Abstract
Walking speed is modulated using propulsive forces (FP) during push-off and both preferred speed and FP decrease with aging. However, even prior to walking slower, reduced FP may be accompanied by potentially unfavorable changes in joint power generation. For example, compared to young adults, older adults exhibit a redistribution of mechanical power generation from the propulsive plantarflexor muscles to more proximal muscles acting across the knee and hip. Here, we used visual biofeedback based on real-time FP measurements to decouple and investigate the interaction between joint-level coordination, whole-body FP, and walking speed. 12 healthy young subjects walked on a dual-belt instrumented treadmill at a range of speeds (0.9 – 1.3 m/s). We immediately calculated the average FP from each speed. Subjects then walked at 1.3 m/s while completing a series of biofeedback trials with instructions to match their instantaneous FP to their averaged FP from slower speeds. Walking slower decreased FP and total positive joint work with little effect on relative joint-level contributions. Conversely, subjects walked at a constant speed with reduced FP, not by reducing total positive joint work, but by redistributing the mechanical demands of each step from the plantarflexor muscles during push-off to more proximal leg muscles during single support. Interestingly, these naturally emergent joint- and limb-level biomechanical changes, in the absence of neuromuscular constraints, resemble those due to aging. Our findings provide important reference data to understand the presumably complex interactions between joint power generation, whole-body FP, and walking speed in our aging population.
Keywords: Plantarflexor, Push-off, Aging, Gait, Biofeedback
1. INTRODUCTION
Walking speed is modulated using propulsive forces generated during push-off (i.e., the anterior component of the ground reaction force vector; FP), and both preferred speed and FP decrease considerably with aging (Nilsson and Thorstensson, 1989). However, even prior to a clinically relevant decline in walking speed, a reduction in FP may be accompanied by potentially unfavorable changes in joint power generation. For example, compared to young adults walking at the same speed, older adults exhibit a redistribution of mechanical power generation from the propulsive plantarflexor (i.e., ankle extensor) muscles to more proximal muscles crossing the knee and hip (DeVita and Hortobagyi, 2000; Franz and Kram, 2013a). In addition to preceding the age-related slowing of preferred speed, these changes may in part mediate the age-related reduction in walking economy (Franz, 2016). Intuitively, reduced FP and slowed preferred speed arise at least in part from altered joint kinetics, in turn governed by joint-level neuromuscular constraints (e.g., muscle weakness). However, the presumably complex interaction between joint-level coordination, whole-body FP, and walking speed is not well understood, even in healthy young adults.
All biomechanical features of walking scale with speed. At the whole-body level, slower walking speeds are accompanied by nearly linear reductions in peak FP, propulsive impulse, and thus total trailing leg positive work performed during push-off (Donelan et al., 2002; Nilsson and Thorstensson, 1989; Peterson et al., 2011). Walking speed effects on leg joint kinetics are also well described in the literature (Ardestani et al., 2016; Denning et al., 2016; Gomenuka et al., 2014; Lelas et al., 2003; Orendurff et al., 2008). For example, Lelas et al. (2003) showed that peak leg joint moments and power generation decreased systematically when walking slower and could be well-predicted in healthy subjects based on their walking speed alone (Lelas et al., 2003). More recently, Farris and Sawicki (2012) revealed that although walking slower reduced peak leg joint kinetics and total positive joint work, the relative contributions from muscles spanning the ankle, knee, or hip to that total was preserved across walking speed (Farris and Sawicki, 2012). We interpret these findings in healthy young adults to suggest that, at least in the absence of joint-level neuromuscular constraints, maintaining these patterns of joint-level coordination is an important and highly functional component of walking.
Cumulative insights underscore the high degree of interdependence among walking speed, FP, and joint kinetics, potentially confounding efforts to understand mechanisms governing the onset and progression of biomechanical changes in aging. Cross-sectional studies suggest that preferred walking speed decreases on average by 16% per decade after age 60 (Abellan van Kan et al., 2009; Bohannon and Williams Andrews, 2011; Himann et al., 1988). However, even prior to walking slower, older adults generate up to 20% smaller FP and up to 26% less trailing limb power during push-off compared to young adults walking at the same speed (Franz and Kram, 2014). Total positive work is largely unaffected by aging, alluding to prerequisite total positive work requirements to walk at a given speed (Mian et al., 2006; Ortega and Farley, 2007). In contrast, aging elicits a redistribution of power generation away from the propulsive plantarflexor muscles during push-off, toward a reliance on more proximal leg muscles during single support (DeVita and Hortobagyi, 2000). However, this characteristic pattern of joint-level coordination in elderly gait, associated with reduced FP and thought to precede the slowing of preferred speed, is fundamentally different from that associated with walking slower. A more complete understanding of the biomechanical changes that precede the slowing of walking speed may have broad implications; similar and simultaneous interdependent changes in walking speed, FP, and joint kinetics also emerge with more acute mobility impairment such as that following stroke (Farris et al., 2015; Hsiao et al., 2015a). However, unlike the well-documented biomechanical changes due to walking speed, to our knowledge no study to date has successfully decoupled the independent effects of reducing FP on joint power generation from the neuromuscular constraints that may precipitate them. Lewis and Ferris (2008) attempted to do so using verbal cues, but these were met with relatively invariant leg joint kinetics. Interestingly, those authors cited a lack of neuromuscular constraints as a likely explanation (Lewis and Ferris, 2008).
