Abstract
Background & objectives:
District-Level Household Survey-4 (DLHS-4) indicated that during 2012-2013, only 56 per cent of children aged 12-23 months in Tamil Nadu were fully vaccinated, which were lesser than those reported in earlier national surveys. We, therefore, conducted cluster surveys to estimate coverage of childhood vaccination in the State, and also to identify the factors associated with low coverage.
Methods:
Cross-sectional surveys were conducted in 15 strata [municipal corporation non-slum (n=1), municipal corporation slum (n=1), hilly (n=1), rural (n=6) and urban (n=6)]. From each stratum, 30 clusters were selected using probability proportional to the population size linear systematic sampling; seven children aged 12-23 months were selected from each cluster and their mothers/care-takers were interviewed to collect information about vaccination status of the child. A child was considered fully vaccinated if he/she received bacillus Calmette-Guérin (BCG), three doses of pentavalent, three doses of oral polio vaccine and one dose of measles vaccine, and appropriately vaccinated if all vaccine doses were given at right age and with right interval. Further, coverage of fully vaccinated children (FVC) as per vaccination cards or mothers’ recall, validated coverage of FVC (V-FVC) among those having cards, and coverage of appropriately vaccinated children (AVC) were estimated using survey data analysis module with appropriate sampling weights.
Results:
A total of 3150 children were surveyed, of them 2528 (80.3%) had vaccination card. The weighted coverage of FVC, V-FVC and AVC in the State was 79.9 per cent [95% confidence interval (CI): 78.2-81.5], 78.8 per cent (95% CI: 76.9-80.5) and 69.7 per cent (95% CI: 67.7-71.7), respectively. The coverage of individual vaccine ranged between 84 per cent (measles) and 99.8 per cent (BCG). About 12 per cent V-FVC were not vaccinated as per the vaccination schedule.
Interpretation & conclusions:
The coverage of FVC in Tamil Nadu was high, with about 80 per cent children completing primary vaccination. Efforts to increase vaccination coverage in the State need to focus on educating vaccinators about the need to adhere to the national vaccination schedule and strengthening supervision to ensure that children are vaccinated appropriately.
Keywords: Children, Tamil Nadu, vaccination coverage, vaccines
Childhood vaccination is one of the most cost-effective public health interventions1. High vaccination coverage is essential to stop transmission of vaccine prevenTable diseases (VPDs) and thereby achieve the benefits offered by vaccination. In India, the Universal Immunization Programme (UIP) targeting six VPDs (tuberculosis, diphtheria, pertussis, tetanus, poliomyelitis and measles) was launched in 1985. Although UIP has partially succeeded in reducing the burden of VPDs2, coverage of primary vaccinations in the country continues to be low, with only 61 per cent children fully vaccinated (defined as receipt of six vaccines by 12 months of age) during 2009 as per the UNICEF Coverage Evaluation Survey3. The coverage of childhood vaccination in the State of Tamil Nadu, India, has been consistently high. As per the National Family Health Surveys (NFHS), District-Level Household Surveys (DLHS) and Coverage Evaluation Survey, during 1998-2010, 77-91 per cent of children aged 12-23 months in the State were fully vaccinated4,5,6,7,8. However, the findings of the DLHS-4 survey indicated that during 2012-2013, only 56 per cent of the children aged 12-23 months in the State were fully vaccinated9. Therefore, the cross-sectional surveys were conducted to estimate the current vaccination coverage in Tamil Nadu and to identify the factors associated with incomplete vaccination.
Material & Methods
Study setting and study population: The cross-sectional surveys were conducted among children aged 12-23 months, residing in Tamil Nadu. The State has 32 revenue districts. All districts, except Chennai, have rural as well as urban areas. There are 10 municipal corporations in the State (Chennai, Vellore, Salem, Erode, Coimbatore, Tiruppur, Tiruchirappalli, Madurai, Tuticorin and Tirunelveli). The entire population of Chennai comes under the municipal corporation.
Sampling procedure: For the purpose of sampling, the State was divided into five strata. This included (i) municipal corporation non-slum stratum (n=1, population of one-year-old children: 145,857) consisting of 670 wards of non-slum areas of 10 municipal corporations, (ii) municipal corporation slum stratum (n=1, population of one-year-old children: 48,837) consisting of 2722 registered slums of 10 municipal corporations, (iii) hilly stratum (n=1, population of one-year-old children: 22,566) comprising the Nilgiris district (rural and urban areas) and other hilly villages in the State as per the 2011 census10, (iv) urban (population of one-year-old children: 366,780), and (v) rural strata (population of one-year-old children: 556,604). To have more precise estimates, the rural and urban areas were further divided into six geographically independent strata by grouping five border-sharing districts (Nilgiris being the hilly district and Chennai being municipal corporation were excluded). Thus, a total of 15 strata were formed. Each urban stratum comprised municipalities, town panchayats, census towns, cantonment board and outgrowths in the five border-sharing districts, whereas the rural strata consisted of revenue villages from these districts.
