Abstract
Background:
Current understanding of tumor biology suggests that breast cancer is a group of diseases with different intrinsic molecular subtypes. Anatomic staging system alone is insufficient to provide future outcome information. The American Joint Committee on Cancer (AJCC) expert panel updated the 8th edition of the staging manual with prognostic stage groups by incorporating biomarkers into the anatomic stage groups. In this study, we retrospectively analyzed the data from our center in China using the anatomic and prognostic staging system based on the AJCC 8th edition staging manual.
Methods:
We reviewed the data from January 2008 to December 2014 for cases with Luminal B Human Epidermal Growth Factor Receptor 2 (HER2)-negative breast cancer in our center. All cases were restaged using the AJCC 8th edition anatomic and prognostic staging system. The Kaplan-Meier method and log-rank test were used to compare the survival differences between different subgroups. SPSS software version 19.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses.
Results:
This study consisted of 796 patients with Luminal B HER-negative breast cancer. The 5-year disease-free survival (DFS) of 769 Stage I–III patients was 89.7%, and the 5-year overall survival (OS) of all 796 patients was 91.7%. Both 5-year DFS and 5-year OS were significantly different in the different anatomic and prognostic stage groups. There were 372 cases (46.7%) assigned to a different group. The prognostic Stage II and III patients restaged from anatomic Stage III had significant differences in 5-year DFS (χ2 = 11.319, P = 0.001) and 5-year OS (χ2 = 5.225, P = 0.022). In addition, cases restaged as prognostic Stage I, II, or III from the anatomic Stage II group had statistically significant differences in 5-year DFS (χ2 = 6.510, P = 0.039) but no significant differences in 5-year OS (χ2 = 5.087, P = 0.079). However, the restaged prognostic Stage I and II cases from anatomic Stage I had no statistically significant differences in either 5-year DFS (χ2 = 0.440, P = 0.507) or 5-year OS (χ2 = 1.530, P = 0.216).
Conclusions:
The prognostic staging system proposed in the AJCC 8th edition refines the anatomic stage group in Luminal B HER2-negative breast cancer and will lead to a more personalized approach to breast cancer treatment.
Keywords: American Joint Committee on Cancer, Biomarker, Breast Cancer, Cancer Stage, Luminal B Human Epidermal Growth Factor Receptor 2-negative, Prognostic Factors
INTRODUCTION
In 1977, the 1st edition of the American Joint Committee for Cancer (AJCC) cancer staging manual was published and reported the TNM (primary tumor [T], regional lymph nodes [N], and distant metastases [M]) staging system.[1] This system is used worldwide as a standardized classification system for malignant tumors. The system is based on a robust set of anatomic disease principles. The TNM staging system is important for prognostic prediction and for treatment recommendations such as adjuvant chemotherapy after surgery.
The extensive research on tumor biology and the 2000 study by Perou et al.[2] have classified breast tumors into subtypes distinguished by pervasive differences in their gene expression patterns. The results supported the proposal that different intrinsic gene subsets could be accompanied by phenotypic diversity in breast tumors. There was consensus that molecular typing was an ideal model for defining the heterogeneity of breast tumors. However, the clinical routine for genetic profiling was not yet established. Thus, surrogate molecular subtypes by immunohistochemical typing were considered the state of the art for assessing risk of relapse and estimating the probable effect of specific therapy in the 2011 St. Gallen International Expert Consensus.[3] The panel supported the clinicopathological determination of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 to define subtypes of primary breast cancer as Luminal A, Luminal B, HER2-positive, and triple negative diseases. The transition to the predominance of tumor biology rather than numerical disease indicators such as tumor size or extent of nodal involvement was finalized by the panel.