The purpose of this study was to investigate the interaction between walking speed, FP, and joint power generation during walking in young adults. Using systematic adjustments in treadmill speed and visual biofeedback based on real-time FP measurements, we tested the hypotheses that: (i) walking slower reduces FP and total positive joint work without affecting joint-level coordination, while conversely, (ii) reducing FP modulates joint-level coordination without affecting total positive joint work. Finally, given their prominent role in generating propulsion (Francis et al., 2013), we hypothesized that plantarflexor muscle activity during push-off would decrease progressively with incremental reductions in walking speed or FP.
2. METHODS
2.1. Participants
12 healthy young subjects participated after providing written, informed consent according to the University of North Carolina Institutional Review Board. Subjects had a mean ± standard deviation age of 26.2 ± 3.1 years, height of 1.75 ± 0.09 m, and body mass of 71.6 ± 8.8 kg. All subjects were free of neurologic impairments and musculoskeletal injury.
2.2. Procedures
Subjects first walked normally on a dual-belt, force-sensing treadmill (Bertec, Columbus, OH) at 1.3 m/s, approximating their preferred speed, and at four slower speeds (0.9 – 1.2 m/s) in 0.1 m/s increments for 1 min each in randomized order. We immediately processed and extracted subject’s average bilateral peak FP from each walking speed for use as target values in subsequent visual biofeedback trials. For trials incorporating visual biofeedback, a custom Matlab (Mathworks, Natick, MA) script continuously computed the average bilateral peak FP from each set of four consecutive steps and visually displayed those values in real-time (Fig. 1). Subjects completed an exploration trial of at least 3 min while freely modulating their instantaneous FP on the projected display to become familiar with the biofeedback paradigm. Finally, subjects walked at 1.3 m/s for 2 min each while matching their instantaneous FP to target values representing the averaged FP extracted from the slower speeds. We sought to investigate naturally emergent biomechanical patterns underlying reductions in FP, and thus did not instruct subjects how to attain target values. We only explained the timing of push-off and that the muscles of the leg generated a force during that time to propel their body forward.
Figure 1.
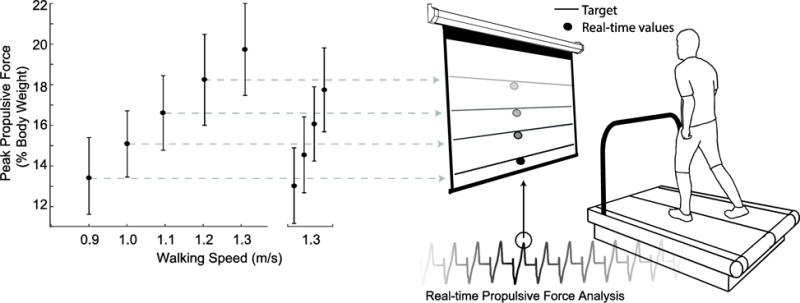
Experimental design using visual biofeedback to decouple the effects of walking speed and propulsive force on joint- and limb-level biomechanical variables. Horizontal lines represent target values extracted from slower speeds while circles represent group average (standard deviation) FP values.
2.3. Measurement and analysis
A 14-camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA) operating at 100 Hz recorded pelvis and lower extremity kinematics via 17 anatomical markers and an additional 14 tracking markers affixed using rigid clusters. A standing trial also included medial knee and ankle markers. We collected bilateral electromyographic (EMG) recordings of muscle activity from the medial gastrocnemius (MG), a major plantarflexor muscle, and its primary antagonist, the tibialis anterior (TA), at 1000 Hz using wireless electrodes (Delsys Trigno, Natick, MA) applied using conductive gel.
We analyzed only the second minute of biofeedback trials to allow subjects time to reach each target. Marker trajectories and ground reaction forces were filtered using 4th order low-pass Butterworth filters with cutoff frequencies of 6 Hz and 100 Hz, respectively. We then used the static standing calibration and functional hip joint centers from a leg circumduction task (Piazza et al., 2001) to scale a seven segment, 18 degree-of-freedom model of the pelvis and right and left legs (Arnold et al., 2010). We used the filtered marker and force data to estimate hip, knee, and ankle joint angles, moments, and powers using an inverse dynamics routine described in detail previously (Silder et al., 2008). From each subject’s average curve, we extracted values corresponding to local minima and maxima for each kinematic and kinetic variable. Further, due to the lack of a distinct peak, we also extracted values corresponding to midstance (i.e., 30% stride). Finally, at the limb level, we calculated the total work performed on the center of mass (CoM) by the leading and trailing legs during double support and the stance leg during single support using the Individual Limbs Method (ILM) (Donelan et al., 2002).