Cluster sampling design was adopted for selecting clusters from each stratum. From each stratum, 30 clusters were selected using probability proportional to the population of each cluster linear systematic sampling.
Sample size: As per the DLHS-4, 56 per cent of the children aged 12-23 months were fully vaccinated9. With this coverage, an absolute precision of 10 per cent, design effect of 2.0 and at 95 per cent confidence level, we needed a minimum sample size of 190 children (rounded off to 210) from each stratum. Thirty clusters were selected from each stratum, and from each cluster, seven children aged between 12 and 23 months were surveyed. Thus, from 15 strata, 3150 children were needed to generate coverage estimates for the State.
Survey procedure: The data were collected between March 1, 2015 and April 1, 2015 by 20 field teams, each consisting of one field supervisor and two field investigators. Before the survey, all field staff underwent a two-day training, covering various aspects of survey such as immunization schedule, informed consent procedure, selection of random household and data collection using the survey questionnaire. The questionnaire was pilot tested before the survey. Each team was given the names of clusters to be surveyed from the given stratum and a random household number for each cluster. This random number was selected based on the total number of households as per 2011 census10 in the selected cluster and was the starting point for the survey in that cluster.
Before beginning the survey, the field supervisor prepared map of the cluster, counted the number of the households in each street and selected the household number corresponding to the assigned random number. The field investigators enquired for the presence of eligible child (aged between 12 and 23 months) in this household and interviewed the respondent (preferably child's mother) if an eligible child was present. If no eligible child was found in this household, the team searched the next nearest household for an eligible child. This procedure was continued till seven children aged 12-23 months were surveyed within the boundary of a selected cluster. In any household, only one eligible child was surveyed. In case more than one eligible child was present in the household, the youngest child was selected.
After obtaining the written informed consent, mothers of the eligible children were interviewed using a structured questionnaire, to collect information about socio-demographic details of the household and vaccination status of the child. Information about vaccination was collected based on information provided by the mother as well as from the vaccination card. Children, whose mothers could not show the vaccination card at the time of survey, were considered as not having the card. Mothers of children who did not receive any one of the eight primary vaccines were interviewed to know the reasons for not vaccination.
Operational definitions: The following operational definitions were used: Eligible child - a child aged 12-23 months at the time of the survey; Fully vaccinated child (FVC): a child who has received bacillus Calmette-Guérin (BCG), three doses of pentavalent (containing vaccine against diphtheria, pertussis, tetanus, hepatitis-B and Haemophilus influenzae B), three doses of oral polio vaccine (OPV) and one dose of measles vaccine (eight vaccine doses) by 12 months of age, as per vaccination cards or history given by mother in case card is not available11,12. This criterion was used by NFHS and DLHS surveys for classifying FVCs. The pentavalent vaccine was introduced in the state since December 2011; Validated FVC (V-FVC): a child who received BCG, three doses of pentavalent, three doses of OPV and one dose of measles vaccine within 12 months of age, as per vaccination card; and Appropriately vaccinated child (AVC): a child was considered appropriately vaccinated if he/she has received all vaccine doses at right age and with right interval, as per the national vaccination schedule. AVC met the following conditions: (a) BCG vaccine – given before attainment of one year of age, (b) pentavalent vaccine - first dose given after six weeks of birth and two subsequent doses with an interval of at least four weeks and receipt of all the three doses before the first year of life, (c) measles vaccine - administered after completion of nine months (270 days) but before the first year of life.
Quality assurance: For the purpose of quality assurance, re-survey of five per cent of children covering 15 strata was planned. Three to four clusters from each stratum were randomly selected and 3-4 children from each selected cluster were re-surveyed.
The Institutional Human Ethics Committee of the National Institute of Epidemiology, Chennai, approved the study. Written informed consent was obtained from the mothers of eligible children or their respondents before interviewing them.
Statistical analysis: Data were double-entered and processed using Epi Info (version 7.1.5, Centers for Disease Control and Prevention, Atlanta, GA, USA) and STATA SE (version 13.0) software (StataCorp LLC, Texas, USA). Using survey data analysis module in STATA, the coverage of (i) FVCs, (ii) V-FVCs and (iii) AVCs along with the corresponding 95 per cent confidence intervals (CIs) for each stratum were estimated13 .
Sampling weights were used to calculate vaccination coverage estimates for each stratum. The coverage for the State was estimated using (i) stratum-level estimates, and (ii) the proportion of one-year-old children in the respective stratum as weights12,13. Using the survey data analysis module in STATA software, multiple logistic regression analysis was done to identify socio-demographic factors associated with non/incomplete vaccination. Variables with P≤0.2 in the univariate analysis were included in the multiple logistic regression analysis.