The Cancer Genome Atlas project, the International Genomic Consortium, and other comprehensive scientific endeavors have allowed us to better understand the molecular underpinnings of cancer in terms of oncogenesis, progression, and resistance.[4] The rapid evolution of knowledge in cancer biology and the discovery and validation of biologic factors can predict cancer outcomes and response to treatment with better accuracy. The AJCC panel recognized the need to incorporate biologic factors such as tumor grade, proliferation rate, ER and PR expression, HER2 expression, and gene expression prognostic panels into the staging system. Thus, the new 8th edition of the AJCC cancer staging system provides a platform for prognostic classification and will remain the worldwide standard for breast cancer staging.[5]
We conducted a retrospective study and survival analysis of the anatomic and prognostic stage groups according to the 8th edition of the AJCC cancer staging system in Luminal B HER2 negative breast cancer using data from our center to understand the application of the updated 8th edition of the AJCC cancer staging system in China.
METHODS
Ethical approval
As a retrospective study and data analysis were performed anonymously, this study was exempt from the ethical approval and informed consent from patients.
Patients
There were 2171 consecutive primary breast cancer patients first diagnosed at the Breast Disease Center, Peking University First Hospital from January 2008 to December 2014. We selected the cases categorized as Luminal B HER2-negative. The cases with incomplete data for the clinical TNM stage, immunohistochemical profile (missing ER, PR, HER2 status, or Ki67) and follow-up data were excluded. The patients with a serious disease such as myocardial, cerebral hemorrhage, renal failure, and so on at the first diagnosis were excluded from the study.
Clinicopathologic data
The following clinicopathologic data were reviewed: age; menopause; body mass index (BMI); anatomic T, N, and M; histological grade; ER; PR; HER2 status; and Ki 67. In the cases with primary systemic therapy, the anatomic T and N were based on physical examination, mammography, and ultrasonography of the breast and regional nodal basins at presentation and status of ER, PR, HER2, and Ki 67. Histological grades were obtained from each patient's diagnostic core needle biopsy. According to the 2011 St. Gallen Consensus,[3] Luminal B HER2-negative tumors were defined as ER-positive and/or PR positive, HER2 negative, and Ki67 >14%.
ER status was classified as negative (lack of any ER immunoreactivity, or <1% immunoreactive tumor cells, with positive inner control) and positive (≥1% immunoreactive tumor cells).[6] Only an intense and complete membrane staining in >10% of the tumor cells qualified for HER2 overexpression (3+). Fluorescence in situ hybridization assays were performed in cases with equivocal (2+) immunohistochemical results to identify cases with gene amplification (HER2 to chromosome 17 centromere ratio ≥2).[7] We used 14% as the threshold to distinguish the Luminal A and B subtypes based on the 2011 St. Gallen Consensus.[3] The tumor histological grade was evaluated according to the Elston-Ellis modification of the Scarff-Bloom-Richardson grading system[8] by assessing morphologic features (tubule formation, nuclear pleomorphism, and mitotic count) and assigning a value of 1 (favorable) to 3 (unfavorable) for each feature. The final score was obtained by adding the scores for all three categories. A combined score of 3–5 points is Grade 1 (G1), a combined score of 6–7 points is Grade 2 (G2), and a combined score of 8–9 points is Grade 3 (G3).
Anatomic and prognostic staging system
All the enrolled cases were restaged using the AJCC 8th edition anatomic and prognostic staging system.[5] Anatomic stage system was based on the anatomic extent of cancer as defined by the T, N, and M categories. Prognostic stage system was based on populations of patients with breast cancer that have been offered – and mostly treated with – appropriate endocrine and/or system chemotherapy, which includes anatomic T, N, and M plus tumor grade and the status of the biomarkers HER2, ER, and PR.
Statistical analysis
The survival data associated with different cancer staging and other clinicopathological data were analyzed with SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). The disease-free survival (DFS) was calculated from the date of surgery to local recurrence or distant metastases, and the overall survival (OS) was calculated from date of diagnosis to death from all causes. The Kaplan-Meier method and log-rank test were used to compare the survival differences between different subgroups. A P < 0.05 was statistical significance.
