Summary
Cocaine users show reduced expression of the metabotropic glutamate receptor (mGluR2), but it is not clear whether this is a predisposing trait for addiction or a consequence of drug exposure. In this study, we found that a nonsense mutation at the mGluR2 gene decreased mGluR2 expression and altered the seeking and taking of cocaine. mGluR2-mutant rats show reduced sensitivity to cocaine reward, requiring more cocaine to reach satiation when it was freely available, and ceasing their drug-seeking behavior sooner than controls when the response requirement was increased. mGluR2-mutant rats also show a lower propensity to relapse after a period of cocaine abstinence, an effect associated with reduced cocaine-induced dopamine and glutamate overflow in the nucleus accumbens. These findings suggest that mGluR2 polymorphisms or reduced availability of mGluR2 might be risk factors for the initial development of cocaine use, but could actually protect against addiction by reducing sensitivity to cocaine reward.
Keywords: etiology of addiction, cocaine reward, glutamate, autoreceptor, mGluR2, self-administration, addiction
eTOC
The etiology and pathophysiology of drug addiction remain poorly understood. Yang et al. show that genetic deletion of mGluR2, a presynaptic glutamate autoreceptor, decreases sensitivity to cocaine reward that causes a compensatory increase in cocaine-taking and a decrease in relapse to cocaine-seeking behavior in rats.
Introduction
Despite intensive research, the etiology and pathophysiology of the development and maintenance of substance use disorders are still not well understood (Kalivas et al., 2005; Wise and Koob, 2014). Although drug use does not lead to addiction in most people, certain individuals are more vulnerable (Sagheddu and Melis, 2015; Swendsen and Le Moal, 2011). Studies in humans suggest that certain vulnerability traits, such as high impulsivity, stress reactivity, novelty-seeking, negative emotionality, and structural abnormalities of the frontostriatal brain system, may predispose individuals to increased drug abuse and the development of addiction (Adams et al., 2003; Ersche et al., 2011; Koob, 2015). However, the cellular and molecular mechanisms by which such traits influence drug-taking and drug-seeking behavior remain unresolved (Swendsen and Le Moal, 2011; Wise and Koob, 2014). Since dopamine (DA) is critically involved in the rewarding and psychostimulant effects of drugs of abuse (Wise, 2008), it has been postulated that innate individual differences in the mesocorticolimbic dopamine (DA) pathway may influence the behavioral response to drugs (Swendsen and Le Moal, 2011). In particular, lower DA D2 and/or D3 receptor availability in the striatum has been considered a risk factor that might facilitate continued drug use and abuse (Song et al., 2012; Volkow et al., 2009; Volkow et al., 2002).
In addition to DA, glutamate also plays an important role in substance use disorders, particularly in relapse to drug-seeking behavior (Kalivas, 2009). However, little is known as to whether an alteration in glutamate receptor expression is also involved in vulnerability to drug-taking and drug-seeking behavior. Glutamate receptors are classified into ionotropic and metabotropic receptors. Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors consisting of eight subtypes that are further classified into three groups (Riedel et al., 2003). Given that group II mGluRs (mGluR2/3) are the presynaptic glutamate autoreceptors that control glutamate release, and that cocaine and heroin have been shown to induce relapse by increasing extracellular levels of dopamine and glutamate in the nucleus accumbens (Moussawi and Kalivas, 2010; Pomierny-Chamiolo et al., 2014), a number of studies have focused on mGluR2/3 agonist therapy as a potential treatment for drug addiction. However, mGluR2/3 agonist therapy may be futile since cocaine-dependent subjects display a significant reduction in mGluR2/3 expression and function in the mesocorticolimbic system (Ghasemzadeh et al., 2009; Huang et al., 2007; Kasanetz et al., 2013; Xi et al., 2002b; Xie and Steketee, 2009). These findings raise the question of whether this reduction in mGluR2/3 expression is a pre-existing trait for the development of addiction or a consequence of drug exposure.
In the present study, we used wild-type (WT) and mGluR2-mutant rats, in which no mGluR2 is expressed, to study whether mGluR2 loss alters cocaine-taking and cocaine-seeking behaviors in different stages of the drug addiction cycle, including acquisition of drug-taking behavior, changes in drug consumption when the dose or response requirement is changed, changes in drug seeking when cocaine is no longer available (extinction), and relapse to drug-seeking behavior upon re-exposure to cocaine or cocaine-associated cues after a period of abstinence. We then used multiple behavioral, neurochemical, immunostaining, and transgenic approaches to study the cellular and molecular mechanisms underlying the changes in drug-taking and drug-seeking behavior observed in rats after mGluR2 deletion.
Results
Characterization of mGluR2-KO rats
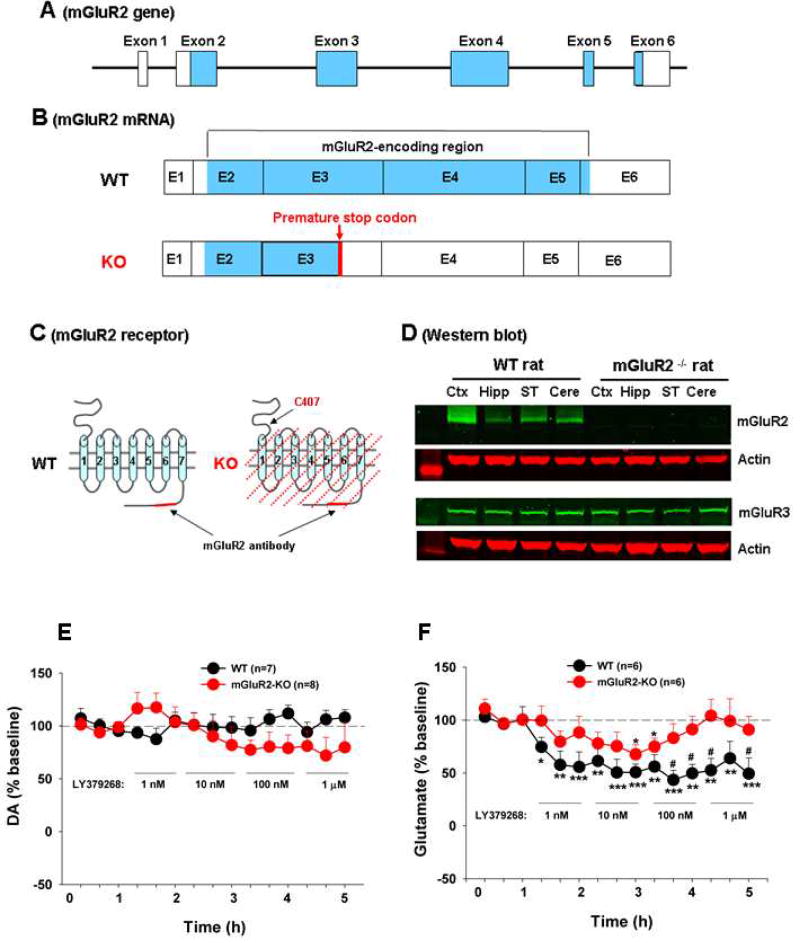
Figure 1 (A, B, C) shows the structure of the mGluR2 gene, transcripts (mRNAs), and receptor proteins in wild-type (WT) and mGluR2-knockout (mGluR2-KO) rats. The KO rats harbor a premature stop codon in exon 3 of the mGluR2 gene (Fig. 1B), causing a nonsense mutation at amino acid position C407 in the extracellular N-terminal domain and truncated mGluR2 expression (Fig. 1C). The truncated protein in mGluR2-KO rats lacks part of the extracellular N-terminal, the agonist binding site, the entire transmembrane and intracellular C-terminal domain (Fig. 1C, slashed area). Therefore, it is likely unstable and rapidly degraded. Single nucleotide polymorphism assays, conducted by Charles River Laboratories Inc. (Frederick, MD, USA), confirmed this nonsense mutation in the mGluR2 genome. Western immunoblot assays with an antibody that targets the deleted intracellular C-terminal of mGluR2 indicated lack of mGluR2 expression in the brain in mGluR2-KO rats (Fig. 1D). Given that mGluR2 shares 66% sequence homology with mGluR3 (Nakanishi et al., 1994), we also examined the expression of mGluR3, and found that mGluR3 expression is not affected in the these mGluR2-KO rats (Fig. 1D).
