Abstract
Background:
Whether cholinergic innervations and/or autophagy have a role in the etiopathology of benign prostatic hyperplasia (BPH) is still unknown. This study aimed to investigate the role of cholinergic innervation and autophagy in the etiopathology of BPH.
Methods:
Male, 13-week-old spontaneous hypertension rats (spontaneous BPH animal model) were divided into three groups: an experimental group (EG, n = 24), a control group (CG, n = 24), and a normal control group (NC, n = 10). The EG animals were intragastrically injected with tolterodine (3.5 mg/kg, twice a day), CG animals were intragastrically injected with physiological saline, and the NC animals did not receive any treatment. Rats were sacrificed every 4 weeks, and the prostatic gross morphological changes, wet weight/body weight (ww/bw), dry weight/wet weight (dw/ww), histological changes, ultrastructural changes, and LC3 immunohistochemistry were continuously observed and compared.
Results:
The gross morphological and ww/bw changes in the three groups were similar at every stage. The dw/ww (mg/mg) values of the EG at week 17, 21, 25, and 29 were 0.1478 ± 0.0034, 0.1653 ± 0.0036, 0.1668 ± 0.0045, and 0.1755 ± 0.0034, respectively, and the CG values were 0.1511 ± 0.0029, 0.1734 ± 0.0020, 0.1837 ± 0.0052, and 0.1968 ± 0.0045, respectively. The difference between EG and CG for dw/ww showed statistical significance after 21 weeks of age (week 21: P = 0.016, week 25: P = 0.008, and week 29: P = 0.001). Both EG and CG, prostatic glandular epithelial cell proliferation, and secretory function improved with age, but in EG, these improvements were slower than those in CG, and all the differences were statistically significant after 21 weeks. An increasing number of autophagosomes in the prostatic glandular cell cytoplasm, attenuation of LC3-I immunohistochemical staining, enhancement of LC3-II staining, and the ratio of LC3-II/LC3-I staining were all progressive in both groups, but the rate of change in EG was faster than that in CG, and these differences gained statistical significance after 25 weeks. Comparisons with regard to the above indexes between CG and NC showed no statistical significance at any stage.
Conclusions:
Cholinergic innervations and activation of autophagy appear to have important functions in the etiopathology of BPH. Drug-mediated blockade of cholinergic innervations could delay the physiopathology processes. Moreover, overactivation of autophagy may also play an important role in this delay.
Keywords: Autophagy, Cholinergic Innervation, Denervation, Etiopathology, Prostatic Hyperplasia
INTRODUCTION
The rich innervation of the mammalian prostate is essential for maintaining its structure and function, and this innervation is mainly composed of the parasympathetic cholinergic nerve and the sympathetic adrenal nerve.[1] Studies indicating a correlation between sympathetic adrenal innervation or its α1-receptor with benign prostatic hyperplasia (BPH) are numerous, but studies concerning parasympathetic cholinergic innervations and its M-receptor are uncommon. Recently, some studies have suggested that injection of botulinum toxin A (a drug that can inhibit acetylcholine release from neuromuscular terminals and autonomous neurons) into BPH tissue can lead to marked atrophy in the prostate gland and noticeable improvement in patients’ symptoms.[2] Thus, cholinergic innervation may also be closely associated with BPH.
In the previous animal studies, after the cholinergic innervation of BPH in rats was cut through microsurgery[3] or blocked by long-term drug administration,[4] the prostatic gland progressively shrank in volume over time. On the other hand, we observed that autophagy in the glandular epithelium of benign hyperplasia decreased along with the changes in glandular atrophy after the blockade of cholinergic innervation.[4] Autophagy is considered a double-edged sword in cell fate determination. Therefore, we speculated that a significant change in autophagy represents a key molecular event in the atrophy of the benign hyperplasic prostatic glandular epithelium induced by cholinergic innervation blockade, although the precise molecular mechanism needs further investigation. This study aimed to investigate the role of cholinergic innervation and autophagy in the etiopathology of BPH.
