Abstract
Brief exposure of skin to near-infrared (NIR) laser light has been shown to augment the immune response to intradermal vaccination and thus act as an immunologic adjuvant. Although evidence indicates that the NIR laser adjuvant has capacity to activate innate subsets including dendritic cells (DCs) in skin as conventional adjuvants do, the precise immunological mechanism by which the NIR laser adjuvant acts is largely unknown.
Here we sought to identify the cellular target of the NIR laser adjuvant by using an established mouse model of intradermal influenza vaccination and examining the alteration of responses resulting from genetic ablation of specific DC populations. We found that a continuous wave (CW) NIR laser adjuvant broadly modulates migratory DC populations, specifically increasing and activating the Lang+ and CD11b−Lang− subsets in skin, and that the antibody responses augmented by the CW NIR laser are dependent on DC subsets expressing CCR2 and Langerin. In comparison, a pulsed wave (PW) NIR laser adjuvant showed limited effects on the migratory DC subsets. Our vaccination study demonstrated that the efficacy of CW NIR laser is significantly better than that of PW laser, indicating that the CW NIR laser offers a desirable immunostimulatory microenvironment for migratory DCs.
These results demonstrate the unique ability of the NIR laser adjuvant to selectively target specific migratory DC populations in skin depending on its parameters, and highlight the importance of optimization of laser parameters for desirable immune protection induced by a NIR laser-adjuvanted vaccine.
Keywords: adjuvant, vaccine, laser, dendritic cells, continuous wave, pulsed wave, near-infrared
Introduction
The development of safe and potent immunologic adjuvants is a key challenge for vaccine development. Typically, chemical or biological adjuvants are used to enhance vaccine efficacy, but most of these elicit side effects, including undesirable local reactogenicity or systemic toxicity due to their ability to stimulate innate immunity. These side effects may prevent approval for clinical use (1–3). An alternative to standard adjuvants is to stimulate the skin with laser light before an intradermally delivered vaccine. We have shown that briefly treating the skin vaccination site with a low power, continuous wave (CW) 1064 nm near-infrared (NIR) laser light immediately before intradermal influenza vaccination significantly enhances immune responses, and results in improved survival in a lethal challenge murine influenza model (4). NIR laser exposures, unlike chemical or biological forms of conventional adjuvants, do not induce prolonged activation of innate immune responses, and the effects typically resolve within 24 hours in exposed tissue (4). This suggests that NIR laser adjuvants have a distinct mechanism of action compared to chemical and biological adjuvants, and may have a more favorable safety profile while maintaining efficacy (4–6).
Multiple mechanisms of action have been described for clinical adjuvants, including establishment of an antigen depot effect, induction of inflammatory cytokines, activation of antigen presenting cells (APCs), recruitment of innate immune cells, and facilitating antigen translocation to draining lymph nodes (7, 8). Although the specific mechanism by which the employed adjuvant enhances protective immunity in many vaccines is often unclear, an in-depth understanding of various adjuvant mechanisms of action is critical for establishing optimal adjuvant-vaccine formulations for induction of effective immunity against infections. Recruitment, activation and maturation of dendritic cells (DCs) appears to play a central role in enhancing adaptive immunity. DCs are critical for the development of innate and adaptive immune responses against pathogens (9). DCs specialize in antigen presentation, directing T cell and humoral immune responses, and maintaining memory responses. An accumulating body of evidence demonstrates that different DC subsets are responsible for specialized immune functions. DCs imprint naïve responses to direct the differentiation of CD4 helper T cells into TH1, TH2, TH17, Tfh, Tr1, and Treg (9–14). Multiple specialized subtypes of DCs that have been identified can be distinguished by surface markers, which have recently been correlated to their unique transcriptome based programs across mice and humans (15–21).