Figure 1.
mGluR2 receptor expression in WT and mGluR2-KO rats. (A) Rat mGluR2 genomic structure, illustrating the 6 exons and the mGluR2-encoding regions (blue color). (B) mGluR2 transcripts (mRNA) and the location of the premature stop codon that causes the termination of mGluR2 protein translation in mGluR2-knockout (mGluR2-KO) rats. (C) mGluR2 receptor structure in WT rats and the predicted receptor deletion region in mGluR2-KO rats. (D) Representative immunoblot bands, showing mGluR2 or mGluR3 expression in cerebral cortex (Ctx), hippocampus (Hipp), striatum (ST) and cerebellum (cere) of WT and mGluR2-KO rats. (E/F) In vivo microdialysis data, showing intra-NAc local perfusion of LY379268 failed to alter extracellular DA efflux (E), but significantly decreased extracellular glutamate levels in both WT and mGluR2-KO rats (F). However, the reduction in extracellular glutamate was significantly attenuated in mGluR2-KO rats than in WT rats. The data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, compared to baseline before LY379268 administration. #p<0.05, compared to mGluR2-KO rats. Also see Figure S1.
To determine whether mGluR2 mutation causes loss of mGluR2 function, we examined the effects of LY-379268, a mGluR2/3 agonist, on presynaptic DA or glutamate release in the nucleus accumbens (NAc). Figure 1-E/F shows that intra-NAc local perfusion of LY-379268 failed to alter extracellular DA (Fig. 1E, two-way ANOVA, the LY-379268 treatment main effect, F14,182 = 1.86, p > 0.05), but significantly decreased extracellular glutamate (Fig. 1F, the LY-379268 treatment main effect, F14,168 = 16.52, p < 0.001) in WT rats in a dose or concentration-dependent manner. However, this inhibitory effect on glutamate release was substantially reduced in mGluR2-KO rats (Fig. 1F, the genotype main effect, F1,13 = 6.52, p < 0.01). These data suggest that mGluR2 is selectively expressed in NAc glutamate terminals, not in DA terminals. mGluR2 deletion caused loss of mGluR2 function in modulating presynaptic glutamate release. mGluR2-KO rats are in general healthy, showing no significant differences from WT rats in fertility, feeding, and body weight. There are no significant changes in basal locomotion and locomotor response to cocaine, rotarod locomotor performance, nociceptive response to heat or morphine, or anxiety-like behavior between WT and KO rats (Fig. S1).
mGluR2 rats require more frequent infusions of cocaine to maintain drug satiety
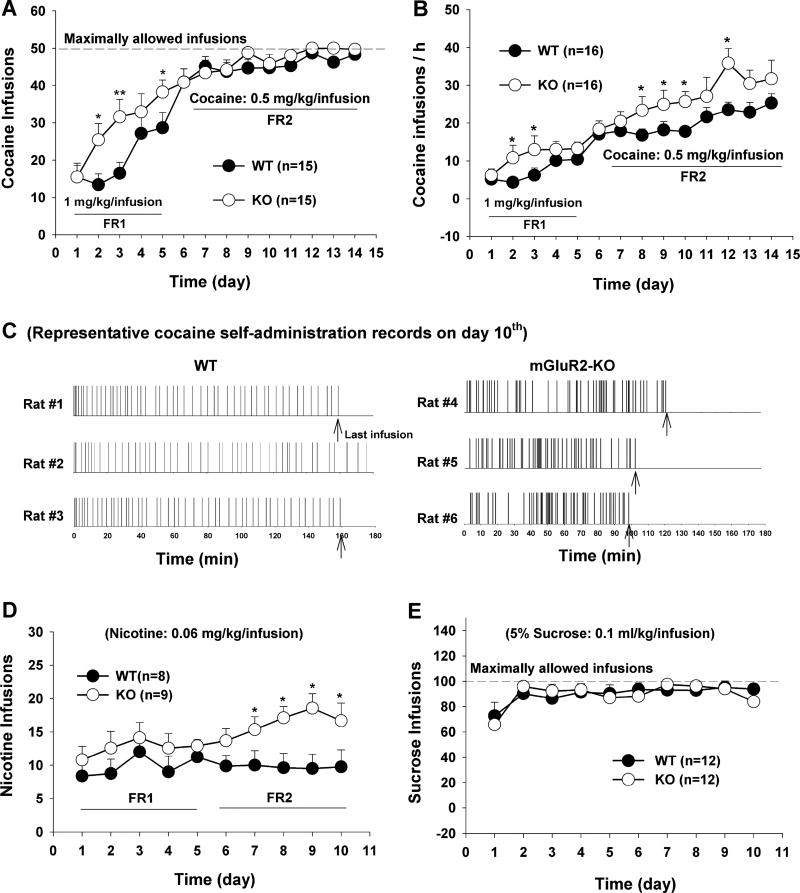
To determine whether mGluR2 deletion alters vulnerability to cocaine use, one group of WT rats (n=12) and one group of mGluR2-KO rats (n=18) were allowed to voluntarily self-administer cocaine, beginning with a dose of 1 mg/kg/infusion for 5 days under a fixed-ratio 1 (FR1) reinforcement schedule (in which one response is required for each infusion), followed by 0.5 mg/kg/infusion under a FR2 reinforcement schedule (in which 2 responses are required for each infusion). Figure 2 (A, B) shows the time course of the acquisition of cocaine self-administration, illustrating that mGluR2-KO rats display enhanced cocaine self-administration as assessed by the total number of cocaine infusions during the daily session (Fig. 2A), which lasted up to 3 hr but was ended early if 50 cocaine infusions were obtained (to prevent overdose). Two-way ANOVA for repeated measures over time revealed a significant strain (WT vs. KO) main effect (Fig. 2A: F1,30=7.28, p<0.05). Individual group comparisons revealed significant differences in the total number of cocaine infusions between WT and mGluR2-KO rats at several time points during acquisition (Fig. 2A).
Figure 2.
Acquisition and maintenance of cocaine, nicotine or sucrose self-administration in WT and mGluR2-KO rats. (A) Total numbers of cocaine infusions within a daily 3 hr session over the initial 14 days of daily cocaine self-administration. (B) Cocaine infusion rates (calculated as the total number of infusions over time spent). (C) Representative records of cocaine self-administration, illustrating that mGluR2-KO rats displayed more rapid cocaine-taking behavior with shorter inter-infusion intervals compared with WT rats. (D) Total numbers of nicotine infusions during the initial 10 days of nicotine self-administration, illustrating enhanced nicotine self-administration in mGluR2-KO rats compared to WT rats. (E) Oral sucrose self-administration during the initial 10 self-administration days, illustrating no difference between two groups of rats. The data are represented as mean ± SEM. *p< 0.05; **p<0.01; compared with WT rats. FR1, fixed-ratio 1; FR2, fixed-ratio 2.
