Summary
It has been appreciated for nearly 100 years that cancer cells are metabolically distinct from resting tissues. More recently understood is that this metabolic phenotype is not unique to cancer cells but instead reflects characteristics of proliferating cells. Similar metabolic transitions also occur in the immune system as cells transition from resting state to stimulated effectors. A key finding in immune metabolism is that the metabolic programs of different cell subsets are distinctly associated with immunological function. Further, interruption of those metabolic pathways can shift immune cell fate to modulate immunity. These studies have identified numerous metabolic similarities between cancer and immune cells, but also critical differences that may be exploited and affect treatment of cancer and immunological diseases.
Introduction
A fundamental requirement for all cells is an ability to obtain and metabolize nutrients to meet basic survival and biosynthetic demands. While cell survival requires efficient energy generation, the metabolic demands of cell proliferation and differentiation can be strikingly different and cells must tightly regulate metabolic pathways accordingly. This has been most notably examined in cancer and immune cells over the past decade. Cell proliferation requires the biosynthesis of nucleotides, lipids and proteins and the generation of reducing power and energy. Elevated uptake of glucose and lactate secretion were the first described metabolic hallmarks of tumors and now form the basis for staging various solid malignancies using 18F-fluorodeoxyglucose (FDG) Positron Emission Tomography (Cori and Cori, 1925; Warburg et al., 1927; Zhu et al., 2011). Cancer metabolism has emerged as a major discipline and revealed new mechanisms and roles for metabolites and metabolic pathways (Pavlova and Thompson, 2016). What has also become apparent is that elevated glucose uptake and rewired cellular metabolism is not only a hallmark of tumors, but a feature of normal proliferating immune and endothelial cells (Cruys et al., 2016; Rathmell et al., 2000; Wang et al., 1976) as well as metabolically active, yet slowly-proliferating macrophages and dendritic cells (O’Neill et al., 2016).
The metabolic and signaling pathways of immune and cancer cells now provide a window to understand metabolic regulation of cell fate. However, while cancer cells are mutated and dysregulated, immune cells follow specific programs as part of normal protections from invading pathogens. The loss of normal physiological regulation in cancer cells comes at a cost of balancing cell growth with the stress of maintaining sufficient nutrient uptake and metabolism. In contrast, immune cells are guided by orchestrated and balanced metabolic programs to support their function. Thus, while cancer cells may display limited metabolic flexibility, immune cells may be highly flexible. However, the coupling of metabolism to transcriptional and signaling programs renders the specific function of immune cells highly dependent on specific metabolic pathways. Here we assess similarities and distinctions between cancer and immune cell metabolism.
Metabolic requirements of cell proliferation
Cell proliferation and anabolic metabolism require the coordinated action of many pathways. Oncogenic mutations and inflammatory activation signals both lead to aerobic glycolysis to accomplish this goal (Pavlova and Thompson, 2016). Aerobic glycolysis is characterized by increased glucose and glutamine uptake and high rates of glycolysis and secretion of lactate even in the presence of oxygen. This metabolic program allows metabolic intermediates to be siphoned off for biosynthesis, but also requires high levels of nutrient input and anaplerosis, or filling of metabolic pathways as metabolites are re-routed into biosynthesis. While inefficient at generating ATP, aerobic glycolysis has been proposed to be well-suited for proliferation. In immune cells, however, cell division is not always an outcome and variations on this program also support distinct immune effector functions.
Glucose utilization, glycolysis and the pentose phosphate pathway
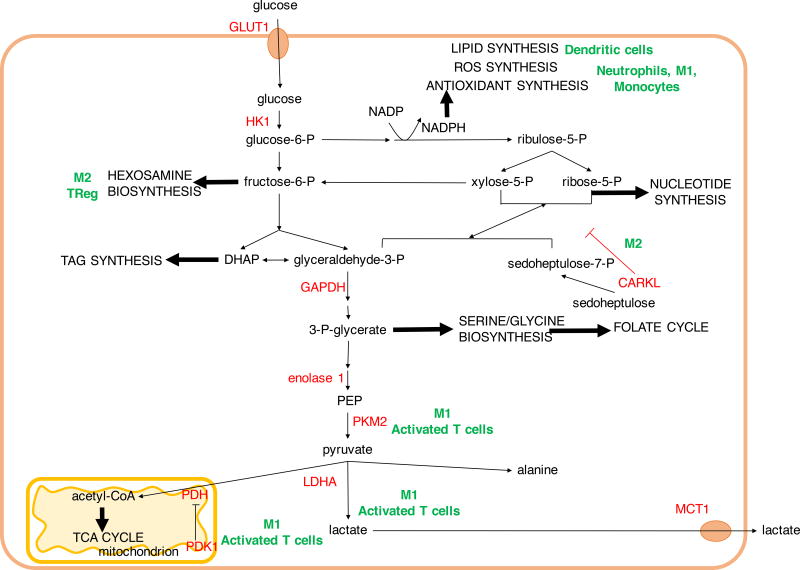
Glucose typically enters the cell via facilitated transport by GLUT family of transporters to initiate glucose metabolism. GLUT proteins can be overexpressed in cancer (Blanco Calvo et al., 2010; Carvalho et al., 2011; Isselbacher, 1972; Krzeslak et al., 2012) and GLUT1 is rapidly upregulated upon T-cell and macrophage activation in an inflammatory setting and contributes towards their glycolytic phenotype (Freemerman et al., 2014; Macintyre et al., 2014a). Glucose then becomes phosphorylated by hexokinases to glucose-6-phosphate (G6P), to be further metabolized in glycolysis or the pentose phosphate pathway (PPP) (Figure 1). Through the PPP, G6P can generate ribose-5-phosphate for the ribose backbone component of nucleotides and NADPH, which plays critical roles in biosynthesis of lipids, nucleic acids and antioxidants. Increased expression of non-oxidative branch PPP enzymes TALDO and TKTL1 (Langbein et al., 2006; Wang et al., 2011a) has been associated with increased tumor metastasis and poor prognosis. Classically activated “M1” macrophages and neutrophils contain also high levels of PPP pathway enzymes, and require abundant NADPH to produce superoxide during respiratory bursts to eliminate extracellular bacteria (Forman and Torres, 2002; Oren et al., 1963; Schnyder, 1978). NADPH is also used in the synthesis of antioxidants glutathione and thioredoxin, which can limit the oxidative damage to phagocytes following respiratory burst (Maeng et al., 2004). PPP flux can be limited by the seduheptalose kinase, carbohydrate kinase-like protein (CARKL), which has been found to be elevated in alternatively activated “M2” macrophages (Haschemi et al., 2012). Inhibiting CARKL increases PPP flux and promotes M1 macrophage polarization (Haschemi et al., 2012). PPP-derived NADPH is also essential for de novo fatty acid synthesis in stimulated dendritic cells, to expand the endoplasmic reticulum and Golgi body for effector cytokine secretion (Everts et al., 2014).
Figure 1. Glycolysis and pentose phosphate pathway (PPP) in biosynthesis and immune function.
Glycolysis and the PPP provide biosynthetic intermediates and reducing power for cell growth and proliferation. In immune cells, select branches provide key metabolites for immune function such as reducing power for the synthesis of reactive oxygen species (ROS) and antioxidants in phagocytic cells and for phospholipid synthesis in dendritic cells. In contrast, suppression of PPP by carbohydrate kinase-like protein (CARKL) induces an anti-inflammatory M2 macrophage phenotype. Hexosamine biosynthesis pathway provides substrates for glycosylation of lipids and proteins important for Treg and M2 macrophage lineages. In rapidly proliferating cells such as T cells and M1 macrophages, glycolysis-derived pyruvate is reduced to lactate to regenerate NAD+ and exported out of the cell. DHAP, dihydroxyacetone phosphate; GLUT1, glucose transporter 1; MCT1, monocarboxylate transporter 1; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PEP, phosphoenol pyruvate; PKM2, pyruvate kinase M2; TAG, triacyl glycerol.
The next glycolytic intermediate, fructose-6-phosphate, can also be diverted to support cell growth by conversion to glucosamine-6-phosphate by fructose-6-phosphate aminotransferase using glutamine-derived amino groups. Hexosamines are substrates for glycosylation reactions and influence cell adhesion and signaling events. Hypoxia-driven hexosamine biosynthetic pathway (HBP) activation correlates with pancreatic adenocarcinoma aggressiveness (Guillaumond et al., 2013). HBP is elevated during M2 macrophage polarization and its inhibition results in defects in M2-specific macrophage functions (Jha et al., 2015). Upon T-cell activation the HBP product UDP-GlcNAc is elevated to promote increased intracellular protein O-GlcNAcylation. This was required not only for clonal expansion of peripheral T cells, but also for T-cell progenitor expansion and their malignant transformation into T cell acute lymphoblastic leukemia (T-ALL) (Swamy et al., 2016). Hexosamine biosynthesis may play a critical role in the balance of Th17 and Treg cells, as increased hexosamines promote Treg in a manner similar to that observed in M2 macrophages (Araujo et al., 2017).
Serine and glycine biosynthesis can account for approximately half of glucose utilization in cancer (Locasale et al., 2011). Glycolysis-derived 3-phosphoglycerate is converted to phospho-hydroxypyruvate by phosphoglycerate dehydrogenase (PHGDH) to initiate serine synthesis. Subsequently phosphoserine transaminase (PSAT1) catalyzes the production of phosphoserine by deamination of glutamate to α-ketoglutarate, supporting anaplerotic flux into the TCA cycle (Possemato et al., 2011). In the folate cycle, serine-derived carbon undergoes a further series of reactions to generate one-carbon units for biosynthetic processes and provides a key source of cellular NADPH, in addition NADPH produced in the PPP (Fan et al., 2014). Serine is required for T cells to traverse the S phase and serine restriction can limit T cell proliferation and effector function (Jun et al., 2014; Ma et al., 2017). The dependence of T cells on serine metabolism may explain why the dihydrofolate reductase inhibitor, methotrexate, has been so successful in combating both cancer and autoimmune disease. Methotrexate is currently in use for rheumatoid arthritis, psoriasis, Crohn’s disease and multiple sclerosis as well as various solid and hematological malignancies (Abolmaali et al., 2013; Gonen and Assaraf, 2012).
The final glycolytic enzyme is pyruvate kinase (PK), which plays a key role to regulate glycolytic flux. PK is expressed in most tissues as two PKM (PK muscle) splice variants, PKM1 and PKM2. PKM2 is overexpressed in cancers and alternates between catalytically slow dimeric and highly active tetrameric states (Christofk et al., 2008). The slow rate of dimeric PKM2 is thought to allow funneling of earlier glycolytic intermediates into the PPP or serine synthesis pathways (Christofk et al., 2008). Consistent with PMK2 as a brake on glycolysis to support biosynthesis, PKM2-deficient cancer cells proliferated only when PKM1 was not upregulated in its place (Israelsen et al., 2013). PKM2 also can promote inflammatory pathways in immune cells, and has been reported to promote phosphorylation of STAT3 and production of inflammatory cytokines as well as interact with Hypoxia Inducible Factor 1α (HIF1α) to induce IL1β (Palsson-McDermott et al., 2015; Shirai et al., 2016). Increased PKM2 isoform has also been found in activated CD4 and CD8 T cells (Cao et al., 2012; Marjanovic et al., 1990).
The end product of glycolysis, pyruvate, has several major fates. Lactate dehydrogenase (LDH)-mediated reduction of pyruvate was first described as a hypoxia mechanism to regenerate NAD+ and sustain glycolysis (Margaria et al., 1933; Meyerhof, 1927; Pasteur, 1858). Utiliztion of this route in normoxia is the experimental basis for the Warburg effect (Warburg et al., 1927). While the Warburg effect was initially attributed to mitochondrial defects, it is now clear that most tumors have functional mitochondria that are essential for proliferation (Cavalli et al., 1997; Warburg, 1956; Weinberg et al., 2010). Warburg metabolism is also the pathway of choice in stimulated proliferating T cells, demonstrating that it is not a metabolic abnormality of cancer (Frauwirth et al., 2002). In addition to ATP and biosynthetic precursors, glycolysis is an important source of reducing power in the form of NADH. However, excess NADH will suppress glycolysis and the channeling of pyruvate into lactate can prevent this accumulation (Vander Heiden et al., 2009; Pavlova and Thompson, 2016). Indeed, inhibiting LDH and lactate export via monocarboxylate transporters (MCTs) results in attenuation of cell growth and has anti-cancer and immunosuppressive properties (Fantin et al., 2006; Le et al., 2010; Murray et al., 2005; Xie et al., 2014). Reduction of pyruvate to lactate may provide a “vent” to dynamically maintain redox balance in addition to mitochondrial oxidation of NADH in the electron transport chain.
Another major pathway for pyruvate is to be oxidized in the mitochondria. Pyruvate dehydrogenase (PDH) mediates conversion of pyruvate into acetyl-CoA and entry into the TCA. PDH is controlled by pyruvate dehydrogenase kinase (PDK)-mediated inhibitory phosphorylation and dephosphorylation by pyruvate dehydrogenase phosphatase (PDP). The PDK1 isoform of PDK is upregulated by cMyc and HIF1α, and inhibiting PDK1 in BRAF-transformed cancer cells resulted in increased respiration and redox stress (Kaplon et al., 2013). PDK inhibition is now being explored in treatment of various malignancies as well as autoimmunity (Bian et al., 2009; Gerriets et al., 2015; Kankotia and Stacpoole, 2014; Kaplon et al., 2013). PDK1 may act as a gatekeeper between the pro-inflammatory Th17 and immunosuppressive Treg cells, as inhibiting PDK1 in Th17 cells resulted in increased ROS and protection of animals from inflammation (Eleftheriadis et al., 2013a; Gerriets et al., 2015; Ostroukhova et al., 2012). However, increased pyruvate oxidation can be pro-inflammatory in some settings. Pyruvate-derived citrate is required for the synthesis of the antimicrobial metabolite itaconate, as well as inducible nitric oxide synthase and cytokine TNFα in M1 macrophages (Meiser et al., 2015).
TCA cycle as a biosynthetic nexus in proliferating cells
The TCA cycle is a biosynthetic and regulatory hub that integrates energy metabolism with epigenetic changes and cell proliferation (Peng et al., 2016; Wellen et al., 2009). While the main reactions of the TCA cycle are well known, recent findings in cancer and immunology fields have shown extensive metabolic remodeling to maintain TCA flux or syphon biosynthetic precursors from the cycle (Figure 2). As glycolysis-derived acetyl-CoA enters the TCA cycle, it is condensed with oxaloacetate to form citrate, which can further be oxidized in the TCA cycle or be exported into cytosol and hydrolyzed by ATP-citrate lyase (ACLY) to generate oxaloacetate and cytosolic acetyl-CoA.