METHODS
Experimental animals and grouping
The following experimental protocol was approved by the Animal Ethics Committee of Beijing Shijitan Hospital, Capital Medical University, China. In total, 58 male spontaneous hypertension rats (SHRs, whose prostate presented spontaneous benign hyperplasia with age and formed a marked BPH at week 29;[5] Beijing Vital River Laboratory Animal Technology Co., Ltd., China), were maintained in a screened environment kept at 22°C with a 12 h light-dark cycle and ad libitum provision of food and water. These mice were 13 weeks old, SPF grade, and possessed body weights of 294.4 ± 5.7 g (range 285–303 g). These SHRs were divided into three groups: an experimental group (EG, n = 24), a control group (CG, n = 24), and a normal control group (NC, n = 10). Tolterodine (an M-receptor blocker, 3.5 mg/kg, dissolved in 1.0 ml physiological saline, given twice a day at a safe dosage for SHR[6]) was intragastrically administered to EG animals. Likewise, CG animals were injected intragastrically with 1.0 ml physiological saline, twice a day, and the NC group did not receive any treatment.
Tissue sample harvesting and processing
At 17, 21, 25, and 29 weeks of age, 6 EG rats, 6 CG rats, and 2 NC rats were weighed and anesthetized (10% chloral hydrate, 300 mg/kg). A long hypogastrium incision was made to fully expose the prostate and the gross morphological changes were closely examined. Subsequently, one side of the prostate ventral lobe was randomly selected and approximately ¼–⅓ was cut off the top portion, immediately sliced into small tissue blocks (1.0 mm × 1.0 mm × 1.0 mm), dropped into a precooled 3% glutaraldehyde fixation solution, and stored at 4°C. The remaining ventral lobe on the same side was excised and fixed in 4% formaldehyde, and the other side of the prostate ventral lobe was excised completely, weighed, and stored at −20°C.
Tissue dry weight measurement
The prostate tissues stored at −20°C were thawed at room temperature (22°C), placed into a small flask, and then baked at 98°C for 48 h. The residual tissues were weighed with a precision balance.
Histology
After fixation in 4% formaldehyde for 24–48 h, the prostate tissues were embedded in paraffin and sectioned continuously (4–5 μm). One section was stained with hematoxylin and eosin, and the other sections were placed on glass slides coated with poly-L-Lysine. Two pathologists independently observed the histomorphological changes under a light microscope.
Electron microscopy
The small tissue blocks fixed in 3% glutaraldehyde were transferred to 1% osmic acid solution for re-fixation. After serial dehydration with acetone, the blocks were embedded in Epon812. In each case, five blocks were prepared. These tissue blocks were first cut in half, stained with methylene blue, and then observed under a light microscope. The Epon812 tissue block was then resculpted. The ultra-thin slices were prepared with an ultramicrotome (75A, UCT, LEICA Ultracut, Germany), double-stained with uranyl acetate and lead citrate, and observed under a Type JEM-1230 electron microscope (JEOL, Japan). In each chip, five glandular epithelial cells were randomly selected and the changes in cellular modality and organelles were observed under × 3000, × 5000, × 8000, × 10,000, and × 20,000 magnifications. The judging standards for the number of autophagic bodies in the cytoplasm were as follows: +, few, diffused distribution; ++, middling, comparatively close-set; and +++, generous, close-set. Two pathologists made independent observations by electron microscopy, and in the case of conflict, the decision was made mutually.
Immunohistochemical staining
The tissue sections were deparaffinized, rehydrated, immersed in an ethylenediaminetetraacetic acid antigen repair solution, and then subjected to antigen retrieval in a microwave oven. The sections were then incubated overnight with the primary antibody (rabbit polyclonal to LC3B, which can detect LC3-I and LC3-II simultaneously, 1:100 dilution, Abcam, MA, USA) at 4°C. After thorough phosphate buffer saline (PBS) washes, a goat anti-rabbit IgG antibody-HRP conjugate (Beijing Zhongshan Golden Bridge Biotech, Inc., Beijing, China) was added and incubated with the tissue sections at 37°C for 30 min. After thorough washing with PBS, the staining was developed with a inspissate-3,3′3olden Bridge Biotech, Inc., BeZhongshan Golden Bridge Biotech, Inc., Beijing, China), and the tissue sections were counterstained and mounted.