As the vaccination status of every child surveyed was collected based on mother's recall as well as vaccination card, sensitivity and specificity of mothers recall was calculated as compared to that of vaccination card. The agreement between the main survey and quality assurance re-survey was estimated by calculating the un-weighted kappa14. Prevalence Adjusted Bias Adjusted Kappa (PABAK) was also calculated as the un-weighted kappa estimates are highly dependent on the prevalence of the condition in the population15,16,17.
Results
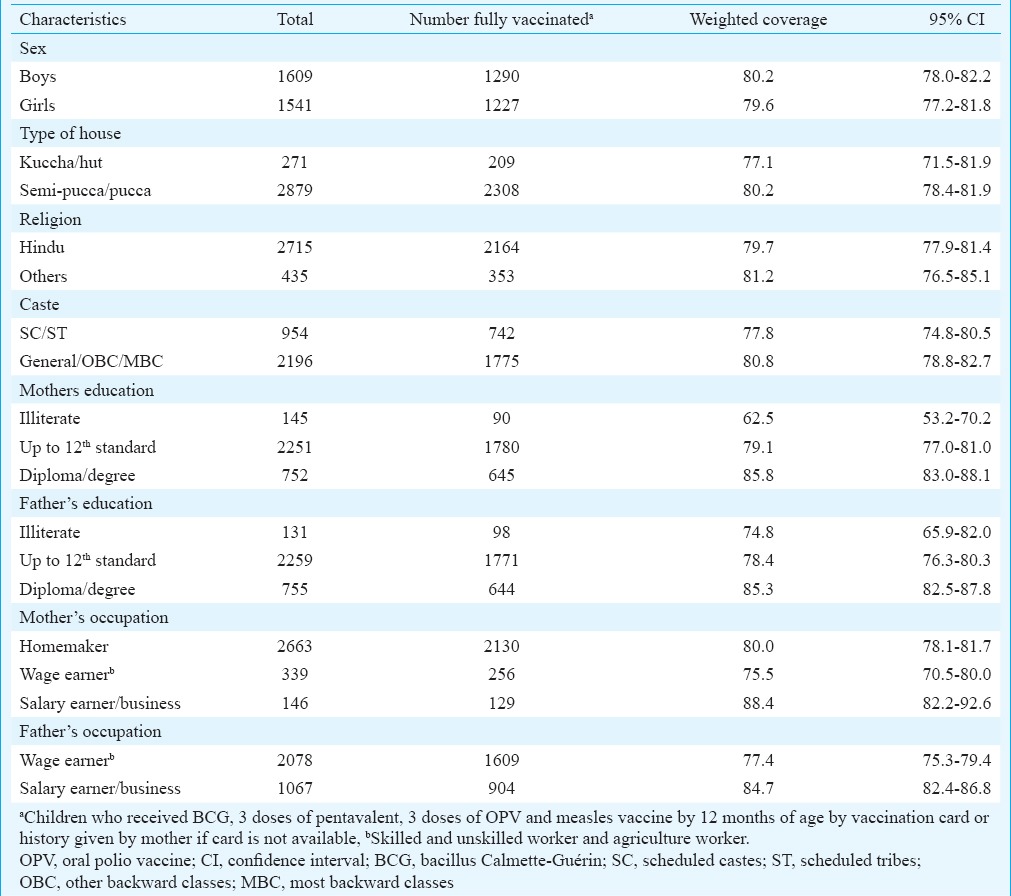
A total of 3150 children from 15 strata were surveyed. The mean age of children was 17.6 months (standard deviation: 3.4, range: 12-23 months). Majority of the children (86.2%) were Hindus and about one-third (30.3%) belonged to scheduled caste/tribe category (Table I). Less than five per cent of the children's parents were illiterate. The median family size of the households surveyed was five (interquartile range 4-6). Most (2528, 80.3%) children had vaccination card. The number of children having vaccination card ranged between 150 (71.4%) and 180 (85.7%) in different strata (P=0.116).
Table I.
Socio-demographic characteristics of children surveyed and coverage of fully vaccinated children by selected variables, Tamil Nadu, India, 2015
Vaccination coverage among children having vaccination card: Coverage of validated fully vaccinated children (V-FVCs): Of the 2528 children having vaccination card, 1991 (78.8%) had received all the eight vaccine doses by the age of 12 months, 89 (3.5%) had received eight vaccine doses but at least one dose was given after age of one year while 448 (17.7%) had received less than eight vaccine doses (partially vaccinated). The weighted V-FVCs in the State was 78.8 per cent (95% CI: 76.9-80.5) and ranged between 73.4 and 82.4 per cent in different strata (Table II). The coverage of V-FVCs was not significantly different across the strata.
Table II.