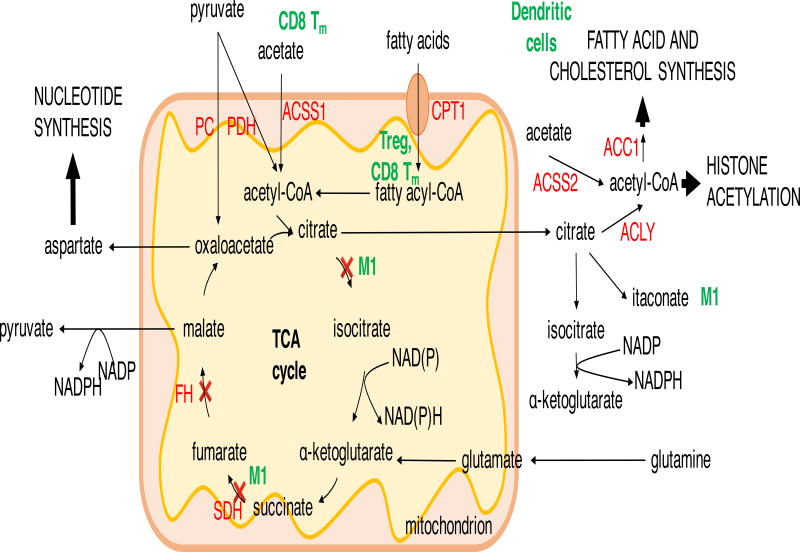
Figure 2. TCA cycle in biosynthesis and epigenetics.
The major TCA cycle inputs from glycolysis, glutaminolysis and fatty acid oxidation provide substrates for ATP production, NADPH generation (via malate and isocitrate) and biosynthetic intermediates for nucleotide, fatty acid and cholesterol synthesis. In M1 macrophages, the break in the TCA cycle after citrate ensures it is fluxed to lipid synthesis and production of antimicrobial itaconate. Breakage of the TCA cycle at succinate dehydrogenase (SDH) in M1 macrophages and in SDH-deficient cancer cells, or fumarate hydratase (FH)-deficient cancer cells leads to accumulation of succinate and/or fumarate, which inhibits α-ketoglutarate-dependent prolyl hydroxylases leading to transcription factor HIF1α stabilization. Apart from metabolic reprogramming, in macrophages this promotes transcription of il1b gene. ACC1, acetyl-CoA carboxylase 1; ACLY, ATP-citrate lyase; ACSS1/2 acetyl-CoA synthetase 1/2; CPT1, carnitine palmitoyltransferase 1; FH, fumarate hydratase; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; SDH, succinate dehydrogenase.
Cytosolic acetyl-CoA is broadly utilized and contributes to protein acetylation, synthesis of cholesterol and fatty acids, or is stored as triacyl-glycerides (TAG). Stimulation of M2 macrophages resulted in ACLY-dependent increase in histone acetylation at M2 macrophage signature gene promoters (Covarrubias et al., 2016). Conversely, glycolysis derived acetyl-CoA is used in activated dendritic cells to generate cellular membranes to expand endoplasmic reticulum and Golgi via acetyl-CoA carboxylase 1 (ACC1) and fatty acid synthase (FAS) (Everts et al., 2014). Acetyl-CoA is also used in inflammatory M1 macrophages for the production of anti-microbial compound itaconate from citrate (Infantino et al., 2011; Jha et al., 2015). In T cells, inhibition of ACC1 and lipid synthesis impairs the function of Th17 cells, instead promoting the development of regulatory T cells, which are characterized by fatty acid oxidation (FAO) (Berod et al., 2014).
Acetate, derived from dietary intake, liver metabolism, intestinal microbiota and intracellular protein and metabolite deacetylation, has emerged as another source of acetyl-CoA for lipid synthesis and protein acetylation (Schug et al., 2016). 13C tracing prior to surgical tumor resection led to the discovery that a large proportion of mitochondrial acetyl-CoA in brain tumors and brain metastasis is derived from plasma acetate (Maher et al., 2012; Mashimo et al., 2014). Mitochondrial acetyl-CoA synthetase 1 (ACSS1) overexpression in hepatocellular carcinoma is correlated with tumor growth and malignancy, especially under hypoxia and cellular stress (Björnson et al., 2015). The knockdown of cytosolic acetyl-CoA synthetase 2 (ASCSS2) reduced tumor burden in mouse models of hepatocellular carcinoma and breast and prostate cancer xenografts (Comerford et al., 2014; Kamphorst et al., 2014; Schug et al., 2015). While it is unclear to what extent basal levels of acetate contribute to cancer metabolism, acetate concentrations can increase during acute bacterial infection, a metabolically stressful condition (Balmer et al., 2016). Exposure of CD8 T cells to acetate during acute infection or development of memory CD8 T cells results in elevated acetyl-CoA levels and increased GAPDH acetylation, promoting glycolytic flux and enhancing rapid effector functions upon antigen re-exposure (Balmer et al., 2016). Acetate has also been shown to promote colonic accumulation of Treg, whereas short chain fatty acids butyrate and propionate, generated by intestinal microbiota, promote induction of peripheral Treg via epigenetic mechanisms (Arpaia et al., 2013).
The diversion of citrate and other TCA cycle intermediates for biosynthetic reactions necessities that adequate supplies of oxaloacetate be maintained for condensation with acetyl-CoA. A major contributor towards this anaplerotic flux is glutamine-derived α-ketoglutarate (Figure 2). Glutamine is the most abundant amino acid in plasma and is metabolized by glutaminase to produce glutamate, which is subsequently converted to α-ketoglutarate by glutamate dehydrogenase (GLUD) or amino acid transferases. Another route for glutamine to support the TCA cycle is through reductive carboxylation, although these reactions can take place within cytosol (Metallo et al., 2011a). In hypoxic conditions or during HIF1α or Myc activation, reductive carboxylation of glutamine-derived α-ketoglutarate produces citrate as well as other TCA cycle metabolites, enabling cells to maintain TCA cycle biosynthetic capacity and proliferate when glucose-derived citrate synthesis is reduced (Metallo et al., 2011b; Wise et al., 2011). Immune cell stimulation has been shown to increase glutaminase activity and glutamine consumption, suggesting an important role for glutamine to fuel lymphocyte proliferation (Ardawi and Newsholme, 1982, 1983). Lack of glutamine resulted not only in reduced proliferation rates but functional impairments of both B and T cells (Crawford and Cohen, 1985; Hörig et al., 1993). More recently, T cells lacking the glutamine transporter ASCT2 (Slc1a5) were shown to be capable of proliferation, but unable to efficiently differentiate into Th17 effectors, but not Treg (Nakaya et al., 2014).
The second major anaplerotic flux into the TCA cycle is the ATP-dependent carboxylation of pyruvate into oxaloacetate by pyruvate carboxylase (PC) (Figure 2). Glutamine deprivation of glioblastoma cells increased PC flux, making these cells reliant on pyruvate carboxylase for lipid and nucleotide synthesis and cell growth (Cheng et al., 2011). In glioblastoma and non-small cell lung cancer isotope tracing identified pyruvate carboxylase as the main origin of TCA cycle intermediates (Christen et al., 2016; Marin-Valencia et al., 2012; Sellers et al., 2015). ASCT2-deficient T cells may rely on this pathway to sustain the TCA cycle, and PC may play an important role in microenvironments where glutamine can become limiting (Nakaya et al., 2014).
In a similar manner as glycolysis and the pentose phosphate pathway, the TCA cycle intermediates can also be used in the production of NADPH for biosynthetic reactions via malic enzyme or isocitrate dehydrogenase (IDH), enzymes that both have mitochondrial and cytosolic isoforms (Figure 2). In order to generate NADPH for fatty acid synthesis, glioblastoma cells were found to engage in glutaminolysis whereby glutamine-derived malate was oxidatively decarboxylated to produce NADPH and pyruvate, which was subsequently secreted as lactate (DeBerardinis et al., 2007). The role of IDH1 in generation of cytosolic NADPH for antioxidant defence has been demonstrated in protecting macrophages against oxidative damage during LPS-induced respiratory bursts (Maeng et al., 2004). Similar roles of both malic enzyme and IDH in NADPH production for antioxidant defense have been found in cancer cells (Bleeker et al., 2010; Lee et al., 2002c; Son et al., 2013).
The TCA cycle is a versatile system, and studies in both cancer and immunity have shown that it can function in modules rather than a single loop. Cancer cells with fumarate hydratase or succinate dehydrogenase deficiencies are able to proliferate despite broken TCA cycles. They engage in enhanced glycolysis for ATP production, reductive carboxylation of glutamine-derived α-ketoglutarate to produce citrate for fatty acid and nucleotide synthesis and also utilize pyruvate carboxylase to generate oxaloacetate, making these pathways metabolic vulnerabilities (Cardaci et al., 2015; Sudarshan et al., 2009; Yang et al., 2010). However, breakage of the TCA cycle is not always associated with mutation and malignancy. M1 macrophages have two breaks in the TCA cycle – after IDH and after SDH, which not only divert flux of citrate to the production of the antimicrobial itaconate and fatty acids (Jha et al., 2015). This breakage also leads to succinate accumulation which contributes to elevated production of mitochondrial ROS via reverse electron flow, resulting in inhibition of HIF-1α degradation (Mills et al., 2016).
Fatty acid oxidation
Lipids are both essential products in anabolism and highly valuable fuels in catabolism. The catabolic process of mitochondrial fatty acid oxidation (FAO) provides a key source of acetyl-CoA, NADH and FADH2 to augment the TCA cycle (Figure 2). Cytosolic fatty acids are first activated by ligation to CoA, which is then exchanged for carnitine by carnitine palmitoyltransferase 1 (CPT-1) upon mitochondrial transport. The fatty acyl group is then ligated from carnitine back to CoA by CPT-2 for FAO in the mitochondrial matrix. Short chain fatty acids are imported into mitochondria directly and do not use the carnitine transport system. Importantly, fatty acid synthesis and oxidation are generally controlled reciprocally by malonyl-CoA, which can inhibit CPT-1a to restrict lipid oxidation in lipid synthesizing cells, ensuring the two processes do not occur in a concurrent futile cycle.
The balance of fatty acid oxidation and synthesis can influence inflammatory and immunosuppressive CD4 T cells and macrophages, as well as the development of long-lived memory CD8 T cells (Tm) from cytotoxic CD8 T cells. Treg, M2 macrophages and Tm have increased FAO compared to their inflammatory counterparts (Teff, M1 macrophages and CD8 Teff, respectively) and inhibiting FAO impairs their function and lineage development (Gerriets et al., 2015; Huang et al., 2014; Michalek et al., 2011; Pearce et al., 2009; Vats et al., 2006). The role of FAO in macrophage polarization is less clear as while the lipid transporter FATP1 promoted M2 macrophages, knockdown of CPT-2 did not affect the development and function of M2 macrophages (Johnson et al., 2016; Nomura et al., 2016). Memory CD8 T cells (Tm) are quiescent long-lived cells that have experienced antigen-stimulation but persist following antigen clearance to mediate a rapid immune response upon antigen re-encounter. These cell exhibit upregulated CPT-1, increased mitochondrial mass and functionality, and spare capacity for respiratory metabolism that aids their long-term survival and withstanding of metabolic stress which is critical for prolonged immunological protection (Buck et al., 2017; Pearce et al., 2009; van der Windt et al., 2012). Tissue resident Tm utilize exogenous fatty acids, taken up by lipid transporters FABP4 and FABP5 that are required for long-term survival of these cells in vivo (Pan et al., 2017). Surprisingly, rather than using extracellular fatty acids, central memory CD8 T cells use cell-intrinsic glucose-derived lipids for FAO in an apparently futile synthesis and oxidation cycle (O’Sullivan et al., 2014). Treg cells seem to also balance between a glycolytic phenotype that promotes their proliferation but compromises their suppressive function, and mitochondrial oxidative metabolism that supports their immunosuppression and lineage commitment (Gerriets et al., 2016; De Rosa et al., 2015).
Recent findings in endothelial cells indicate that fatty acid oxidation can also be a part of an anabolic metabolic program. While potentially endothelial-specific, fatty acids can contribute to aspartate and deoxyribonucleoside triphosphate generation in proliferating endothelial cells (Schoors et al., 2015). More generally, fatty acid-derived acetyl-CoA entering the TCA cycle also contributes to citrate production, which can generate NADPH for biosynthetic reactions and the antioxidant glutathione production (Carracedo et al., 2013; Pike et al., 2011). A number of cancers do actively uptake and oxidize fatty acids, especially during metastasis and cellular stress (Caro et al., 2012; Carracedo et al., 2013; Monti et al., 2005). Examples include diffuse large B-cell lymphoma, prostate cancer and glioblastoma (Caro et al., 2012; Cirillo et al., 2014; Liu, 2006; Monti et al., 2005). In triple negative breast cancer cells etomoxir treatment or knockdown of CPT-1B or CPT-2 reduced ATP production and proliferation and reduced tumor growth and metastasis in vivo (Camarda et al., 2016; Park et al., 2016). Interestingly, silencing of the mitochondrial biogenesis transcription factor peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1α) in invasive cancer cells attenuates their invasive potential and metastasis without affecting cell proliferation (LeBleu et al., 2014).
Amino acid metabolism and nucleotide synthesis
The biosynthesis of nucleotides for DNA replication RNA, ATP and NAD/NADP as well as synthesis of non-essential amino acids and polyamines require a supply of reduced nitrogen. Glutamine is the primary nitrogen source and most proliferating cells rely on glutamine uptake for anabolic growth (Fontenelle and Henderson, 1969; Hörig et al., 1993). In support of the biosynthetic precursor role for glutamine, deprivation of glutamine in T cell activation resulted in a proliferative block which could be partially alleviated by an exogenous supply of nucleotides and polyamines (Wang et al., 2011b). Similarly, in K-ras transformed fibroblasts, glutamine deficiency-induced cell cycle block could be rescued by addition of deoxyribonucleotides (Gaglio et al., 2009).