The following scoring system was used to assess the stained tissue sections, determined by the density and extent: ① density: 0, no staining; 1, nankeen; 2, buffy; and 3, brown; ② extent: 0, no staining; 1, <30% cytoplasm; 2, 30–60% cytoplasm; and 3, >60% cytoplasm. The final score was expressed as follows: −, 0; +, 1–2; ++, 3–5; and +++, 6–9. LC3-II/LC3-I: −, ≤1, +, >1. Scoring was done independently by two pathologists, and in case of conflict, the decision was made mutually.
Statistical analysis
The SPSS version 19.0 statistical software (SPSS Inc., Chicago, Illinois, USA) was used for analysis. The data of the prostate wet weight/rat body weight (ww/bw) and prostate dry weight/wet weight (dw/ww) were presented as mean ± standard deviation, and analysis was performed using analysis of variance. The data of the autophagic body distribution and LC3 immunohistochemistry staining in rats’ prostate glandular epithelium cells were analyzed with the Chi-square test. P < 0.05 was considered statistically significant.
RESULTS
Gross morphological changes
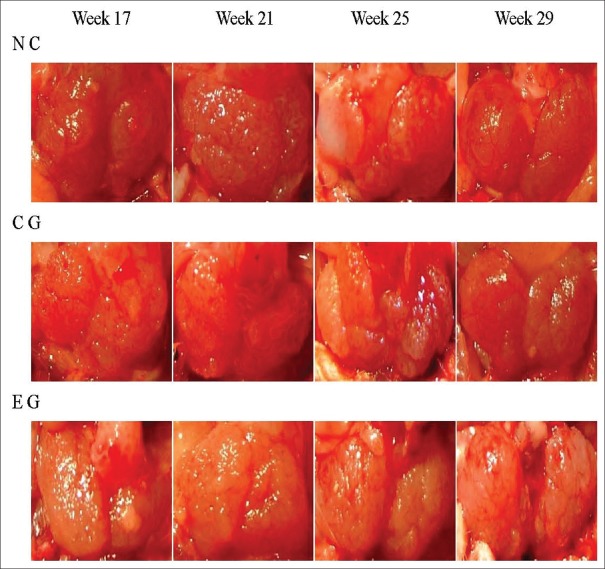
In all three groups (EG, CG, and NC), the prostate volume progressively accreted with rat age, and the differences between the volumes in the weeks 17, 21, 25, and 29 were not significant [Figure 1].
Figure 1.
The gross morphological changes of the prostate of spontaneous hypertension rats in every group in the weeks 17, 21, 25, and 29. NC: Normal control; CG: Control group; EG: Experimental group.
Prostate wet weight/rat body weight and prostate dry weight/wet weight
The prostate ww/bw of the rats in the three groups was elevated at weeks 17, 21, 25, and 29 [Table 1], with no statistical significance being present between the groups (P > 0.05 for all comparisons). The dw/ww of the three groups of rats also increased with age [Table 2], and a comparison of this ratio from the CG and NC groups revealed no statistical difference (P > 0.05) at every phase. However, the tendency for the dw/ww ratio to increase with age in the EG group was slower than that in the other two groups, and the difference between EG/CG and EG/NC showed statistical significance after 21 weeks of age (week 21: F = 12.348, P = 0.016, 0.001; week 25: F = 20.471, P = 0.008, <0.0001; week 29: F = 44.134, P = 0.001, <0.0001, respectively).
Table 1.
The prostate wet weight/body weight of the rats in the three groups (mg/g)
| Subgroups | NC (n = 2) | CG (n = 6) | EG (n = 6) | F | P |
|---|---|---|---|---|---|
| Week 17 | 0.4537 ± 0.0302 | 0.4566 ± 0.0546 | 0.4731 ± 0.0498 | 0.182 | 0.836 |
| Week 21 | 0.5159 ± 0.0067 | 0.4895 ± 0.0473 | 0.5169 ± 0.0577 | 0.531 | 0.602 |
| Week 25 | 0.4518 ± 0.0038 | 0.4948 ± 0.0712 | 0.4563 ± 0.0839 | 0.492 | 0.624 |
| Week 29 | 0.5104 ± 0.0375 | 0.5298 ± 0.0560 | 0.5113 ± 0.0307 | 0.303 | 0.744 |
Data were shown as mean ± SD. SD: Standard deviation; NC: Normal control; CG: Control group; EG: Experimental group.
Table 2.