Various types of clinical lasers can induce an immunostimulatory microenvironment that recruits and activates DCs, similar to that induced by classical chemical and biological adjuvants (5, 6). Wang et al. demonstrated that treatment of the vaccine inoculation site on the skin with a commercial non-ablative fractional laser followed by intradermal vaccination augmented humoral immune responses, and induced cross-protective immunity in a lethal challenge murine model of influenza (22, 23). Dying skin cells killed by laser release damage-associated molecular patterns (DAMPs), attracting APCs, in particular plasmacytoid DCs (pDCs), resulting in augmentation of immune responses. Terhorst et al. observed that skin micropores generated by ablative fractional laser using the P.L.E.A.S.E.® (Precise Laser Epidermal System) device enhanced the anti-tumor immune responses induced by prophylactic and therapeutic cancer vaccines (24). The local inflammatory milieu created by the death of keratinocytes during skin laser microporation was similarly responsible for activation of XCR1+ DCs, thereby inducing tumor antigen-specific CD8+ and CD4+ T cell responses. We and others demonstrated that application of non-tissue damaging lasers in skin also induces an immunostimulatory microenvironment that activates DCs (6, 25). The application of a pulsed wave (PW) 532 nm laser increases the motility of APCs in skin and increases antigen-positive CD11c+ DCs in the skin-draining lymph node (dLN) (26), augmenting an anti-influenza TH1-skewed immunity, resulting in suboptimal protection (4). We also previously noted that exposure to a low power, CW 1064 nm NIR laser induced an upregulation of a selective set of chemokines in skin, accumulation of CD11c+ cells and activation of DCs in skin-dLN, resulting in significant enhancement of immune responses and improved survival in a lethal influenza challenge murine model (4). However, in vivo cellular immunological responses to NIR laser adjuvants, including identification of the DC subsets that generate the distinct immune response to each laser, remain poorly characterized. A more precise description of these responses is needed to optimize design of an adjuvanted vaccine incorporating the NIR laser to induce effective protection. Here we show that the NIR lasers target specific DC subsets and augment immune responses to an influenza vaccine. These findings advance our mechanistic understanding of combinatorial vaccine and laser adjuvant.
Materials and Methods
Animals
Six to eight-week-old female C57BL/6 mice (stock no:000664) were purchased from Jackson Laboratories. All animals were acclimated for two weeks prior to the beginning of the experiments. CCR2−/− (004999), CCR7−/− (006621) and Lang-GFP/DTR (016940) mice were purchased from Jackson Laboratories and bred at Massachusetts General Hospital (MGH). All animal procedures were performed following the Public Health Service Policy on Humane Care of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of MGH.
Systemic depletion of cells harboring diphtheria toxin receptor (DTR)
Langerin-GFP/DTR animals were injected intraperitoneally with 4 ng/g of diphtheria toxin (DT, Sigma-Aldrich) 24 h before immunization as described previously (27, 28). Control C57BL/6 mice were also treated with 4 ng/g of DT within the same experiment.
Skin damage study
For visual inspection, we observed for any signs of skin damage including blistering, bruising, crusting, edema, redness or swelling at 0, 1, 2, and 4 days after laser illumination as previously described (4). For skin histology, mice were heart-perfused with 4% paraformaldehyde before, or at 2, 6, and 24 h after laser illumination. Five µm-thick paraffin-embedded sections were hematoxylin and eosin-stained and examined for microscopic tissue damage, and polymorphonuclear infiltration was quantitated on the slides in 5 randomized fields using Image J freeware (NIH) as previously described (4).
Laser adjuvant illumination and influenza vaccinations
A Nd:YVO4 1064 nm laser (RMI laser, Lafayette, CO) was used as previously described (4). The 1064 nm laser can be set to emit either continuous wave (CW) or nanosecond pulsed wave (PW) at a repetition rate of 10 kHz. The irradiance (power density) of both the CW and PW 1064 nm lasers at the skin surface was 5 W/cm2, as this irradiance was shown previously to be non-tissue damaging over an extended period of time, to maintain skin temperature less than 43°C, and to induce optimal adjuvant effects (4). The 532 nm laser output was PW only at 10 kHz at an irradiance of 1 W/cm2. All lasers were adjusted to illuminate a circular exposure on the skin of approximately 5 mm (0.2 cm2) with less than a 50% difference in beam intensity from center to edge. Laser exposures at 1064 nm were one minute with a total dose of 300 J/cm2, and at 532 nm were four minutes with a total dose 240 J/cm2.