Coverage of validated fully vaccinated children (V-FVC), appropriately vaccinated children (AVC) and fully vaccinated children (FVC), Tamil Nadu, 2015
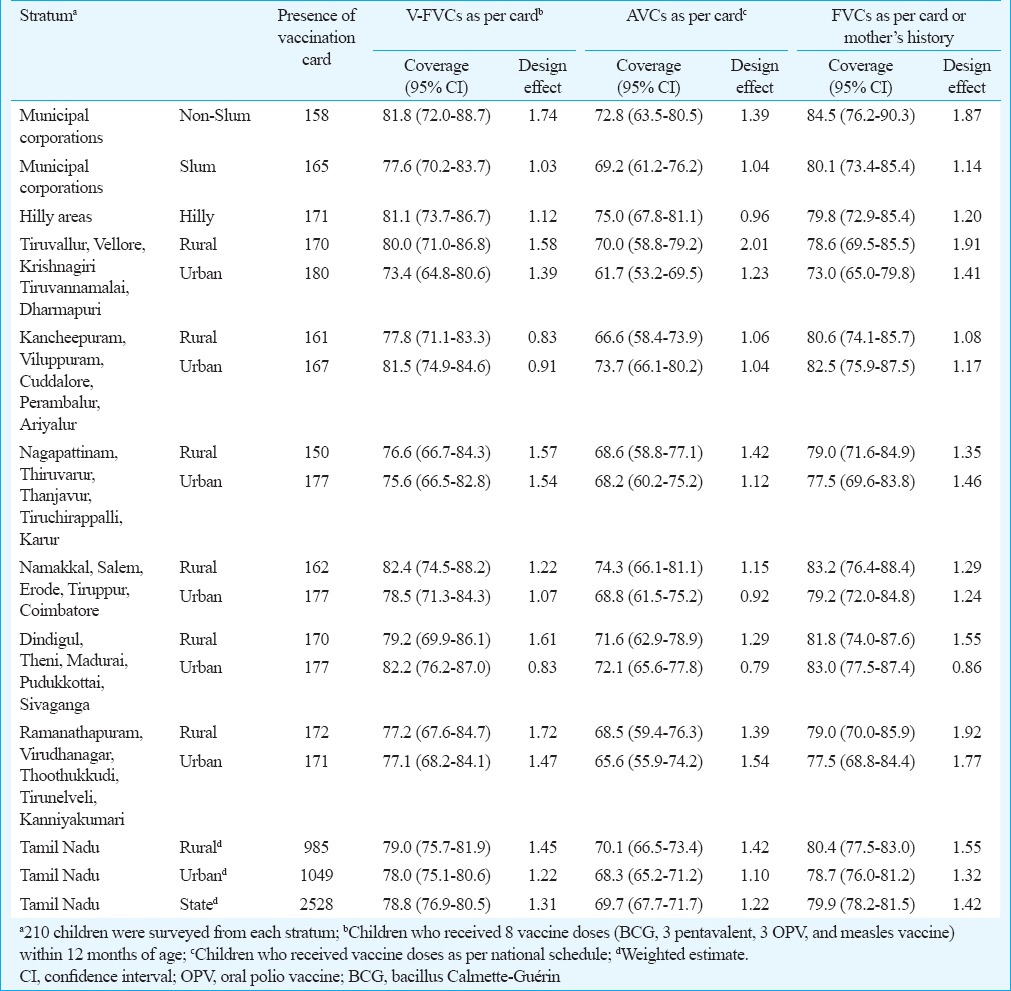
Coverage of appropriately vaccinated children (AVCs): Of the 2,528 children having vaccination card, 1763 (69.7%) had received all the eight vaccine doses as per the vaccination schedule, 317 (12.5%) had received eight doses but at least one dose was not as per the schedule while 448 (17.7%) were partially vaccinated. The coverage of appropriately vaccinated children in the State was 69.7 per cent (95% CI: 67.7-71.7) (Table II). Among the 1991 FVCs as per cards, 1763 (88.5%) had received all the vaccine doses as per vaccination schedule. The common observations for inappropriate vaccination among the remaining 228 children included first dose of pentavalent vaccine given before 42 days (n=48, 21.1%), interval between the two doses of pentavalent vaccine <28 days (n=103, 45.2%) and measles vaccine given before 270 days (n=137, 60.1%).
Vaccination coverage among children not having vaccination card:
Sensitivity and specificity of mother's recall about vaccination status: The vaccination status of 2528 children as per vaccination card was compared with mother's recall history. Considering the vaccination status as per card as gold standard, the sensitivity and specificity of mother's recall ranged between 99.1 and 100 per cent for BCG; 90.7 and 36.7 per cent for three doses of pentavalent; 91.2 and 34.3 per cent for three doses of OPV and 93.5 and 42.5 per cent for measles vaccine. The sensitivity and specificity of the recall method for full vaccination status were 87.7 and 41.7 per cent, respectively.
Of the 622 FVCs who did not have vaccination card, 526 (84.6%) had received all the eight vaccine doses within 12 months as per mothers’ recall; 94 (15.1%) were partially vaccinated while 2 (0.3%) were unvaccinated.