We note that there was no difference between groups in the total number of cocaine infusions per session after the initial 5 days of cocaine self-administration. This is a ceiling effect due to the maximum of 50 cocaine infusions allowed per session, which most animals reached consistently after the 5th day of training. Therefore, we normalized the total number of cocaine infusions with respect to the amount of time spent (i.e., infusions per hr). Figure 2B shows that mGluR2-KO rats displayed a significantly higher rate of cocaine self-administration over the 14 days of training. Two-way ANOVA for repeated measures over time revealed a significant strain (WT vs. KO) main effect (Fig.2B, F1,30=24.62, p<0.001). Figure 2C shows the patterns of cocaine self-administration, illustrating that mGluR2-KO rats reached the maximal 50 infusions in a shorter period of time than WT rats (i.e., with shorter inter-infusion intervals), requiring more cocaine to reach a state of satiety and displaying a tolerance-like insensitivity to the rewarding effects of cocaine (Panlilio et al., 2003).
To study whether this reduced sensitivity to cocaine reward generalizes to other addictive drugs, we examined the effects of mGluR2 deletion on intravenous nicotine self-administration. Figure 2D shows that mGluR2-KO displayed higher rates of nicotine self-administration as compared to WT rats (F1,15=7.82, p<0.01), consistent with a reduced sensitivity to nicotine reward. In contrast to cocaine and nicotine, mGluR2 deletion did not alter oral self-administration of sucrose, a nondrug reinforcer (Fig. 2E).
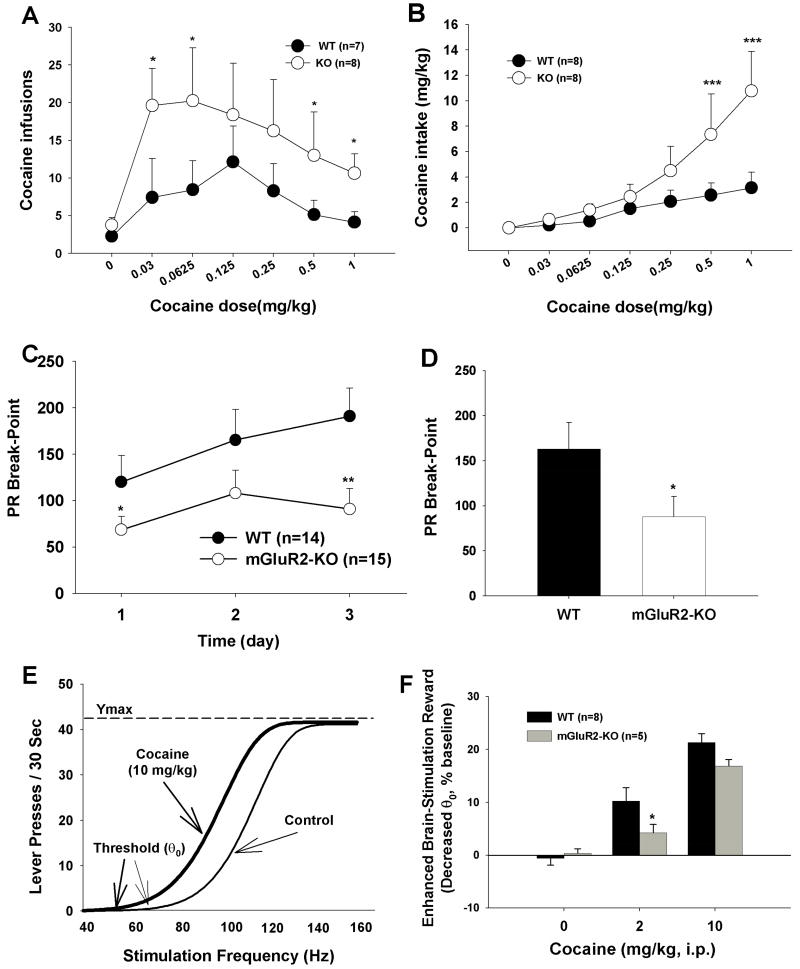
We then compared cocaine self-administration dose-response curves between KO and WT rats. Figure 3A shows that mGluR2 deletion significantly shifted the cocaine dose-response curve upward as compared to WT rats (F1,13=9.88, p<0.01). Paired comparisons between KO and WT rats revealed significant increases in cocaine self-administration in mGluR2-KO rats at several unit doses of cocaine (Fig. 3A). Figure 3B shows cumulative cocaine intake (e.g., infusion number × unit cocaine dose, mg/kg), illustrating a significant upward shift in the cocaine dose-response intake curve in mGluR2-KO rats (F1,13=9.12, p<0.01).
Figure 3.
Effects of mGluR2 deletion on cocaine self-administration and on cocaine-enhanced electrical brain-stimulation reward. (A and B) Cocaine self-administration and cocaine intake under different cocaine doses, illustrating that mGluR2 deletion shifted the cocaine dose-response curve upward. (C and D) Cocaine self-administration under progressive-ratio (PR) reinforcement conditions, illustrating a reduction in break-point for cocaine self-administration in mGluR2-KO rats compared to WT rats. (E) Representative rate-frequency curves for electrical brain-stimulation reward (BSR), indicating that cocaine (10 mg/kg i.p.) produced a leftward shift in the rate-frequency function and lowered the BSR θ0 value. (F) Mean percent changes in BSR θ0, indicating that mGluR2 deletion significantly attenuated the enhanced BSR produced by 2 mg/kg cocaine. The data are represented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001 compared with WT control group.
mGluR2 deletion attenuates cocaine reward
Drug intake under FR1 and FR2 schedules is determined largely by drug satiety and the duration of rewarding effects, but can also be affected by other factors not related to reward (Lynch and Carroll, 2001). Therefore, we obtained a more direct measure of the effects of mGluR2 deletion on the rewarding strength of cocaine by using a progressive-ratio (PR) schedule, in which the break-point (i.e., the maximal number of responses the subject will emit to obtain a reward when the response requirement is increased) indicates the efficacy of the reward in that subject (Richardson and Roberts, 1996). Figure 3C shows that mGluR2 deletion significantly lowers the break-point for cocaine self-administration (F1,27=5.88, p<0.05). Figure 3D shows the averaged breakpoint levels over the last 3 days, indicating a significant reduction in break-point in mGluR2-KO rats compared to WT rats. These findings indicate a reduced sensitivity to cocaine’s rewarding effects after mGluR2 deletion.
To obtain further evidence concerning the effects of mGluR2 deletion on the rewarding effects of cocaine, we used electrical intracranial self-stimulation (ICSS) to directly compare the effects of cocaine on brain reward function between KO and WT rats. We found that mGluR2 deletion significantly reduced the ability of cocaine to enhance brain-stimulation reward (Fig. 3F: F1,11=5.92, p<0.05, two-way ANOVA), as assessed by stimulation threshold (θ0) for maintaining ICSS behavior (Fig. 3E). Paired comparisons revealed a significant reduction in cocaine-enhanced brain-stimulation reward in mGluR2-KO rats compared to WT rats after 2 mg/kg, not 10 mg/kg, cocaine administration.
mGluR2 deletion reduces relapse to drug-seeking behavior
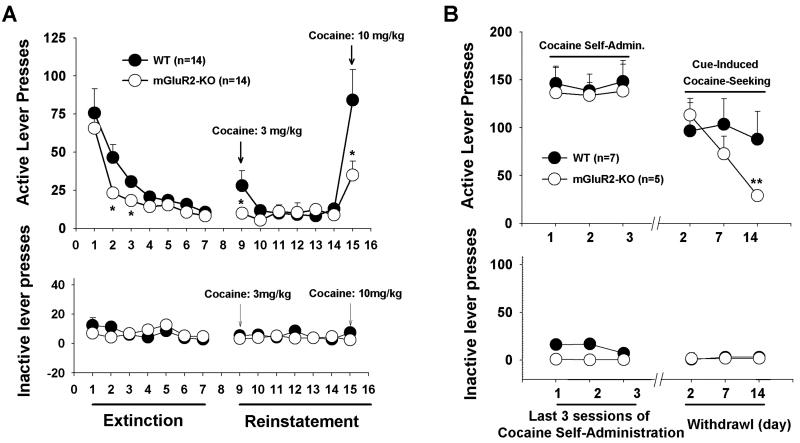
We then examined the effects of mGluR2 deletion on cocaine-induced relapse to drug-seeking after cocaine extinction. Figure 4A shows extinction responding over 2 weeks, during which active lever pressing did not produce cocaine infusions or the presentation of the cocaine-associated cue-light and tone. mGluR2-KO rats responded less (i.e., showed faster extinction) on days 2 and 3 of extinction training. After extinction criteria were met, we assessed cocaine-induced reinstatement of drug-seeking on days 9 and 15 (Fig. 4A). We found that mGluR2 deletion significantly attenuated the reinstatement of responding induced by cocaine (3, 10 mg/kg, i.p.) (Fig. 4A; F1,26=5.06, p<0.05).