Mice were depilated (Nair, Church & Dwight) 2–3 days before laser adjuvant illumination and immunization. The inactivated influenza virus A/PR/8/34 (H1N1, Charles River) vaccine was delivered intradermally (i.d.) using 1 µg in a total volume of 10 µl of saline per mouse. Vaccine was injected in the center of the laser-treated spot on the back of the mouse within 5 minutes of the laser treatment. Vaccination groups included: no vaccine (saline/sham treated), 1 µg vaccine only, CW 1064 nm illuminated for 1 min at 5 W/cm2 prior to 1 µg i.d. vaccine, PW 1064 nm illuminated for 1 min at 5 W/cm2 followed by 1 µg i.d., PW 532 nm laser illuminated for 4 min at 1 W/cm2 followed by 1 µg i.d., 1 µg influenza vaccine delivered intramuscularly (i.m.), 1 µg influenza vaccine mixed with Alum (Imject®, Thermo-Fisher) delivered i.d., and 1 µg influenza vaccine mixed with an oil-in-water emulsion adjuvant with a formulation similar to MF59® (AddaVax, Invivogen) delivered i.d. As described previously, 28 days later mice were homologously challenged intranasally with live A/PR/8/34 (H1N1) virus at a dose of 2 × 105 50% egg infectious doses (EID50). Four days after challenge mice were sacrificed and blood and spleen samples were taken for further analysis (4).
Anti-influenza antibody responses and determination of Hemagglutination inhibition (HAI) titers
Anti-influenza IgG, IgG1, and IgG2c humoral responses were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (4). Briefly, ELISA plates (Immulon) were coated with 100 ng of inactivated influenza virus. Serially diluted mouse serum samples were added to the wells, and bound immunoglobulins were detected with the appropriate secondary antibody (goat anti-mouse IgG [1:10,000, Sigma-Aldrich], rat anti-mouse IgG1 [1:2,000, SouthernBiotech], or goat anti-mouse IgG2c [1:4,000, SouthernBiotech]. A titer was designated as the serum dilution corresponding to the inflection point of the plot of the optical density vs. dilution of serum. Hemagglutination inhibition (HAI) titers in sera samples were determined by SRI International (Harrisburg, VA).
Assessment of T cell responses
Splenocytes were harvested 4 days post live influenza challenge and immediately processed for assessment of T cell responses. Splenocyte preparations were each divided into two duplicate wells within a round bottom 96-well plate containing 1 × 106 cells and incubated with or without 1 µg/ml inactive influenza for 60 hours for determination of cytokine release from splenocytes. Splenocyte culture supernatants were collected and the amounts of IFN-γ or IL-4 (pg/ml) were determined using DuoSet ELISA kits (R&D Systems) following manufacturer’s instructions.
Influenza virus challenge study
Immunized mice were anesthetized and challenged intra-nasally with live influenza A/PR/8/34 at a dose of 1.5×106 50% egg infectious doses (EID50), which is equivalent to 150×50% mouse lethal dose (MLD50), in 30 µl saline 28 days after vaccination as previously described (4). Survival and body weight were monitored for 15 days post-challenge. Mice showing a hunched posture, ruffled fur, or greater than 20% body weight loss, or mice which were not eating or drinking, were considered to have reached the experimental endpoint.
Immunization with OVA and DC isolation from lymph nodes (LNs)
In order to quantitate DC migration and function in vivo, mice were injected i.d. with a total of 40 µg of endotoxin-free ovalbumin (OVA) conjugated to Alexa Fluor 488 (OVA-A488) (Life Technologies) as described previously (4). Briefly, the prepared mouse back was illuminated with the NIR laser in four locations, as described above. This was followed by i.d. injections of OVA-A488 at each treatment site (10 µg in 10 µl saline per spot, 4 spots in total).
To quantify DC subsets, skin-draining lymph nodes (inguinal, brachial, and axial) were harvested and pooled from each animal 24 h after laser administration and OVA-A488 injections. LN were teased apart and incubated with collagenase D (2.5mg/ml and 0.45U/ml, Roche) at 37°C for 25min in HBSS (Invitrogen). 10 mM EDTA was added for an additional 5 min incubation as previously described (29). Five million cells per well were plated for subsequent labeling for flow cytometry.
