. 2015;18:268–77. doi: 10.4103/0972-2327.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Combination therapy in epilepsy: When and what to use. Drugs. 2006;66:1817–29. doi: 10.2165/00003495-200666140-00004. [DOI] [PubMed] [Google Scholar]
- 5.St Louis EK. Truly “rational” polytherapy: Maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009;7:96–105. doi: 10.2174/157015909788848929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan B, Schlesinger M, Rodemer W, Pollard J, Hesdorffer D, Allen Hauser W, et al. Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia. 2011;52:619–26. doi: 10.1111/j.1528-1167.2010.02929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SV, Koshy S, Nair CR, Sarma SP. Frequent seizures and polytherapy can impair quality of life in persons with epilepsy. Neurol India. 2005;53:46–50. doi: 10.4103/0028-3886.15054. [DOI] [PubMed] [Google Scholar]
- 8.Hasan SS, Bahari MB, Babar ZU, Ganesan V. Antiepileptic drug utilisation and seizure outcome among paediatric patients in a Malaysian public hospital. Singapore Med J. 2010;51:21–7. [PubMed] [Google Scholar]
- 9.Haroon A, Tripathi M, Khanam R, Vohora D. Antiepileptic drugs prescription utilization behavior and direct costs of treatment in a national hospital of India. Ann Indian Acad Neurol. 2012;15:289–93. doi: 10.4103/0972-2327.104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasperaviciute D, Sisodiya SM. Epilepsy pharmacogenetics. Pharmacogenomics. 2009;10:817–36. doi: 10.2217/pgs.09.34. [DOI] [PubMed] [Google Scholar]
- 11.Cramer JA, Fisher R, Ben-Menachem E, French J, Mattson RH. New antiepileptic drugs: Comparison of key clinical trials. Epilepsia. 1999;40:590–600. doi: 10.1111/j.1528-1157.1999.tb05561.x. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovic M, Jocic-Jakubi B, Stevanovic D. Adverse effects of antiepileptic drugs and quality of life in pediatric epilepsy. Neurol India. 2015;63:353–9. doi: 10.4103/0028-3886.158203. [DOI] [PubMed] [Google Scholar]
- 13.Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: Can a natural history be developed? Lancet Neurol. 2008;7:151–60. doi: 10.1016/S1474-4422(08)70018-8. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology 2005-2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Collaborating Centre for Drug Statistics Methodology about the ATC/DDD System. [accessed on February 22 2013]. Available from http://www.whocc.no/atcddd/
- 16.Canevini MP, De Sarro G, Galimberti CA, Gatti G, Licchetta L, Malerba A, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51:797–804. doi: 10.1111/j.1528-1167.2010.02520.x. [DOI] [PubMed] [Google Scholar]
- 17.Yue L, Yu PM, Zhao DH, Wu DY, Zhu GX, Wu XY, et al. Determinants of quality of life in people with epilepsy and their gender differences. Epilepsy Behav. 2011;22:692–6. doi: 10.1016/j.yebeh.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA, Steinborn B, Striano P, Hlinkova L, Bergmann A, Bacos I, et al. Non-interventional surveillance study of adverse events in patients with epilepsy. Acta Neurol Scand. 2011;124:13–21. doi: 10.1111/j.1600-0404.2010.01440.x. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Brandenburg NA, Xu X, Vera-Llonch M, Oster G. The impact of seizures and adverse effects on global health ratings. Epilepsy Behav. 2007;11:179–84. doi: 10.1016/j.yebeh.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Garnett WR, St Louis EK, Henry TR, Bramley T. Transitional polytherapy: Tricks of the trade for monotherapy to monotherapy AED conversions. Curr Neuropharmacol. 2009;7:83–95. doi: 10.2174/157015909788848884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11:803–13. doi: 10.1016/S1474-4422(12)70103-5. [DOI] [PubMed] [Google Scholar]
- 22.Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: A prospective cohort study. Neurology. 2012;79:1384–91. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serrano-Castro PJ, Alonso-Morillejo E, Pozo-Munoz C, Payan-Ortiz M, Quiroga-Subirana P, Fernandez-Perez J. Characteristics of temporal lobe epilepsy with no ictal impairment of consciousness. Clin Neurol Neurosurg. 2013;115:1338–42. doi: 10.1016/j.clineuro.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Poolos NP, Warner LN, Humphreys SZ, Williams S. Comparative efficacy of combination drug therapy in refractory epilepsy. Neurology. 2012;78:62–8. doi: 10.1212/WNL.0b013e31823ed0dd. [DOI] [PubMed] [Google Scholar]
- 26.Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: Response to sequential treatment schedules. Eur J Neurol. 2006;13:277–82. doi: 10.1111/j.1468-1331.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 27.Pirio Richardson S, Farias ST, Lima AR, 3rd, Alsaadi TM. Improvement in seizure control and quality of life in medically refractory epilepsy patients converted from polypharmacy to monotherapy. Epilepsy Behav. 2004;5:343–7. doi: 10.1016/j.yebeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Piazzini A, Beghi E, Turner K, Ferraroni M, LICE Quality of Life Group Health-related quality of life in epilepsy: Findings obtained with a new Italian instrument. Epilepsy Behav. 2008;13:119–26. doi: 10.1016/j.yebeh.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Perucca E, Kwan P. Overtreatment in epilepsy: How it occurs and how it can be avoided. CNS Drugs. 2005;19:897–908. doi: 10.2165/00023210-200519110-00001. [DOI] [PubMed] [Google Scholar]
- 30.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk?A combined analysis. Epilepsia. 2012;53:249–52. doi: 10.1111/j.1528-1167.2011.03354.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaminski RM, Matagne A, Patsalos PN, Klitgaard H. Benefit of combination therapy in epilepsy: A review of the preclinical evidence with levetiracetam. Epilepsia. 2009;50:387–97. doi: 10.1111/j.1528-1167.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann T, Bertheussen KH, Svalheim S, Rauchenzauner M, Luef G, Gjerstad L, et al. Haematological side effects of antiepileptic drug treatment in patients with epilepsy. Acta Neurol Scand Suppl. 2011;191:23–7. doi: 10.1111/j.1600-0404.2011.01539.x. [DOI] [PubMed] [Google Scholar]
- 33.Kesavan R, Kukreti R, Adithan C. Genetic polymorphism of drug refractory epilepsy. Indian J Med Res. 2011;134:253–5. [PMC free article] [PubMed] [Google Scholar]


