Abstract
Species-area relationships have been observed for virtually all major groups of macroorganisms that have been studied to date but have not been explored for microscopic phytoplankton algae, which are the dominant producers in many freshwater and marine ecosystems. Our analyses of data from 142 different natural ponds, lakes, and oceans and 239 experimental ecosystems reveal a strong species-area relationship with an exponent that is invariant across ecosystems that span >15 orders of magnitude in spatial extent. A striking result is that the species-area relationship derived from small-scale experimental studies correctly scales up to natural aquatic ecosystems. These results significantly broaden our knowledge of the effects of island size on biodiversity and also confirm the relevance of experimentally derived data to the analysis and understanding of larger-scale ecological patterns. In addition, they confirm that patterns in microbial diversity are strongly consistent with those that have been repeatedly reported in the literature for macroorganisms.
Keywords: biodiversity, island biogeography, species-area, scale-invariance
Patterns in local species diversity are very strongly influenced by scale, and different processes may determine biodiversity at different spatial scales (1). Although the ecological problems associated with scale are well known, many subdisciplines of ecology are still struggling with two critically important questions (2). First, how do the absolute dimensions of time, space, and levels of organization influence ecological patterns and processes? Second, how can the results from small, local, experimental-scale systems be used to gain insight about larger spatial scales?
The number of scientific laws emerging from community ecology is still relatively small (3). However, it has been known for more than a century that more species are found to be present when sampling is extended to a larger spatial area (4). This species-area relationship is commonly expressed as a power law,
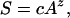 |
[1] |
where S is species richness, A is the area of the system sampled, z quantifies the scaling of richness with area, and c is a taxon- and environment-dependent constant, which is the slope of a graph whose x axis is Az and whose y axis is S (4). The species-area relationship was a fundamental stimulant (5) in the development of MacArthur and Wilson's (6) island biogeography theory.
The exponent of species-area relationships (z) has frequently been found to vary with changes in the scale being considered. For example, both flowering plants (4) and terrestrial mammals (7) exhibit strong variations in the value of z as one moves from very large to very small spatial scales. Lomolino (5) has also developed a resource- and body size-based model that predicts a small island effect in which there is a tendency for species richness to be independent of surface area for very small islands. In addition, Anderson and Wait (8) have suggested that diversity on small islands may be more strongly modified than large islands by spatial subsidies from the surrounding matrix. Other factors influencing the shape of species-area relationships include species minimum resource or space requirements, resource competition, food web structure, and species pools (9). These studies reflect a strong uncertainty in the literature about the nature of ecology's most general, yet protean, pattern (10).
Most of what we know of species-area curves is derived from analyses of terrestrial systems. Yet, phytoplankton typically occupy discrete habitats with definable borders that are comparable in some ways to oceanic islands and isolated patches of terrestrial vegetation. Moreover, over geological time scales, many of these aquatic habitats can be frequently quite ephemeral, and the biota that they currently contain must reflect the dynamics of colonization and extinction, at least over historical, biogeographic time scales.
It is striking that despite intense study of many groups of organisms, evidence supporting the existence of species-area curves for phytoplankton has not generally been reported in the literature. Indeed, Hutchinson (11) presented data indicating that algal diversity in lakes could decrease weakly with lake surface area, and Dodson et al. (12) were unable to detect a simple species richness-area relationship for lake phytoplankton. In a laboratory study of different-sized beaker ecosystems receiving a standardized species inoculum, Dickerson and Robinson (13) were surprised to find significantly higher algal diversity in the smaller systems.
This apparent lack of evidence for a consistent phytoplankton species-area relationship could, in fact, reflect the absence of such a relationship in natural aquatic ecosystems, given the highly vagile and nearly ubiquitous nature of phytoplankton. For example, closely related members of the same planktonic genera having very similar morphologies are known to occur in similar habitats worldwide (14), suggesting extremely rapid and effective dispersal. Many species of phytoplankton thus can be considered to be cosmopolitan (15), and the same floras and keys can be used in Europe, Asia, and the Americas (16). However, other potentially cosmopolitan microbial taxa with a similarly small body size do nonetheless exhibit significant species-area relationships (soil fungi, ref. 17; ciliated protozoa, ref. 18). The exponent in Eq. 1 for ciliates has been found to be small but measurable, suggesting the hypothesis that a low but nonzero value of z might also exist for phytoplankton.