Stride-averaged EMG data were demeaned and rectified, band-pass filtered (20–400Hz), and then low-pass filtered (10Hz) to compute a linear envelope before averaging between left and right legs. We normalized all EMG data to the average signal across a stride during the normal, 1.3 m/s walking trial. Finally, for each subject we computed phase-averaged EMG activity according to the following: loading response (0–10%), mid-stance (10–30%), terminal stance (30–60%), initial swing (60–73%), mid-swing (73–87%), and terminal swing (87–100%) (Perry and Burfield, 2014). Due to a measurement error confirmed by statistical tests for outliers, we excluded two subjects’ MG activity.
2.4. Statistical Analysis
Shapiro-Wilks tests confirmed normal distributions for each outcome measure (i.e., FP, hip, knee, and ankle joint angles, moments, and powers, total positive joint work and relative joint contributions, CoM work, and EMG activity). We then tested for main effects of speed and FP on all outcome measures using two one-way repeated measures analyses of variance. When a significant main effect was found, planned post-hoc pairwise comparisons were focused between normal walking at 1.3 m/s and either the slower speed conditions (Hypotheses 1 and 3) or the reduced FP conditions (Hypotheses 2 and 3). Finally, paired t-tests determined the success of subjects’ reaching FP targets. Significance was defined using an alpha level of 0.05.
3. RESULTS
3.1. Propulsive Force
FP decreased linearly with slower walking speed by up to 32% across the range of speeds tested (main effect, p<0.001) (Fig. 1). When walking at 1.3 m/s, subjects also significantly and systematically reduced their average FP to match the prescribed targets (main effect, p<0.001) (Fig. 1). Subjects average FP differed significantly from the target values only for those extracted from the 1.0 m/s (p=0.011) and 1.1 m/s (p<0.001) conditions, and in both cases subjects undershot the target values. For the same reduction in FP, walking slower and walking with smaller FP elicited comparable reductions in stride length (p’s<0.001) (Table 1).
Table 1.
Mean ± standard deviation stride length during normal and biofeedback trials (m)
| Paired Speed (m/s) | Normal | Biofeedback |
|---|---|---|
| 0.9 | 1.15 ± 0.06 | 1.16 ± 0.08 |
| 1.0 | 1.21 ± 0.06 | 1.19 ± 0.08 |
| 1.1 | 1.27 ± 0.07 | 1.24 ± 0.06 |
| 1.2 | 1.34 ± 0.06 | 1.32 ± 0.06* |
| 1.3 | 1.40 ± 0.07 | N/A |
significantly different between Normal and Biofeedback (p<0.05)
3.2. Joint and Whole Body Mechanics
Walking slower decreased total positive joint work by up to 34% across the range of speeds tested (p<0.001). However, walking speed had little effect on the relative contributions from the individual leg joints; walking slower elicited only a modest 9% decrease in the hip’s contribution to total positive work (p=0.005) (Fig. 2A). Positive CoM work, both that performed by the trailing leg during push-off and by the stance leg during single support, decreased systematically with slower speed (p’s<0.001) (Fig. 2B). For example, compared to walking at 1.3 m/s, positive push-off work and positive single support work decreased by 27% and 48%, respectively, when walking at 0.9 m/s. Finally, walking slower elicited modest but statistically significant reductions in knee extension during stance (p=0.001) and ankle plantarflexion during push-off (p<0.001) (Fig. 3).
Figure 2.
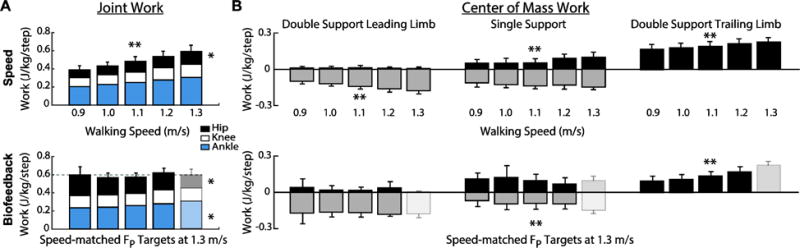
Group average (A) joint work and (B) center of mass work across walking speeds and prescribed values of propulsive force. Single asterisks (*) represent significant main effects of speed or reduced FP on leg joint work. Double asterisks (**) represent significant main effects of speed or reduced FP on total work.
Figure 3.