The prostate dry weight/wet weight of the rats in the three groups (mg/mg)
| Subgroups | NC (n = 2) | CG (n = 6) | EG (n = 6) | F | P |
|---|---|---|---|---|---|
| Week 17 | 0.1527 ± 0.0044 | 0.1511 ± 0.0029 | 0.1478 ± 0.0034 | 2.315 | 0.145 |
| Week 21 | 0.1721 ± 0.0028 | 0.1734 ± 0.0020 | 0.1653 ± 0.0036 | 12.348 | 0.002 |
| Week 25 | 0.1791 ± 0.0013 | 0.1837 ± 0.0052 | 0.1668 ± 0.0045 | 20.471 | <0.0001 |
| Week 29 | 0.1911 ± 0.0038 | 0.1968 ± 0.0045 | 0.1755 ± 0.0034 | 44.134 | <0.0001 |
Data were shown as mean ± SD. SD: Standard deviation; NC: Normal control; CG: Control group; EG: Experimental group.
Histological changes
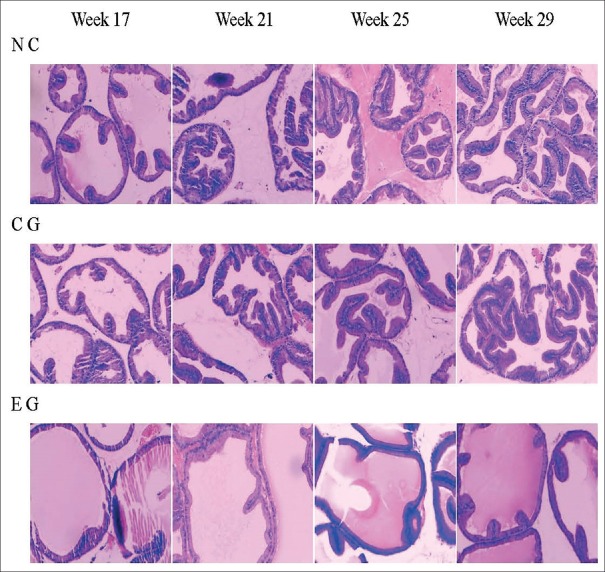
The prostate glandular epithelium of the NC group of rats gradually became highly stylolitic with age; the cell layer increased progressively and pooled up as mounds, and after 21 weeks, the mounds were scattered throughout the glandular lumen. The prostate glandular cells in the CG group of rats showed the same changes as those in the NC group. However, in the EG group, the rate of cell proliferation in the glandular epithelium with age was slower than that in the CG or NC group. Specifically, the appearance of high columnar cells was observed at later stages, and the increase in the cell layer was slower and there were fewer mounds. Furthermore, when we lengthened drug administration time, the difference between the EG group and the CG or NC groups grew progressively significant, and after 21 weeks, there were marked differences [Figure 2].
Figure 2.
The continuous histological changes of the spontaneous hypertension rats prostate in every group in the weeks 17, 21, 25, and 29. Along with age increase, prostate glandular epithelium cell gradually became high stylolitic, and the cell layer progressively increased and poled up as mounds. However, the hyperplasia expression in EG group was not as significant as in NC and CG (H and E, ×100). NC: Normal control; CG: Control group; EG: Experimental group.
Ultrastructural changes
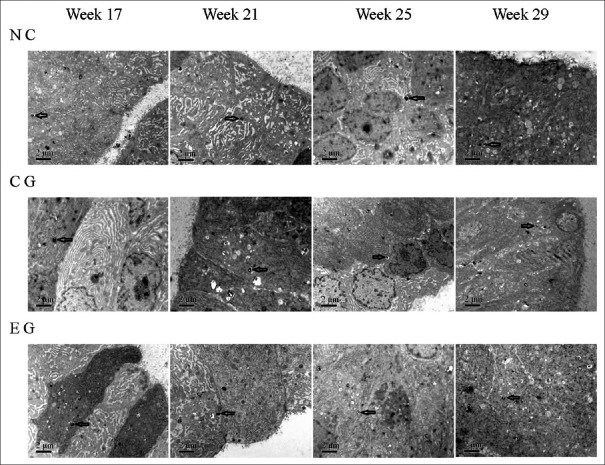
With aging in the NC group, the prostate glandular epithelial cells gradually became high columnar, with multiple layer arrangement; the number of organelles such as rough endoplasmic reticulum and Golgi apparatus progressively increased, and more and more secretory vesicles and lamellar secretions appeared at the cavosurface. The prostate glandular cells in the CG group of rats showed the same cellular proliferation rate and active secretion function as those in the NC group. Many autophagic bodies were detected in cytoplasm of the prostate glandular cells of the NC and CG groups of rats, which increased slightly with age. However, in the EG group, cell proliferation and appearance of active secretion function in prostate glandular cells were less significant compared to that in the CG or NC groups of the same age. However, the cytoplasmic distribution of autophagic bodies in the EG group at every phase seemed to be greater than that in the two groups; the difference increased with age, and at 29 weeks, the difference was statistically significant (χ2 = 5.333, P = 0.021). However, the manifestation of classical apoptosis was not observed in all three groups [Figure 3 and Table 3].