Figure 4.
Cocaine- or cocaine-associated cue-induced drug-seeking behavior in WT and mGluR2-KO rats. (A) Active and inactive lever responding during extinction, illustrating lower extinction responding and lower cocaine-triggered reinstatement responding in mGluR2-KO rats. (B) Active and inactive lever responding during the last 3 sessions of cocaine self-administration and when animals were re-exposed to the previous cocaine self-administration chambers after 2, 7 or 14 days of withdrawal, illustrating a progressive reduction in cue-induced cocaine-seeking over time of withdrawal in mGluR2-KO rats. The data are represented as mean ± SEM. *p<0.05; **p<0.01 compared with WT control group.
We also compared cocaine-associated cue-induced drug-seeking in KO and WT rats after different durations of withdrawal in the home cage (i.e., ‘incubation of craving’). Figure 4B shows cue-induced drug-seeking (lever presses) after 2, 7, and 14 days of withdrawal, illustrating a progressive reduction in responding over time in mGluR2-KO rats, but not WT rats (Fig. 4B, F1,10=5.05, p<0.05). Paired comparisons revealed a significant reduction in active lever presses on day 14 in mGluR2-KO rats compared to WT rats.
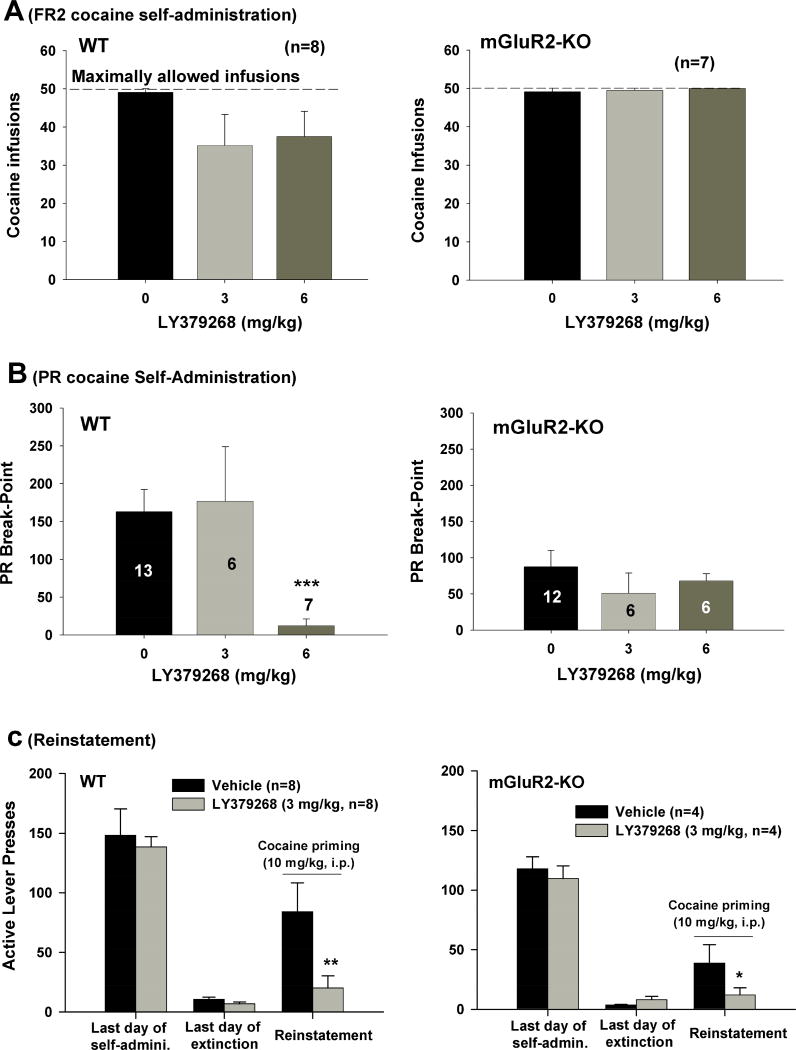
We note that the above findings that mGluR2 deletion reduces cocaine reward and relapse appears to conflict with the finding that pharmacological activation of mGluR2 with LY-379268 also inhibits cocaine self-administration and cocaine- or cue-induced reinstatement of drug-seeking behavior (Baptista et al., 2004; Justinova et al., 2016; Lu et al., 2007; Moussawi and Kalivas, 2010; Peters and Kalivas, 2006). To address this question, we observed the effects of LY-379268 on cocaine self-administration and relapse to cocaine seeking in both WT and mGluR2-KO rats. Figure 5A shows that systemic administration of LY-379268 (3, 6 mg/kg, i.p.) did not significantly alter cocaine self-administration under a fixed-ratio (FR2) schedule of reinforcement in either mGluR2-KO or WT rats. We also observed the time courses of cocaine self-administration within daily 3-h session, illustrating that pretreatment with LY379268 slightly, but not statistically significantly, decreased cocaine self-administration within the first hour of cocaine self-administration in WT rats, not in mGluR2-KO rats (Fig. S2).
Figure 5.
Effects of LY-379268 on cocaine self-administration and cocaine-induced reinstatement of drug-seeking behavior. Systemic administration of LY-379268 (3, 6 mg/kg, i.p.) failed to alter cocaine self-administration under FR2 reinforcement schedule in either WT or mGluR2-KO rats (A), but significantly inhibited cocaine self-administration under progressive-ratio (PR) reinforcement schedule in WT rats, not in mGluR2-KO rats (B). mGluR2-KO rats displayed lower reinstatement response to cocaine priming than WT rats (C). Pretreatment with LY-379268 (3 mg/kg, i.p.) produced a significant reduction in cocaine-induced reinstatement of drug-seeking behavior in both WT and mGluR2-KO rats (C). The data are represented as mean ± SEM. * p<0.05, *** p<0.001, compared to the vehicle control group. Also see Figure S2.