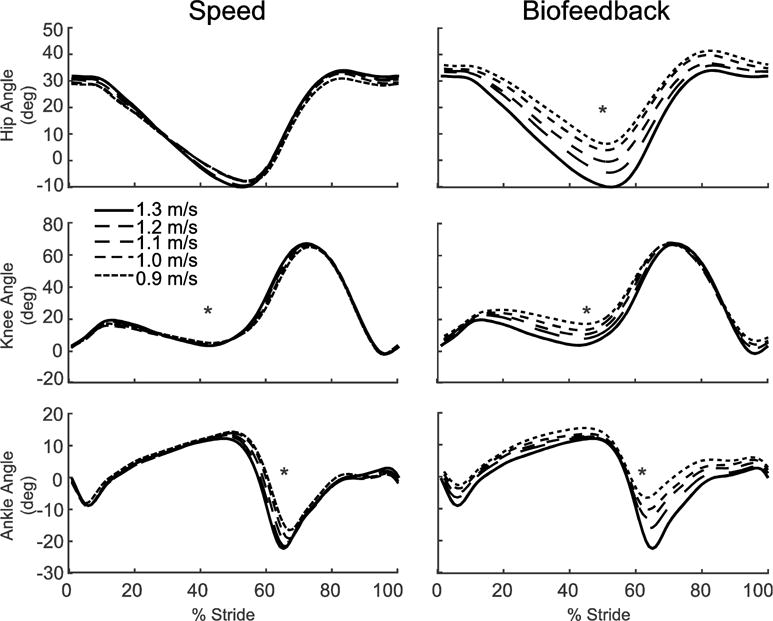
Group average lower extremity joint angles as modified by reductions in speed (left) and FP (right). Positive values indicate flexion. Gray dashed lines represent biofeedback trials at 1.3 m/s with FP target values prescribed from the corresponding walking speeds. Asterisks (*) represent significant main effects of speed or reduced FP.
Despite exerting identical FP during push-off, walking at 1.3 m/s while independently reducing FP using biofeedback elicited substantially different biomechanical changes from those observed when walking slower. With smaller FP, total positive joint work remained unchanged, regardless of reduction in force (Fig. 2A). However, for the lowest FP condition compared to walking normally, the ankle’s contribution to total positive joint work per step decreased from 51% to 39% (p<0.001) and the hip’s contribution increased from 25% to 39% (p<0.001) when walking with smaller FP. These changes were accompanied by significant decreases in ankle moment (p<0.001) and increases in hip moment (p<0.001) (Fig. 4). To reduce FP, subjects progressively decreased positive CoM work performed by the trailing leg during push-off by up to 58% (p’s<0.001) (Fig. 2B). Moreover, and in contrast to walking slower, reducing FP tended to increase positive CoM work during single support (p=0.057), also the phase in which we observed the most prominent increase in hip joint power generation (Fig. 5). Finally, regarding kinematic changes, reducing FP decreased extension at all leg joints (p’s<0.001) (Fig. 3).
Figure 4.
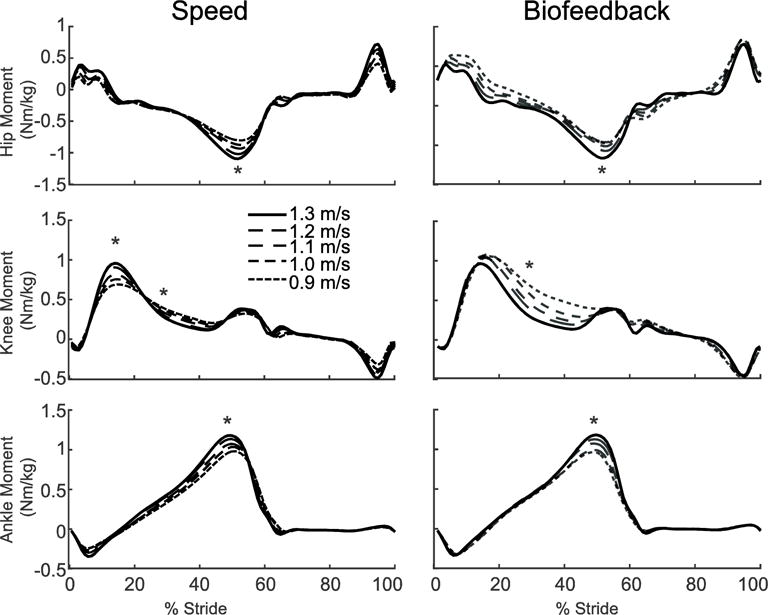
Group average lower extremity joint moments as modified by reductions in speed (left) and FP (right). Positive values indicate external extension moments. Gray dashed lines represent biofeedback trials at 1.3 m/s with FP target values prescribed from the corresponding walking speeds. Asterisks (*) represent significant main effects of speed or reduced FP.
Figure 5.
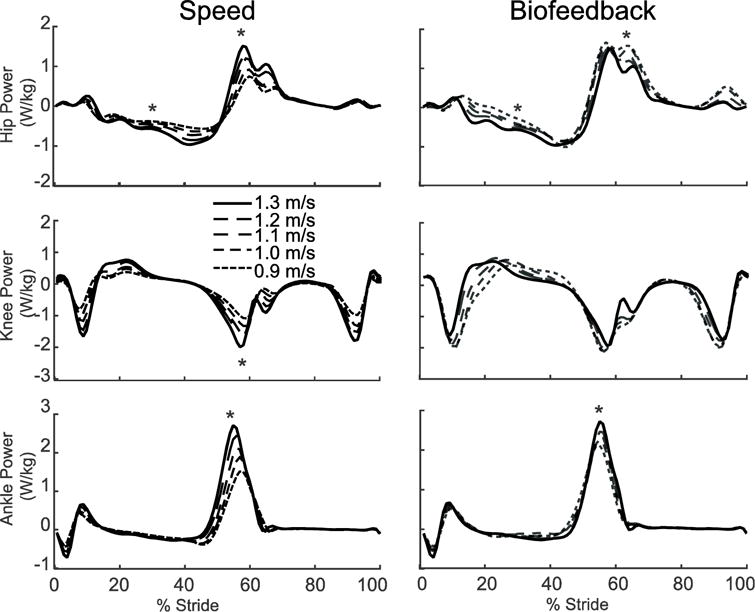
Group average lower extremity joint powers as modified by reductions in speed (left) and FP (right). Positive values indicate power generation. Gray dashed lines represent biofeedback trials at 1.3 m/s with FP target values prescribed from corresponding walking speeds. Asterisks (*) represent significant main effects of speed or reduced FP.