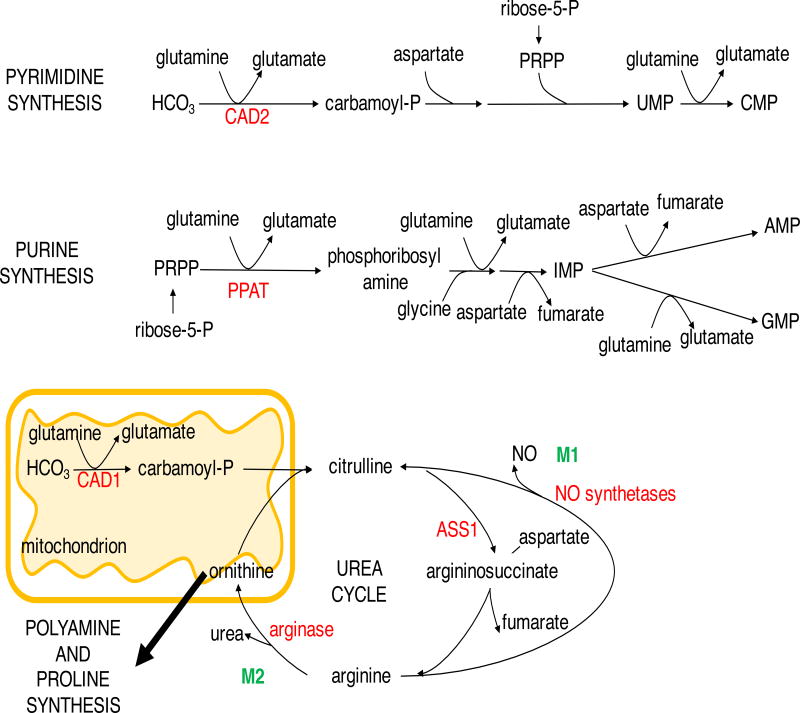
Glutamine carries two amide groups, both of which are used for biosynthetic purposes. The transfer of the amide nitrogen of glutamine yields glutamate and the biosynthesis of asparagine, hexosamine, purines and pyrimidines. Asparagine synthetase (ASNS) is frequently overexpressed in cancer and asparagine itself promotes cell proliferation as well as cell survival in the absence of glutamine (Krall et al., 2016; Sircar et al., 2012; Zhang et al., 2014). Low ASNS expression in acute lymphoblastic leukemia (ALL), however, makes these cells reliant on exogenous asparagine (Broome, 1963; Egler et al., 2016; Scherf et al., 2000). This metabolic vulnerability is exploited for patient treatment with bacterial asparaginase to reduce the blood asparagine levels and induce ALL cell apoptosis (Egler et al., 2016). Glutamine phosphoribosylpyrophosphate amidotransferase (PPAT) and carbamolyl phosphate synthetase (CAD2) initiate the formation of purine and pyrimidine rings, respectively, in the committed step in nucleobase synthesis (Figure 3) (Lane and Fan, 2015). Aspartate provides an additional nitrogen for purine biosynthesis, both nitrogen and carbon for pyrimidines and it can restore nucleotide synthesis and cell proliferation in glutamine-deprived K-ras-driven cancer cell lines (Patel et al., 2016).
Figure 3. Nitrogen metabolism in cell proliferation and immune function.
Purine and pyrimidine nucleotide synthesis requires glutamine and aspartate-derived nitrogen in proliferating cancer cells and effector T cells. Arginine metabolism provides polyamines and nitric oxide for proliferating cells. Arginine is differentially utilized by inflammatory M1 macrophages for NO synthesis while the anti-inflammatory M2 macrophages divert arginine to polyamine and hydroxyproline synthesis, inhibiting NO production. ASS1, argininosuccinate synthetase 1; CAD1/2, carbamoyl phosphate synthetase 1/2; IMP, inosine monophosphate; PPAT, phosphoribosyl pyrophosphate amidotransferase; PRPP, phosphoribosyl pyrophosphate.
Purine and pyrimidine pools expand several fold in T cell activation (Fairbanks et al., 1995). Indeed, the dependence of lymphocytes on de novo nucleotide synthesis may account for the immunosuppressive action of inosine 5’-monophosphate dehydrogenase (IMPDH) inhibitors, mizoribin and mycophenolate mofetil, the dihydroorotate dehydrogenase inhibitor, leflunomide, and azathioprine, which was initially discovered as an antileukemic drug (Allison, 2000). Additionally, methotrexate, which depletes purine biosynthesis enzymes of substrate, is used in cancer and inflammatory diseases (Abolmaali et al., 2013; Asselin et al., 2011). The immunosuppressive effects of these drugs arise not only due to reduced proliferation of inflammatory cells, but targeting nucleotide biosynthesis can tilt the balance between inflammatory and non-inflammatory immune cell development. Glutamine restriction, or inhibition of purine and pyrimidine synthesis by pharmacological agents or CAD or PPAT knockdown enriched CD4 T cells with regulatory properties while reducing the numbers of CD4 T cells that do not express Treg transcription factor FOXP3 (Metzler et al., 2016).
Glutamine can also be deamidated to glutamate by glutaminases, with the release of ammonia and the resultant glutamate participates in transamination reactions to produce non-essential amino acids and α-ketoglutarate. Nitrogen balancing in cell proliferation was elegantly demonstrated in epithelial cells, which during proliferative phases preferentially used glutamate aminotransferases rather than the nitrogen-wasting GLUD to produce α-ketoglutarate (Coloff et al., 2016). In this manner, nitrogen from the transamination of glutamate was transferred to keto acids such as pyruvate, oxaloacatete and 3-phosphohydroxy pyruvate and conserved in the respective non-essential amino acids (NEAA) alanine, aspartate and serine (Coloff et al., 2016). In contrast, the GLUD reaction was preferred during quiescent phases to prevent the syphoning of keto acids from glycolysis and the TCA cycle when NEAA are not needed (Coloff et al., 2016).
Arginine, another non-essential amino acid participates in the biosynthesis of polyamines, creatine, glutamate, proline and the production of nitric oxide. Due to downregulation of arginine biosynthesis enzymes, argininosuccinate synthetase 1 (ASS1), ornithine transcarbamylase and/or argininosuccinate lyase, cancers like hepatocellular carcinoma, melanoma, renal cell and prostate cancers become auxotrophic for arginine and susceptible to arginine-depletion therapies (Patil et al., 2016; Phillips et al., 2013). Arginine depletion in ASS1-deficient cancer cells resulted in reduced polyamine levels and synthetic lethality (Locke et al., 2016). Likewise, ASS1 plays an important role in T cells as ASS1 deficiency reduced differentiation and numbers of peripheral T cells, which may explain the sensitivity of T cells to arginase (Tarasenko et al., 2015).
Signaling cascades involved in achieving the proliferative state
The metabolic pathways deregulated in cancer that permit dysregulated proliferation reflect normal cellular growth programs and therefore are similar between cancer and the normal proliferating cells of the immune system (Figure 4A). While mutations drive cell intrinsic growth factor independence of cancer cells, immune cells undergo metabolic reprogramming following cell extrinsic activation signals. Engagement of the T-cell receptor (TCR) with the peptide-MHC complexes are required to set T cells for entry into the cell cycle and proliferation. Co-stimulatory signals, such as CD28, then activate a cascade of proliferative and biosynthetic programs, which are further modulated by the cytokine mileu in the microenvironment (Acuto and Michel, 2003). Similarly, activation of the B-cell receptor and cytokine or T cell mediated co-stimulatory signals activate B cells (Yuseff et al., 2013). The cells of the innate immune system, such as macrophages and dendritic cells become activated in response to foreign pathogens or endogenous danger signals via pattern recognition receptors (Kelly and O’Neill, 2015).
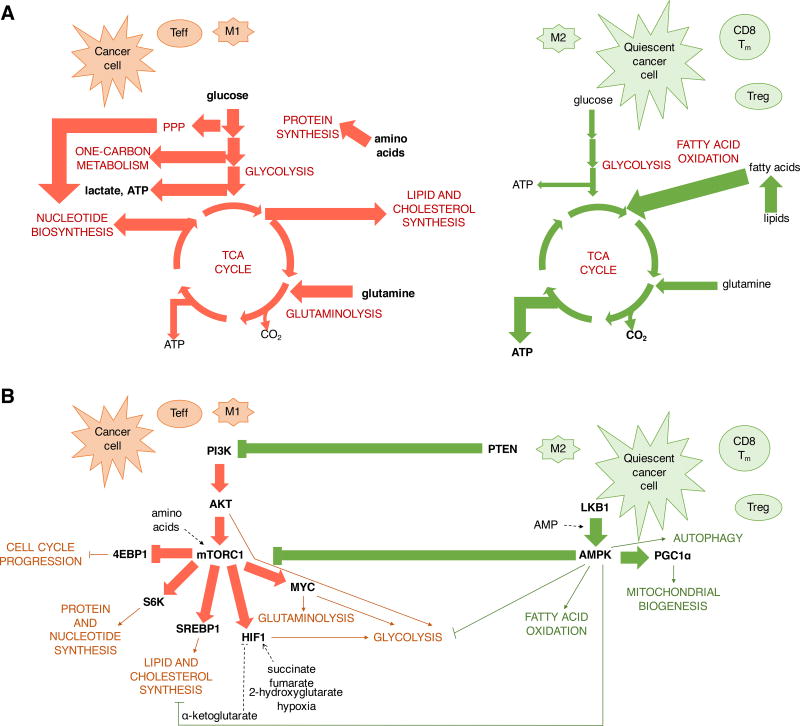
Figure 4. Proliferative metabolic programming and signaling exhibited by effector T cells, inflammatory macrophages and cancer cells versus the catabolic metabolism and signaling in Treg, M2 macrophages, memory T cells and quiescent cancer cells.
(A) M1 macrophages, effector T cells and cancer cells are characterized by biosynthetic metabolism to support proliferation and anabolism with high rates of glycolysis and glutaminolysis for the synthesis of proteins, nucleic acids and lipids. In contrast, Treg, M2 macrophages, memory T cells and quiescent cancer cells are primarily characterized by catabolic metabolism and utilization of fatty acid oxidation for ATP synthesis. (B) Activation of growth factor receptors leads to Akt-mediated upregulation of glucose uptake and glycolytic flux and promotes lipid synthesis. Proliferative signals and metabolic hues are balanced with biosynthetic metabolism by mTORC1, which orchestrates nucleotide and protein synthesis via ribosomal protein S6 kinase with progression through the cell cycle via 4EBP1. Transcription factors Myc, HIF1α and SREBPs mediate glutamine and glucose metabolism and promote lipid and cholesterol synthesis. These pathways are opposed by PTEN and AMPK which inhibit PI3K and mTORC1 signaling and promote fatty acid oxidation, autophagy and transcriptional co-activator PGC1α-mediated mitochondrial biogenesis. PPP, pentose phosphate pathway.
PI3K/Akt/mTOR signaling axis
The phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is commonly activated in cancer due to frequent mutation or amplifications in its components (Yuan and Cantley, 2008). Normally, PI3K is activated on receiving signals from receptor tyrosine kinases such as epidermal growth factor receptor and insulin-like growth factor 1 receptor. The activation of PI3K takes place within a few second after T cell activation and is mediated by both TCR and CD28 signaling-induced events (Appleman et al., 2002; Costello et al., 2002; Frauwirth et al., 2002; Gold and Aebersold, 1994; Harriague and Bismuth, 2002; Ward et al., 1993). Downstream of the PI3K, Akt is a major regulator of glucose metabolism (Figure 4B). Akt promotes aerobic glycolysis and increases the cell surface membrane expression of glucose transporter GLUT1 by promoting its recycling and reducing its internalization (Elstrom et al., 2004; Jacobs et al., 2008; Wieman et al., 2007). Akt also facilitates the phosphorylation of hexokinase 2 (HK2) and phosphofructokinase 2, which promotes the activation of the rate-controlling enzyme of glycolysis, phosphofructokinase 1 (Deprez et al., 1997; Miyamoto et al., 2007). Apart from glucose metabolism, Akt is involved in the upregulation of fatty acid and cholesterol synthesis and expression of the low density lipoprotein via sterol regulatory element binding protein 1 (SREBP1) although the involvement of Akt in SREBP1-mediated lipogenesis in T cells is unclear (Guo et al., 2009; Kidani et al., 2013).
Although frequently dysregulated and constitutively active in cancer, proper control of PI3K/Akt/mTOR pathway is essential in immunity. Inhibition of this pathway is both immunosuppressive and supports generation of quiescent memory T cells, while excessive activation can promote autoimmunity. During T cell development in the thymus, Notch-mediated PI3K/Akt signaling is required for pre-T cell viability, size and glycolytic rate, which protects them from apoptosis (Ciofani and Zúñiga-Pflücker, 2005). Expression of constitutively activate form of Akt in the T cells of transgenic mice resulted in increased glycolytic rate and heightened incidence of autoimmunity and lymphoma, accompanied by a reduced requirement for growth factor signaling (Rathmell et al., 2003; Waickman and Powell, 2012). Similarly, deletion of the tumor suppressor gene and negative upstream regulator of PI3K/Akt PTEN in T cells results in autoimmune disease and lymphoma (Liu et al., 2010; Suzuki et al., 2001). PTEN deletion impairs elimination of autoreactive T cells in the thymus, enhances PI3K/Akt signaling, hyperproliferation, Th1 and Th2 cytokine secretion and resistance to apoptosis in T cells (Suzuki et al., 2001). Deletion of PTEN in mature T cells however does not lead to malignancy but leads to autoimmunity (Liu et al., 2010). Treg-specific deletion of PTEN or TSC1 resulted in increased Akt and mTORC1 signaling and impaired Treg functionality with dysregulation of transcriptional and metabolic programs and upsetting of the balance between glycolysis and mitochondrial metabolism (Huynh et al., 2015; Park et al., 2013; Shrestha et al., 2015).
Downstream of Akt the mTOR Complex 1 (mTORC1) is a central regulator of anabolic metabolism (Figure 4B). The mTORC1 inhibitor rapamycin reduced Akt-mediated increase in the uptake of amino acids, low density lipoprotein and iron and led to reduced cell size and survival (Edinger and Thompson, 2002). mTORC1 signals via 4EBP1 and S6 kinase to increase ribosomal protein translation and progression through the cell cycle and activates SREBP1 and HIF-1α to induce fatty acid and cholesterol synthesis and glycolytic reprogramming, respectively (Düvel et al., 2010; Peterson et al., 2011; Porstmann et al., 2008; Yecies et al., 2011). SREBP1 is required for lipogenesis and inflammasome function in LPS-stimulated macrophages and failure to activate SREBP1 prevents activation-induced T cell proliferation (Im et al., 2011; Kidani et al., 2013). mTORC1 and SREBP1 also coordinate the production of NADPH for lipid synthesis and other biosynthetic functions via inducing expression of PPP enzymes, concomitantly ensuring ribose-1-phosphate supply for nucleotides (Dibble and Manning, 2013). mTORC1 signaling also coordinates cell growth with nucleotide synthesis via S6 kinase promotion of CAD, the rate limiting enzyme of pyrimidine synthesis (Ben-Sahra et al., 2013; Robitaille et al., 2013).