However, under the progressive-ratio schedule, LY-379268 pretreatment significantly decreased cocaine self-administration and lowered PR break-points in WT rats (Fig. 5B, left panel, F2,23 = 4.78, p<0.05), but not in mGluR2-KO rats (Fig. 5B, right panel, F2,21 = 0.69, p>0.05). Replicating the finding obtained earlier (Fig. 3C/D), mGluR2-KO rats also displayed a significant reduction in PR breakpoint for cocaine (in the vehicle control group), compared to WT rats (162.5 ± 20.6 versus 71.8 ± 16.2, p<0.01). Figure 5C shows the effects of LY-379268 on cocaine-induced reinstatement of drug-seeking behavior. Again, mGluR2-KO rats displayed significantly lower reinstatement to drug seeking behavior than WT rats. However, systemic administration of LY-379268 (3 mg/kg, i.p.) significantly inhibited cocaine-induced reinstatement in both WT (F1,14 = 4.02, p<0.01) and mGluR2-KO (F1,6 = 3.61, p<0.05) rats.
mGluR2 deletion attenuates DA/glutamate response to cocaine
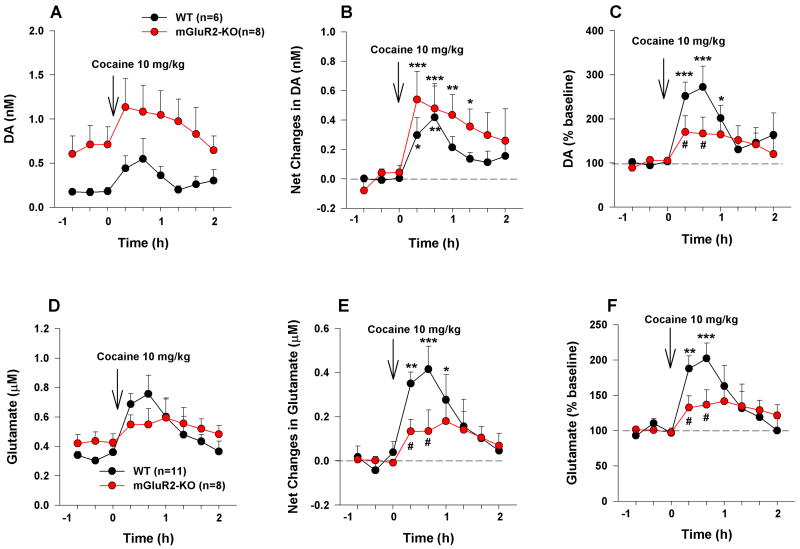
It is well documented that cocaine-induced reinstatement of drug-seeking behavior is associated with increased extracellular DA and glutamate in the NAc (Kalivas and Volkow, 2005; Wise and Koob, 2014; Xi et al., 2006a). Therefore, we further compared NAc DA/glutamate responses to cocaine in rats during reinstatement testing. Figure 6 shows microdialysis data, illustrating that basal levels of extracellular DA (before cocaine administration) are significantly higher in mGluR2 KO rats than in WT rats. Cocaine priming produced a significant increase in extracellular DA in both KO and WT rats. Since basal levels of extracellular DA were higher in KO rats, we calculated cocaine-induced net increases in extracellular DA (nM) by subtracting the basal level of DA from the DA levels after cocaine administration (Fig. 6B). We found that mGluR2 deletion did not significantly alter the absolute amount by which cocaine increased DA (Fig. 6B, F1,12=1.82, p>0.05). However, when cocaine-induced increases in DA were normalized by dividing the post-cocaine level by the basal pre-cocaine level (% baseline), we found that mGluR2 deletion significantly reduced the relative amount of cocaine-induced change in DA level (Fig. 6C: F1,12=4.43, p<0.05). To confirm these findings, we repeated the same experiments with a lower dose of cocaine (3 mg/kg instead of 10 mg/kg (Fig. S3-A), in view of the fact that low unit doses of cocaine were used in the above-mentioned cocaine self-administration experiments. With this lower dose, we found again that mGluR2 deletion failed to alter the amplitude (or net increase) of cocaine-enhanced extracellular DA (Fig. S3-B), but significantly decreased the % change in cocaine-enhanced extracellular DA in mGluR2-KO rats (which again had higher basal levels of extracellular DA) (Fig. S3-C).
Figure 6.
Cocaine-induced changes in extracellular DA and glutamate in WT and mGluR2-KO rats measured during reinstatement testing. (A, B, and C) Cocaine-induced changes in extracellular DA, expressed as DA concentrations (nM) (A), net changes (postcocaine DA – precocaine DA, nM) (B), or percent of precocaine baseline (C). (D, E, and F) Cocaine-induced changes in extracellular glutamate, expressed as glutamate concentrations (µM) (D), net changes (postcocaine glutamate – precocaine glutamate, µM) (E), or percent of precocaine baseline (F). The data are represented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001 compared with precocaine baseline. #p<0.05 compared with WT rats. Also see Figures S3–S4.
We also analyzed extracellular glutamate in the same dialysis samples. We found that KO and WT rats did not differ in basal levels of glutamate (Fig. 6D, Fig. S4), but mGluR2 deletion significantly attenuated cocaine-induced net changes in extracellular glutamate (Fig. 6E, F1,12=5.11, p<0.05; Fig. S3-E) and in cocaine-induced % changes in extracellular glutamate over pre-cocaine baseline (Fig. 6F, F1,12=4.55, p<0.05; Fig. S3-F).
mGluR2 deletion elevates basal levels of extracellular DA and glutamate
Although the basal levels of extracellular DA or glutamate shown in Figure 5 (and Suppl. Fig. S1) appear higher in mGluR2-KO rats than in WT rats, this difference was not statistically significant. This may be related to the fact that DA (or glutamate) levels in the basal dialysate samples are very low and also quite variable across subjects. Therefore, we used no-net-flux microdialysis, a more sensitive and reliable microdialysis method (Hershey and Kennedy, 2013; Xi et al., 2003), to quantitatively measure and compare basal levels of extracellular DA and glutamate in both groups of rats. For this, a series of different concentrations of DA or glutamate (added to the dialysis buffer) were locally perfused into the NAc. In theory, when DA or glutamate levels in the dialysis probe are equivalent to extracellular DA or glutamate in the brain, no net DA or glutamate flux occurs (i.e., the influx and efflux across the dialysis membrane are equivalent). Figure S4 shows basal levels of extracellular DA or glutamate as measured using no-net-flux microdialysis [i.e., when Y-axis value (DAinput - DAoutput) = 0], illustrating a significantly higher basal level of extracellular DA in mGluR2-KO rats (2.11 ± 0.25 nM) than in WT rats (1.22 ± 0.19 nM) (Fig. S4-A: p<0.05). Similarly, basal levels of glutamate were also significantly higher in mGluR2-KO rats (0.63 ± 0.08 µM) than in WT rats (0.36 ± 0.072 µM) (Fig. S4-B: p<0.05).