Subtyping of DCs in LNs by flow cytometry
Cells isolated from skin-draining LN were labeled on ice in 2–5 % FBS/PBS using the Cytofix/ Cytoperm™ kit (BD Biosciences) for multiparameter flow cytometry. The following antibodies were obtained from BD, eBioscience, or BioLegend: hamster anti-mouse CD11c (PE; N418), rat anti-mouse MHC class II (I-A/I-E) (A700; M5/114.15.2), rat anti-mouse CD11b (PerCP Cy5.5; M1/70), hamster anti-mouse CD103 (PE Cy7; 2E7), mouse anti-mouse langerin (CD207) (APC; 4C7), rat anti-mouse CD8a (APC e780, 53-6.7) and rat anti-mouse CD86 (BV605; GL1). Live/dead®-Aqua and OVA conjugated to A488 were obtained from Life Technologies. Data acquisition was performed on a Fortessa cytometer (BD, 4 lasers, capable of acquiring 13 colors, DIVA software for automatic compensation) followed by analysis on FlowJo software (TreeStar).
Dendritic cell subsets from dLN were gated similarly to a previous description (29) using the following strategy: scatter, exclusion of dead cells, singlets, and CD11c+ versus MHC class II. Classical lymph node resident cells (cDC) were selected on CD11chi status and I-A/I-E intermediate levels, and were further sub-gated for CD11b+ vs. CD103+ populations. Migratory dendritic cells (migDC) were selected for CD11c intermediate levels and I-A/I-Ehi status as distinct from cDC, and further gated for Langerin (Lang+) vs. CD11b+. From cells within the migDC+ Lang+ gate, CD11b+ vs. CD103+ subpopulations were further gated (fig. S1).
Cutaneous fluorescein isothiocyanate (FITC) painting assay
Mice were shaved and depilated as described above. Four hours prior to the intradermal vaccination and laser treatment, mice were painted on the 4 spots of flank back skin (approximately 5 mm in diameter per spot) with 10 µl of a 1% FITC solution (Isomer I, Sigma) prepared in acetone:dibutyl phthalate (1:1, vol/vol; Sigma) as previously described (30). NIR laser treatment followed by vaccination with OVA (EndoFit™ Ovalbumin, Invivogen) at each treatment site (10 µg in 10 µl saline per spot, 4 spots in total) was performed on the FITC-painted sites. We then harvested skin-dLN 24 and 48 h after the vaccination and laser treatment, isolated DCs from LN, and analyzed using the same strategy as described above on the basis of surface markers of DCs and FITC fluorescence by flow cytometry.
Statistical analyses
A log transformation of antibody titers was applied for antibody titer analysis in order to reduce positive skewing in the distribution of the raw antibody titers which would violate parametric test assumptions. We ran a mixed within and between subject (mouse) repeated measures analysis of variance (ANOVA) where the 3 antibody titers of IgG and subclasses (IgG, IgG1, IgG2c) were treated as a 3 level within subject factor, crossed with the between subject factors of 4 Genotypes (WT, Lang-GFP/DTR, CCR2−/−, CCR7−/−) and 3 Treatments (vaccine i.d., vaccine i.d. + CW 1064 nm, vaccine i.d. + PW 1064 nm). All 2 and 3 way interactions of within and between factors were included in the initial run. Nonsignificant terms were subsequently removed in a backward elimination algorithm using a cutoff of P = 0.05. The degrees of freedom were adjusted for correlated error with the Huynh-Feldt correction. Tukey post hoc multiple comparison tests were run as needed.
A similar analysis restricted to the WT genotype was also run crossing the 3 level within subject antibody factor (IgG, IgG1, IgG2c) with the 7 level between subject Treatment factor (vaccine i.d., vaccine i.d. + CW 1064 nm, vaccine i.d. + PW 1064 nm, vaccine i.d. + PW 532 nm, vaccine i.m., vaccine + Alum i.d., and vaccine + MF59 i.d.). Since the IgG, IgG1 and IgG2c responses of WT mice injected with or without DT for all treatments were not statistically significant, we pooled the results within the same treatment groups.
For the assessment of T cell responses, a similar analysis was run where the 2 cytokines (IFN-γ, IL-4) were treated as a 2 level within subject factor, crossed with the between subject factors of 4 genotypes (WT, Lang-GFP/DTR, CCR2−/−, CCR7−/−) and 3 Treatments (vaccine i.d., vaccine i.d. + CW 1064 nm, vaccine i.d. + PW 1064 nm). A similar analysis restricted to the WT genotype was also run crossing the 2 level within subject cytokine factor (IFN-γ, IL-4) with the 7 level between subject Treatment factor (vaccine i.d., vaccine i.d. + CW 1064 nm, vaccine i.d. + PW 1064 nm, vaccine i.d. + PW 532 nm, vaccine i.m., vaccine + Alum i.d., and vaccine + MF59 i.d.).