RESULTS
Patients characteristics
There were 871 patients with Luminal B HER2-negative breast cancer, and the following cases were excluded: 34 cases underwent surgery in other hospitals or refused follow-up, 23 cases had incomplete TNM data, and 18 cases had incomplete histopathological data. Therefore, the study consisted of 796 patients, including 785 females and 11 males. The median patient age was 55 (range: 22–92) years, and the median follow-up time was 38 (range: 5–107) months. During the follow-up period, there were 49 cases categorized as anatomic Stage group I–III who had recurrence and distant metastasis, and 45 patients died. Furthermore, three patients with de novo Stage IV disease died. The 5-year DFS of the 769 Stage I–III patients was 89.7%, and the 5-year OS of all 796 patients was 91.7%. The survival analysis showed that age, tumor size, lymph node status, BMI, chemotherapy, and endocrine therapy were correlated with OS. Furthermore, tumor size and lymph nodes status were also correlated with DFS [Table 1].
Table 1.
Clinicopathological characteristics of the patients with Luminal B HER2-negative breast cancer
| Varieties | Cases (n) | Cases of recurrence and metastasis (n) | 5-year DFS (%) | χ2 | P‡ | Cases of death (n) | 5-year OS (%) | χ2 | P‡ |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| <35 years | 31 | 4 | 77.9 | 5.883 | 0.054 | 2 | 91.8 | 9.639 | 0.008 |
| 35–65 years | 589 | 30 | 91.2 | 27 | 93.6 | ||||
| >65 years | 176 | 15 | 84.6 | 19 | 84.0 | ||||
| Menstrual status | |||||||||
| Perimenopause | 343 | 17 | 93.2 | 1.738 | 0.419 | 16 | 92.0 | 2.112 | 0.348 |
| Postmenopause | 442 | 31 | 86.8 | 31 | 90.7 | ||||
| Male | 11 | 1 | 90.0 | 1 | 90.9 | ||||
| Body mass index | |||||||||
| <18.5 kg/m2 | 15 | 2 | 84.6 | 2.107 | 0.349 | 3 | 76.4 | 6.365 | 0.041 |
| 18.5–25.0 kg/m2 | 460 | 25 | 90.6 | 27 | 91.3 | ||||
| >25.0 kg/m2 | 321 | 22 | 88.6 | 18 | 92.1 | ||||
| Tumor | |||||||||
| T1 | 395 | 15 | 93.9 | 13.976 | 0.003 | 18 | 94.3 | 14.091 | 0.003 |
| T2 | 352 | 28 | 86.3 | 23 | 90.0 | ||||
| T3 | 39 | 5 | 72.6 | 5 | 78.9 | ||||
| T4 | 10 | 1 | 80.0 | 2 | 40.0 | ||||
| Lymph nodes | |||||||||
| N0 | 429 | 18 | 93.2 | 45.225 | <0.001 | 18 | 94.5 | 16.000 | 0.001 |
| N1 | 236 | 16 | 90.4 | 18 | 87.7 | ||||
| N2 | 78 | 4 | 88.1 | 5 | 90.5 | ||||
| N3 | 53 | 11 | 42.3 | 7 | 65.6 | ||||
| Histological grade | |||||||||
| I | 141 | 6 | 91.2 | 2.183 | 0.336 | 9 | 90.9 | 0.685 | 0.710 |
| II | 461 | 27 | 90.0 | 25 | 91.0 | ||||
| III | 194 | 16 | 87.8 | 14 | 92.3 | ||||
| Surgical therapy | |||||||||
| Lumpectomy | 199 | 10 | 90.9 | 0.632 | 0.427 | 9 | 90.0 | 0.863 | 0.353 |
| Mastectomy | 590 | 39 | 88.8 | 39 | 92.2 | ||||
| No surgery* | 7 | 0 | 100.0 | 0 | 100.0 | ||||