The activation of mTORC1 integrates growth factor signaling and nutrient sensing (Dibble and Manning, 2013). Amino acids in particular, especially leucine, arginine and glutamine are needed for robust activation of mTORC1 (Han et al., 2012; Nicklin et al., 2009; Sancak et al., 2008, 2010; Wang et al., 2015). Indeed, depletion of essential amino acids by upregulation of catabolic enzymes in dendritic cells results in reduced mTORC1 signaling and T cell proliferation and promotion of Treg lineage (Cobbold et al., 2009). Furthermore, in response to activation, T cells upregulate a number of amino acid transporters (Sinclair et al., 2013a). Notably, T cells deficient for large neutral amino acid transporter SLC7A5 failed to activate mTOR pathway in response to TCR signaling and did not clonally expand or differentiate into effector cells (Sinclair et al., 2013a).
Inhibition of the PI3K/Akt/mTOR pathway has been explored in both cancer treatment and immunosuppression. Rapamycin treatment during acute viral infection or immunization in mice increased the numbers of CD8 memory T cells and the expression of memory cell markers (Araki et al., 2009). Transient high-dose rapamycin treatment reduced activation, proliferation and effector function of CD8 T cells, but provided these cells with superior generation of immune memory that resulted in increased tumor clearance (Li et al., 2012). However, rapamycin treatment is detrimental to CD8 recall response, as activation of mTORC1 is needed for enhanced recall ability and anti-tumor functionality (Li et al., 2012). Another study found that inhibition of mTORC2 activity via deficiency of Rictor, a component of complex 2, also promoted memory T cell formation, enhanced fatty acid oxidation and increased spare respiratory capacity via reduced Akt activation, which is downstream of mTORC2 (Zhang et al., 2016).
AMPK
AMP-activated protein kinase (AMPK) is activated to counter mTORC1 and relieve bioenergetic stress, such as in unrestrained anabolic growth (Figure 4B) (Hardie, 2014). An increased ratio of AMP/ATP in metabolically stressed cells enhances phosphorylation of AMPK by the tumor suppressor liver kinase B1 (LKB1), which then results in AMPK activation. AMPK can also be activated by calcium/calmodulin-dependent protein kinase kinase-β (CamKKβ), and TGF-β-activated kinase-1 (TAK1) (Woods et al., 2005). AMPK is a negative regulator of mTORC1 and promotes catabolic processes and mitochondrial oxidation while inhibiting biosynthesis to preserve ATP, redox balance, and prolong cell survival under metabolic stress. AMPK also promotes a switch to fatty acid oxidation by activating phosphorylation of PGC-1α to induce mitochondrial biogenesis and by inhibitory phosphorylation of ACC1 and ACC2 to block fatty acid synthesis (Jäger et al., 2007). AMPK also activates autophagy, a catabolic process whereby cellular proteins and organelles are degraded inside lysosome-fused vesicles to supply critical nutrients during starvation (Kim et al., 2011; Shang et al., 2011). Loss of AMPK results in elevated mTORC1 and HIF-1α-mediated anabolic metabolism and diversion of glucose towards glycolysis and lipid synthesis rather than ATP production (Faubert et al., 2012; Gwinn et al., 2008; Inoki et al., 2003; Kishton et al., 2016).
Given the roles of AMPK to suppress mTORC1 and anabolic metabolism while also relieving metabolic stress through increased catabolic metabolism, AMPK can have conflicting impacts on cancer and T cells. While several therapies that indirectly promote AMPK activation like metformin, the complex I inhibitor that inhibits ATP synthesis in ETC, and aminoimidazole carboxamide ribonucleotide (AICAR), an AMP analog, were shown to inhibit cancer development and progression, AMPK enacts pro-survival advantages in cases of metabolic stress (Vincent et al., 2015). The survival-promoting stress responses orchestrated by AMPK demonstrate how metabolic plasticity enables tumor cells to withstand metabolically stressful conditions such as nutrient depletion, drug treatment and hypoxia in the tumor microenvironment (Faubert et al., 2015). Conversely, unrestrained anabolic metabolism can lead to insufficient ATP generation and AMPK can play dual roles in T and B cell acute lymphoblastic leukemia (Chan et al., 2017; Kishton et al., 2016). AMPK both restrains cancer cell growth in a tumor suppressing fashion while also stimulating mitochondrial oxidative metabolism to alleviate stress and maintain cancer cell survival (Chan et al., 2017; Kishton et al., 2016).
Similarly, AMPK-activated metabolic reprogramming is an important response to cellular stress in T cells. LKB1-deficient T cells had reduced AMPK activation and survival upon cytokine deprivation (MacIver et al., 2011). Glucose-deprived effector T cells activate AMPK, which downregulates genes associated with glycolysis and increases glutamine flux into the TCA cycle, maintaining ATP balance and enabling effector cell proliferation (Blagih et al., 2015). Loss of AMPK signaling resulted in reduced ATP level and elevated cell death upon glucose withdrawal in both effector CD4 and CD8 T cells. Furthermore, while AMPK-deficient CD4 T cells could develop into Th1 and Th17 lineages and maintain their effector function, the total number of antigen-specific CD4 and CD8 T cells was reduced in vivo, leading to diminished immune responses (Blagih et al., 2015). AMPK-directed reprogramming of fatty acid oxidation, mitochondrial function and suppression of mTORC1 activity not only improves the metabolic fitness of CD8 T cells to metabolic stress, but also is instrumental in generating long-lived memory cells (Pearce et al., 2009; Rolf et al., 2013).
Hypoxia and HIF1α
Hypoxia-inducible factors (HIFs), of which HIF-1α is the most ubiquitously expressed, mediate the switch from oxidative phosphorylation to glycolytic ATP production in response to hypoxia (LaGory and Giaccia, 2016). HIFs are short-lived proteins that are hydroxylated by α-ketoglutarate-dependent prolyl hydroxylases (PHDs) in normoxia that targets them for degradation by Von Hippel–Lindau (VHL) E3 ubiquitin ligase complex (Huang et al., 1998; Maxwell et al., 1999). Independently of oxygen availability, HIF1α expression is induced by growth factor signaling from PI3K/Akt/mTORC1 pathway which promotes cap-dependent translation of HIF-1α mRNA (Figure 4B) (Düvel et al., 2010; Hudson et al., 2002; Jiang et al., 2001; Zhong et al., 2000). Apart from PI3K/Akt/mTORC1, metabolic cues are relayed to HIF-1α by succinate, fumarate, lactate and pyruvate, which inhibit PHDs, thereby preventing HIF-1α degradation (Lu et al., 2005; Selak et al., 2005). HIF-mediated transcriptional program both in hypoxia and in response to growth factor signaling results in increased expression of glucose and lactate transporters, glycolytic enzymes and LDHA, and reduced mitochondrial oxygen consumption by activation of pyruvate dehydrogenase kinase, which inhibits pyruvate conversion to acetyl-CoA (Calvert et al., 2006; Hayashi et al., 2004; Kim et al., 2006; Papandreou et al., 2006; Semenza et al., 1996; Ullah et al., 2006).
Due to the potential for low oxygen availability in inflammatory sites and direct HIF1α-regulating signaling events, HIF1α can play a crucial role in immunity by modulating the ability of immune cells to adapt to hypoxic conditions (Makino et al., 2003). Upon TCR engagement, HIF-1α is activated downstream of mTORC1, although the initial T cell activation does not seem to depend on HIF-1α signaling (Nakamura et al., 2005; Wang et al., 2011b). HIF-1α is important in determining the balance of inflammatory Th17 and immunosuppressive Treg cells as it increases he expression of Th17 signature transcription factor Rorγt and promotes the glycolytic phenotype of Th17 while targeting the Treg transcription factor FoxP3 for proteasomal degradation (Dang et al., 2011; Shi et al., 2011). Similarly, HIF-1α enhances the cytolytic capacity of CD8 T cells, delays the onset of exhaustion caused by continued antigen exposure in instances like chronic inflammation and cancer (Doedens et al., 2013). Even in normoxia, stabilization of HIF1α by succinate/ROS-mediated inhibition of PHD activity that can occur in macrophages, or increased HIF1α protein synthesis due to enhanced mTORC1 activity can increase aerobic glycolysis (Tannahill et al., 2013).
Myc-driven metabolic events
The transcription factor Myc orchestrates a number of transcriptional programs involved in glucose and glutamine metabolism and nucleotide synthesis (Figure 4B) (Stine et al., 2015). Myc is translocated or rearranged in a number of hematological malignancies and amplified in a large number of solid human cancers, with its overexpression estimated in 50% of tumors (Beroukhim et al., 2010; Vennstrom et al., 1982; Vita and Henriksson, 2006). Myc was identified as an early response gene to proliferative signals in lymphocytes, stimulated with lipopolysaccharide or concavalin A, and fibroblasts, exposed to growth factors (Kelly et al., 1983). Ornithine decarboxylase, the first enzyme in polyamine biosynthesis pathway, was the first identified transcriptional target of c-Myc (Bello-Fernandez et al., 1993). Overexpression of Myc promoted aerobic glycolysis and successively several genes involved in glycolysis were found to be direct transcriptional targets of c-Myc (Kim et al., 2004; Osthus et al., 2000; Shim et al., 1997). Subsequently, nucleotide biosynthesis enzymes CAD, PRPS2, IMPDH1, IMPDH2 and TS were all identified as Myc target genes (Eberhardy and Farnham, 2001; Liu et al., 2008; Mannava et al., 2008). The discovery that oncogenic Myc levels activate a transcriptional program that promotes mitochondrial glutaminolysis for bioenergetic and biosynthetic processes established its role as a driver of glutamine consumption by proliferating cells (Pavlova and Thompson, 2016; Wise et al., 2008). Myc was also shown to increase expression of glutamine transporters SLC1A5 (ASCT2) and SLC38A5 (SN2) and glutaminase, leading to increased glutaminolytic flux and an addiction of Myc-transformed cells to glutamine (Wise et al., 2008). Myc is thus an integrator of bioenergetics and biosynthesis pathways with cell growth (Perna et al., 2012).
Analysis of transcription factors involved in the metabolic reprogramming revealed an early requirement for Myc following T cell activation (Wang et al., 2011b). Acute loss of Myc suppressed the metabolic reprogramming of glucose, glutamine and polyamine metabolism and prevented T cell growth (Wang et al., 2011b). Activation of the T-cell receptor of CD8 T cells by APC can result in asymmetric division with the daughter cell proximal to the APC having higher level of amino acid transporters, amino acids and mTORC1 than the distal cell (Pollizzi et al., 2016; Verbist et al., 2016). Because Myc is a very short-lived protein this asymmetric partitioning of mTORC1 signaling led to asymmetric expression of Myc (Verbist et al., 2016). Cells with high expression of Myc had enhanced glycolysis, PPP and glutamine metabolism and were present at higher numbers in mice following an acute infection with influenza (Verbist et al., 2016). However, low Myc-expressing cells had a superior recall ability in response to re-challenge, supporting the idea that asymmetric division and partitioning of activation signals can give rise to daughters displaying metabolic characteristics of effector and memory CD8 T cells (Verbist et al., 2016).
In macrophages, the situation is more complex with mitogenic stimulus colony-stimulating factor 1 (CSF-1) promoting Myc-dependent metabolic program in bone marrow-derived macrophages and their entry into the cell cycle and proliferation (Liu et al., 2016). However, pro-inflammatory stimulation with LPS led to downregulation of c-Myc, reduced proliferation and upregulation of HIF-1α and glycolysis (Liu et al., 2016). Consistent with Myc expression being anti-inflammatory in macrophages, c-Myc was upregulated during alternative differentiation of macrophages into the M2 lineage (Jablonski et al., 2015; Pello et al., 2012).
Metabolic plasticity and stress in cancer
Mutation and oncogenic transformation can place critical boundaries on the behavior of cancer cells. Driver mutations can induce signaling, genetic, and metabolic conditions that lead to oncogene addiction and dysregulated growth. Distinct combinations of mutations and oncogenic signaling pathways will lead to specific metabolic programs, which may not reflect an overall balance in essential pathways. This lack of concerted metabolic reprogramming can lead to over-reliance on certain metabolic pathway and substrates, and the need for damage control mechanisms. Indeed, aberrant activation of oncogenes such as Myc and Ras can lead to oncogene-induced cellular senescence and downregulation of anabolic pathways - a hurdle that cancer cells must overcome for tumorigenesis (Aird and Zhang, 2014). The recognition that cellular stress pathways enable cancer cell survival in response to nutrient depletion and hypoxia in the tumor microenvironment has fueled the development of drugs targeting these pathways (Luo et al., 2009). However, even in the absence of environmental stresses, cancer cells can exhibit metabolic inflexibility, imbalances, and metabolic addictions that can be exploited for therapy.
Comparison of normal proliferating T cells to proliferating T cell acute lymphoblastic leukemia cells demonstrates a clear metabolic stress that can be induced by oncogenic signals. Like activated T cells, T-ALL cells have increased glucose uptake and aerobic glycolysis, and depend on these for cell survival (Kishton et al., 2016). Despite selection for cancer cells with rapid proliferation, however, normal activated T cells had higher rates of glucose uptake, glycolytic and pentose phosphate pathway fluxes, and faster rates of proliferation than T-ALL cells (Kishton et al., 2016). While both cMyc and mTOR were activated in both T-ALL and normal activated T cells, the signaling downstream of mTORC1 was suppressed in T-ALL (Kishton et al., 2016). This inhibition of mTOR signaling was removed by deletion of AMPK, suggesting that T-ALL cells were under metabolic stress that AMPK moderated. Indeed, AMPK-deficient T-ALL had higher rates of aerobic glycolysis and anabolic metabolism, yet were highly susceptible to apoptosis in vivo (Kishton et al., 2016). Consistent with the reliance of Notch transformed T-ALL cells for oxidative phosphorylation and AMPK signaling to maintain ATP levels and manage metabolic stress, rotenone or phenformin treatment induced cell death in T-ALL cells but not in naïve or activated T cells (Kishton et al., 2016).