3.3. Electromyography
Walking slower decreased MG and TA muscle activations (Fig. 6). Compared to normal walking at 1.3 m/s, MG activity decreased with speed throughout stance (p’s<0.036) and during terminal swing (p=0.020). TA activity decreased during the loading response (p<0.001) and throughout swing (p’s<0.018). When walking with reduced FP, changes in MG activity resembled those observed for walking slower; compared to walking normally, MG activity decreased during terminal stance and pre-swing (p<0.001). Pairwise comparisons of terminal stance and pre-swing MG activity showed a significant reduction (p’s<0.003) for each reduced FP trial compared to normal walking at 1.3 m/s with no differences between any other reduced FP condition. Conversely, and in contrast to walking slower, TA activity tended to increase during the loading response (p=0.065) and from mid-stance through terminal swing (p’s<0.015).
Figure 6.
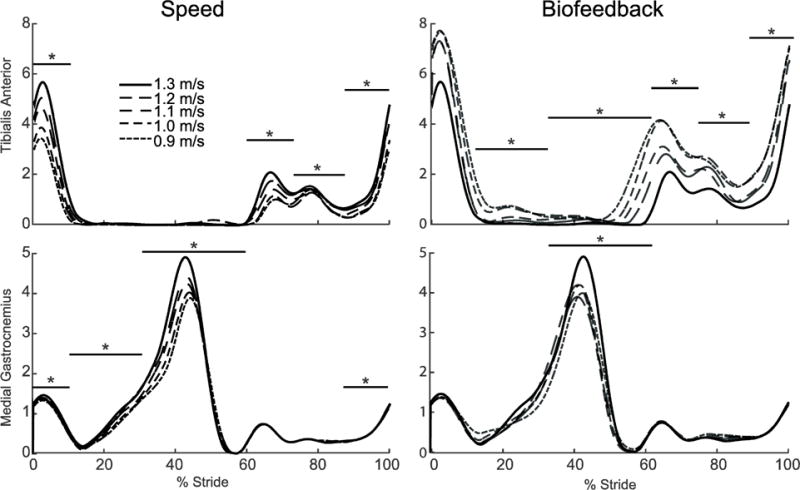
Group average electromyographic (EMG) recordings from the medial gastrocnemius and tibialis anterior as modified by reductions in speed (left) and FP (right). Gray dashed lines represent biofeedback trials at 1.3 m/s with FP target values prescribed from corresponding walking speeds. Asterisks (*) and horizontal bars represent gait cycle phases with significant main effects of speed or reduced FP.
DISCUSSION
Humans modulate their walking speed using propulsive forces (FP) generated during push-off. Accordingly, clinically relevant reductions in preferred speed in old age are universally accompanied by smaller peak FP, which may in turn be governed by complex changes in leg joint kinetics. This high degree of interdependence among walking speed, FP, and underlying joint kinetics may confound efforts to investigate the onset and progression of biomechanical changes in aging. Therefore, in this study, we decoupled and quantified the independent effects of walking slower and reducing FP on leg joint power generation during the stance phase of walking. First, our results reveal that, despite being highly interdependent, reducing FP elicits profound effects on leg joint kinetics that differ substantially from those due to reducing walking speed. Second, and much more surprisingly, we found that young adults, in the absence of joint-level neuromuscular constraints, reduced FP using naturally emergent joint- and limb-level biomechanical patterns that very closely resemble those due to aging.
We largely accept our first hypothesis; walking slower reduced FP and total positive joint work with negligible effects on coordination between the hip, knee, and ankle joint musculature. Our findings are in agreement with the abundant literature demonstrating relatively linear reductions in peak joint moments and powers with slower walking speed (Ardestani et al., 2016; Denning et al., 2016; Donelan et al., 2002; Gomenuka et al., 2014; Lelas et al., 2003; Nilsson and Thorstensson, 1989; Orendurff et al., 2008; Peterson et al., 2011). Confirming their high degree of interdependence, these biomechanical changes were accompanied by systematic reductions in FP and also with shorter steps and smaller peak leg joint ranges of motion. Also in agreement with prior studies (Farris and Sawicki, 2012), we found that the relative contributions from muscles spanning the hip, knee, and ankle to total positive joint work was generally preserved across the walking speeds tested. We observed only a subtle (<10%) decrease in the net contribution of muscles spanning the hip. We interpret these relatively consistent contributions to suggest that, at least in the absence of joint-level neuromuscular constraints, the maintenance of joint-level coordination is a functionally relevant component of normal, unimpaired walking. Thus, we posit that the presence of altered joint-level coordination, regularly observed with aging but not independently associated with walking speed, is more closely associated foremost with reductions in FP.