| Neo-/adjuvant chemotherapy | |||||||||
| Yes | 492 | 29 | 91.7 | 2.899 | 0.235 | 22 | 93.7 | 9.607 | 0.008 |
| No | 222 | 17 | 84.6 | 27 | 87.1 | ||||
| Unknown | 82 | 3 | 87.6 | 9 | 81.7 | ||||
| Adjuvant endocrine therapy | |||||||||
| Yes | 708 | 46 | 89.8 | 0.845 | 0.358 | 39 | 92.3 | 4.398 | 0.036 |
| Unknown | 88 | 3 | 87.6 | 9 | 81.9 | ||||
| Adjuvant radiotherapy | |||||||||
| No (without radiotherapy indications†) | 321 | 14 | 93.0 | 5.076 | 0.079 | 17 | 93.0 | 1.334 | 0.513 |
| Yes (with radiotherapy indications†) | 433 | 34 | 86.2 | 29 | 89.5 | ||||
| No (with radiotherapy indications†) | 42 | 1 | 80.0 | 2 | 82.8 |
*No surgery: All the seven patients who did not undergo surgery were de novo Stage IV. †Radiotherapy indications include patients with positive lymph node and/or lumpectomy. ‡P: Log-rank test was used to estimate the survival differences between different subgroups. DFS: Disease-free survival; OS: Overall survival; HER2: Human epidermal growth factor receptor 2.
Stage group by anatomic and prognostic staging system
The distribution of cases for each stage group is listed in Table 2. The 796 enrolled Luminal B HER2-negative patients were staged using the anatomic staging system. There were 268 patients (33.7%) grouped as Stage I, 369 patients (46.4%) grouped as Stage II, 132 patients (16.6%) grouped as Stage III, and 27 patients (3.4%) grouped as Stage IV.
Table 2.
Five-year DFS and OS of Luminal B HER2-negative patients using the anatomic and prognostic stage groups in the AJCC 8th edition
| Staging system | Stage | Cases (n) | Events* | Percentage | 5-year DFS | Deaths (n) | Percentage | 5-year OS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | P† | χ2 | P† | |||||||
| Anatomic staging system | I | 268 | 7 | 95.7 | 16.234 | <0.001 | 12 | 94.5 | 12.486 | 0.006 |
| II | 369 | 26 | 89.8 | 20 | 91.6 | |||||
| III | 132 | 16 | 76.0 | 13 | 87.5 | |||||
| IV | 27 | 3 | 40.0 | |||||||
| Prognostic staging system | I | 422 | 16 | 93.9 | 20.766 | <0.001 | 17 | 94.0 | 14.813 | 0.002 |
| II | 236 | 16 | 88.2 | 15 | 90.8 | |||||
| III | 111 | 17 | 74.2 | 13 | 86.2 | |||||
| IV | 27 | 3 | 40.0 | |||||||
*Events: Including recurrence and metastasis events; †P: Log-rank test was used to estimate the survival differences between different subgroups. DFS: Disease-free survival; OS: Overall survival; HER2: Human epidermal growth factor receptor 2; AJCC: American Joint Committee on Cancer.
When the same cohort was restaged by combined ER, PR, HER2, and histological grade status according to the prognostic grading system, we found 422 patients (53.0%) grouped as Stage I, 236 patients (29.6%) grouped as Stage II, 111 patients (13.9%) grouped as Stage III, and 27 patients (3.4%) grouped as Stage IV.