Similarly, comparing pre-B-cell acute lymphoblastic leukemia (pre-B-ALL) with myeloid leukemia cells, both of which are derived from the same progenitor, revealed low levels of ATP, glycolytic reserve and spare respirator capacity accompanied by activation of the energy stress sensor LKB1 and AMPK in B-ALL and this correlated with poor patient outcome (Chan et al., 2017). Pre-B-ALL cells but not myeloid cells or mature B cells were sensitive to loss of AMPK activity, and AMPK inhibition in patient-derived pre-B-ALL samples was synergistic with glucocorticoid treatment, the standard therapy for pre-B-ALL (Chan et al., 2017). Myc-transformed cells were shown to display dependence on AMPK-related kinase 5 (ARK5), an upstream regulator of AMPK (Liu et al., 2012). ARK5 enabled oncogenic Myc-expressing cells to sustain glutamine metabolism by maintaining mitochondrial respiratory capacity and expression of ETC complexes, maintaining cellular ATP levels and preventing energy depletion-induced apoptosis (Liu et al., 2012).
In addition to AMPK, autophagy is a homeostatic cellular recycling mechanism for damaged organelles and misfolded proteins that is rapidly upregulated under metabolic stress to provide energy needed to maintain essential functions. Autophagy has become a signature addiction of oncogenic Ras-driven tumors such as non-small cell lung cancer (NSCLC) and pancreatic cancer in which defective autophagy even in nutrient-replete conditions results in accumulation of damaged mitochondria, impaired oxidative phosphorylation and reduced rate of proliferation both in vitro and in vivo (Guo et al., 2011; White, 2013; Yang et al., 2011). Furthermore, autophagy deficiency in Kras-driven NSCLC models leads to benign oncocytomas rather than carcinomas and adenocarcinomas (Guo et al., 2013). Autophagy-defective Braf-driven cancer cells are also unable to drive malignant tumor growth due to accumulation of damaged mitochondria, oxidative stress and onset of senescence (Strohecker et al., 2013; Xie et al., 2015). Apart from supporting oxidative phosphorylation, FAO and limiting ROS via mitochondrial quality control, autophagy participates in amino acid homeostasis via degradation of intracellular proteins and supporting macropinocytosis, the uptake of bulk extracellular nutrients (Seo et al., 2016; Strohecker et al., 2013). Thus, autophagy is critical for overcoming oncogene-induced metabolic stress that leads to oncogene-induced senescence. Another function of autophagy together with the unfolded protein response is to prevent protein aggregates and endoplasmic reticulum stress in cancer where aneuploidy and gene copy number changes result in imbalances in transcript levels and protein expression (Santaguida et al., 2015; Santanam et al., 2016; Suh et al., 2012).
Oncogene-induced biosynthetic programs induce high glycolytic and TCA cycle fluxes which need to be balanced with the cellular redox state. Excess NADH can block downstream TCA cycle reactions and creates pressure on the electron transport chain and ATP synthetase resulting in formation of reactive oxygen species (ROS). Some of the excess reducing power is channeled towards production of lactate and citrate and ROS are important signaling molecules promoting cell proliferation, glycolysis, activation of HIF-1α, PI3K and MAPK signaling, and are produced by ETC as well as NADPH oxidases (Kamata et al., 2005; Lambeth, 2004; Lee et al., 2002b; Richard et al., 2000). This growth promoting-function of ROS promotes tumorigenesis, but the ROS level in cancer needs to be balanced as excessive ROS can lead to the oxidative damage to proteins, lipids and DNA, leading to senescence (Sullivan and Chandel, 2014; Weinberg et al., 2010). Hypoxia-reperfusion of tumors as well as oncogenic overexpression of Ras and Braf can lead to excessive ROS, making cancer cells reliant on antioxidant mechanisms (DeNicola et al., 2011; Irani et al., 1997). ROS detoxification mechanisms such as the glutathione system and superoxide dismutases are required for cancer cell survival, and impairing them can lead to selective killing of cancer cells (Chuang et al., 2003; Kerr et al., 2016; Yun et al., 2015). One of the substrates for glutathione synthesis is serine, its deprivation can lead to ROS-induced cell death in p53 deficient cancer cells, which are unable to direct limited serine from nucleotide synthesis to glutathione (Maddocks et al., 2013). This example again highlights a cancer vulnerability that has dual effects in cancer – one the one hand ROS detoxification mechanisms suppress tumor formation by suppressing growth-promoting increases in ROS, but they protect transformed cells from excess ROS that would lead to cellular damage and cancer cell death.
Tumor microenvironment and metabolic vulnerabilities of immune cells
Given the similar metabolic requirements for cell proliferation and anabolic metabolism between cancer and immune cells (Figure 4A), the tumor microenvironment represents a tug-o-war for nutrients. Direct measurement of glucose uptake and glycolysis of activated T cells and leukemic T cells showed a significantly higher rate of glucose metabolism in T cells (Kishton et al., 2016). Thus, in a direct cellular nutrient-competition setting, T cells are likely to out-compete tumor cells. However, the abundance of tumor cells lead to nutrient depletion, hypoxia and accumulation of metabolic waste products in the tumor microenvironment that pose a challenge for mounting a successful anticancer attack (Chang et al., 2015; Ho et al., 2015; Siska and Rathmell, 2015). The tumor microenvironment is heterogeneous with studies reporting high concentrations of lactic acid, derived from glycolysis and glutaminoysis, accompanied by reduced pH and low glucose concentration in tumor interstitial fluid compared to blood or spleen (Gullino et al., 1964; Ho et al., 2015; Wagner and Wiig, 2015; Wiig et al., 2010). Aberrant tumor vasculature further results in hypoxic regions and increased concentration of CO2 (Lee et al., 2014; Thomlinson and Gray, 1955; Wagner and Wiig, 2015; Wiig et al., 2010).
Glucose limitation not only impairs effector cell proliferation and biosynthetic capacity needed to perform their effector function, it also affects energy-sensing growth-modulating signaling pathways important for immune cell activation (Cham and Gajewski, 2005; Chang et al., 2015; Macintyre et al., 2014b). Limiting glucose or inhibiting glycolysis increases AMP/ATP ratio, which activates AMPK, and represses mTOR and HIF-1α activity, favoring immunosuppressive Treg over Teff CD4 T cells, anti-inflammatory M2 macrophages over M1 and impairs dendritic cell function (Blagih et al., 2015; Delgoffe et al., 2009; Krawczyk et al., 2010; Sag et al., 2008; Shi et al., 2011). Other nutrients such as amino acid leucine, are needed to maintain anabolic mTOR signaling to promote immune function (Sinclair et al., 2013b; Zheng et al., 2009).
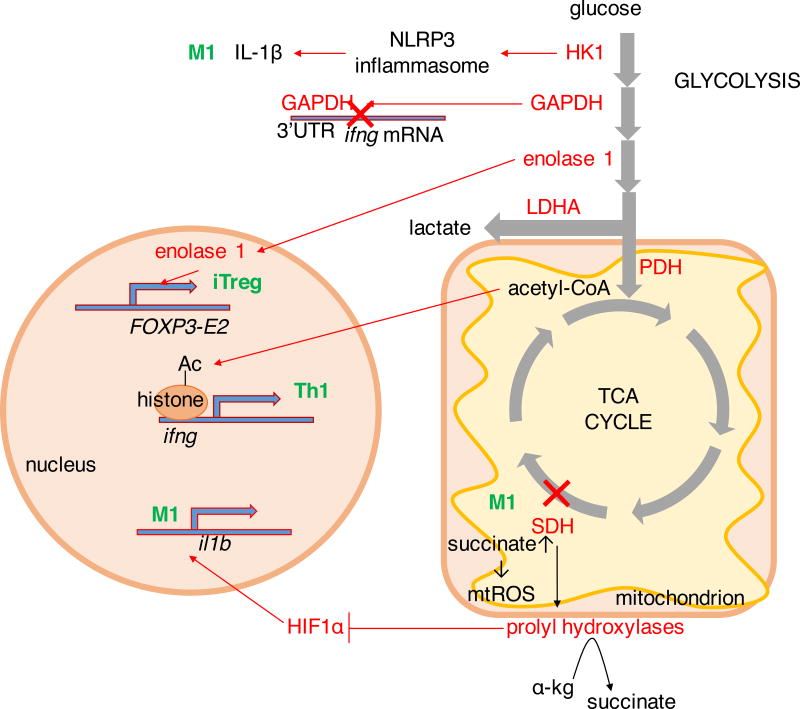
The different functional populations of immune cells each use a distinct metabolic program and altering those pathways can shift cellular differentiation and function. Glucose metabolism is a potent regulator of immune cell function. Glucose limitation or inhibition of glycolysis by 2-deoxyglucose in CD8 T cells resulted in reduced cytolytic activity and diminished production of IFNγ, granzyme B and GM-CSF (Cham and Gajewski, 2005; Cham et al., 2008). Apart from altering functionality, glucose limitation in activated T cells induces anergy, a state of unresponsiveness under activating conditions, characterized by inability to upregulate cytokine secretion and proliferate (Zheng et al., 2009). Tumor-infiltrating T cells (TILS) in mouse melanoma models demonstrated signatures of glucose deprivation and displayed reduced effector function, and this effect was exacerbated by overexpression of the glycolytic enzyme HK1 in melanoma xenografts, demonstrating a metabolic advantage for increased glucose metabolism in the tumor microenvironment (Ho et al., 2015). Restriction of melanoma growth in mice could be also achieved by overexpression of the glycolytic enzyme phosphoenolpyruvate carboxykinase in T cells, which sustained T cell receptor signaling (Ho et al., 2015). In xenograft models of antigenic sarcoma tumors that become rejected by T cell-mediated responses, increasing the tumor’s glycolytic capacity enhances tumor progression (Chang et al., 2015). TIL in these tumors had lower glucose uptake and reduced IFNγ production which in co-cultures could be rescued by glucose addition (Chang et al., 2015). Furthermore, checkpoint blockade therapy in progressive sarcoma models resulted in tumor regression, increased glucose availability in the tumor microenvironment, reduced glycolysis and mTORC1 activity in tumor cells with increased aerobic glycolysis and IFNγ production by TILS (Chang et al., 2015). Switching activated Th1 cells from glucose to galactose, a sugar that promotes oxidative phosphorylation did not impair their proliferation but abrogated the production of IFNγ (Chang et al., 2013). With reduced flux the glycolytic enzyme GAPDH directly inhibited IFNγ production by binding the 3’ untranslated region of ifng mRNA, which was rescued by addition of GAPDH substrate, 3-phosphoglycerate (Figure 5) (Chang et al., 2013). An alternate or parallel mechanism by which reduced glucose metabolism inhibits IFNγ production is through regulation of histone acetylation at the ifng locus (Figure 5) (Peng et al., 2016). The glycolytic enzyme hexokinase 1 has also been shown in macrophages to regulate inflammation and interact with the NOD-, leucine-rich region-, and pyrin domain-containing 3 (NLRP3) inflammasome, which activates caspase-1 to produce mature IL-1β and IL-18 (Figure 5) (Moon et al., 2015). The secretion of pro-inflammatory cytokines was reduced LPS-stimulated macrophages by glycolysis inhibitor 2-DG (Moon et al., 2015).
Figure 5. Metabolic enzymes in immune function.
Metabolic enzymes directly impact immunity. When glycolysis is low, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) binds the 3’untranslated region of ifng gene, suppressing its transcription. In contrast, lactate dehydrogenase A (LDHA) promotes cytoplasmic accumulation of acetyl-CoA, a substrate for histone acetylation which promotes transcription of ifng gene important in Th1-mediated immune responses. Enolase 1 is involved in splicing of the Treg signature transcription factor FOXP3 into FOXP3-E2 splice variant, while hexokinase 1 (HK1) promotes caspase-dependent cleavage of IL1β into its active form. Breakage of the TCA cycle at succinate dehydrogenase (SDH) in M1 macrophages leads to accumulation of succinate and production of mitochondrial ROS, inhibiting α-ketoglutarate-dependent prolyl hydroxylases that leads to stabilization of transcription factor HIF1α. In M1 macrophages this promotes transcription of il1b gene. α-kg, α-ketoglutarate; PDH, pyruvate dehydrogenase.
Glycolysis may play dual roles to regulate Treg. While Treg can be highly proliferative, they have also been shown to not require mTOR or Glut1 that promote anabolic metabolism and to instead primarily utilize an oxidative metabolism of lipids and pyruvate (Beier et al., 2015; Delgoffe et al., 2009; Gerriets et al., 2015; Macintyre et al., 2014b; Michalek et al., 2011). However, Treg can have considerable metabolic plasticity to increase glycolysis and respond to inflammatory cues with mTORC1 activation and Glut1 upregulation (Gerriets et al., 2016; Procaccini et al., 2010). In these cases, the Treg transcription factor FoxP3 can be downregulated, leading to reduced Treg stability and function (Gerriets et al., 2016; Huynh et al., 2015; Park et al., 2013; Shrestha et al., 2015). Indeed, Treg with elevated glycolysis were more proliferative, yet had reduced ability to maintain FoxP3 and suppress inflammation (Gerriets et al., 2016). Conversely, glycolysis inhibition has also been shown to result in localization of the glycolytic enzyme enolase-1 to the nucleus where it suppressed the expression of FoxP3-E2 splicing variant, impairing the development and suppressive capacity of Treg (Figure 5) (De Rosa et al., 2015).
Hypoxia is not only associated with tumors but is also occurs in inflamed tissue and lymphoid organs. In vitro studies indicated that oxygen limitation negatively impacted T cell proliferation and reduced the production of cytokines IFNγ and IL-2 (McNamee et al., 2013). The impact of hypoxia on CD8 T cells has been controversial. CD8 T cells were shown to have reduced proliferation and cytokine secretion at low oxygen, their cytolytic capacity was higher compared to normoxia, while other studies supported the conclusion that hypoxia is detrimental to CD8 T cell function (Caldwell et al., 2001; Vuillefroy de Silly et al., 2016). In vivo findings with T cell activation in hypoxic, normoxic and hyperoxic conditions revealed that T cell activation increased with increasing oxygen saturation, and was highest in T cells exposed to more oxygenated environments (Ohta et al., 2011). Hypoxia also promotes the production of S-2-hydroxyglutarate in CD8 T cells, which reduced effector cytokine secretion and cytolytic capacity of CD8 T cells but promoted their long-term in vivo persistence, proliferation and anti-tumor capacity (Tyrakis et al., 2016). Dendritic cells differentiated in hypoxic conditions expressed reduced levels of MHC-II, co-stimulatory molecules and produced less pro-inflammatory cytokines IL-1β, IL-6, and TNFα, which could be reversed and even enhanced by re-oxygenation (Wang et al., 2010). Hypoxic DC also produced higher levels of the immunosuppressive cytokine TGFβ, implying their role in immunosuppressive Treg generation (Wang et al., 2010). Macrophages accumulate in hypoxic tumor regions, these tumor-associated macrophages secrete vascular endothelial growth factor and epidermal growth factor, promoting tumor invasion, angiogenesis and metastasis and are correlated with poor disease outcome (Murdoch et al., 2004). Some of the effects of hypoxia are due to the induction of HIF1α. In CD4 T cells, HIF1α stimulates the development of Th17 lineage by promoting transcription of Th17 signature transcription factor RORγt and cytokine IL-17 (Dang et al., 2011). Conversely, HIF1α may inhibit the development of Treg by targeting the Treg transcription factor FoxP3 for degradation (Dang et al., 2011).