Coverage of fully vaccinated children (FVCs): The coverage of FVCs in the State as per card or mother's recall in case card was not available was 79.9 per cent (95% CI: 78.2-81.5). The coverage was comparable across the 15 strata. The coverage in the rural, urban, non-slum, slum and hilly areas was 80.4 per cent (95% CI: 77.5-83.0), 78.7 per cent (95% CI: 76.0-81.2), 84.5 per cent (95% CI: 76.2-90.3), 80.1 per cent (95% CI: 73.4-85.4) and 79.8 per cent (95% CI: 72.9-85.4), respectively (Table II).
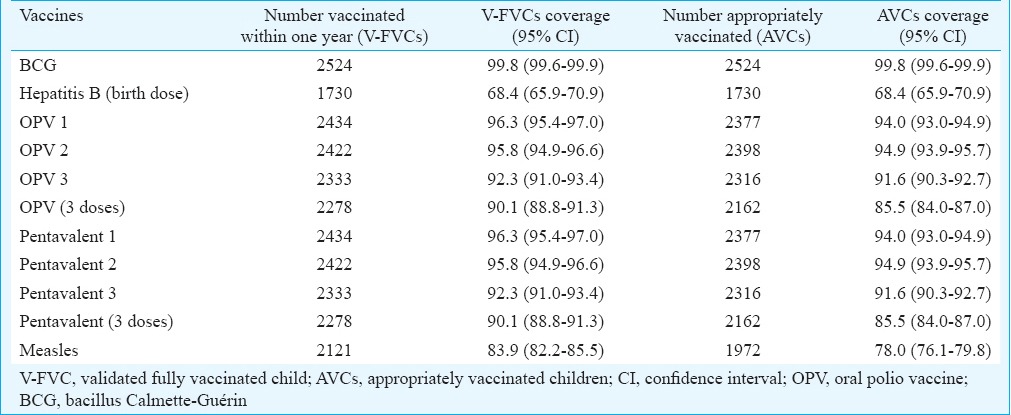
Coverage of different vaccines: The coverage of BCG, three doses of OPV/three doses of pentavalent and measles vaccine among children having vaccination card in the State was 99.8 per cent (95% CI: 99.6-99.9), 90.1 per cent (95% CI: 88.8-91.3), and 83.9 per cent (95% CI: 82.2-85.5), respectively (Table III). The dropout rate from BCG to measles, pentavalent 1 to measles and pentavalent 1 to pentavalent 3 was 15.9, 12.9 and 4.1 per cent, respectively.
Table III.
Coverage of different vaccines among children with vaccination cards (n=2528), Tamil Nadu, India, 2015
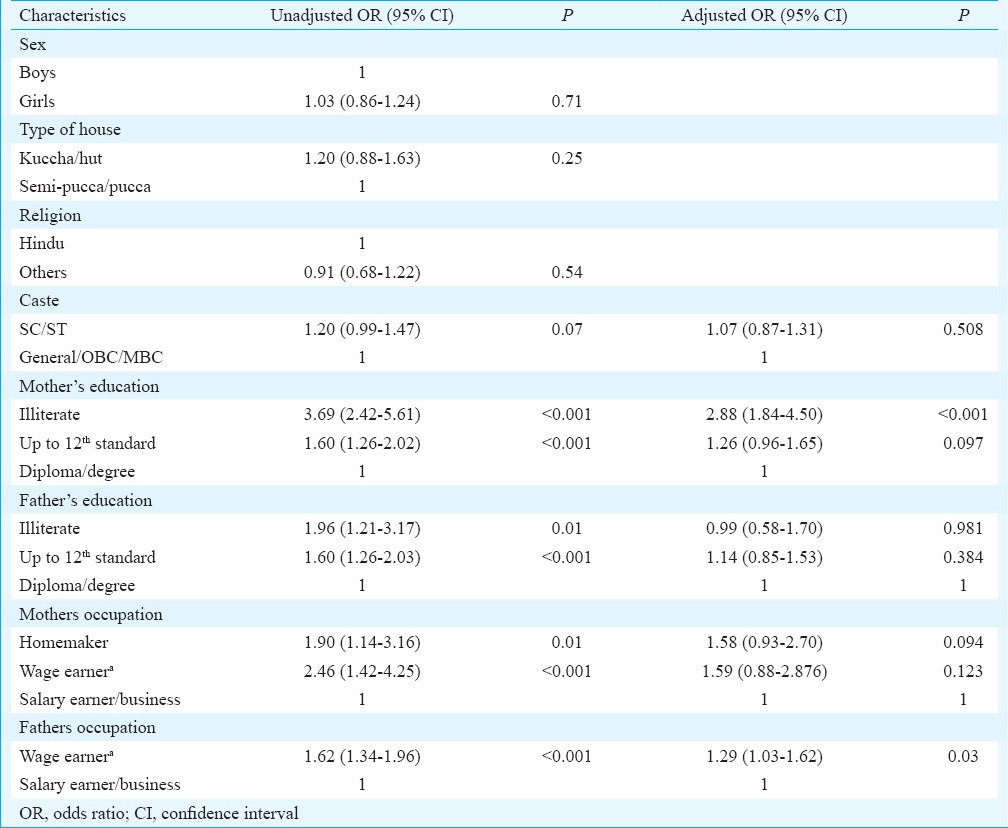
Coverage of fully vaccinated children (FVCs) by selected socio-demographic characteristics: Table I describes the coverage of FVCs by selected socio-demographic characteristics. The coverage was not different among children by sex, religion or caste. Coverage was significantly lower among children whose parents were illiterate and whose fathers were wage earners. On multiple logistic regression analysis, children whose mothers were illiterate and whose fathers were wage earners were more likely to be incompletely vaccinated (Table IV).