Despite identical effects on step length, independently reducing FP during walking elicited joint- and limb-level biomechanical adaptations that were fundamentally different from those due to walking slower. We fully accept our second hypothesis; reducing FP while maintaining walking speed modulated joint-level coordination without affecting total positive joint work. Subjects significantly and systematically reduced FP, not by reducing total positive joint work, but by redistributing the mechanical demands of each step from the plantarflexor muscles to more proximal muscles crossing the hip. Indeed, a post-hoc linear regression revealed a significant correlation underlying this distal to proximal redistribution of joint power generation (R2=0.45, p<0.001, Figure 7). We also discovered a series of joint-level kinematic and limb-level kinetic changes that we can also link to walking with reduced FP. For example, we observed unique and considerable decreases in hip, knee, and ankle joint extension, most notably during late stance (i.e., hip and knee) and early swing (i.e., ankle). Reduced joint extension, specifically at the hip, is not an altogether surprising modification to reduce FP. Hsiao et. al. (2015) used models to predict that trailing limb angle, a value closely related to peak hip extension, is one major determinant of FP (Hsiao et al., 2015b). Interestingly, at the knee, the increased joint flexion and thus flexor moment during single support served only to delay the timing of power absorption and generation without altering the relative contribution of muscles crossing the knee to total positive joint work. We also observed a phase-dependent redistribution of work performed on the CoM at the individual limb level unique to reducing FP. Specifically, subjects progressively reduced trailing limb positive work during push-off which, given the requirement to preserve their walking speed, subsequently precipitated an increase in net positive work performed during single support. Cumulatively, and as we elaborate more below, these joint- and limb-level biomechanical changes elicited solely by prescribing smaller FP are precisely those most ascribed to elderly gait.
Figure 7.
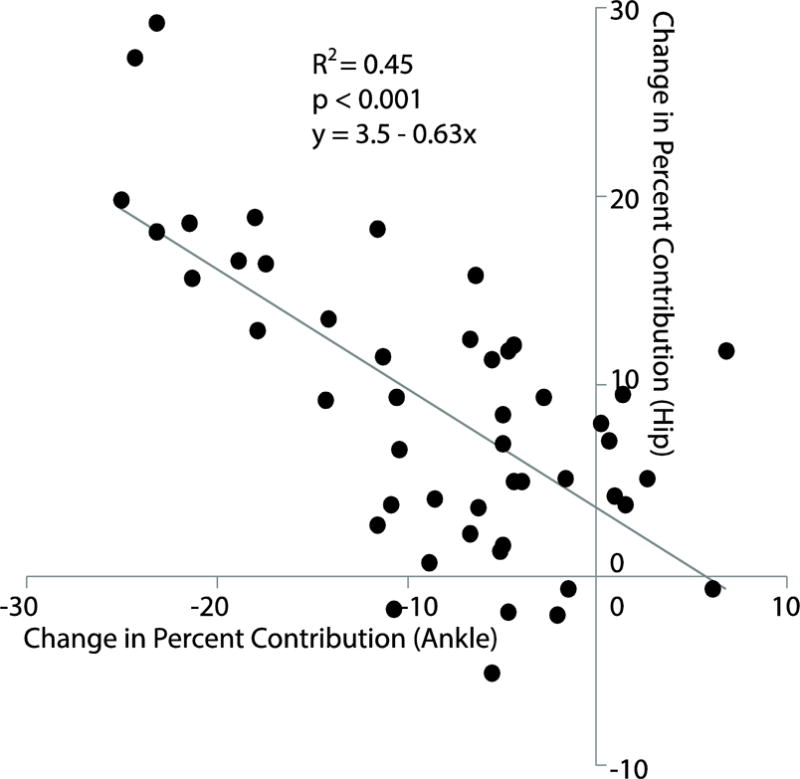
Bivariate correlation between the change in percent contribution from muscles spanning the hip versus ankle to total positive joint work relative to normal walking for all subjects walking with reduced FP targets. The gray line represents the best-fit linear regression.
Young adults walking at a given speed but with smaller FP elicited a multitude of biomechanical adaptations reminiscent of elderly gait. Consistent with our young subjects walking with smaller FP, older adults walk with a well-described distal to proximal redistribution of power generation during walking compared to young adults walking normally (DeVita and Hortobagyi, 2000; Franz and Kram, 2014) In addition, Franz and Kram (2014) reported that older adults exhibit the phase-dependent redistribution of positive CoM during walking, from the trailing limb during push off to the stance limb during single support (Franz and Kram, 2013a), also evident in our young subjects. We also noted several kinematic similarities between our young subjects responding to biofeedback and characteristic age-related changes in walking, including reduced peak hip extension and shorter step lengths. Thus, our findings may allude to complex associations between joint- and limb-level biomechanical changes that emerge with age-related mobility impairment and the onset of reductions in FP during push-off.