Lactic acid, another result of cancer glycolysis, suppresses glycolytic metabolism and results in lowering of the pH in the tumor microenvironment. It has suppressive effect on CD8 T cell proliferation and cytokine secretion by inhibiting the MAP kinase signaling (Fischer et al., 2007; Mendler et al., 2012). Tumor-derived lactic acid was taken up by macrophages and promoted their polarization to the suppressive M2 lineage via HIF-1α-mediated expression of M2-characteristic arginase 1 (Colegio et al., 2014). Lowered pH, which is associated with lactic acidosis, also leads to CD8 T cell anergy and elevation of tumor pH with proton pump inhibitors delayed cancer progression in both active and adoptive immunotherapy (Calcinotto et al., 2012).
Arginase I expression by myeloid-derived suppressor cells in the tumor environment is one of the mechanisms of T cell suppression (Rodriguez et al., 2004, 2009). Arginine supplementation promoted a central-memory like phenotype with enhanced IFNγ secretion upon restimulation, improved in vivo survival and enhanced anti-tumor immunity (Geiger et al., 2016). The pro-inflammatory M1 macrophages and the more tolerogenic M2 macrophages flux arginine into different and opposing pathways, antagonizing each other (Jha et al., 2015). M1 macrophages utilize inducible nitric oxide synthase to generate NO, which mediates antimicrobial and tumor-killing effects (Figure 3) (Mills et al., 1992; Rahat and Hemmerlein, 2013; Weiss et al., 2010). In comparison, M2 macrophages divert arginine to ornithine via arginase to synthesize hydroxyproline and polyamines, important in wound healing and tissue repair (Mills et al., 1992). Polyamines, produced by both cancer cells and anti-inflammatory immune cells are abundant in the tumor microenvironment and inhibit T cell function (Nowotarski et al., 2013; Ye et al., 2016). Blocking polyamine synthesis by targeting ornithine decarboxylase augments CD8 T cell tumor infiltration and IFNγ production (Ye et al., 2016).
The first immunosuppressive effects of the depletion of the essential amino acid tryptophan on T cells were observed when inhibition of tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) induced rejection of fetal allografts (Munn et al., 1998). It was subsequently shown that IFNγ and CD40-stimulated macrophages express high levels of IDO, and that stimulation of T cells in the absence of tryptophan results in cell cycle arrest and a state of anergy mediated in part by sensing of uncharged tryptophan tRNA (Lee et al., 2002a; Munn et al., 1999, 2005). Increased expression of IDO in cancer correlates with poor disease outcome and reduced function of T cells, which can be rescued by pharmacologically blocking IDO with 1-methyltryptophan (Platten et al., 2012; Uyttenhove et al., 2003). Other immunosuppressive effects mediated by IDO as well as the alternative enzyme tryptophan-2,3-dioxygenase 2 (TDO), include tryptophan catabolites which activate aryl hydrocarbon receptor and apoptosis in select T cell subsets (Fallarino et al., 2002; Ninomiya et al., 2016; Platten et al., 2012).
Exploiting metabolic vulnerabilities in cancer and immunity
Increased glycolysis has been associated with inflammatory effector phenotypes in a variety of activated immune cells (O’Neill et al., 2016). Conversely, mitochondrial oxidative programs are associated with memory, suppressive, or wound healing immune cell phenotypes. Each subset of immune cells has now been shown to have distinct metabolic requirements (Buck et al., 2015; O’Neill and Pearce, 2016; O’Neill et al., 2016). Thus, although T cells, macrophages, and dendritic cells each have significant plasticity to engage in other metabolic pathways for energy generation, survival and proliferation, these changes alter or impair immune function. These alterations in cell function are due to requirements of each immunological subset for specific metabolites, signaling intermediates, or epigenetic modifications mediated by metabolite levels. As metabolic intermediates change, cell differentiation is not properly induced or stress response pathways feed-back to restrict or alter cell fate.
Unlike immune cells that are activated by antigen and inflammatory cues that yield coordinated metabolic programs, tumorigenic transformations do not activate metabolic pathways in a concerted manner. Thus, cancer cells can develop substrate dependencies guided by oncogene-driven metabolic programs. The reprograming of cellular metabolism by Myc towards glutaminolytic TCA cycle with minimized glucose contribution creates a glutamine addiction in Myc transformed cells (Wise and Thompson, 2010; Wise et al., 2008). Similarly, cancer cells with deregulated Akt signaling are glucose addicted (Elstrom et al., 2004). A special case is cancers with FH and SDH mutations. The lack of a functional TCA cycle in these cells makes them reliant on glycolysis and pyruvate carboxylase anaplerotic fluxes to sustain biosynthetic reactions (Cardaci et al., 2015; Tong et al., 2011).
Studies that examine the metabolism of cancer and immune cells have led to a remarkable reshaping of our understanding of cell metabolism and the response of each to metabolic manipulation. Indeed, therapies such as methotrexate and metformin do target cell metabolism, and other pre-clinical treatments with 2-deoxyglucose, DCA, or DON to target glycolysis, pyruvate oxidation, and glutamine metabolism can protect animal models from a variety of inflammatory disease models (Eleftheriadis et al., 2013b; Gerriets et al., 2015; Lee et al., 2015; Michalek et al., 2011; Ohashi et al., 2013; Ostroukhova et al., 2012; Shi et al., 2011; Yin et al., 2015). While targeting cancer metabolism holds promise, results in vivo have been thus far disappointing and less robust than those obtained by targeting immune cell metabolism. This discrepancy may be because while immunity shifts away from inflammatory responses upon mild metabolic inhibition, cancer cells must be metabolically inhibited to an extent sufficient to induce apoptosis if the cancer is to be eliminated. A further area where these two distinct modes of response to metabolic stress may influence cell fate is in the tumor microenvironment. T cells and inflammatory macrophages must function to drive immunity in the context of tumors where the microenvironment is metabolically hostile. Better understanding of metabolic requirements, metabolic pathway influence on cell signaling and differentiation, and of how different cell populations respond to metabolic stress will now provide improved approaches to modulate immunity in both inflammatory diseases and in cancer.
Acknowledgments
We thank members of the Rathmell lab for their conversations and intellectual input. This work was supported by R01DK105550 (J.C.R.) and R21CA194829 (J.C.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors of this manuscript have a financial interest related to this work.
References
- Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013;71:1115–1130. doi: 10.1007/s00280-012-2062-0. [DOI] [PubMed] [Google Scholar]
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Aird KM, Zhang R. Metabolic alterations accompanying oncogene-induced senescence. Mol. Cell. Oncol. 2014;1:e963481. doi: 10.4161/23723548.2014.963481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison AC. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47:63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- Appleman LJ, van Puijenbroek AAFL, Shu KM, Nadler LM, Boussiotis VA. CD28 Costimulation Mediates Down-Regulation of p27kip1 and Cell Cycle Progression by Activation of the PI3K/PKB Signaling Pathway in Primary Human T Cells. J. Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo L, Khim P, Mkhikian H, Mortales C-L, Demetriou M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife. 2017;6:e21330. doi: 10.7554/eLife.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi MSM, Newsholme EA. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem. J. 1982;208:743–748. doi: 10.1042/bj2080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi MSM, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Biochem. J. 1983;212:835–842. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, DeRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, Lipshultz SE, Camitta BM. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118:874 LP–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, et al. Memory CD8+ T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity. 2016;44:1312–1324. doi: 10.1016/j.immuni.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Beier UH, Angelin A, Akimova T, Wang L, Liu Y, Xiao H, Koike MA, Hancock SA, Bhatti TR, Han R, et al. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de Novo Pyrimidine Synthesis by Growth Signaling Through mTOR and S6K1. Science (80-.) 2013;339:1323 LP–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bahre H, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Josefsson E, Jonsson I-M, Verdrengh M, Ohlsson C, Bokarewa M, Tarkowski A, Magnusson M. Dichloroacetate alleviates development of collagen II-induced arthritis in female DBA/1 mice. Arthritis Res. {&} Ther. 2009;11:R132. doi: 10.1186/ar2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnson E, Mukhopadhyay B, Asplund A, Pristovsek N, Cinar R, Romeo S, Uhlen M, Kunos G, Nielsen J, Mardinoglu A. Stratification of Hepatocellular Carcinoma Patients Based on Acetate Utilization. Cell Rep. 2015;13:2014–2026. doi: 10.1016/j.celrep.2015.10.045. [DOI] [PubMed] [Google Scholar]
- Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vázquez G, Yurchenko E, Raissi TC, van der Windt GJW, Viollet B, Pearce EL, et al. The Energy Sensor AMPK Regulates T Cell Metabolic Adaptation and Effector Responses In Vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Blanco Calvo M, Figueroa A, Grande Pulido E, García Campelo R, Antón Aparicio Luís Potential Role of Sugar Transporters in Cancer and Their Relationship with Anticancer Therapy. Int. J. Endocrinol. 2010;2010:14. doi: 10.1155/2010/205357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker FE, Atai NA, Lamba S, Jonker A, Rijkeboer D, Bosch KS, Tigchelaar W, Troost D, Vandertop WP, Bardelli A, et al. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome JD. EVIDENCE THAT THE L-ASPARAGINASE OF GUINEA PIG SERUM IS RESPONSIBLE FOR ITS ANTILYMPHOMA EFFECTS. J. Exp. Med. 1963;118:121–148. doi: 10.1084/jem.118.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345 LP–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang C-H, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJW, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2017;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M, et al. Modulation of Microenvironment Acidity Reverses Anergy in Human and Murine Tumor-Infiltrating T Lymphocytes. Cancer Res. 2012;72:2746 LP–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential Effects of Physiologically Relevant Hypoxic Conditions on T Lymphocyte Development and Effector Functions. J. Immunol. 2001;167:6140 LP–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Cahill J, Yamaguchi-Okada M, Zhang JH. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1α and its downstream target genes. J. Appl. Physiol. 2006;101:853 LP–865. doi: 10.1152/japplphysiol.00268.2006. [DOI] [PubMed] [Google Scholar]
- Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings G, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22:427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao MD, Sitter B, Bathen TF, Bofin A, Lønning PE, Lundgren S, Gribbestad IS. Predicting long-term survival and treatment response in breast cancer patients receiving neoadjuvant chemotherapy by MR metabolic profiling. NMR Biomed. 2012;25:369–378. doi: 10.1002/nbm.1762. [DOI] [PubMed] [Google Scholar]
- Cardaci S, Zheng L, MacKay G, van den Broek NJF, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V, Kamphorst JJ, et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol. 2015;17:1317–1326. doi: 10.1038/ncb3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, et al. Metabolic Signatures Uncover Distinct Targets in Molecular Subsets of Diffuse Large B Cell Lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG, Soares FA. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics. 2011;66:965–972. doi: 10.1590/S1807-59322011000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli LR, Varella-Garcia M, Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 1997;8:1189–1198. [PubMed] [Google Scholar]
- Cham CM, Gajewski TF. Glucose Availability Regulates IFN-γ Production and p70S6 Kinase Activation in CD8+ Effector T Cells. J. Immunol. 2005;174:4670 LP–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LN, Chen Z, Braas D, Lee J-W, Xiao G, Geng H, Cosgun KN, Hurtz C, Shojaee S, Cazzaniga V, et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature. 2017;542:479–483. doi: 10.1038/nature21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC-C, van der Windt GJW, Blagih J, Qiu J, et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJW, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Matés JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl. Acad. Sci. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen S, Lorendeau D, Schmieder R, Broekaert D, Metzger K, Veys K, Elia I, Buescher JM, Orth MF, Davidson SM, et al. Breast Cancer-Derived Lung Metastases Show Increased Pyruvate Carboxylase-Dependent Anaplerosis. Cell Rep. 2016;17:837–848. doi: 10.1016/j.celrep.2016.09.042. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Chuang JI, Chang TY, Liu HS. Glutathione depletion-induced apoptosis of Ha-ras-transformed NIH3T3 cells can be prevented by melatonin. Oncogene. 2003;22:1349–1357. doi: 10.1038/sj.onc.1206289. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Zúñiga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Cirillo A, Salle ADi, Petillo O, Melone MAB, Grimaldi G, Bellotti A, Torelli G, de’ Santi MS, Cantatore G, Marinelli A, et al. High grade glioblastoma is associated with aberrant expression of ZFP57, a protein involved in gene imprinting, and of CPT1A and CPT1C that regulate fatty acid metabolism. Cancer Biol. Ther. 2014;15:735–741. doi: 10.4161/cbt.28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloff JL, Murphy JP, Braun CR, Harris IS, Shelton LM, Kami K, Gygi SP, Selfors LM, Brugge JS. Differential Glutamate Metabolism in Proliferating and Quiescent Mammary Epithelial Cells. Cell Metab. 2016;23:867–880. doi: 10.1016/j.cmet.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, et al. Acetate Dependence of Tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori CF, Cori GT. THE CARBOHYDRATE METABOLISM OF TUMORS: I. THE FREE SUGAR, LACTIC ACID, AND GLYCOGEN CONTENT OF MALIGNANT TUMORS. J. Biol. Chem. 1925;64:11–22. [Google Scholar]
- Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, Wang J, Ben-Sahra I, Byles V, Polynne-Stapornkul T, et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5:e11612. doi: 10.7554/eLife.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, Cohen HJ. The essential role of L-glutamine in lymphocyte differentiation in vitro. J. Cell. Physiol. 1985;124:275–282. doi: 10.1002/jcp.1041240216. [DOI] [PubMed] [Google Scholar]
- Cruys B, Wong BW, Kuchnio A, Verdegem D, Cantelmo AR, Conradi L-C, Vandekeere S, Bouche A, Cornelissen I, Vinckier S, et al. Glycolytic regulation of cell rearrangement in angiogenesis. Nat. Commun. 2016;7:12240. doi: 10.1038/ncomms12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad.Sci. U. S. A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR Kinase Differentially Regulates Effector and Regulatory T Cell Lineage Commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and Activation of Heart 6-Phosphofructo-2-kinase by Protein Kinase B and Other Protein Kinases of the Insulin Signaling Cascades. J. Biol. Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat. Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a Metabolic Gene Regulatory Network Downstream of mTOR Complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardy SR, Farnham PJ. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J. Biol. Chem. 2001 doi: 10.1074/jbc.M109014200. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Akt Maintains Cell Size and Survival by Increasing mTOR-dependent Nutrient Uptake. Mol. Biol. Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egler RA, Ahuja SP, Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016;7:62–71. doi: 10.4103/0976-500X.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis T, Pissas G, Karioti A, Antoniadi G, Antoniadis N, Liakopoulos V, Stefanidis I. Dichloroacetate at therapeutic concentration alters glucose metabolism and induces regulatory T-cell differentiation in alloreactive human lymphocytes. J. Basic Clin. Physiol. Pharmacol. 2013a;24:271. doi: 10.1515/jbcpp-2013-0001. [DOI] [PubMed] [Google Scholar]