In this article, we explore the scale dependence of phytoplankton biodiversity by analyzing published data from aquatic systems ranging in size from the Arctic Ocean down to some of the largest and oldest lakes on Earth, ponds, small outdoor mesocosms, and tiny laboratory microcosms.
Methods
We collected literature data from 142 different oceans, lakes, and natural ponds worldwide for which estimates of area (A, km2) and investigator-reported phytoplankton species richness (S) were available (a list of citations is available upon request). Species richness values were derived from investigator-reported microscope counts and were based on either species lists or tabulated values. The 239 experimental systems involved manipulations of natural marine and freshwater phytoplankton assemblages and ranged in size from outdoor ponds down to artificial outdoor mesocosms, steady-state laboratory chemostats, and semicontinuous cultures. The size of all ecosystems examined here varied from 3.8 × 10–9 km2 to 1.41 × 107 km2 in surface area. The duration of all experiments exceeded 30 days, and the reported species richness values are based on investigator-reported measurements of the final communities. All analyses were performed with spss statistical software.
Results
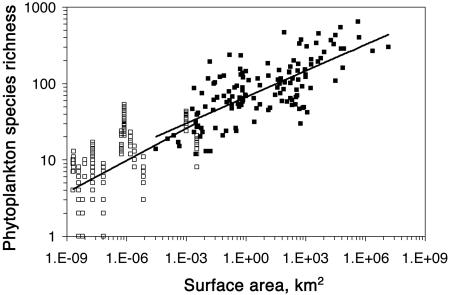
The data presented in Fig. 1 revealed the presence of highly significant, and strikingly similar, power relationships between phytoplankton species richness and surface area in both natural and experimental aquatic ecosystems:
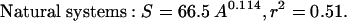 |
[2] |
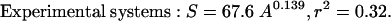 |
[3] |
The exponents (z) in both Eqs. 2 and 3 are very small and lie at the lower end of the values that have been observed for most taxa having larger body sizes (the range of z reported in ref. 4 is 0.116–0.669). The relatively small magnitude of z for phytoplankton is generally consistent with the z values that would be expected to occur in the presence of high immigration rates from regional species pools (6). However, these values considerably exceed the value of z found for ciliates (0.043; ref. 18).
Fig. 1.
Relationship between phytoplankton species richness and ecosystem surface area (km2) in natural (▪) and experimental aquatic ecosystems (□).
The natural and experimental systems described jointly by Eqs. 2 and 3 differ, however, in one important aspect: the natural systems were either directly contiguous with other water masses (e.g., the ocean basins) or frequently connected to river channels (the lake basins) that can provide a constant inflow of potential colonists through advection or fluvial inputs. Cottenie et al. (19) and Chase and Ryberg (20) have demonstrated that metacommunity structure and propagule subsidies can modify the composition and species diversity of animal communities within ponds located within single watersheds, despite the presence of strong differences in local-scale environmental conditions, and it is very likely that this mechanism can similarly influence natural phytoplankton communities.
In contrast, the experimental systems studied here were much less permeable to propagule flows across their outer boundaries: the ponds and outdoor mesocosms were exposed only to atmospheric deposition or overland transport by mobile animals, and the laboratory microcosms were completely closed systems. All three sets of experimental systems thus lacked a source of water-transported, immigrating propagules after their initial filling, and the laboratory microcosms were completely isolated from further immigration once a natural community inoculum had been added at the beginning of the experiment. The artificial mesocosms and microcosms also lacked a sedimentary seed bank containing resting stages from previous communities, such as those that are almost always present in natural waterbodies.