For the assessment of CD86 expression, we normalized on a scale of 0–1 to account for variations of signal intensity, instrumental settings, and fluorescence settings to pool data generated by flow cytometry from multiple experiments. Data noted as relative median fluorescence were analyzed using non-parametric Kruskal-Wallis with Dunn’s correction for multiple comparisons.
The data analysis for this paper was conducted using SAS/STAT® software for Windows (SAS Institute Inc., Cary, NC) and Prism 6 (GraphPad software 2015). Data were pooled from at least two independent experiments for each treatment that was evaluated.
Supplemental materials
The following is a list of the supplemental figures.
Fig. S1 Multi-parameter flow cytometry gating schematics
Fig. S2 Effect of NIR laser on skin tissue
Fig. S3 Depletion of cells harboring DTR in Langerin-GFP/DTR animals
Fig. S4 Effect of laser illumination on body weight following viral challenge
Results
Nanosecond-pulsed wave (PW) near-infrared (NIR) laser is non-tissue damaging
We first determined if the previously established maximum dosage of the pulsed wave (PW) near-infrared (NIR) laser (4) was non-tissue damaging, as it was not determined in the previous study. Mice received exposures at 5.0 W/cm2 for 1 minute, at which the maximum safe irradiances with no visual skin damage was confirmed and skin temperatures did not exceed 43 °C. Skin damage was evaluated after illumination by visual inspection and histology. No visual damage such as blistering, bruising, crusting, edema, redness or swelling damage was seen at any time point for both the 1064 nm PW laser (fig. S2a), which is consistent with our previous report (4). On histological examination, no tissue damage or inflammatory response at any given time point was detected by hematoxylin and eosin staining (fig. S2b) as evidenced by minimal polymorphonuclear cell-infiltration in the skin (less than 6 /mm2 on average, fig. S2c). Thus, we concluded that the dosage for the PW NIR laser was non-tissue damaging and non-inflammatory.
CW and PW 1064 nm lasers augment immune responses to influenza vaccination
We next compared the adjuvant effect of PW NIR laser with previously explored visible and CW NIR lasers and conventional adjuvants including Alum and an oil-in-water emulsion, AddaVax®, in a murine influenza vaccination model. Mice received a single laser dose and were injected i.d. with whole inactivated influenza virus A/PR/8/34.
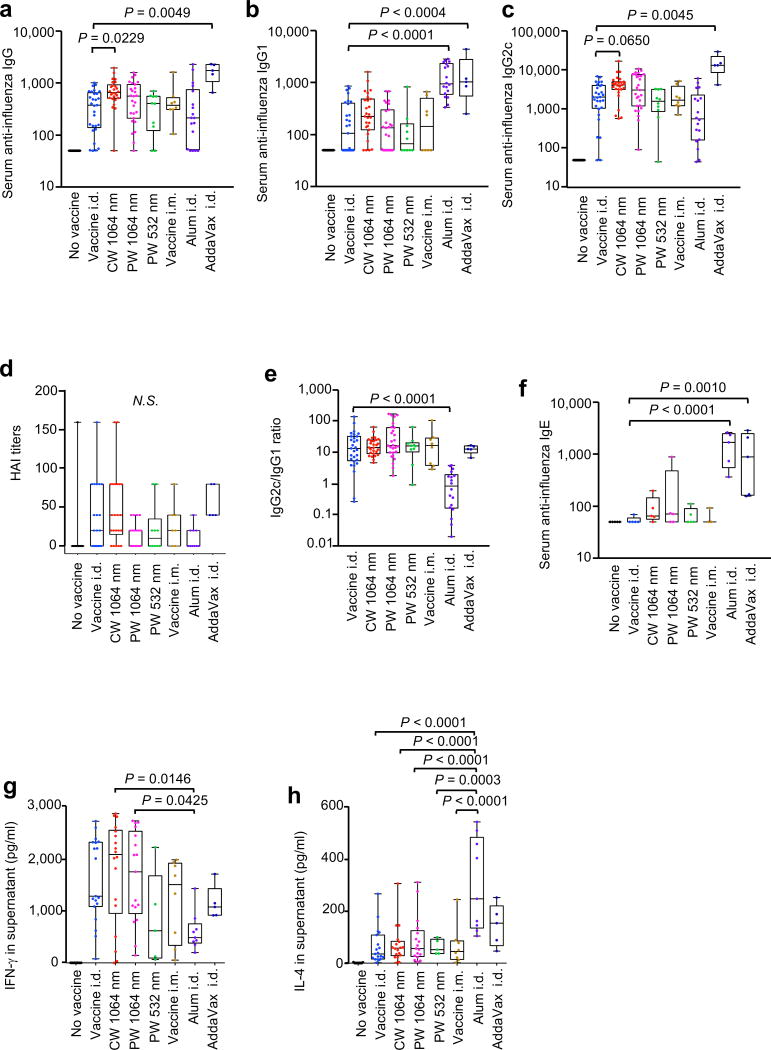