Figure 3.
The dynamic ultrastructural changes of the prostate glandular epithelium cells of spontaneous hypertension rats in every group. Along with the growth of age, the prostate glandular epithelium cells gradually become high columnar, multiple layer arrangement, the number of organelles such as rough endoplasmic reticulum and Golgi apparatus progressively rose, more and more secretory vesicles and lamellar secretions appeared at the cavosurface. These changes in EG were less significant than in CG and NC. Autophagic body (arrows) appeared in cytoplasm of the prostate glandular cells and increased with age; the number increased faster in EG than in CG and NC (×10,000). NC: Normal control; CG: Control group; EG: Experimental group.
Table 3.
The autophagic body distribution in prostate glandular epithelium cells of rats
| Groups | Subgroups | + | ++ | +++ |
|---|---|---|---|---|
| NC | Week 17 (n = 2) | 2 | ||
| Week 21 (n = 2) | 1 | 1 | ||
| Week 25 (n = 2) | 2 | |||
| Week 29 (n = 2) | 1 | 1 | ||
| CG | Week 17 (n = 6) | 5 | 1 | |
| Week 21 (n = 6) | 2 | 4 | ||
| Week 25 (n = 6) | 1 | 4 | 1 | |
| Week 29 (n = 6)* | 4 | 2 | ||
| EG | Week 17 (n = 6) | 4 | 2 | |
| Week 21 (n = 6) | 1 | 3 | 2 | |
| Week 25 (n = 6) | 3 | 3 | ||
| Week 29 (n = 6)* | 2 | 4 |
*Chi-square test, χ2 = 5.333; P = 0.021. +, few, diffused distribution; ++, middling, comparatively close-set; +++, generous, close-set. NC: Normal control; CG: Control group; EG: Experimental group.
Immunohistochemical staining
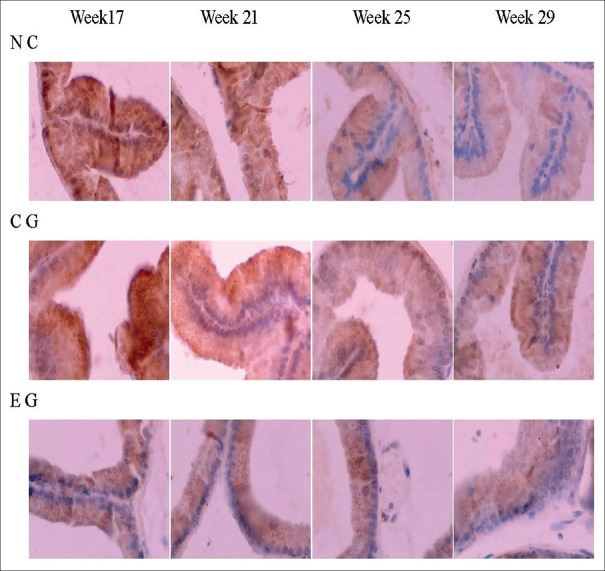
Staining of cytoplasmic LC3-I of the prostatic epithelium weakened, whereas LC3-II staining strengthened with the age of the rats in all three groups. However, in the EG group, the rate of the changes in the staining of the two LC3 isoforms with time was more significant compared to those in the other two groups. Concerning LC3-II/LC3-I staining, the difference between the EG and CG groups become increasingly significant with age, and showed statistical significance after 25 weeks (χ2 = 4.000, P = 0.046), whereas the difference between CG and NC groups showed no statistical significance at any age (P > 0.05, for all comparisons) [Figure 4 and Table 4].