To assess for an effect of ecosystem permeability on the relationship between species richness and area, we performed an analysis of covariance (Table 1) of the pooled data, in which the natural systems were coded as 0 and the experimental systems were coded as 1. This statistical analysis (21) revealed no significant difference either in the regression slope z (as revealed by the high P value for the log10 AREA × CODE term) or intercept c (as revealed by the high P value for the CODE term) for the two datasets; the effect of area on species richness was thus indistinguishable between these two kinds of systems. Phytoplankton species richness thus scales smoothly and consistently from laboratory microcosms to the world's oceans, with 74% of the observed variance in S being attributable to variations in ecosystem surface area alone:
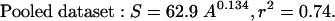 |
[4] |
Table 1. Analysis of covariance for log10 phytoplankton species richness.
| Source | Type III sum of squares | df | Mean square | F | Significance level |
|---|---|---|---|---|---|
| Corrected model | 114.734 | 3 | 38.245 | 364.149 | <0.001 |
| Intercept | 178.164 | 1 | 178.164 | 1696.395 | <0.001 |
| CODE | 0.001 | 1 | 0.001 | 0.007 | 0.934 |
| Log10 AREA | 24.340 | 1 | 24.340 | 231.758 | <0.001 |
| CODE × log10 AREA | 0.235 | 1 | 0.235 | 2.236 | 0.136 |
| Error | 39.805 | 379 | 0.105 | ||
| Total | 774.272 | 383 | |||
| Corrected total | 154.539 | 382 |
log10 lake area (AREA, km2) is the covariate, and the data is coded as either 0 (natural aquatic ecosystems) or 1 (experimental ecosystems). R2 = 0.742 (adjusted R2 = 0.740).
Discussion
Although the z values for phytoplankton are modest (ref. 4), the tremendous variation in surface area of the natural water bodies studied here is accompanied by a very substantial covariation in local phytoplankton species richness: when comparing the oceans or large lakes to small ponds, species richness drop from ≈750 species to ≈100 or fewer. It is an open question whether this broad pattern reflects the dynamics inherent in island biogeographic theory, namely the coupling of local ecosystems to regional species pools through recurrent immigration, balanced against extinctions. Given the enormous absolute population sizes of phytoplankton species even in individual water bodies as small as several deciliters, however, we conclude that it is unlikely that the patterns that we document in Fig. 1 are because of extinctions caused by stochastic demography of small populations.
One interpretation of this result is that the natural aquatic systems are acting like islands with respect to their algal floras: the relationship shown in Fig. 1 results from differences in species richness among spatially segregated islands that differ in areal extent, with each island appearing only once in the analysis and never being combined with other islands to form nested units of a larger size. Fig. 1 is thus a Type IV curve in the classification system of Scheiner (22). Variations in ecosystem size among natural islands such as these could enhance immigration rates and reduce extinction rates through at least six mechanisms: (i) larger surface areas provide larger targets for the deposition of viable algal cells and propagules from the atmosphere, as well as immigration through transport on migratory waterfowl; (ii) larger basins have larger watersheds and higher water inflow rates and, thus, should receive a greater mass flux of viable algal cells and propagules through fluvial sources; (iii) larger basins may be more likely to have multiple inflowing river channels and, thus, are likely to receive viable algal cells and propagules derived from a greater diversity of fluvially derived sources; (iv) larger basins will likely enclose more different types of habitats, which facilitates colonization by species that have specialized habitat requirements; (v) larger basins will be able to harbor larger absolute population sizes and, for this reason alone, may enjoy lower extinction rates; and (vi) larger basins may tend to have a larger spatial extent of sedimentary algal seed banks (23) that can act as an internal buffer, reducing the rate of species extinction through localized rescue effects. In addition to these six mechanisms, some (but not all) large lakes are ancient and thus may have had greater time to accumulate endemic species through speciation.
An alternative perspective arises from considering the results from closed microcosm studies. These systems, too, reveal a strong area effect on richness. However, because they are deliberately set up to preclude most avenues of immigration, the effect of ecosystem size must emerge from in situ dynamics. In effect, larger bodies of water must be able to sustain even as closed ecosystems a richer array of species. Effects iv–vi above could all have a role in permitting the maintenance of richer communities at larger spatial scales, even without recurrent immigration and recolonization from external sources.