Table IV.
Socio-demographic factors associated with incomplete vaccination
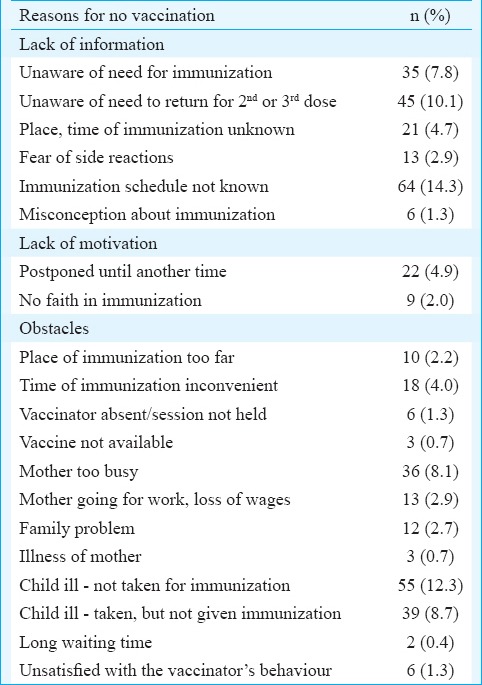
Reasons for non-vaccination: Lack of information and obstacles for vaccination were common reasons for not vaccination among the 283 mothers whose children were un/partially vaccinated (Table V).
Table V.
Reasons for no vaccination among 283 mothers, Tamil Nadu
Quality assurance: A total of 186 (6%) children from 54 clusters covering 15 strata were re-surveyed, and information about their vaccination status was collected. On re-survey, 161 (86.6%) had vaccination cards as against 153 (82.3%) during the original survey. Among the 147 children who had cards during both surveys, 116 (79%) and 119 (81%) children were fully vaccinated as per the main survey and re-survey, respectively (P=0.67). Among the 19 children who did not have vaccination cards during both the surveys, 14 and 16 children were fully vaccinated as per the main and re-survey, respectively (P=0.46).
Discussion
Although vaccines have made an important contribution to public health, VPDs contribute significantly towards under-five mortality globally2. Achieving high rates of vaccination has been the greatest challenge to the immunization programme managers in many developing countries. Periodic estimation of vaccination coverage is necessary to monitor the progress in achieving the targets and guide the programme managers in improving the immunization services. The findings of our survey indicated that the coverage of FVCs in Tamil Nadu was about 80 per cent. The coverage for FVCs was not different across rural, urban, hilly, slum and non-slum areas. About 16 per cent of children who received BCG and about 13 per cent of children who received first dose of DPT did not complete the full course of vaccination. Lack of awareness about vaccination and obstacles such as child's illness and inconvenient timing of vaccination were the main reasons for incomplete or non-vaccination.
The DLHS-4 conducted during 2012-2013 indicated low coverage of FVCs in Tamil Nadu9. This survey covered children born during 2010-2011. The low vaccination coverage could be on account of the change in the vaccination strategy in the State. In April 2008, following death of four infants following measles vaccination, Government of Tamil Nadu had stopped outreach vaccination and decided that all children were vaccinated in health facilities (facility-based vaccination)18,19. This strategy was reversed in October 2011, followed by introduction of pentavalent vaccine. Compared to DLHS-4 coverage in 2010-2011, the coverage of FVCs during 2013-2014 in the State has increased9. The State also has achieved a target of 90 per cent coverage against three doses of DPT set by the Global Vaccine Action Plan20. MCV1 coverage of 83.9 per cent however, was lower than the global goal of 90 per cent recommended as per Global Measles and Rubella Strategic Action Plan21.