Figure 4.
The dynamic changes of LC3 immunohistochemistry staining in the prostate tissue of spontaneous hypertension rats. The staining of LC3-I (diffuse staining in cytoplasm) in the prostatic epithelium cell became weaker and weaker with age, while staining of LC3-II (granular staining in cytoplasm) became stronger and stronger. The variation tendency in EG was faster than in CG and NC (×400). NC: Normal control; CG: Control group; EG: Experimental group.
Table 4.
The LC3 immunohistochemistry staining in prostate glandular epithelium cells of spontaneous hypertension rats
| Groups | Subgroups | LC3-I | LC3-II | LC3-II/LC3-I | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| – | + | ++ | +++ | – | + | ++ | +++ | ≤1 | >1 | ||
| NC | Week 17 (n = 2) | 2 | 1 | 1 | 2 | ||||||
| Week 21 (n = 2) | 1 | 1 | 2 | 2 | |||||||
| Week 25 (n = 2) | 2 | 1 | 1 | 1 | 1 | ||||||
| Week 29 (n = 2) | 1 | 1 | 2 | 2 | |||||||
| CG | Week 17 (n = 6) | 1 | 5 | 3 | 3 | 6 | |||||
| Week 21 (n = 6) | 2 | 4 | 1 | 3 | 2 | 5 | 1 | ||||
| Week 25 (n = 6)* | 2 | 3 | 1 | 2 | 3 | 1 | 3 | 3 | |||
| Week 29 (n = 6) | 1 | 4 | 1 | 1 | 3 | 2 | 1 | 5 | |||
| EG | Week 17 (n = 6) | 1 | 3 | 2 | 2 | 3 | 1 | 4 | 2 | ||
| Week 21 (n = 6) | 1 | 4 | 1 | 1 | 3 | 2 | 2 | 4 | |||
| Week 25 (n = 6)* | 3 | 3 | 3 | 3 | 6 | ||||||
| Week 29 (n = 6) | 4 | 2 | 2 | 4 | 6 | ||||||
*Chi-square test for LC3II/I, χ2 = 4.000; P = 0.046. NC: Normal control; CG: Control group; EG: Experimental group; -, stain score 0; +, stain score 1–2; ++, stain score 3–5; +++, stain score 6–9.
DISCUSSION
The presence of normal, intact innervation is essential for maintaining the normal modality and function of mammalian organs. The mammalian prostate has a rich nerve supply composed mainly of the adrenergic sympathetic nerve and the cholinergic parasympathetic nerve.[1] Among these, the adrenergic sympathetic nerve is distributed chiefly in the capsule and in the smooth muscles around the glandular lumens and the crypt, whereas the cholinergic parasympathetic nerve is distributed in both the glandular epithelium and the stromal smooth muscles.[7] Furthermore, all types of cholinergic receptor isoforms are proven to be distributed universally in the mammalian prostatic glandular epithelium.[8,9,10] Therefore, the cholinergic innervation and the distribution of the cholinergic receptors may have an intimate relationship with the growth, metabolism, and function of mammalian prostatic glandular epithelium.[11]
SHR is an animal model for spontaneous BPH, as its prostatic glandular epithelium presents a naturally occurring benign hyperplasic change with age and forms a typical BPH at 29 weeks.[5] In the current study, the changes in the prostatic glandular epithelium of the NC and CG rats are consistent with those reported previously. However, in the EG group, this natural benign prostatic hyperplasia process was postponed by the long-term, continued use of tolterodine, an unselective M-receptor blocker. Thus, cholinergic innervation may play an important role in the genesis and development of BPH in rats, and blocking cholinergic innervation may delay this pathologic process.