General ecological theory suggests that larger ecosystems can sustain more complex food webs for a variety of reasons (e.g., refs. 24 and 25), and it is feasible for such food web effects to lead to a species-area relationship at lower trophic levels (e.g., because of keystone predation effects; ref. 26). Moreover, even if competitive exclusion is occurring, if it is sufficiently slow, one might observe long transient phases of coexistence; such transients are likely to last longer in larger systems. Conceivably, comparable explanations arise at all spatial scales, so it is tempting to suggest that the consistency of the z values between the set of closed artificial ecosystems and the larger, open, natural ecosystems reflects a fundamental effect of ecosystem size on internal processes that regulate species persistence and coexistence. The influence of these within-system processes (i.e., those processes that do not directly depend on the immigration-emigration balance and coupling/connectivity with regional systems) on species richness thus may scale similarly in all aquatic ecosystems. We do not yet have a fully developed theory for why this finding might be the case, but such consistency in scaling may have to do with the basic properties of hydrodynamics, the mechanics of resource exploitation, food web interactions, and the fact that a greater degree of local differentiation can develop in turbulent fluids occupying a domain of greater spatial extent.
Our results thus suggest that ecosystem size has a strong impact on local species richness by setting an upper bound to the achievable diversity. However, the controlling effects of ecosystem size can be further modified at the local scale by abiotic and biotic processes; as stressed by Adler et al. (27), we also cannot ignore the influence of time (both ecological and evolutionary) in determining species diversity patterns. Nonetheless, it is striking that phytoplankton species richness in experimental microcosms has the same scaling relationship as phytoplankton inhabiting much larger, natural, aquatic ecosystems. This congruence suggests the existence of a spatial signal that is discernable despite the presence of significant environmental noise and inevitable sampling error.
We suggest that the results shown in Fig. 1 may help to bring closure to two major hypotheses proposed in the 1970s to account for G. Evelyn Hutchinson's (28) Paradox of the Plankton. Both nonequilibrium (29) and equilibrium-based (30–32) mechanisms have been proposed to account for the patterns of species diversity that we observe in natural phytoplankton communities. We suggest here that ecosystem size may operate to constrain maximum potential species richness primarily through nonequilibrium mechanisms. Most ecosystems are temporally variable and spatially heterogeneous, and this conclusion is surely important in explaining how they can harbor rich, complex biological communities (33). Such heterogeneity should act to determine the number of available habitats and the diversity of habitat qualities, both of which govern the number of species that can coexist in the absence of dispersal and immigration.
Ecosystem size also influences metacommunity and source-sink dynamics through the migration or translocation of one or more species from one habitat to another within the system (34). Reducing ecosystem size should tend to reduce the absolute magnitude of this spatiotemporal heterogeneity and, thus, the maximum number of species that can be supported within the boundaries of the system. The mixing that occurs in small water bodies between its heterogeneous habitats also can reduce diversity through a homogenizing effect relative to source-sink relations (35, 36). In larger water bodies, such mixing and dispersal may not be sufficiently extensive or rapid enough to lead to homogenization, and diversity can thus be higher. We hypothesize that species sorting sufficient to result in the very small species richness values that are predicted by pure equilibrium-based theory for predator-free communities (approximately four to five species or fewer) may only be possible either in extremely small, closed systems such as the Indian temple tank reported by Hutchinson (11) or in natural systems that are driven to extremely low diversity levels by exceptionally high nutrient supply rates.
The strong coherence that we have observed in the values of z and c for both natural and artificial systems (Fig. 1) leads us to conclude that model aquatic ecosystems can successfully inform us about potential determinants of biodiversity change at the whole-ecosystem level (37), just as experiments performed at multiple scales successfully informed us three decades ago about the ecosystem-level effects of anthropogenic nutrient enrichment (38). Fig. 1 also provides evidence that natural patterns in microbial diversity are strongly consistent with those reported in the literature for macroorganisms (39).