Survival analysis of anatomic and prognostic stage group
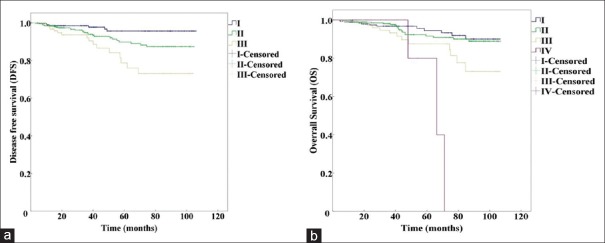
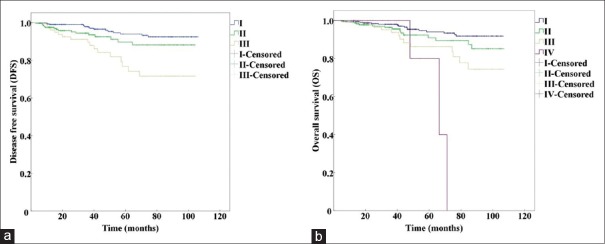
We analyzed patient survival using the log-rank test and found that there were significant differences for the 5-year DFS (χ2 = 16.234, P < 0.01) and 5-year OS (χ2 = 12.486, P < 0.01) in different anatomic stages [Table 2]. Furthermore, the different prognostic stages were significantly different with respect to the 5-year DFS (χ2 = 20.766, P < 0.01) and 5-year OS [χ2 = 14.813, P < 0.01, Table 2]. The Kaplan-Meier curves for the different anatomic stage groups are shown in Figure 1, and the prognostic stage groups are presented in Figure 2.
Figure 1.
Kaplan-Meier survival curves using the anatomic staging system in the AJCC 8th edition. (a) DFS of anatomic Stage I, II, and III (P<0.001). (b) OS of anatomic Stage I, II, III, and IV (P=0.006). DFS: Disease-free survival; OS: Overall survival; AJCC: American Joint Committee on Cancer.
Figure 2.
Kaplan-Meier survival curves using the prognostic staging system in the AJCC 8th edition. (a) DFS of anatomic Stage I, II, and III (P<0.001). (b) OS of prognostic Stage I, II, III, and IV (P=0.002). DFS: Disease-free survival; OS: Overall survival; AJCC: American Joint Committee on Cancer.
Reclassifications from anatomic stage group to prognostic stage group
The combination of biomarkers and prognostic stage group for the Luminal B HER2-negative cohort in this study was altered compared to anatomic stage group. The majority of anatomic Stage I patients (254 cases, 94.8%) remained prognostic Stage I, and 14 cases (5.2%) were altered to prognostic Stage II. In the group of anatomic Stage II patients, approximately, half of the patients (168 cases, 45.5%) were downstaged to prognostic Stage I. There were 170 cases (46.1%) that remained Stage II, and 31 cases (8.4%) were upstaged to prognostic Stage III. In the group of anatomic Stage III patients, there were 52 cases (39.4%) downstaged to prognostic Stage II, and the other 80 cases (60.6%) remained Stage III. Within the 796 cases, there were 251 cases (31.5%) assigned to a better prognostic group, 121 cases (15.2%) assigned to a worse group, and 372 cases (46.7%) assigned to a different group. The changes in staging are shown in Table 3.
Table 3.