Although the coverage of full vaccination in the State was 80 per cent, the coverage of individual vaccination in the State was high, ranging between 84 and 99.8 per cent. About 12 per cent FVCs were inappropriately vaccinated - either before the recommended age of vaccination or the interval between the two doses was less than the recommended interval. Most of the inappropriately vaccinated children in Tamil Nadu received vaccines before the recommended age. Vaccinating at an appropriate age and correctly spacing the vaccine doses are the two most important predictors of vaccine-induced protection. Vaccine doses administered at intervals less than the minimum intervals or earlier than the minimum age are likely to interfere with antibody response and thereby affect protection conferred by these vaccines22. A study based on the analysis of DLHS-3 data identified age-inappropriate vaccination as an important weakness in the national immunization programme in India23. Only one-third of the infants had received measles and DPT3 vaccine at the recommended age23. In India, the national level health surveys conducted periodically provide the coverage of FVCs. These surveys show a rising trend of FVCs in many Indian States3,4,5,6,7,8,9. However, high vaccination coverage rates do not necessarily indicate age-appropriate vaccination3,4,5,6,7,8,9. Collecting information about age-appropriate coverage of measles and DPT/pentavalent vaccination along with overall coverage would be useful for the State- and district-level immunization programme managers to improve the quality of vaccination.
In Tamil Nadu, lack of parental knowledge about vaccination schedule, need for vaccination and need to complete the vaccination schedule were important reasons for non/partial vaccination. Children whose mothers were illiterate were more likely to be incompletely vaccinated. These findings were consistent with the studies conducted in India and elsewhere about the reasons for non-vaccination27. Interventions aimed at improving health workers’ communication with illiterate mothers at the time of vaccination would further improve the vaccination coverage in the State28,29.
Our study had certain limitations. First, vaccination card was not available with 20 per cent of children at the time of survey. For logistical reasons, village health nurses (VHN) were not contacted in case mothers did not have the card. The vaccination status of these children was assessed based on mother's recall history, which might not be reliable. However, in the remaining 80 per cent children who had vaccination cards, the sensitivity of mother's recall for full vaccination status was high. Second, although there was a good agreement in the vaccination coverage between the original survey and the quality-assurance survey, the percentage of children with cards was different during the two surveys. It is a common practice that VHNs after vaccination retains the vaccination card with her and returns it to the mother after updating the vaccination details. Thus, the actual proportion of children with cards could be higher than 80 per cent observed in the study.
In conclusion, our study showed that the coverage of different vaccines among children aged 12-23 months in Tamil Nadu was high, ranging between 84 and 99.8 per cent.; 80 per cent of the children had received all the vaccines. About 16 per cent of children who received BCG and about 13 per cent of children who received first dose of DPT did not complete the full course of vaccination. About one in ten FVCs was inappropriately vaccinated. It is necessary to strengthen the immunization programme in the State to increase the vaccination coverage and decrease the dropout rates. The efforts to increase the vaccination coverage in the State need to focus on educating health workers about the correct national vaccination schedule and need for adhering to the schedule, and strengthening supportive supervision to ensure that children are vaccinated appropriately at right age and with right interval. It is also necessary to educate mothers about the benefit of complete vaccination.
Acknowledgment
Authors thank Dr K. Kolandaswamy, Director of Public Health and Preventive Medicine, Government of Tamil Nadu, for his critical inputs in planning the survey, and acknowledge the support and cooperation extended by Drs S. Somasundaram, C. Sekar, G. K. Dorairaj, K.Surendran, R. Mohan Kumar, Shri S. Sampath, and the Deputy Directors of Health Services of all the health unit districts. Authors also thank the MPH trainees of 2014 cohort for conducting the re-survey, and Drs B. Viduthulai Virumbi and Vinay Kumar for coordinating the movement of study teams.
Footnotes
Conflicts of Interest: None.
References
- 1.World Bank 1993. World Development Report 1993: Investing in Health. New York: Oxford University Press; [accessed on February 23 2016]. Available from https://openknowledge.worldbank.org/handle/10986/5976 . [Google Scholar]
- 2.Lahariya C. A brief history of vaccines & vaccination in India. Indian J Med Res. 2014;139:491–511. [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF. Coverage Evaluation Survey. 2009. [accessed on February 23, 2016]. Available from http://www.unfpa.org/sowmy/resources/docs/library/R309_UNICEF_2010_INDIA_2009CoverageSurvey.pdf .