The CW NIR laser significantly augmented anti-influenza IgG response (P = 0.0229, fig. 1a) and induced a statistically marginal increase in IgG2c (P = 0.0650, fig. 1c) compared to the non-adjuvanted i.d. only group. The HAI geometric mean titer (GMTs) for the CW laser group was higher (20.25, 95% CI, 9.17–44.69) than that of the non-adjuvanted i.d. only group (8.484, 95% CI, 3.407–21.13), although this difference was not statistically significant (fig. 1d). The CW laser adjuvant induced a similar anti-influenza IgG2:IgG1 ratio (fig. 1e) and IFN-γ and IL-4 secretion levels from ex vivo stimulated splenocytes compared to the non-adjuvanted i.d. only group (fig. 1g–h). Our results confirm that the CW NIR laser adjuvants an influenza vaccine, inducing a mixed T helper type 1 (TH1) and TH2 immunity as previously reported (4). The PW laser adjuvant similarly augmented anti-influenza IgG titer (fig. 1a–c) with no appreciable increase in HAI titer and a similar IgG2:IgG1 ratio (fig. 1d) and IFN-γ and IL-4 secretion levels from ex vivo stimulated splenocytes (fig. 1g–h) compared to the non-adjuvanted group (fig. 1e). None of these observations were statistically significant. Taken together, these results suggest that the CW NIR laser is a more effective adjuvant than the PW laser for intradermal influenza vaccine.
Figure 1. Effect of the laser adjuvant on anti-influenza immune responses.
(a–f) Influenza-specific humoral responses 4 days after challenge. Mice were vaccinated with 1 µg of inactivated influenza virus (A/PR/8/34) with or without laser illumination or representative chemical adjuvant (alum or AddaVax) and challenged intranasally with live homologous virus 4 weeks after vaccination. Titer of influenza-specific serum IgG subclass was determined by ELISA, plates were coated with inactivated influenza virus. (a) IgG, (b) IgG1 and (c) IgG2c titers. (d) HAI titers. (e) IgG2c/IgG1 ratio. (f) IgE titers. (g–h), Systemic T-cell responses were measured 4 days after challenge by re-stimulating 1×106 splenocytes with inactivated influenza vaccine antigen. Levels of (g) IFN-γ and (h) IL-4 in splenocyte culture supernatants are shown. Experimental and control groups: (a–c, e) n = 30, 29, 26, 27, 10, 8, 18, 5, (d) n = 14, 22, 22, 22, 10, 7, 10, 5, (f) n = 5, 5, 5, 5, 5, 3, 5, 5, (g–h) n = 20, 19, 18, 17, 5, 8, 9, 5, for no vaccine, vaccine i.d., vaccine i.d. + CW 1064 nm, vaccine i.d. + PW 1064 nm, vaccine i.d. + PW 532 nm, vaccine i.m., vaccine + Alum i.d., and vaccine + AddaVax i.d. vaccine groups, respectively. Results were pooled from three independent experiments and analyzed using two-way ANOVA with Tukey’s correction.