The analyses presented here also emphasize the pivotal role that space plays in generating the patterns of biodiversity that we observe in nature (40). We conclude that ecosystem size controls the maximum local phytoplankton species richness attainable in any given aquatic ecosystem. However, local communities typically are greatly restricted subsets of regional species pools (41) because of the species sorting that results from the operation of local environmental filters. For example, evidence by Worm et al. (42) indicates that both nutrient availability (fertility) and predation can act as powerful environmental sieves at the local level. Local phytoplankton species richness in open natural aquatic ecosystems of different surface areas thus may simply represent a balance between the opposing processes of immigration and extinction, as predicted by MacArthur and Wilson (6). However, the presence of an essentially identical z value in less permeable experimental systems that differed equally strongly in surface area provides intriguing indirect evidence for the importance of internal ecological processes as regulators of local phytoplankton species diversity. A challenge for future work will be to disentangle the relative importance of local processes versus immigration-colonization balances, and the overall importance of equilibrium versus nonequilibrium mechanisms, in explaining observed species-area relationships in phytoplankton and the diversity patterns that are observed for most other organisms as well (24, 34, 43, 44).
Acknowledgments
We thank I. Ahlgren, K. Ashton, S. Bennett, J. Brown, G. Cronberg, J. Makarewicz, P. Leavitt, G. Morabito, H. Paerl, F. Pick, P.J. Schembri, E. Welch, and three anonymous reviewers for their constructive comments. This work was conducted as part of the Patterns in Microbial Diversity Working Group supported by the National Center for Ecological Analysis and Synthesis, a center funded by the National Science Foundation Grant DEB-9421535, the University of California, Santa Barbara, and the State of California. This work was also supported by National Science Foundation Grants DEB-0108302 and DEB-9520882.
Author contributions: V.H.S. designed research; V.H.S., J.P.G., R.D.H., M.A.L., and F.d.N. performed research; J.P.G., M.A.L., V.H.S., and B.L.F. analyzed data; and V.H.S., B.L.F., R.D.H., and M.A.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Willis, K. J. & Whittaker, R. J. (2002) Science 295, 1245–1248. [DOI] [PubMed] [Google Scholar]
- 2.Bengtsson, J., Engelhardt, K., Giller, P., Hobbie, S., Lawrence, D., Levine, J., Vilá, M. & Wolters, V. (2002) in Biodiversity and Ecosystem Functioning: Synthesis and Perspectives, eds. Loreau, M., Naeem, S. & Inchausti, P. (Oxford Univ. Press, New York), pp. 209–220.
- 3.Lawton, J. H. (1998) Oikos 84, 177–192. [Google Scholar]
- 4.Rosenzweig, M. L. (1995) Species Diversity in Space and Time (Cambridge Univ. Press, New York).
- 5.Lomolino, M. V. (1999) in Ecological Assembly Rules: Perspectives, Advances, Retreats, Eds. Weiher, E. & Keddy, P. (Cambridge Univ. Press, New York).
- 6.MacArthur, R. H. & Wilson, E. O. (1967) The Theory of Island Biogeography (Princeton Univ. Press, Princeton).
- 7.Brown, J. H. (1995) Macroecology (Univ. of Chicago Press, Chicago).
- 8.Anderson, W. B. & Wait, D. A. (2001) Ecol. Lett. 4, 289–291. [Google Scholar]
- 9.Tjørve, E. (2003) J. Biogeogr. 30, 827–835. [Google Scholar]
- 10.Lomolino, M. V. (2000) J. Biogeogr. 27, 17–26. [Google Scholar]
- 11.Hutchinson, G. E. (1966) A Treatise on Limnology: Introduction to Lake Biology and the Limnoplankton (Wiley, New York), Vol. 2.
- 12.Dodson, S. I., Arnott, S. E. & Cottingham, K. L. (2000) Ecology 81, 2662–2679. [Google Scholar]
- 13.Dickerson, J. E. & Robinson, J. V. (1985) Ecology 66, 966–980. [Google Scholar]
- 14.Reynolds, C. S. & Elliot, J. A. (2002) Verh. Int. Ver. Theor. Angew. Limnol. 28, 336–334. [Google Scholar]
- 15.Ichimura, T. (1996) Hydrobiologia 336, 1–17. [Google Scholar]
- 16.Mann, D. G. & Droop, S. J. M. (1996) Hydrobiologia 336, 19–32. [Google Scholar]
- 17.Wildman, H. G. (1987) Trans. Br. Mycol. Soc. 88, 291–297. [Google Scholar]
- 18.Finlay, B. J. (2002) Science 296, 1061–1063. [DOI] [PubMed] [Google Scholar]
- 19.Cottenie, K., Michels, E., Nuytten, N. & DeMeester, L. (2003) Ecology 84, 991–1000. [Google Scholar]
- 20.Chase, J. M. & Ryberg, W. A. (2004) Ecol. Lett. 7, 676–683. [Google Scholar]
- 21.Kleinbaum, D. G. & Kupper, L. L. (1978) Applied Regression Analysis and Other Multivariable Methods (Duxbury, North Scituate, MA).