- Eleftheriadis T, Pissas G, Karioti A, Antoniadi G, Antoniadis N, Liakopoulos V, Stefanidis I. Dichloroacetate at therapeutic concentration alters glucose metabolism and induces regulatory T-cell differentiation in alloreactive human lymphocytes. J. Basic Clin. Physiol. Pharmacol. 2013b;24:271. doi: 10.1515/jbcpp-2013-0001. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Everts B, Amiel E, Huang SC-C, Smith AM, Chang C-H, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJW, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKK[epsiv] supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of Ribonucleotide Availability to Proliferating T-lymphocytes from Healthy Humans: DISPROPORTIONATE EXPANSION OF PYRIMIDINE POOLS AND CONTRASTING EFFECTS OF DE NOVO SYNTHESIS INHIBITORS. J. Biol. Chem. 1995;270:29682–29689. [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et al. AMPK Is a Negative Regulator of the Warburg Effect and Suppresses Tumor Growth In Vivo. Cell Metab. 2012;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: Many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109:3812 LP–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Fontenelle LJ, Henderson JF. Sources of nitrogen as rate-limiting factors for purine biosynthesis de novo in Ehrlich ascites tumor cells. Biochim. Biophys. Acta - Gen. Subj. 1969;177:88–93. doi: 10.1016/0304-4165(69)90067-1. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 Signaling Pathway Regulates Glucose Metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic Reprogramming of Macrophages: GLUCOSE TRANSPORTER 1 (GLUT1)-MEDIATED GLUCOSE METABOLISM DRIVES A PROINFLAMMATORY PHENOTYPE. J. Biol. Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine Deprivation Induces Abortive S-Phase Rescued by Deoxyribonucleotides in K-Ras Transformed Fibroblasts. PLoS One. 2009;4:1–17. doi: 10.1371/journal.pone.0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17:1459–1466. doi: 10.1038/ni.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Aebersold R. Both phosphatidylinositol 3-kinase and phosphatidylinositol 4-kinase products are increased by antigen receptor signaling in B cells. J. Immunol. 1994;152:42–50. [PubMed] [Google Scholar]
- Gonen N, Assaraf YG. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resist. Updat. 2012;15:183–210. doi: 10.1016/j.drup.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Leca J, Olivares O, Lavaut M-N, Vidal N, Berthezéne P, Dusetti NJ, Loncle C, Calvo E, Turrini O, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. 2013;110:3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullino PM, Clark SH, Grantham FH. The interstitial fluid of solid tumors. Cancer Res. 1964;24:780–794. [PubMed] [Google Scholar]
- Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, Lin KY, Huang TT, Akhavan D, Hock MB, et al. EGFR Signaling Through an Akt-SREBP-1-Dependent, Rapamycin-Resistant Pathway Sensitizes Glioblastomas to Antilipogenic Therapy. Sci. Signal. 2009;2:ra82–ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Chen H-Y, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JMS, Karantza V, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK—Sensing Energy while Talking to Other Signaling Pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al. The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1α under hypoxic conditions in trophoblast-derived cells. J. Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P-C, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui Y-C, Cui G, Micevic G, Perales JC, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörig H, Spagnoli GC, Filgueira L, Babst R, Gallati H, Harder F, Juretic A, Heberer M. Exogenous glutamine requirement is confined to late events of T cell activation. J. Cell. Biochem. 1993;53:343–351. doi: 10.1002/jcb.240530412. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC-C, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill CM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of Hypoxia-Inducible Factor 1α Expression and Function by the Mammalian Target of Rapamycin. Mol. Cell. Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S-S, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, Raffatellu M, Osborne TF. Linking Lipid Metabolism to the Innate Immune Response in Macrophages through Sterol Regulatory Element Binding Protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R, Palmieri F, Iacobazzi V. The mitochondrial citrate carrier: a new player in inflammation. Biochem. J. 2011;438:433 LP–436. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan K-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic Signaling Mediated by Oxidants in Ras-Transformed Fibroblasts. Science (80-.) 1997;275:1649 LP–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, et al. PKM2 Isoform-Specific Deletion Reveals a Differential Requirement for Pyruvate Kinase in Tumor Cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher KJ. Sugar and amino acid transport by cells in culture—differences between normal and malignant cells. N. Engl. J. Med. 1972;286:929–933. doi: 10.1056/NEJM197204272861707. [DOI] [PubMed] [Google Scholar]
- Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado J, de D, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SC-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Jiang B-H, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-Kinase Signaling Controls Levels of Hypoxia-inducible Factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- Johnson AR, Qin Y, Cozzo AJ, Freemerman AJ, Huang MJ, Zhao L, Sampey BP, Milner JJ, Beck MA, Damania B, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol. Metab. 2016;5:506–526. doi: 10.1016/j.molmet.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun DY, Taub D, Chrest FJ, Kim YH. Requirement of the expression of 3-phosphoglycerate dehydrogenase for traversing S phase in murine T lymphocytes following immobilized anti-CD3 activation. Cell. Immunol. 2014;287:78–85. doi: 10.1016/j.cellimm.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive Oxygen Species Promote TNFα-Induced Death and Sustained JNK Activation by Inhibiting MAP Kinase Phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kamphorst JJ, Chung MK, Fan J, Rabinowitz JD. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer {&} Metab. 2014;2:23. doi: 10.1186/2049-3002-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankotia S, Stacpoole PW. Dichloroacetate and cancer: New home for an orphan drug? Biochim. Biophys. Acta - Rev. Cancer. 2014;1846:617–629. doi: 10.1016/j.bbcan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EME, Cascante M, Shlomi T, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- Kelly B, O’Neill LAJ. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35:603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kerr EM, Gaude E, Turrell FK, Frezza C, Martins CP. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature. 2016;531:110–113. doi: 10.1038/nature16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, et al. The sterol regulatory element binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. Evaluation of Myc E-Box Phylogenetic Footprints in Glycolytic Genes by Chromatin Immunoprecipitation Assays. Mol. Cell. Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishton RJ, Barnes CE, Nichols AG, Cohen S, Gerriets VA, Siska PJ, Macintyre AN, Goraksha-Hicks P, de Cubas AA, Liu T, et al. AMPK Is Essential to Balance Glycolysis and Mitochondrial Metabolism to Control T-ALL Cell Stress and Survival. Cell Metab. 2016;23:649–662. doi: 10.1016/j.cmet.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016;7:11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, et al. Toll-like receptor–induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742 LP–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 Glucose Transporters in Endometrial and Breast Cancers. Pathol. Oncol. Res. 2012;18:721–728. doi: 10.1007/s12253-012-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18:356–365. doi: 10.1038/ncb3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lane AN, Fan TW-M. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43:2466. doi: 10.1093/nar/gkv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein S, Zerilli M, zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br. J. Cancer. 2006;94:578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16 doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-F, Lo Y-C, Cheng C-H, Furtmuller GJ, Oh B, Andrade-Oliveira V, Thomas AG, Bowman CE, Slusher BS, Wolfgang MJ, et al. Preventing Allograft Rejection by Targeting Immune Metabolism. Cell Rep. 2015;13:760–770. doi: 10.1016/j.celrep.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-T, Boss M-K, Dewhirst MW. Imaging Tumor Hypoxia to Advance Radiation Oncology. Antioxid. Redox Signal. 2014;21:313–337. doi: 10.1089/ars.2013.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002a;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-R, Yang K-S, Kwon J, Lee C, Jeong W, Rhee SG. Reversible Inactivation of the Tumor Suppressor PTEN by H2O2. J. Biol. Chem. 2002b;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Lee SM, Koh H-J, Park D-C, Song BJ, Huh T-L, Park J-W. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic. Biol. Med. 2002c;32:1185–1196. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- Li Q, Rao R, Vazzana J, Goedegebuure P, Odunsi K, Gillanders W, Shrikant PA. Regulating Mammalian Target of Rapamycin To Tune Vaccination-Induced CD8 T Cell Responses for Tumor Immunity. J. Immunol. 2012;188:3080 LP–3087. doi: 10.4049/jimmunol.1103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- Liu L, Ulbrich J, Muller J, Wustefeld T, Aeberhard L, Kress TR, Muthalagu N, Rycak L, Rudalska R, Moll R, et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- Liu L, Lu Y, Martinez J, Bi Y, Lian G, Wang T, Milasta S, Wang J, Yang M, Liu G, et al. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1α-dependent. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1564–1569. doi: 10.1073/pnas.1518000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Karnell JL, Yin B, Zhang R, Zhang J, Li P, Choi Y, Maltzman JS, Pear WS, Bassing CH, et al. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J. Clin. Invest. 2010;120:2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-C, Li F, Handler J, Huang CRL, Xiang Y, Neretti N, Sedivy JM, Zeller KI, Dang CV. Global Regulation of Nucleotide Biosynthetic Genes by c-Myc. PLoS One. 2008;3:e2722. doi: 10.1371/journal.pone.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Ghazaly E, Freitas MO, Mitsinga M, Lattanzio L, Lo Nigro C, Nagano A, Wang J, Chelala C, Szlosarek P, et al. Inhibition of the Polyamine Synthesis Pathway Is Synthetically Lethal with Loss of Argininosuccinate Synthase 1. Cell Rep. 2016;16:1604–1613. doi: 10.1016/j.celrep.2016.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible Inactivation of HIF-1 Prolyl Hydroxylases Allows Cell Metabolism to Control Basal HIF-1. J. Biol. Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, Mainolfi N, Suri V, Guak H, Balmer ML, et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017;25:345–357. doi: 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014a;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, et al. The Glucose Transporter Glut1 Is Selectively Essential for CD4 T Cell Activation and Effector Function. Cell Metab. 2014b;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The Liver Kinase B1 Is a Central Regulator of T Cell Development, Activation, and Metabolism. J. Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks ODK, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng O, Kim YC, Shin H-J, Lee J-O, Huh T-L, Kang K, Kim YS, Paik S-G, Lee H. Cytosolic NADP(+)-dependent isocitrate dehydrogenase protects macrophages from LPS-induced nitric oxide and reactive oxygen species. Biochem. Biophys. Res. Commun. 2004;317:558–564. doi: 10.1016/j.bbrc.2004.03.075. [DOI] [PubMed] [Google Scholar]
- Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, et al. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. doi: 10.1002/nbm.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Nakamura H, Ikeda E, Ohnuma K, Yamauchi K, Yabe Y, Poellinger L, Okada Y, Morimoto C, Tanaka H. Hypoxia-Inducible Factor Regulates Survival of Antigen Receptor-Driven T Cells. J. Immunol. 2003;171:6534 LP–6540. doi: 10.4049/jimmunol.171.12.6534. [DOI] [PubMed] [Google Scholar]
- Mannava S, Grachtchouk V, Wheeler LJ, Im M, Zhuang D, Slavina EG, Mathews CK, Shewach DS, Nikiforov MA. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle. 2008;7:2392–2400. doi: 10.4161/cc.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaria R, Edwards HT, Dill DB. THE POSSIBLE MECHANISMS OF CONTRACTING AND PAYING THE OXYGEN DEBT AND THE R{O}LE OF LACTIC ACID IN MUSCULAR CONTRACTION. Am. J. Physiol. -- Leg. Content. 1933;106:689–715. [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang X-L, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, et al. Analysis of Tumor Metabolism Reveals Mitochondrial Glucose Oxidation in Genetically Diverse Human Glioblastomas in the Mouse Brain In Vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanovic S, Eriksson I, Nelson BD. Expression of a new set of glycolytic isozymes in activated human peripheral lymphocytes. Biochim. Biophys. Acta - Gene Struct. Expr. 1990;1087:1–6. doi: 10.1016/0167-4781(90)90113-g. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, et al. Acetate Is a Bioenergetic Substrate for Human Glioblastoma and Brain Metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang G-W, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- McNamee EN, Johnson DK, Homann D, Clambey ET. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 2013;55:58–70. doi: 10.1007/s12026-012-8349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Krämer L, Sapcariu SC, Battello N, Ghelfi J, D’Herouel AF, Skupin A, Hiller K. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J. Biol. Chem. 2015 doi: 10.1074/jbc.M115.676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011a;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011b;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B, Gfeller P, Guinet E. Restricting Glutamine or Glutamine-Dependent Purine and Pyrimidine Syntheses Promotes Human T Cells with High FOXP3 Expression and Regulatory Properties. J. Immunol. 2016;196:3618–3630. doi: 10.4049/jimmunol.1501756. [DOI] [PubMed] [Google Scholar]
- Meyerhof O. RECENT INVESTIGATIONS ON THE AEROBIC AND AN-AEROBIC METABOLISM OF CARBOHYDRATES. J. Gen. Physiol. 1927;8:531–542. doi: 10.1085/jgp.8.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J. Immunol. 1992;149:2709 LP–2714. [PubMed] [Google Scholar]
- Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457–470.e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2007;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RCT, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- Moon J-S, Hisata S, Park M-A, DeNicola GM, Ryter SW, Nakahira K, Choi AMK. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015;12:102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science (80-.) 1998;281:1191 LP–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T Cell Proliferation by Macrophage Tryptophan Catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- Murray CM, Hutchinson R, Bantick JR, Belfield GP, Benjamin AD, Brazma D, Bundick RV, Cook ID, Craggs RI, Edwards S, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H. TCR Engagement Increases Hypoxia-Inducible Factor-1α Protein Synthesis via Rapamycin-Sensitive Pathway under Hypoxic Conditions in Human Peripheral T Cells. J. Immunol. 2005;174:7592 LP–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Xiao Y, Zhou X, Chang J-H, Chang M, Cheng X, Blonska M, Lin X, Sun S-C. Inflammatory T Cell Responses Rely on Amino Acid Transporter ASCT2 Facilitation of Glutamine Uptake and mTORC1 Kinase Activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya S, Nakamura N, Kitagawa J, Hara T, Shimizu M, Tsurumi H. The Roles of Aryl Hydrocarbon Receptor in T Cells at IDO-Positive Tumor Microenvironment. Blood. 2016;128:3693 LP–3693. [Google Scholar]
- Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, Finkel T. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17:216–217. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotarski SL, Woster PM, Casero RA. Polyamines and cancer: Implications for chemoprevention and chemotherapy. Expert Rev. Mol. Med. 2013;15:e3–e3. doi: 10.1017/erm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213:15 LP–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, van der Windt GJW, Huang SC-C, Curtis JD, Chang C-H, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. Memory CD8+ T Cells Use Cell-Intrinsic Lipolysis to Support the Metabolic Programming Necessary for Development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Akazawa T, Aoki M, Kuze B, Mizuta K, Ito Y, Inoue N. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. Int. J. Cancer. 2013;133:1107–1118. doi: 10.1002/ijc.28114. [DOI] [PubMed] [Google Scholar]
- Ohta A, Diwanji R, Kini R, Subramanian M, Ohta A, Sitkovsky M. In vivo T Cell Activation in Lymphoid Tissues is Inhibited in the Oxygen-Poor Microenvironment. Front. Immunol. 2011;2:27. doi: 10.3389/fimmu.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML. METABOLIC PATTERNS IN THREE TYPES OF PHAGOCYTIZING CELLS. J. Cell Biol. 1963;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of Glucose Transporter 1 and Glycolytic Gene Expression by c-Myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang Q, Alam R. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol.- Lung Cell. Mol. Physiol. 2012;302:L300–L307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR, Domingo-Fernandez R, Johnston DGW, et al. Pyruvate Kinase M2 Regulates Hif-1a; Activity and IL-1b Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Park JH, Vithayathil S, Kumar S, Sung P-L, Dobrolecki LE, Putluri V, Bhat VB, Bhowmik SK, Gupta V, Arora K, et al. Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer. Cell Rep. 2016;14:2154–2165. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Jin H-S, Lopez J, Elly C, Kim G, Murai M, Kronenberg M, Liu Y-C. TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 2013;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteur L. Memoire sur la fermentation appeleelactique. Ann Chim Phys. 1858;52:404–418. [Google Scholar]
- Patel D, Menon D, Bernfeld E, Mroz V, Kalan S, Loayza D, Foster DA. Aspartate Rescues S-phase Arrest Caused by Suppression of Glutamine Utilization in KRas-driven Cancer Cells. J. Biol. Chem. 2016;291:9322–9329. doi: 10.1074/jbc.M115.710145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil MD, Bhaumik J, Babykutty S, Banerjee UC, Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer/’s armor. Oncogene. 2016;35:4957–4972. doi: 10.1038/onc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang L-S, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A, Doni A, Nebuloni M, Swigart LB, Evan GI, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–421. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science (80-.) 2016;354:481–484. doi: 10.1126/science.aaf6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna D, Faga G, Verrecchia A, Gorski MM, Barozzi I, Narang V, Khng J, Lim KC, Sung W-K, Sanges R, et al. Genome-wide mapping of Myc binding and gene regulation in serum-stimulated fibroblasts. Oncogene. 2012;31:1695–1709. doi: 10.1038/onc.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MM, Sheaff MT, Szlosarek PW. Targeting Arginine-Dependent Cancers with Arginine-Degrading Enzymes: Opportunities and Challenges. Cancer Res. Treat. 2013;45:251–262. doi: 10.4143/crt.2013.45.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs {NADPH} production and increases reactive oxygen species resulting in {ATP} depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta - Bioenerg. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Platten M, Wick W, den Eynde BJ. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012;72:5435–5440. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- Pollizzi KN, Sun I-H, Patel CH, Lo Y-C, Oh M-H, Waickman AT, Tam AJ, Blosser RL, Wen J, Delgoffe GM, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8+ T cell differentiation. Nat Immunol. 2016;17:704–711. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung Y-L, Schulze A. SREBP Activity Is Regulated by mTORC1 and Contributes to Akt-Dependent Cell Growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo H-K, Jang HG, Jha AK, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccini C, De Rosa V, Galgani M, Abanni L, Cali G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, et al. An Oscillatory Switch in mTOR Kinase Activity Sets Regulatory T Cell Responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front. Physiol. 2013;4:144. doi: 10.3389/fphys.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Heiden MGV, Harris MH, Frauwirth KA, Thompson CB. In the Absence of Extrinsic Signals, Nutrient Utilization by Lymphocytes Is Insufficient to Maintain Either Cell Size or Viability. Mol. Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur. J. Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouysségur J. Non-hypoxic pathway mediates the induction of hypoxia inducible factor 1 alpha (HIF-1α) in vascular smooth muscle cells. J. Biol. Chem. 2000 doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative Phosphoproteomics Reveal mTORC1 Activates de Novo Pyrimidine Synthesis. Science (80-.) 2013;339:1320 LP–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I–Producing Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma Are a Subpopulation of Activated Granulocytes. Cancer Res. 2009;69:1553 LP–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKα1: A glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, Romano A, Simone SDe, Procaccini C, Rocca CLa, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Vasile E, White E, Amon A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 2015;29:2010–2021. doi: 10.1101/gad.269118.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanam U, Banach-Petrosky W, Abate-Shen C, Shen MM, White E, DiPaola RS. Atg7 cooperates with Pten loss to drive prostate cancer tumor growth. Genes Dev. 2016;30:399–407. doi: 10.1101/gad.274134.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, et al. A gene expression database for the molecular pharmacology of cancer. Nat Genet. 2000;24:236–244. doi: 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- Schnyder J. Role of phagocytosis in the activation of macrophages. J. Exp. Med. 1978;148:1449–1457. doi: 10.1084/jem.148.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, Bruning U, Missiaen R, Queiroz KCS, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, et al. Acetyl-CoA Synthetase 2 Promotes Acetate Utilization and Maintains Cancer Cell Growth under Metabolic Stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16:708–717. doi: 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Sellers K, Fox MP, II MB, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. Pyruvate carboxylase is critical for non–small-cell lung cancer proliferation. J. Clin. Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Jiang B-H, Leung SW, Passantino R, Concordet J-P, Maire P, Giallongo A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-inducible Factor 1. J. Biol. Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- Seo J-W, Choi J, Lee S-Y, Sung S, Yoo HJ, Kang M-J, Cheong H, Son J. Autophagy is required for PDAC glutamine metabolism. Sci. Rep. 2016;6:37594. doi: 10.1038/srep37594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Chen S, Du F, Li S, Zhao L, Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc. Natl. Acad. Sci. U. S. A. 2011;108:4788–4793. doi: 10.1073/pnas.1100844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Dolde C, Lewis BC, Wu C-S, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016;213:337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi Y-B, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013a;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi Y-B, Taylor PM, Cantrell DA. Antigen receptor control of amino acid transport coordinates the metabolic re-programming that is essential for T cell differentiation. Nat. Immunol. 2013b;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar K, Huang H, Hu L, Cogdell D, Dhillon J, Tzelepi V, Efstathiou E, Koumakpayi IH, Saad F, Luo D, et al. Integrative molecular profiling reveals asparagine synthetase is a target in castration-resistant prostate cancer. Am. J. Pathol. 2012;180:895–903. doi: 10.1016/j.ajpath.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015;36:257–264. doi: 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, Metabolism, and Cancer. Cancer Discov. 2015 doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E. Autophagy Sustains Mitochondrial Glutamine Metabolism and Growth of BRAF(V600E)–Driven Lung Tumors. Cancer Discov. 2013;3 doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarshan S, Sourbier C, Kong H-S, Block K, Romero VV, Yang Y, Galindo C, Mollapour M, Scroggins B, Goode N, et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and HIF-1α stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 2009;29:4080–4090. doi: 10.1128/MCB.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh DH, Kim M-K, Kim HS, Chung HH, Song YS. Unfolded protein response to autophagy as a promising druggable target for anticancer therapy. Ann. N. Y. Acad. Sci. 2012;1271:20–32. doi: 10.1111/j.1749-6632.2012.06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer {&} Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DMF, Cantrell DA. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 2016;17:712–720. doi: 10.1038/ni.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is a danger signal that induces IL-1β via HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko TN, Gomez-Rodriguez J, McGuire PJ. Impaired T cell function in argininosuccinate synthetase deficiency. J. Leukoc. Biol. 2015;97:273–278. doi: 10.1189/jlb.1AB0714-365R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomlinson RH, Gray LH. The Histological Structure of Some Human Lung Cancers and the Possible Implications for Radiotherapy. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W-H, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, Romero VV, Sougrat R, Vaulont S, Viollet B, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah MS, Davies AJ, Halestrap AP. The Plasma Membrane Lactate Transporter MCT4, but Not MCT1, Is Up-regulated by Hypoxia through a HIF-1α-dependent Mechanism. J. Biol. Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennstrom B, Sheiness D, Zabielski J, Bishop JM. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J. Virol. 1982;42:773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist KC, Guy CS, Milasta S, Liedmann S, Kamiński MM, Wang R, Green DR. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389–393. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent EE, Coelho PP, Blagih J, Griss T, Viollet B, Jones RG. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene. 2015;34:3627–3639. doi: 10.1038/onc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin. Cancer Biol. 2006;16:318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Vuillefroy de Silly R, Dietrich P-Y, Walker PR. Hypoxia and antitumor CD8(+) T cells: An incompatible alliance? Oncoimmunology. 2016;5:e1232236. doi: 10.1080/2162402X.2016.1232236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Wiig H. Tumor Interstitial Fluid Formation, Characterization, and Clinical Implications. Front. Oncol. 2015;5:115. doi: 10.3389/fonc.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Guo K, Gao D, Kang X, Jiang K, Li Y, Sun L, Zhang S, Sun C, Liu X, et al. Identification of transaldolase as a novel serum biomarker for hepatocellular carcinoma metastasis using xenografted mouse model and clinic samples. Cancer Lett. 2011a;313:154–166. doi: 10.1016/j.canlet.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu C, Zhu F, Liu F, Zhang P, Guo C, Wang X, Li H, Ma C, Sun W, et al. Reoxygenation of hypoxia-differentiated dentritic cells induces Th1 and Th17 cell differentiation. Mol. Immunol. 2010;47:922. doi: 10.1016/j.molimm.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011b;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tsun Z-Y, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Sci. 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the Origin of Cancer Cells. Science (80-.) 1956;123:309 LP–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The metabolis of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SG, Westwick J, Hall ND, Sansom DM. Ligation of CD28 receptor by B7 induces formation of D-3 phosphoinositides in T lymphocytes independently of T cell receptor/CD3 activation. Eur. J. Immunol. 1993;23:2572–2577. doi: 10.1002/eji.1830231029. [DOI] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GRS, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, et al. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J. Exp. Med. 2010;207:2455 LP–2467. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev. 2013;27:2065–2071. doi: 10.1101/gad.228122.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman HL, Wofford JA, Rathmell JC. Cytokine Stimulation Promotes Glucose Uptake via Phosphatidylinositol-3 Kinase/Akt Regulation of Glut1 Activity and Trafficking. Mol. Biol. Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Tenstad O, Iversen PO, Kalluri R, Bjerkvig R. Interstitial fluid: the overlooked component of the tumor microenvironment? Fibrogenes. {&} Tissue Repair. 2010;3:12. doi: 10.1186/1755-1536-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial Respiratory Capacity Is A Critical Regulator Of CD8(+) T Cell Memory Development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang X-Y, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, Ward PS, Shay JES, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong S-P, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Xie H, Hanai J, Ren J-G, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, et al. Targeting Lactate Dehydrogenase-A Inhibits Tumorigenesis and Tumor Progression in Mouse Models of Lung Cancer and Impacts Tumor-Initiating Cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Koh JY, Price S, White E, Mehnert JM. Atg7 Overcomes Senescence and Promotes Growth of BrafV600E-Driven Melanoma. Cancer Discov. 2015;5:410–423. doi: 10.1158/2159-8290.CD-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’Antonio G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA, Abu-Asab MS, Bratslavsky G, Tsokos M, Merino MJ, et al. UOK 262: Fumarate Hydratase (−/−) Hereditary Leiomyomatosis Renal Cell Carcinoma: In Vitro and In Vivo Model of an Aberrant Energy Metabolic Pathway in Human Cancer. Cancer Genet. Cytogenet. 2010;196:45–55. doi: 10.1016/j.cancergencyto.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, Geng Z, Dominguez D, Chen S, Fan J, Qin L, Long A, Zhang Y, Kuzel TM, Zhang B. Targeting Ornithine Decarboxylase by α-Difluoromethylornithine Inhibits Tumor Growth by Impairing Myeloid-Derived Suppressor Cells. J. Immunol. 2016;196:915 LP–923. doi: 10.4049/jimmunol.1500729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee C-H, et al. Akt Stimulates Hepatic SREBP1c and Lipogenesis through Parallel mTORC1-Dependent and Independent Pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Choi S-C, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015;7:274ra18–274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio IIC, Giannopoulou EG, Rago C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science (80-.) 2015;350:1391 LP–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuseff M-I, Pierobon P, Reversat A, Lennon-Dumenil A-M. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol. 2013;13:475–486. doi: 10.1038/nri3469. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, et al. Asparagine Plays a Critical Role in Regulating Cellular Adaptation to Glutamine Depletion. Mol. Cell. 2014;56:205–218. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tschumi BO, Lopez-Mejia IC, Oberle SG, Meyer M, Samson G, Ruegg MA, Hall MN, Fajas L, Zehn D, et al. Mammalian Target of Rapamycin Complex 2 Controls CD8 T Cell Memory Differentiation in a Foxo1-Dependent Manner. Cell Rep. 2016;14:1206–1217. doi: 10.1016/j.celrep.2015.12.095. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T Cells Are Metabolically Anergic. J. Immunol. 2009;183:6095 LP–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu M-M, Simons JW, Semenza GL. Modulation of Hypoxia-inducible Factor 1α Expression by the Epidermal Growth Factor/Phosphatidylinositol 3-Kinase/PTEN/AKT/FRAP Pathway in Human Prostate Cancer Cells: Implications for Tumor Angiogenesis and Therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- Zhu A, Lee D, Shim H. Metabolic PET Imaging in Cancer Detection and Therapy Response. Semin. Oncol. 2011;38:55–69. doi: 10.1053/j.seminoncol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]