We also compared the efficacy of the PW 532 nm green laser adjuvant as well as conventional adjuvants, Alum and Addavax®. In our current study, PW 532 nm laser administration produced neither significant anti-influenza antibody responses nor T cell responses (fig. 1a–d, g–h), as previously reported (4). A mixture of influenza vaccine with Alum resulted in elevated anti-influenza IgG1 (P < 0.0001, fig. 1b), significantly lower IgG2:IgG1 ratio than the non-adjuvanted group (P < 0.0001, fig. 1e), increased IL-4 (P = 0.0006, fig. 1h) and decreased IFN-γ (fig. 1g) influenza-specific responses compared to the non-adjuvanted group. The finding is consistent with published data showing that alum induces a profoundly polarized TH2 response (8, 31). We also show that the alum adjuvant induces a significant anti-influenza IgE response (fig. 1f, P < 0.0001 compared to the non-adjuvanted i.d. only group). A mixture of the influenza vaccine with Addavax®, delivered i.d., significantly increased anti-influenza IgG, IgG1, and IgG2c responses (IgG: P < 0.0049, IgG1: P = 0.0004, IgG2c: P < 0.0045, fig. 1a–c) with a similar IgG2:IgG1 ratio to the non-adjuvanted group (fig. 1e). The HAI GMT for Addavax® group was the highest among the test groups (52.78, 95% CI, 32.94–84.57), although this difference is not statistically significant (fig. 1d). However, Addavax® generated a significantly increased IgE (fig. 1f, P = 0.0010), resembling an allergic response. These results indicate that the use of chemical adjuvants including Addavax®, for i.d. vaccination, may lead to unexpected induction of hypersensitivity. In contrast, NIR laser adjuvants, including the newly tested PW NIR laser, did not induce appreciable IgE responses (fig. 1f). Together, these data indicate that NIR laser treatment produces a mixed TH1-TH2 immune response to influenza vaccination depending on laser parameter without inducing any hypersensitivity.
The NIR laser adjuvant modulates migDC population within the skin-dLN
We have shown that the CW NIR laser induces migrational and functional changes of CD11c+ cells in skin and dLN (4). In order to further identify which specific DC subsets are activated by the CW and PW NIR laser adjuvants, we harvested skin-dLN 24 h after laser treatment and i.d. injection of fluorescently labeled OVA.
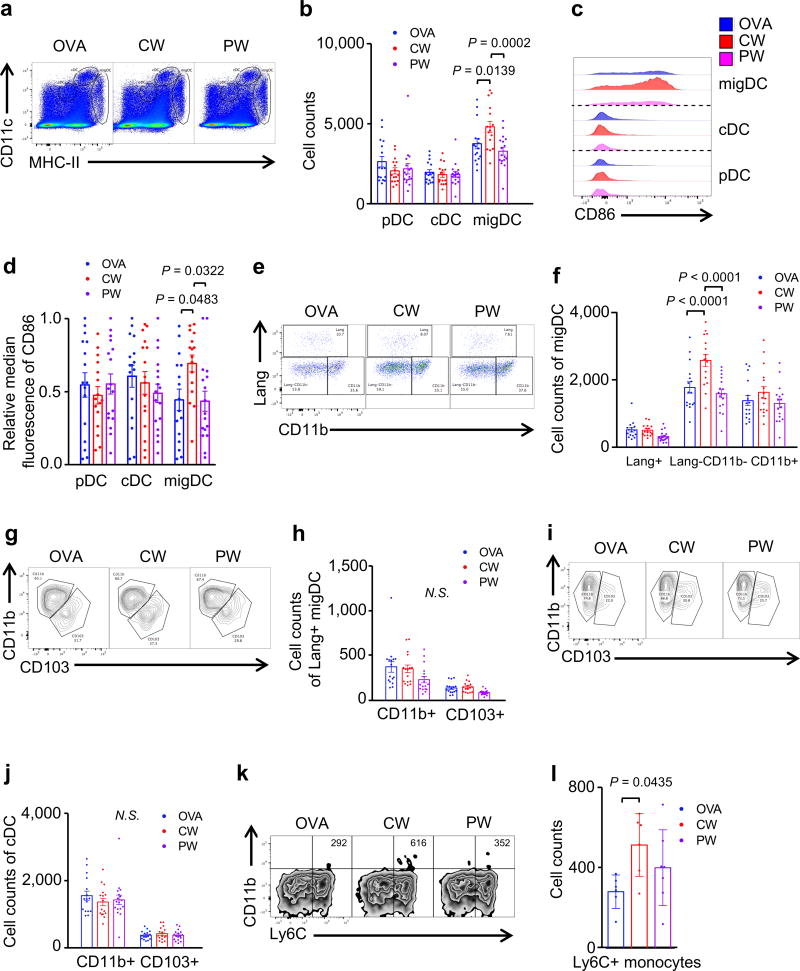
Tissue migratory DC (migDC) in skin-dLN, defined by MHC class IIhi-CD11cint expression, can be further divided into the following functionally distinct subsets: Langerin−CD11b−, Langerin−CD11b+, Langerin+CD103+CD11b−, and Langerin+CD103−CD11b+ Langerhans cells (LC) (32–35). In addition to migDC, classical DC (cDC) that are resident within LN originate from the bone marrow. cDC are characterized by MHC-IIintCD11chi expression and contain CD11b+ (CD8α−) and CD103+ (CD8α+) subsets. Plasmacytoid DCs (pDCs) are characterized by MHC-IIlowCD11clow expression and are capable of priming T cells after infection or immunization in