- 4.International Institute for Population Sciences (IIPS) National Family Health Survey (MCH and Family Planning), India 1992-93. Bombay: International Institute for Population Sciences; 1995. [accessed on February 23 2016]. Available from http://www.rchiips.org/nfhs/data/india1/iachap9.pdf . [Google Scholar]
- 5.International Institute for Population Sciences (IIPS), ORC Macro. National Family Health Survey (NFHS-2) 1998-1999: India. Mumbai: International Institute for Population Sciences; 2000. [accessed on February 23 2016]. Available from http://www.nfhsindia.org/data/india/indch8.pdf . [Google Scholar]
- 6.National Family Health Survey (NFHS-3) 2005-2006: India. Vol. 1. Mumbai, India: International Institute for Population Sciences; 2007. [accessed on February 23 2016]. International Institute for Population Sciences (IIPS) and Macro International. Available from http://www.rchiips.org/nfhs/NFHS-3%20Data/TamilNadu_report.pdf . [Google Scholar]
- 7.Reproductive and child health-district level household survey 2 (RCH-DLHS-2), India 2002-2004. Mumbai: International Institute for Population Sciences; 2006. [accessed on February 23 2016]. International Institute for Population Sciences (IIPS), Government of India. Available from http://www.rchiips.org/pdf/rch2/National_Report_RCH-II.pdf . [Google Scholar]
- 8.District level household and facility survey (DLHS-3) 2007-2008: India. Mumbai: International Institute for Population Sciences; 2010. [accessed on February 23 2016]. International Institute for Population Sciences (IIPS) Available from http://www.rchiips.org/pdf/rch3/state/Tamil.pdf . [Google Scholar]
- 9.District level household and facility survey-4 State fact sheet Tamil Nadu (2012-13) Mumbai: International Institute for Population Sciences; 2010. [accessed on February 23, 2016]. International Institute for Population Sciences (IIPS) Available from http://www.rchiips.org/pdf/dlhs4/report/TN.pdf . [Google Scholar]
- 10.Ministry of Home Affairs, Government of India Census of India 2011. Office of the Registrar General and Census Commissioner. [accessed on February 23 2016]. Available from http://www.censusindia.gov.in .
- 11.Ministry of Health and Family Welfare, Government of India. Immunization handbook for medical officers. 2008. [accessed on February 15 2016]. Available from http://www.nihfw.org/pdf/nchrc-publications/immunihandbook.pdf .
- 12.WHO. Immunization coverage cluster survey: Reference manual. Geneva: WHO; 2005. [Google Scholar]
- 13.Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat Q. 1991;44:98–106. [PubMed] [Google Scholar]
- 14.Cohen J. A coefficients of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 15.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46:423–9. doi: 10.1016/0895-4356(93)90018-v. [DOI] [PubMed] [Google Scholar]
- 16.Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43:551–8. doi: 10.1016/0895-4356(90)90159-m. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–9. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- 18.Amdekar YK, Singhal T, Committee on Immunization Indian Academy of Pediatrics Measles vaccine deaths: The IAP-COI stand. Indian Pediatr. 2008;45:479–80. [PubMed] [Google Scholar]
- 19.John TJ. Death of children after measles vaccination. Indian Pediatr. 2008;45:477–8. [PubMed] [Google Scholar]
- 20.WHO. Global Vaccine Action Plan 2011-2020. Geneva: WHO; 2013. [accessed on February 23 2016]. Available from http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ [Google Scholar]
- 21.WHO. Global measles and rubella strategic plan 2012-2020. [accessed on February 23, 2016]. Available from http://www.unicef.org/immunization/files/Measles_Rubella_StrategicPlan_2012_2020.pdf .
- 22.National Center for Immunization and Respiratory Diseases. General recommendations on immunization – Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- 23.Awofeso N, Rammohan A, Iqbal K. Age-appropriate vaccination against measles and DPT-3 in India – Closing the gaps. BMC Public Health. 2013;13:358. doi: 10.1186/1471-2458-13-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlopoulou ID, Michail KA, Samoli E, Tsiftis G, Tsoumakas K. Immunization coverage and predictive factors for complete and age-appropriate vaccination among preschoolers in Athens, Greece: A cross – sectional study. BMC Public Health. 2013;13:908. doi: 10.1186/1471-2458-13-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadnes LT, Nankabirwa V, Sommerfelt H, Tylleskär T, Tumwine JK, Engebretsen IM, PROMISE-EBF Study Group Is vaccination coverage a good indicator of age-appropriate vaccination?A prospective study from Uganda. Vaccine. 2011;29:3564–70. doi: 10.1016/j.vaccine.2011.02.093. [DOI] [PubMed] [Google Scholar]
- 26.Fadnes LT, Jackson D, Engebretsen IM, Zembe W, Sanders D, Sommerfelt H, et al. Vaccination coverage and timeliness in three South African areas: A prospective study. BMC Public Health. 2011;11:404. doi: 10.1186/1471-2458-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: A review of the grey literature. Int Health. 2012;4:229–38. doi: 10.1016/j.inhe.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Johri M, Pérez MC, Arsenault C, Sharma JK, Pai NP, Pahwa S, et al. Strategies to increase the demand for childhood vaccination in low- and middle-income countries: A systematic review and meta-analysis. Bull World Health Organ. 2015;93:339–46. doi: 10.2471/BLT.14.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel AR, Nowalk MP. Expanding immunization coverage in rural India: A review of evidence for the role of community health workers. Vaccine. 2010;28:604–13. doi: 10.1016/j.vaccine.2009.10.108. [DOI] [PubMed] [Google Scholar]