Finally, we partially accept our hypothesis that plantarflexor muscle activity during push-off would decrease progressively with incremental reductions in walking speed or FP. Indeed, MG activity decreased significantly when subjects walked slower or were encouraged to reduce their FP during push-off. We suspected that reductions in MG activity would be proportional to those for FP. However, compared to walking normally, we found that subjects reduced their push-off MG activity by a relatively constant amount for all biofeedback conditions, despite considerable differences in the prescribed FP. Instead, we observed that the significant but constant reduction in MG activity was systematically coupled with a step-wise increase in TA activity with smaller FP. Increased TA activity, and presumably muscle force, would facilitate a reduction in the net plantarflexor moment during push-off, a second major determinant of FP (Hsiao et al., 2015b). Moreover, these neuromuscular changes used to reduce FP were distinct from the simultaneous reductions in both MG and TA activity used to walk slower, despite very similar consequences on the net ankle moment during push-off. Thus, in the absence of neuromuscular constraints (i.e., muscle weakness, etc.), our findings suggest that changes to muscle recruitment patterns are capable of contributing to reduced ankle power generation and FP during push-off. This is particularly relevant given the profound changes in leg muscle recruitment patterns that emerge in old age (Franz and Kram, 2013b; Schmitz et al., 2009).
This study provides no direct insight into the origins of age-related mobility impairment. However, the unanticipated similarities between joint- and limb-level biomechanical changes elicited by reduced FP and those commonly ascribed to older adults do point to FP as an important and potentially relevant target for intervention. Although they are commonly prescribed and consistently improve muscle strength in older adults, resistance training interventions often fail to directly translate to improving propulsive power generation or walking speed (Beijersbergen et al., 2013). As an alternative or perhaps complementary approach, our prior work has demonstrated the clinical viability of visual biofeedback to encourage older adults with propulsive deficits to enhance their FP (Franz et al., 2014). Our present findings allude to potentially favorable joint- and limb-level changes that may accompany those enhancements in older adults, including but not limited to a potential reversal of the characteristic distal to proximal redistribution of power generation. In addition, and admittedly speculative, these gait changes may have consequences for walking economy. Anecdotally, our subjects reported that it was aerobically challenging to match the prescribed FP, especially those extracted from the slowest speeds. One possible explanation is that a redistribution to more proximal leg muscles for power generation is energetically costly (Huang et al., 2015). We note that older adults consume oxygen 15–20% faster than young adults during walking. Thus, future work should investigate the extent to which age-related declines in walking economy are biomechanically mediated as well as the energetic consequences of targeted interventions designed to enhance push-off power generation via FP (Franz, 2016).
We acknowledge several limitations of this study. First, we recorded EMG activity for only the medial gastrocnemius and tibialis anterior. Thus, we were unable to discern how changes in muscle activities of the quadriceps or hamstrings contributed to reported changes net leg joint kinetics. Further, consistent with our clinical motivation, our protocol for these young adult subjects did not include biofeedback targets representing increases in FP. Future studies may use a similar paradigm to investigate the independent effects of walking faster or with larger FP, providing valuable reference data for potential improvements in gait biomechanics following intervention. Our primary research focus pertained to leg joint power generation and forward propulsion. Thus, we did not record upper body kinematics such as trunk position or arm swing that may have systematically varied with biofeedback and thus indirectly influenced our outcome measures. We also interpret our findings primarily in the context of their implications for age-related gait changes, but did not include a cohort of older adults in our study design. Lastly, we interpret the distal to proximal redistribution of joint power generation in elderly gait as unfavorable for its potential role in governing age-related changes in walking performance and economy, but alternative interpretations exist. For example, increased trunk flexion in older adults may require increased hip extensor moments to prevent falls.
CONCLUSION
We used visual biofeedback to decouple the effects of reduced FP and slower speed on joint power generation in walking. Our findings largely supported our hypotheses and revealed that, despite being fundamentally interdependent, reducing FP elicits profound effects on leg joint kinetics that differ substantially from those due to walking slower. Moreover, we found that reducing FP in healthy young adults elicited naturally emergent joint- and limb-level biomechanical patterns that resemble those due to aging. In the absence of joint-level neuromuscular constraints, these changes simultaneously included reduced joint extension, reduced push-off work, increased single support work, and a redistribution of the mechanical demands of each step to muscles acting across the hip. Our findings provide important reference data for isolating the presumably complex interactions between joint power, whole-body FP, and walking speed in our aging population.