Autophagy is a basic and vital phenomenon in eukaryotic cells. Typically, there is a basal level of autophagic activity in normal cells, but autophagic activity is enhanced when cells encounter stresses such as nutrition deficiency, oxidative stress, hyperthermia, injuries, and tissue structural adjustments. Under these circumstances, a free double-membrane vesicle, derived from a mitochondrion or the rough endoplasmic reticulum, encapsulates cytoplasmic materials such as paraprotein, cellular metabolites, or injured organelles, to form autophagosomes. These vesicles then combine with lysosomes to form autophagolysosomes that degrade their contents into amino acids, nucleotides, and free fatty acids, which are secreted into the cytoplasm and reused by cells to maintain homeostasis. Therefore, maintaining a moderate level of autophagic activity is very important for cell survival. Conversely, excessive autophagic activity will “digest” an excessive amount of cytoplasmic components, such as important proteins and key organelles, which will be detrimental to cell survival. Similarly, suboptimal levels of autophagic activity cannot provide sufficient material and energy by removing metabolic wastes and damaged organelles, and thus, this leads to the induction of cell death (Type II programmed cell death).[12,13]
Recently, an increasing number of studies have suggested that autophagy plays an important role in the pathophysiological process of carcinogenesis, development, neurodegenerative diseases, pathogen infection, cardiovascular and cerebrovascular diseases, and aging.[14,15] Autophagy may enhance, postpone, or inhibit such disease depending on the type of disease or even the stage of the disease. Autophagy is now being regarded as a new pathogenic mechanism for many diseases and a new therapeutic target.[16,17,18] However, studies concerning the role of autophagy in BPH are rare. In our previous animal study, Li et al.[19] found that androgen deprivation could markedly increase the autophagic puncta in PWR-1E prostate epithelial cells, and the apoptosis rate of PWR-1E cells was promoted significantly by a 3-methyladenine-induced blockade in autophagy. In addition, the immunohistochemical study by Liu et al.[20] and Li et al.[19] showed that autophagic activity in human BPH tissue was elevated significantly after treatment with a 5α-reductase inhibitor. However, we could not find any study concerning autophagic activity and BPH etiopathology.
The present study showed that in the natural progression of BPH in rats, the autophagosome in the prostatic glandular epithelium increased progressively with age. LC3-II/LC3-I staining, which marks the formation of autophagic vacuoles,[21] is also elevated progressively with rat age. These phenomena indicate that the autophagic activity in the rat prostate glandular epithelium was activated and upregulated in the benign hyperplastic processes. In benign hyperplasia, the characteristics of rat prostate glandular epithelia include hypermetabolism, metabolic waste accumulation, acceleration of organelle regeneration and reconstitution, and an increase in paraprotein and abnormal organelles, which all require autophagy for quick and efficient cleaning to satisfy the requirements of hypertrophic cells. Therefore, the adequate activation of autophagy may be a key point in benign hyperplasia of the prostate glandular epithelium.
However, with the benign hyperplastic process in rat prostate glandular epithelium being slowed down by the drug-induced blockade of cholinergic innervation, autophagy in the glandular cells was activated significantly. Thus, it is unknown if autophagic overactivity in the prostate epithelium, as induced by tolterodine, is a self-protection mechanism or a trigger for cell death caused by long-term blockade of cholinergic innervations. However, this molecular event likely has a close relationship with the delay in the genesis of BPH and in the development of processes induced by blocking cholinergic innervations. Given this relationship, further investigations may be able to reveal a new direction for study on the pathogenesis and prevention of BPH.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81070601) and the Beijing Natural Science Foundation (No. 7102106).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Xiao-Song Rao and Prof. Xiu-Qing Tang for their effort on the histological and electron microscopic observations.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.McVary KT, McKenna KE, Lee C. Prostate innervation. Prostate Suppl. 1998;8:2–13. doi: 10.1002/(SICI)1097-0045(1998) 8+<2::AID-PROS2>3.0.CO;2-U. [PubMed] [Google Scholar]