Cocaine self-administration down-regulates NR2B expression in mGluR2-KO rats
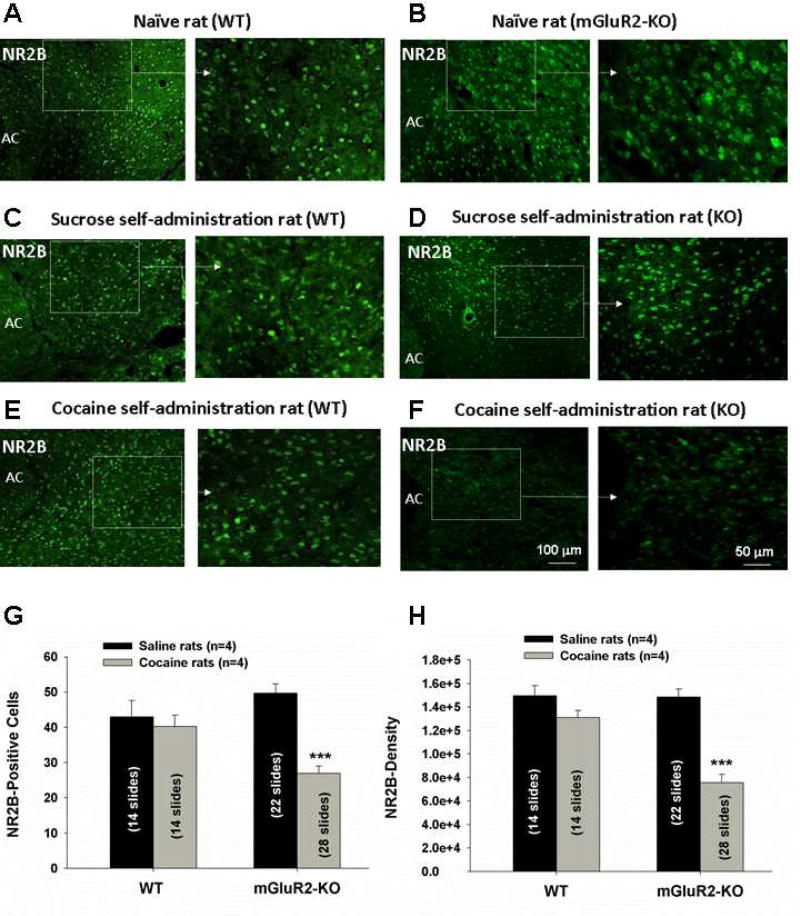
We next investigated whether a similar DA/glutamate deficiency also underlies the reductions in cue-induced cocaine-seeking behavior observed in mGluR2 KO rats (Fig. 4) Unfortunately, the changes in extracellular DA and glutamate induced by cocaine-associated cues were too small to be detected by in vivo microdialysis in our pilot study. We therefore used immunostaining methods to compare NMDA receptor (NR2A and NR2B subunits) expression in the NAc between two groups of rats based on a recent finding that incubation of cocaine craving is associated with the generation of NR2B-rich silent synapse in the NAc (Huang et al., 2015; Lee et al., 2013). Figure S5 shows that the majority of NR2B-immunostaining cells are positive for DARPP-32, a neuronal marker of medium-spiny neurons in the striatum (Borgkvist and Fisone, 2007), suggesting a postsynaptic distribution of NR2B. mGluR2 deletion did not significantly alter basal NR2A (Fig. S6) or NR2B (Fig. 7A–B) expression in the NAc, measured 14 days after withdrawal from the last session of cocaine self-administration. However, cocaine self-administration significantly down-regulated expression of NR2B (Fig. 7F), but not NR2A (Fig. S6), in mGluR2-KO rats (Fig. 7F). In contrast, cocaine self-administration did not affect NR2B or NR2A expression in WT rats (Fig. 7E; Fig. S6). This reduction in NR2B expression in mGluR2 KO rats is cocaine-specific since oral sucrose self-administration did not alter NR2B expression in either WT rats (Fig. 7C) or KO rats (Fig. 7D). Quantitative assays of NR2B-immunostaining indicate a significant reduction in the total number of NR2B-positive cells (Fig. 7G) and in the density of NR2B-immunostaining (Fig. 7H) in mGluR2-KO rats, compared to WT rats.
Figure 7.
NR2B-immunostaining in the NAc. (A and B), NR2B-immunostaining in naïve rats; (C and D), NR2B-immunostaining in rats 14 days after sucrose self-administration; (E and F), NR2B-immunostaining in rats 14 days after cocaine self-administration. (G), Mean numbers of NR2B-positive striatal neurons per field under 20 × magnification; (H) Mean densities of NR2B-immunostaining per neuron in WT and mGluR2-KO rats 14 days after cocaine self-administration. The data are represented as mean ± SEM. ***P < 0.001 compared with saline control rats. AC: Anterior commissure. Also see Figures S5–S6.
Discussion
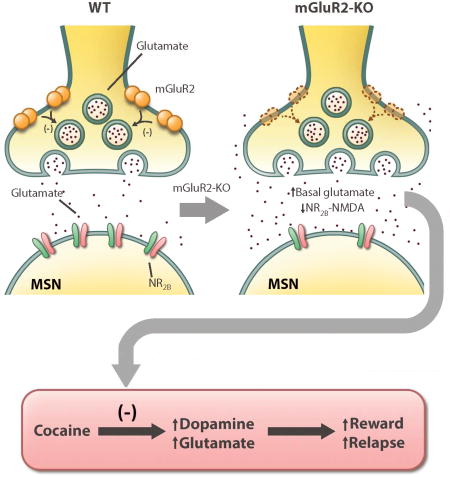
The major findings of the present study are that mGluR2 deletion increased rates of cocaine self-administration when the response requirement was low, decreased cocaine self-administration when the response requirement was progressively increased, and attenuated relapse to cocaine-seeking behavior. All of these behavioral effects can be explained by a reduction in the rewarding efficacy of cocaine. That is, high rates of cocaine intake might compensate for reduced rewarding effects of low doses, and decreased intake at high response requirements suggests that mGluR2 KO rats will not pay as high a “price” as WT rats to obtain cocaine (Hursh and Silberberg, 2008). The attenuated relapse to drug-seeking behavior was accompanied by a reduction in DA/glutamate response to cocaine after cocaine extinction and a reduction in NR2B expression in the NAc in the same rats after cocaine withdrawal, suggesting a protective effect against relapse to drug-seeking behavior.
Vulnerability to drugs of abuse depends upon multiple factors – including the biological state and trait of the individual, the environment, and age (Belin and Deroche-Gamonet, 2012; Everitt et al., 2008; Homberg et al., 2014). Currently prevailing theories on the etiology of drug abuse conceptualize addiction as an executive function/inhibitory control deficit (Volkow and Morales, 2015), increased incentive salience attributed to drug-related stimuli (Singer et al., 2016), a compulsive habit (Everitt et al., 2008), and a hyperactive stress system and removal of negative reinforcement (Koob, 2015). Since DA is critically involved in multiple aspects of drug abuse and addiction, the majority of studies seeking to identify biomarkers for vulnerability to drug abuse have focused on the mesocorticolimbic DA system (Sagheddu and Melis, 2015; Volkow et al., 2009). For example, lower D2/3 receptor availability in the striatum has been considered a risk factor that may influence susceptibility to continued drug abuse and addiction (Song et al., 2012; Volkow et al., 2002). By the use of mGluR2-KO rats in the present study, we found that genetic deletion of a presynaptic glutamate autoreceptor – mGluR2, significantly provokes naïve rats to rapidly acquire self-administration and to take more cocaine when the price is low, suggesting that mGluR2 loss may be an important risk factor for the initial development of cocaine abuse. This finding is consistent with recent reports that alcohol-dependent patients display a significant reduction in mGluR2 expression in the anterior cingulate cortex (Meinhardt et al., 2013) and that rats selectively bred for having high intake of alcohol lack mGluR2 expression (Zhou et al., 2013).
How does mGluR2 deletion alter cocaine self-administration? The present findings support the hypothesis that mGluR2 deletion attenuates cocaine’s rewarding effects, which leads to compensatory increases in drug intake when the price is low. First, under a fixed-ratio 2 schedule mGluR2 deletion shifted the cocaine self-administration dose-response curve upward. Since the majority of cocaine doses used in self-administration are located on the descending limb of the inverted “U”-shaped dose-response curve, where the rate of self-injection is inversely related to the duration of satiating effects, an upward shift in the cocaine self-administration curve is reasonably interpreted as a reduction in cocaine reward (Song et al., 2012). Second, mGluR2 deletion decreased break-point for cocaine self-administration when the “price” or work required was progressively increased, indicating a reduced sensitivity to cocaine reward (Richardson and Roberts, 1996). Third, mGluR2 deletion decreased cocaine-enhanced electrical brain-stimulation reward, consistent with cocaine having a reduced effect on reward circuitry. Fourth, mGluR2 deletion decreased cocaine’s effects on DA in the NAc, a neurotransmitter and brain region critically involved in drug reward (Wise, 2008).
Another important finding in the present study is that mGluR2 deletion decreased the likelihood of relapse to drug-seeking. This was indicated by reduced cocaine-seeking responses during extinction, lower levels of reinstatement when rats were re-exposed to cocaine, and progressively lower levels of cue-induced drug-seeking when rats were tested after increasing amounts of time spent with no access to cocaine or the self-administration context. This decreased relapse response is fully consistent with the finding that mGluR2-KO rats are less persistent in seeking cocaine under the PR schedule, further supporting the hypothesis that cocaine is less rewarding and addictive after mGluR2 deletion.