Acknowledgments
We thank Randall Bissette for assisting with data collection. This work was supported by grants from NIH (R01AG051748) and the University of North Carolina University Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
References
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Ardestani MM, Ferrigno C, Moazen M, Wimmer MA. From normal to fast walking: Impact of cadence and stride length on lower extremity joint moments. Gait Posture. 2016;46:118–125. doi: 10.1016/j.gaitpost.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Annals of biomedical engineering. 2010;38:269–279. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijersbergen CM, Granacher U, Vandervoort AA, DeVita P, Hortobagyi T. The biomechanical mechanism of how strength and power training improves walking speed in old adults remains unknown. Ageing research reviews. 2013;12:618–627. doi: 10.1016/j.arr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Denning WM, Becker Pardo M, Winward JG, Hunter I, Ridge S, Hopkins JT, Reese CS, Parcell AC, Seeley MK. Ambulation speed and corresponding mechanics are associated with changes in serum cartilage oligomeric matrix protein. Gait Posture. 2016;44:131–136. doi: 10.1016/j.gaitpost.2015.11.007. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol (1985) 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Simultaneous positive and negative external mechanical work in human walking. J Biomech. 2002;35:117–124. doi: 10.1016/s0021-9290(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Farris DJ, Hampton A, Lewek MD, Sawicki GS. Revisiting the mechanics and energetics of walking in individuals with chronic hemiparesis following stroke: from individual limbs to lower limb joints. J Neuroeng Rehabil. 2015;12:24. doi: 10.1186/s12984-015-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris DJ, Sawicki GS. The mechanics and energetics of human walking and running: a joint level perspective. J R Soc Interface. 2012;9:110–118. doi: 10.1098/rsif.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CA, Lenz AL, Lenhart RL, Thelen DG. The modulation of forward propulsion, vertical support, and center of pressure by the plantarflexors during human walking. Gait Posture. 2013;38:993–997. doi: 10.1016/j.gaitpost.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR. The Age-Associated Reduction in Propulsive Power Generation in Walking. Exercise and sport sciences reviews. 2016;44:129–136. doi: 10.1249/JES.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Kram R. Advanced age affects the individual leg mechanics of level, uphill, and downhill walking. J Biomech. 2013a;46:535–540. doi: 10.1016/j.jbiomech.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Kram R. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture. 2013b;37:378–384. doi: 10.1016/j.gaitpost.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Kram R. Advanced age and the mechanics of uphill walking: a joint-level, inverse dynamic analysis. Gait Posture. 2014;39:135–140. doi: 10.1016/j.gaitpost.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Maletis M, Kram R. Real-time feedback enhances forward propulsion during walking in old adults. Clin Biomech (Bristol, Avon) 2014;29:68–74. doi: 10.1016/j.clinbiomech.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Gomenuka NA, Bona RL, da Rosa RG, Peyre-Tartaruga LA. Adaptations to changing speed, load, and gradient in human walking: cost of transport, optimal speed, and pendulum. Scandinavian journal of medicine & science in sports. 2014;24:e165–173. doi: 10.1111/sms.12129. [DOI] [PubMed] [Google Scholar]
- Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20:161–166. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- Hsiao H, Awad LN, Palmer JA, Higginson JS, Binder-Macleod SA. Contribution of Paretic and Nonparetic Limb Peak Propulsive Forces to Changes in Walking Speed in Individuals Poststroke. Neurorehabil Neural Repair. 2015a doi: 10.1177/1545968315624780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. The relative contribution of ankle moment and trailing limb angle to propulsive force during gait. Hum Mov Sci. 2015b;39:212–221. doi: 10.1016/j.humov.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TW, Shorter KA, Adamczyk PG, Kuo AD. Mechanical and energetic consequences of reduced ankle plantar-flexion in human walking. J Exp Biol. 2015;218:3541–3550. doi: 10.1242/jeb.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelas JL, Merriman GJ, Riley PO, Kerrigan DC. Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture. 2003;17:106–112. doi: 10.1016/s0966-6362(02)00060-7. [DOI] [PubMed] [Google Scholar]
- Lewis CL, Ferris DP. Walking with increased ankle pushoff decreases hip muscle moments. J Biomech. 2008;41:2082–2089. doi: 10.1016/j.jbiomech.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta physiologica (Oxford, England) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Thorstensson A. Ground reaction forces at different speeds of human walking and running. Acta physiologica Scandinavica. 1989;136:217–227. doi: 10.1111/j.1748-1716.1989.tb08655.x. [DOI] [PubMed] [Google Scholar]
- Orendurff MS, Bernatz GC, Schoen JA, Klute GK. Kinetic mechanisms to alter walking speed. Gait Posture. 2008;27:603–610. doi: 10.1016/j.gaitpost.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol (1985) 2007;102:2266–2273. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- Perry J, Burfield J. Gait Analysis: Normal and Pathological Function, Second ed. SLACK Incorporated; 2014. [Google Scholar]
- Peterson CL, Kautz SA, Neptune RR. Braking and propulsive impulses increase with speed during accelerated and decelerated walking. Gait Posture. 2011;33:562–567. doi: 10.1016/j.gaitpost.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza SJ, Okita N, Cavanagh PR. Accuracy of the functional method of hip joint center location: effects of limited motion and varied implementation. J Biomech. 2001;34:967–973. doi: 10.1016/s0021-9290(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silder A, Heiderscheit B, Thelen DG. Active and passive contributions to joint kinetics during walking in older adults. J Biomech. 2008;41:1520–1527. doi: 10.1016/j.jbiomech.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