- 2.Shim SR, Cho YJ, Shin IS, Kim JH. Efficacy and safety of botulinum toxin injection for benign prostatic hyperplasia: A systematic review and meta-analysis. Int Urol Nephrol. 2016;48:19–30. doi: 10.1007/s11255-015-1153-3. doi: 10.1007/s11255-015-1153-3. [DOI] [PubMed] [Google Scholar]
- 3.Cai JL, Xin DQ, He Q, Tang XQ, Na YQ. Pathological changes of benign hyperplastic prostate after removal of innervation of cholinergic pelvic nerve: Experiment with spontaneous hypertension rats (in Chinese) Natl Med J China. 2008;18:1284–8. [PubMed] [Google Scholar]
- 4.Cai JL, Yao WM, Yuan XL, Xia M, Na YQ. Changes of rat's benign hyperplasia prostate glandular epithelium cells after interfering cholinergic innervations by drug (in Chinese) Chin J Exp Surg. 2014;31:1231–4. [Google Scholar]
- 5.Zhang X, Na Y, Guo Y. Biologic feature of prostatic hyperplasia developed in spontaneously hypertensive rats. Urology. 2004;63:983–8. doi: 10.1016/j.urology.2003.11.024. doi: 10.1016/j.urology.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Cai JL, Yao WM, Li NC, Chen ZH, Ye ZH, Na YQ. Medium-and-long-term tolerance of tolterodine to spontaneous hypertension rats. J Clin Urol. 2010;25:945–7. [Google Scholar]
- 7.Pennefather JN, Lau WA, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: A review of pharmacological and histological studies. J Auton Pharmacol. 2000;20:193–206. doi: 10.1046/j.1365-2680.2000.00195.x. doi: 10.1046/j.1365-2680.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 8.Lau WA, Pennefather JN. Muscarinic receptor subtypes in the rat prostate gland. Eur J Pharmacol. 1998;343:151–6. doi: 10.1016/s0014-2999(97)01535-5. doi: 10.1016/S0014-2999(97)01535-5. [DOI] [PubMed] [Google Scholar]
- 9.Fernández JL, Rivera L, López PG, Recio P, Vela-Navarrete R, García-Sacristán A. Characterization of the muscarinic receptor mediating contraction of the dog prostate. J Auton Pharmacol. 1998;18:205–11. doi: 10.1046/j.1365-2680.1998.18486.x. [DOI] [PubMed] [Google Scholar]
- 10.Obara K, Arai K, Miyajima N, Hatano A, Tomita Y, Takahashi K. Expression of m2 muscarinic acetylcholine receptor mRNA in primary culture of human prostate stromal cells. Urol Res. 2000;28:196–200. doi: 10.1007/s002400000113. doi: 10.1007/s002400000113. [DOI] [PubMed] [Google Scholar]
- 11.Ventura S, Pennefather J, Mitchelson F. Cholinergic innervation and function in the prostate gland. Pharmacol Ther. 2002;94:93–112. doi: 10.1016/s0163-7258(02)00174-2. doi: 10.1016/S0163-7258(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh D, Walton JL, Roepe PD, Sinai AP. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol. 2012;14:589–607. doi: 10.1111/j.1462-5822.2011.01745.x. doi: 10.1111/j.1462-5822.2011.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: A troika governing cancer and its treatment. Cell. 2016;166:288–98. doi: 10.1016/j.cell.2016.05.051. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Cao Y, Tong T, Shi J, Zhang Y, Yang Y, et al. Autophagy in atherosclerosis: A phenomenon found in human carotid atherosclerotic plaques. Chin Med J. 2015;128:69–74. doi: 10.4103/0366-6999.147815. doi: 10.4103/0366-6999.147815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ntsapi C, Loos B. Caloric restriction and the precision-control of autophagy: A strategy for delaying neurodegenerative disease progression. Exp Gerontol. 2016;83:97–111. doi: 10.1016/j.exger.2016.07.014. doi: 10.1016/j.exger.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Netea-Maier RT, Plantinga TS, van de Veerdonk FL, Smit JW, Netea MG. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy. 2016;12:245–60. doi: 10.1080/15548627.2015.1071759. doi: 10.1080/15548627.2015.1071759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang JC, Feng YL, Liang X, Cai XJ. Autophagy in 5-fluorouracil therapy in gastrointestinal cancer: Trends and challenges. Chin Med J. 2016;129:456–63. doi: 10.4103/0366-6999.176069. doi: 10.4103/0366-6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Yang X, Wang H, Xu E, Xi Z. Inhibition of androgen induces autophagy in benign prostate epithelial cells. Int J Urol. 2014;21:195–9. doi: 10.1111/iju.12210. doi: 10.1111/iju.12210. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Xu P, Chen D, Fan X, Xu Y, Li M, et al. Roles of autophagy-related genes Beclin-1 and LC3 in the development and progression of prostate cancer and benign prostatic hyperplasia. Biomed Rep. 2013;1:855–60. doi: 10.3892/br.2013.171. doi: 10.3892/br.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]