Reclassifications between anatomic stage group and prognostic stage group
| Anatomic stage group | Different prognostic stage group altered from the same anatomic stage subgroup | Different prognostic stage group altered from the same anatomic stage group | 5-year DFS | 5-year OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage | Cases (n) | Stage | Cases (n) | Alteration | Cases (n) | Alteration | Cases, n (%) | χ2 | P* | χ2 | P* |
| I | 268 | IA | 264 | (a) IA→(p) IA | 210 | (a) I→(p) I | 254 (94.8) | 0.440 | 0.507 | 1.530 | 0.216 |
| (a) IA→(p) IB | 40 | ||||||||||
| (a) IA→(p) IIA | 14 | (a) I→(p) II | 14 (5.2) | ||||||||
| IB | 4 | (a) IB→(p) IA | 2 | ||||||||
| (a) IB→(p) IB | 2 | ||||||||||
| II | 369 | IIA | 246 | (a) IIAI→(p) IB | 154 | (a) II→(p) I | 168 (45.5) | 6.510 | 0.039 | 5.087 | 0.079 |
| (a) IIA→(p) IIA | 61 | ||||||||||
| (a) IIA→(p) IIB | 10 | ||||||||||
| (a) IIA→(p) IIIA | 21 | (a) II→(p) II | 170 (46.1) | ||||||||
| IIB | 123 | (a) IIB→(p) IB | 14 | ||||||||
| (a) IIB→(p) IIB | 99 | ||||||||||
| (a) IIB→(p) IIIA | 4 | (a) II→(p) III | 31 (8.4) | ||||||||
| (a) IIB→(p) IIIB | 3 | ||||||||||
| (a) IIB→(p) IIIC | 3 | ||||||||||
| III | 132 | IIIA | 75 | (a) IIIA→(p) IIA | 14 | (a) III→(p) II | 52 (39.4) | 11.319 | 0.001 | 5.225 | 0.022 |
| (a) IIIA→(p) IIB | 38 | ||||||||||
| (a) IIIA→(p) IIIA | 2 | ||||||||||
| (a) IIIA→(p) IIIB | 19 | ||||||||||
| (a) IIIA→(p) IIIC | 2 | ||||||||||
| IIIB | 12 | (a) IIIB→(p) IIIB | 7 | (a) III→(p) III | 80 (60.6) | ||||||
| (a) IIIB→(p) IIIC | 5 | ||||||||||
| IIIC | 45 | (a) IIIC→(p) IIIA | 7 | ||||||||
| (a) IIIC→(p) IIIB | 22 | ||||||||||
| (a) IIIC→(p) IIIC | 16 | ||||||||||
| IV | 27 | (a) IV→(p) IV | 27 (100) | ||||||||
*P: Log-rank test was used to estimate the survival differences between different prognostic stage group altered from the same anatomic stage group. DFS: Disease-free survival; OS: Overall survival; (a): Anatomic stage; (p): Prognostic stage.
Survival analysis of different prognostic stage groups in the same anatomic stage group
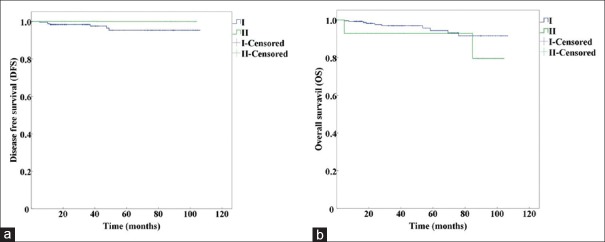
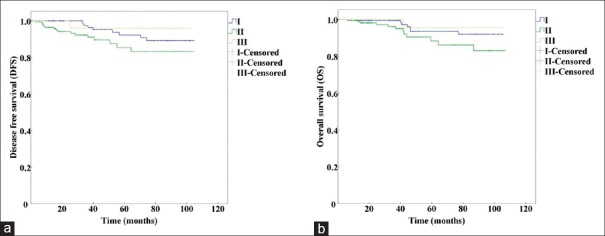
We compared the survival among different prognostic stage groups from the same anatomic stage group [Table 3]. The results showed that prognostic Stages II and III from anatomic Stage III had significant differences in 5-year DFS (χ2 = 11.319, P = 0.001) and 5-year OS (χ2 = 5.225, P = 0.022). The prognostic Stages I, II, and III from the anatomic Stage II group had statistically significant differences in 5-year DFS (χ2 = 6.510, P = 0.039) and no significant differences in 5-year OS (χ2 = 5.087, P = 0.079). The prognostic Stage I and II cases from the anatomic Stage I group had no statistically significant differences in 5-year DFS (χ2 = 0.440, P = 0.507) or 5-year OS (χ2 = 1.530, P = 0.216).