A possible mechanism for the blunted effects of cocaine in these animal models of reward and relapse is that cocaine priming was less effective in elevating extracellular DA and glutamate levels in the NAc in the mGluR2-KO rats. Since cocaine-enhanced extracellular DA and glutamate are positively correlated with reinstatement responding to cocaine (Gipson and Kalivas, 2014), a reduction in cocaine-enhanced extracellular DA and glutamate may directly lead to a reduction in relapse to drug-seeking behavior. Furthermore, mGluR2-KO rats displayed higher basal levels of extracellular DA and glutamate, which may also contribute to attenuated relapse since drug craving and relapse have been shown to be associated with a reduction in basal levels of extracellular DA and glutamate or in basal glutamate transmission in the NAc after chronic cocaine administration (Kalivas, 2009; Thomas and Malenka, 2003). Accordingly, elevation of extracellular DA and glutamate observed in mGluR2-KO rats may normalize reduced basal levels of extracellular DA and glutamate, upon chronic exposure to cocaine, and might therefore relieve drug craving that drives relapse to drug-seeking behavior. Lastly, cocaine self-administration significantly decreased NR2B-containing NMDA receptor expression in the NAc of mGluR2-KO rats, suggesting that attenuated NMDA receptor-mediated synaptic transmission may also contribute to the reduction in drug- or cue-induced drug-seeking behavior. We note the previous reports that cocaine self-administration up-regulates NR2B expression in the NAc (Ben-Shahar et al., 2009; Hemby et al., 2005). However, we did not see significant changes in NR2A or NR2B expression in WT rats. One possibility is that cocaine-induced changes in NR2B expression recovered after 24 hours of withdrawal from the last cocaine self-administration.
The mechanism(s) through which cocaine self-administration down-regulates NR2B expression in mGluR2-KO rats has not been elucidated. One hypothesis is that enhanced basal extracellular glutamate, combined with cocaine-enhanced glutamate release, causes NMDA receptor internalization and down-regulation in mGluR2-KO rats.
mGluR2 and mGluR3 are highly expressed in presynaptic glutamate terminals (Testa et al., 1998) and functionally act as glutamate autoreceptors regulating presynaptic glutamate release (Baker et al., 2002; Xi et al., 2002a). This is further supported by the finding in the present study that intra-NAc local perfusion of the mGluR2 agonist LY-379268 significantly lowered extracellular glutamate levels in WT rats, but not in mGluR2-KO rats. We note that mGluR2 deletion did not completely block the effects of LY-379267 on extracellular glutamate. This may be related to the fact that LY-379268 is a dual mGluR2 and mGluR3 agonist. Hence, the effects of LY-379268 observed in mGluR2-KO mice are most likely mediated by activation of mGluR3 receptor. In contrast to glutamate, little is known about how mGluR2 deletion elevates basal levels of extracellular DA. mGluR2/3s are not found in midbrain DA neurons or striatal DA terminals (Golembiowska et al., 2002). This is also supported by our finding that intra-NAc local perfusion of LY-379268 failed to alter extracellular DA in the NAc. Therefore, it is unlikely that mGluR2 directly modulates presynaptic DA release. It was recently reported that a subpopulation (~30%) of midbrain DA neurons co-release DA and glutamate in the striatum (Berube-Carriere et al., 2009; Yamaguchi et al., 2015). Thus, one possibility is that mGluR2 is also expressed in such dual phenotype (glutamate-DA) neurons, and therefore mGluR2 deletion causes an increase in both DA and glutamate via a disinhibition mechanism. Another possibility is that enhanced extracellular glutamate in mGluR2-KO rats may stimulate DA release directly by activation of glutamate receptors on VTA DA neurons or their terminals or indirectly by other neural circuits based upon the global nature of the mGluR2 deletion.
We note that the present finding that cocaine is less rewarding and less addictive in mGluR2-KO rats appears opposite to the finding mGluR2 deletion in mice enhanced some behavioral effects of cocaine (locomotion, conditioned place preference) (Morishima et al., 2005). The precise reasons for such conflicting findings are unclear. It may be related to different species (rats vs. mice), different drug use history (cocaine self-administration rats vs. drug naïve mice), and different basal levels of extracellular DA and glutamate that are well characterized in mGluR2-KO rats in the present study, but not characterized in mGluR2-KO mice (Morishima et al., 2005).
We also note that the above finding that mGluR2 deletion causes a reduction in cocaine reward and relapse might appear to conflict with the pharmacological action produced by LY-379268. As we have demonstrated above, the reductions in cocaine reward and relapse to cocaine-seeking behavior in mGluR2 KO rats is largely due to increases in basal levels of extracellular DA and glutamate that subsequently blunts cocaine-induced (%) changes in extracellular DA or glutamate. In contrast, LY-379268-induced reductions in cocaine self-administration and reinstatement are mediated by direct activation of presynaptic mGluR2 that blocks cocaine- or nicotine-induced increases in extracellular DA and glutamate (Bauzo et al., 2009; D’Souza et al., 2011; Moussawi and Kalivas, 2010; Xi et al., 2010).
In conclusion, in the present study we systematically examined the role of mGluR2 in animal models of the acquisition, maintenance and extinction of cocaine self-administration, and in the reinstatement of cocaine-seeking behavior. We found that mGluR2 deletion significantly increased cocaine intake when the price (i.e., response requirement) was low, most likely as a compensatory response to reduced cocaine reward. Also consistent with reduced sensitivity to cocaine reward, mGluR2-KO rats stopped seeking cocaine sooner than WT rats when the price was increased or when cocaine was unavailable, and they also showed attenuated relapse to drug-seeking behavior, attenuated DA/glutamate response to cocaine after extinction, and attenuated postsynaptic NR2B expression after cocaine withdrawal. Taken together, these findings suggest that reduced mGluR2 availability diminishes cocaine reward such that intake might be high (to compensate) when drug is freely available, but addiction and relapse might actually be less likely to occur because drug seeking ceases more easily when the price is high or the drug is unavailable.
Experimental Procedures
Animals
Male wild-type (WT) and mGluR2-KO rats with Wistar strain genetic background (250–300 g) were bred at the National Institute on Drug Abuse (NIDA) from three mGluR2+/− breeding pairs purchased from Transposagen Biopharmaceutical Inc. They were individually housed in a climate-controlled room on a reverse light–dark cycle with ad libitum access to food and water. Genotyping was performed by Charles River Laboratories. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the US National Institutes of Health.
Intravenous cocaine or nicotine self-administration
Intravenous catheterization surgery and cocaine self-administration have been described previously (Xi et al., 2006a). In brief, after 5–7 d of recovery from i.v. surgery, each rat was placed into a standard operant chamber and allowed to lever-press for i.v. cocaine (1 mg/kg/infusion) self-administration under a fixed-ratio 1 (FR1) reinforcement schedule first and then switched to a low dose of cocaine (0.5 mg/kg/infusion) under FR2 reinforcement schedule until stable day-to-day self-administration was established. The self-administration patterns, total lever-pressing numbers, and rates of cocaine infusion and lever responses were compared in WT and mGluR2-KO rats. The effects of systemic administration of LY-379268 on cocaine self-administration under FR2 reinforcement were evaluated.
After stable cocaine self-administration was achieved, additional groups of animals were used to compare the break-point levels under progressive-ratio reinforcement, the dose-response self-administration curves, extinction response, cocaine-induced reinstatement of drug-seeking behavior, and cue-induced cocaine-seeking behavior after different periods of time of withdrawal between WT and mGluR2-KO rats. More details about the experimental methods are provided in the Supplement.