- 22.Scheiner, S. M. (2004) Glob. Ecol. Biogeogr. 13, 479–484. [Google Scholar]
- 23.Reynolds, C. S. (1997) Vegetation Processes in the Pelagic: A Model for Ecosystem Study, Excellence in Ecology, ed. Kinne, O. (Ecology Inst., Oldendorf, Germany), Vol. 9.
- 24.Holt, R. D. (1993) in Species Diversity in Ecological Communities: Historical and Geographical Perspectives, eds. Ricklefs, R. E. & Schluter D. (Univ. of Chicago Press, Chicago), pp. 77–88.
- 25.Holt, R. D., Lawton, J. H., Polis, G. A. & Martinez, N. (1999) Ecology 80, 1495–1504. [Google Scholar]
- 26.Leibold, M. A. (1996) Am. Nat. 147, 784–812. [Google Scholar]
- 27.Adler, P. B. & Lauenroth, W. K. (2003) Ecol. Lett. 6, 749–756. [Google Scholar]
- 28.Hutchinson, G. E. (1961) Am. Nat. 95, 137–145. [Google Scholar]
- 29.Richerson, P., Armstrong, R. & Goldman, C. R. (1970) Proc. Natl. Acad. Sci. USA 67, 1710–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen, R. (1975) Am. Nat. 109, 35–49. [Google Scholar]
- 31.Tilman, D. (1982) Resource Competition and Community Structure (Princeton Univ. Press, Princeton). [PubMed]
- 32.Interlandi, S. J. & Kilham, S. S. (2001) Ecology 82, 1270–1282. [Google Scholar]
- 33.Holt, R. D. (2001) in Encyclopedia of Biodiversity, ed. Levin, S. (Academic, New York), Vol. 5, pp. 413–426. [Google Scholar]
- 34.Holt, R. D. (1992) Theor. Popul. Biol. 41, 354–371. [Google Scholar]
- 35.Amarasekare, P. & Nisbet, R. M. (2001) Am. Nat. 158, 572–584. [DOI] [PubMed] [Google Scholar]
- 36.Mouquet, N. & Loreau, M. (2002) Am. Nat. 159, 420–426. [DOI] [PubMed] [Google Scholar]
- 37.Petchey, O. L., Morin, P. J., Hulot, F. D., Loreau, M., McGrady-Steed, J. & Naeem, S. (2002) in Biodiversity and Ecosystem Functioning: Synthesis and Perspectives, eds. Loreau, M., Naeem, S. & Inchausti, P. (Oxford Univ. Press, New York), pp. 127–138.
- 38.Smith, V. H. (1998) in Successes, Limitations, and Frontiers in Ecosystem Science, eds. Pace, M. L. & Groffman, P. M. (Springer, New York).
- 39.Horner-Devine, M. C., Lage, M., Hughes, J. B. & Bohannan, B. J. M. (2004) Nature 432, 750–753. [DOI] [PubMed] [Google Scholar]
- 40.Tilman, D. & Karieva, P. (1997) Spatial Ecology (Princeton Univ. Press, Princeton).
- 41.Ricklefs, R. (1987) Science 235, 167–171. [DOI] [PubMed] [Google Scholar]
- 42.Worm, B., Lotze, H. K., Hillebrand, H. & Sommer, U. (2002) Nature 417, 848–851. [DOI] [PubMed] [Google Scholar]
- 43.Hillebrand, H. & Blenckner, T. (2002) Oecologia 132, 497–491. [DOI] [PubMed] [Google Scholar]
- 44.Crawley, M. J. & Harral, J. E. (2001) Science 291, 864–868. [DOI] [PubMed] [Google Scholar]