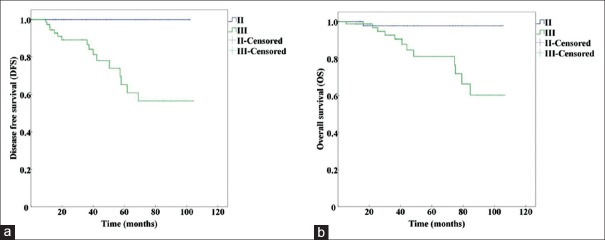
The Kaplan-Meier curves of prognostic Stage I and II from anatomic Stage I are shown in Figure 3. The curves of prognostic Stage I, II, and III from anatomic Stage II are presented in Figure 4, and the curves of prognostic Stage II and III from anatomic Stage III are shown in Figure 5.
Figure 3.
Kaplan-Meier survival curves of prognostic Stage I and II altered from anatomic Stage I by using the AJCC 8th edition. (a) Kaplan-Meier survival curves for DFS (P=0.507). (b) Kaplan-Meier survival curves for OS (P=0.216). DFS: Disease-free survival; OS: Overall survival; AJCC: American Joint Committee on Cancer.
Figure 4.
Kaplan-Meier survival curves of prognostic Stage I, II, and III altered from anatomic Stage II by using the AJCC 8th edition. (a) Kaplan-Meier survival curves for DFS (P=0.039). (b) Kaplan-Meier survival curves for OS (P=0.079). DFS: Disease-free survival; OS: Overall survival; AJCC: American Joint Committee on Cancer.
Figure 5.
Kaplan-Meier survival curves of prognostic Stage II and III altered from anatomic Stage III by using the AJCC 8th edition. (a) Kaplan-Meier survival curves for DFS (P=0.001). (b) Kaplan-Meier survival curves for OS (P=0.022). DFS: Disease-free survival; OS: Overall survival; AJCC: American Joint Committee on Cancer.
DISCUSSION
St. Gallen Symposium was a very efficient consensus panel discussion, and the panel recommendations provide a standard for up-to-date breast cancer treatment following the subtype classification.[3] The data showed there were more patients with Luminal B breast cancer than Luminal A disease.[9,10] Luminal B breast cancer is complicated, and the patients were divided into two subgroups based on status: HER2-positive and HER2-negative. This study enrolled only patients with Luminal B HER2-negative breast cancer to avoid the interference of nonregular trastuzumab administration in HER2-positive patients in China. The AJCC 8th edition[5] noted that the prognostic value of these stage groups is based on populations with breast cancer that have been offered and treated with appropriate systemic therapy. There was prior evidence indicating that HER2-positive patients treated with trastuzumab have improved survival in the adjuvant setting[11,12] and that HER2-positive patients without trastuzumab treatment had poorer outcomes than HER2-negative patients.[13,14]
A study of patients treated in our Breast Disease Center from 2011 to 2012 showed that 53.8% of primary breast cancers were Luminal B subtype, and among this subtype, 80.4% of patients were HER2-negative.[15] The patients with Luminal B HER2-negative breast cancer had higher histological grade, less responsiveness to endocrine therapy, and worse prognosis than Luminal A patients.[15,16] We analyzed the survival data with more refined prognostic stage groups because this setting accounted for a substantial proportion of breast cancer patients with a relatively poor prognosis.
Our comparison of anatomic stage groups revealed there were more cases in the prognostic Stage I group and fewer cases in Stages II and III. In anatomic Stage group II, approximately half of the cases (45.5%) were downstaged to prognostic Stage I. These results suggest that the therapy de-escalation of these patients should be considered. In addition, it is valuable to discuss the use of multigene profile assays in the cases downstaged to the prognostic Stage I group. The genetic results could be used to explore the intrinsic gene subtypes and estimate patient prognosis and response to therapy.