Intracranial electrical brain-stimulation reward
The general procedures for electrical brain-stimulation reward (BSR) were as we have reported previously (Xi et al., 2006b; Xi et al., 2008). Briefly, rats were anesthetized under sodium pentobarbital (65 mg/kg i.p.), and unilateral monopolar stainless-steel stimulating electrodes (Plastics One, Roanoke, VA, USA) were surgically placed into the lateral hypothalamus (AP −2.56, ML ±1.9, and DV −8.6). After 7 days of recovery from surgery, rats were allowed to self-train (autoshape) to lever-press for rewarding BSR. Following establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. Throughout the experiment, animals were run for 3 sessions per day. Since lever-pressing behavior was variable during the first session (the “warm up” session), but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively.
The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. Ymax was defined as the maximal rate of response. The BSR threshold (θ0) and Ymax were mathematically derived for each baseline run and each test session run by analyzing each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies using best-fit mathematical algorithms as reported previously (Xi et al., 2008). Once stable baseline θ0 and Ymax values were achieved (<10% variation over 5 continuous days), the effects of cocaine (2, 10 mg/kg, i.p.) on BSR were assessed. After each test, animals received an additional 5–7 days of BSR re-stabilization until a new baseline θ0 was established. The order of testing for various doses of cocaine was counterbalanced. The effect of cocaine on BSR was compared between WT and mGluR2-KO rats.
In vivo microdialysis
Intracranial guide cannula implantation surgery and in vivo microdialysis procedures were as described previously (Xi et al., 2002a). After 7 d of recovery from surgery, a microdialysis probe (SciPro) was inserted into the nucleus accumbens (NAc) at least 12 h before experiments began, to minimize damage-induced dopamine (DA) release during experimentation. On the experimental day, dialysis buffer was perfused through the probe (2.0 µL/min) via a syringe pump (Bioanalytical Systems) starting at least 2 h before sample collection. To prevent DA degradation, dialysis samples were collected every 20 min into 10 µL of 0.5 M perchloric acid. After 1 h of baseline collection, animals received vehicle, cocaine (3, 10 mg/kg i.p.). In the no-net flux microdialysis experiment, a series of different concentrations of DA (0.1, 0.5, 2, 4 nM) or glutamate (2, 4, 8 µM) was locally perfused into the NAc (1 hour each concentration). Additional groups of rats were used to study the effects of LY-379268 on basal levels of extracellular DA and glutamate in the NAc. After collection, all samples were frozen at −80 °C until analysis. DA and glutamate in the dialysis samples were measured with an electrochemical detection system and fluorescence spectrophotometer, respectively.
Immunoblot and Immunohistochemistry Assays
We used a Western blot assay to compare mGluR2 and mGluR3 expression in the cortex, striatum, hippocampus and cerebellum between WT and mGluR2-KO rats. We then used fluorescent immunochemistry to compare expression of NR2A and NR2B in the NAc. Methods are described in detail in the Supplement.
Data Analysis
All data are presented as mean ± SEM. One-way ANOVA for repeated measures was used to analyze the difference between WT and mGluR2-KO rats in terms of break-point, cocaine-enhanced brain-stimulation reward, basal levels of extracellular DA and glutamate, and in terms of density/cell count of NR2B-immunostaining. Two-way ANOVA for repeated measures over time was used to analyze the difference between WT and mGluR2-KO rats with respect to drug-taking and drug-seeking and locomotor and extracellular DA responses to cocaine. Paired comparisons between KO and WT groups were carried out using the Student–Newman–Keuls method.
Supplementary Material
Highlights.
mGluR2 deletion reduces sensitivity to cocaine reward
mGluR2 deletion causes an increase in cocaine self-administration
mGluR2 deletion causes a reduction in relapse to drug-seeking behavior
mGluR2 deletion causes a reduction in dopamine and glutamate responses to cocaine
Acknowledgments
This work was supported by National Institute on Drug Abuse, Intramural Research Program (Z1A DA000389). We thank Dr. Eliot L. Gardner for his full support of this research project and Drs. Amy Newman, Leigh Panlilio and Eliot L. Gardner for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information includes Supplemental Experimental Procedures, Supplemental References, and 6 Supplemental figures.
Author Contributions:
HJY, HYZ, GHB, YH, and JTG carried out the experiments and analyzed data. HJY and ZXX designed the experiments and wrote the manuscript.
The authors declare no competing financial interest conflict.
References
- Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. The American journal of drug and alcohol abuse. 2003;29:691–712. doi: 10.1081/ada-120023465. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacology, biochemistry, and behavior. 2009;94:204–210. doi: 10.1016/j.pbb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. The Journal of comparative neurology. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neuroscience and biobehavioral reviews. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Liechti ME, Ramirez-Nino AM, Kuczenski R, Markou A. The metabotropic glutamate 2/3 receptor agonist LY379268 blocked nicotine-induced increases in nucleus accumbens shell dopamine only in the presence of a nicotine-associated context in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2111–2124. doi: 10.1038/npp.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain : a journal of neurology. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kalivas PW. More cocaine-more glutamate-more addiction. Biological psychiatry. 2014;76:765–766. doi: 10.1016/j.biopsych.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Konieczny J, Ossowska K, Wolfarth S. The role of striatal metabotropic glutamate receptors in degeneration of dopamine neurons: review article. Amino acids. 2002;23:199–205. doi: 10.1007/s00726-001-0129-z. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain research. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey ND, Kennedy RT. In vivo calibration of microdialysis using infusion of stable-isotope labeled neurotransmitters. ACS chemical neuroscience. 2013;4:729–736. doi: 10.1021/cn300199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Karel P, Verheij MM. Individual differences in cocaine addiction: maladaptive behavioural traits. Addiction biology. 2014;19:517–528. doi: 10.1111/adb.12036. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schluter OM, Dong Y. Silent Synapses Speak Up: Updates of the Neural Rejuvenation Hypothesis of Drug Addiction. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2015;21:451–459. doi: 10.1177/1073858415579405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Le Foll B, Redhi GH, Markou A, Goldberg SR. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology. 2016;233:1791–1800. doi: 10.1007/s00213-015-3994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Molecular psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Koob GF. The dark side of emotion: the addiction perspective. European journal of pharmacology. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nature neuroscience. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biological psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Experimental and clinical psychopharmacology. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. European journal of pharmacology. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Masu M, Bessho Y, Nakajima Y, Hayashi Y, Shigemoto R. Molecular diversity of glutamate receptors and their physiological functions. Exs. 1994;71:71–80. doi: 10.1007/978-3-0348-7330-7_8. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology. 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Rup K, Pomierny B, Niedzielska E, Kalivas PW, Filip M. Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharmacology & therapeutics. 2014;142:281–305. doi: 10.1016/j.pharmthera.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behavioural brain research. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Sagheddu C, Melis M. Individual differences and vulnerability to drug addiction: a focus on the endocannabinoid system. CNS & neurological disorders drug targets. 2015;14:502–517. doi: 10.2174/1871527314666150225143748. [DOI] [PubMed] [Google Scholar]
- Singer BF, Guptaroy B, Austin CJ, Wohl I, Lovic V, Seiler JL, Vaughan RA, Gnegy ME, Robinson TE, Aragona BJ. Individual variation in incentive salience attribution and accumbens dopamine transporter expression and function. The European journal of neuroscience. 2016;43:662–670. doi: 10.1111/ejn.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Le Moal M. Individual vulnerability to addiction. Annals of the New York Academy of Sciences. 2011;1216:73–85. doi: 10.1111/j.1749-6632.2010.05894.x. [DOI] [PubMed] [Google Scholar]
- Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. The Journal of comparative neurology. 1998;390:5–19. [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2003;358:815–819. doi: 10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity research. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. The Journal of pharmacology and experimental therapeutics. 2002a;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006a;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. Journal of neurochemistry. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006b;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. The Journal of pharmacology and experimental therapeutics. 2002b;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng XQ, Gardner EL. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology. 2009;203:501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. The European journal of neuroscience. 2015;41:760–772. doi: 10.1111/ejn.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, et al. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16963–16968. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.