Considering the differences of pathological characteristics, risk of relapse, the sensitivities to available therapies between each individual breast cancer patients, cancer staging is so important that it should not only provide the information of biological features of the breast cancer, but also reflect the molecular characteristics of the breast cancer. Personalized-medicine approach to breast cancer also requires more precisely cancer staging. Based on all the currently available knowledge including both biological and molecular of breast cancer, the evidence-based anatomic TNM staging is supplemented, as appropriate, by selected molecular markers and newly acquired insights into the molecular underpinnings of cancer, the 8th AJCC cancer staging system develops prognostic staging system. As shown in the study, the survival analysis of different prognostic stage group within the same anatomic stage group showed that prognostic stage refined the survival prognosis. And, especially in poor prognosis anatomic group, there are statistic differences between different prognostic stage groups from the same anatomic group. This finding indicates that systemic treatment might be changed in several anatomic stage groups. For example, a patient in the anatomic Stage I cohort that was altered to Stage II in the prognostic staging system should consider therapy escalation.
This study had several limitations. First, this was a retrospective study. However, the study was useful because we examined an Asian patient cohort to validate the prognostic staging system suggested in the AJCC 8th edition. In the revised guidelines, a prognostic group stage was established based on the National Cancer Data Base analysis.[5] A second limitation of the study was that only a portion of all molecular subtypes were enrolled. We did not see the correlation between several biomarkers and survival prognosis. The histological grade of most patients (82.4%) in the Luminal B HER2-negative cohort was assigned grades II and III. It is noteworthy that BMI had a correlation with OS. However, the best prognosis was in the group with BMI >25.0 kg/m2. The guidelines for cancer survivors[17] recommend that individuals should strive to achieve and maintain a healthy weight as defined by a BMI between 18.5 and 25.0 kg/m2. This finding is contradictory due to sample bias or race and requires further study.
In conclusion, the prognostic staging system reported in the AJCC 8th edition can refine the anatomic stage group in patients with Luminal B HER2 negative breast cancer, and will lead to a more personalized approach for breast cancer treatment.
Financial support and sponsorship
This study was supported by grants from the Precision Medicine Special Project of National Key Research and Development Program (No. 2016YFC0901302), and the Beijing Municipal Science and Technology Commission (No. Z131107002213007).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.American Joint Committee for Cancer Staging and End Results Reporting. Manual for Staging of Cancer. Chicago, IL, USA: American Joint Committee; 1977. [Google Scholar]
- 2.Perou CM, SÂrlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel Members. Strategies for subtypes – Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9. doi: 10.3322/caac.21388. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 5.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al., editors. American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 6.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 8.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 2002;41:154–61. doi: 10.1046/j.1365-2559.2002.14892.x. [PubMed] [Google Scholar]
- 9.Hu H, Liu YH, Xu L, Zhao JX, Duan XN, Ye JM, et al. Clinicopathological classification and individualized treatment of breast cancer. Chin Med J. 2013;126:3921–5. doi: 10.3760/cma.j.issn.0366-6999.20123368. [PubMed] [Google Scholar]
- 10.Fountzilas G, Dafni U, Bobos M, Batistatou A, Kotoula V, Trihia H, et al. Differential response of immunohistochemically defined breast cancer subtypes to anthracycline-based adjuvant chemotherapy with or without paclitaxel. PLoS One. 2012;7:e37946. doi: 10.1371/journal.pone.0037946. doi: 10.1371/journal.pone.0037946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–8. doi: 10.1200/JCO.2008.19.9844. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fokter Dovnik N, Dovnik A, Cas Sikošek N, Ravnik M, Arko D, Takac I. Prognostic role of HER2 status and adjuvant trastuzumab treatment in lymph node-negative breast cancer patients – A retrospective single center analysis. Breast Care (Basel) 2016;11:406–10. doi: 10.1159/000454690. doi: 10.1159/000454690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H, Liu Q, Xu L, Ye J, Zhao J, Duan X, et al. Study on clinicopathological classification and clinical and pathological characteristics in breast cancer (in Chinese) Chin J Surg. 2014;52:113–6. doi: 10.3760/cma.j.issn.0529-5815.2014.02.008. [PubMed] [Google Scholar]
- 16.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–7. doi: 10.1016/j.breast.2011.07.008. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–74. doi: 10.3322/caac.21142. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]