LN (36). The pDC, cDC, and migDC populations were evaluated as defined by their varying MHC class II and CD11c expression and their surface markers including Langerin, CD11b and CD103 as previously described (29). The CW NIR laser adjuvant increased the number of migDC populations compared to the no laser-OVA i.d. only control group (P = 0.0139, fig. 2a and b). These migDC were also preferentially activated by the CW NIR laser treatment, as indicated by the expression of CD86 (P = 0.0483, fig. 2c and d). Amongst all the DC subsets in skin, Lang−CD11b− DCs preferentially expanded following NIR CW laser illumination (P < 0.0001, fig. 2e and f), accompanied by a marginal increase in CD11b+ migDC subset (fig. 2e and f). The NIR laser did not induce appreciable changes in the number of cDC or pDC in LN (fig. 2a and b). Further analysis indicated that the CW NIR laser adjuvant induced no appreciable change in Lang+ migDC (fig. 2g and h) or the cDC subsets (fig. 2i and j).
Figure 2. The effect of NIR laser adjuvant on DCs within the skin draining lymph nodes.
DCs in skin-draining lymph nodes (skin-dLN) were processed and stained for multi-parameter flow cytometry 24 hours after intradermal vaccination with 40 µg Alexa Fluor-488-labeled OVA with or without one minute CW or PW 1064 nm NIR laser treatment. (a) Representative gates of plasmacytoid DCs (pDC), classical lymphoid tissue-resident DCs (cDCs), and migratory DCs (migDC); numbers indicate percent of total lymphocytes. (b) Cell counts. (c) Representative histograms of CD86 expression. (d) Median fluorescent intensity of CD86 expression for pDC, cDC, and migDC population. (e) Representative gates of migDC subsets. (f) Cell counts of migDC subpopulation within skin-dLN. (g) Representative gates of Lang+migDC subsets, numbers indicate percent parent. (h) Cell counts of Lang+migDC subpopulation within skin-dLN. (i) Representative gates of cDC subsets, number representing percent parent. (j) Cell counts of cDC subpopulation within skin-dLN. (k) Representative gates of CD11b+Ly6C+ monocytes. (l) Cell counts of CD11b+Ly6C+ monocytes within skin-dLN. Data were analyzed with (b, f, h, j and l) two-way ANOVA followed by the Tukey's honestly significant difference (HSD) tests or (d) Kruskal-Wallis with Dunn’s correction for multiple comparisons. Experimental and control groups: (a–j) n = 16, 16, 17, (k–l) n = 6, 6, 7, for OVA i.d., OVA i.d. + CW 1064 nm, OVA i.d. + PW 1064 nm, respectively. Data are derived from three independent experiments.
The PW NIR laser adjuvant did not induce appreciable changes in the number of migDC in LN (fig. 2a and b) nor significantly alter the expression of activation markers of migDC subsets (fig. 2c and d). The PW NIR laser adjuvant led to a marginal decrease in Lang+ migDC subsets compared to OVA i.d. only group (fig. 2e and f), while inducing no change in other DC subsets. Further analysis showed the PW NIR laser decreased the number of both Lang+CD11b+ and Lang+CD103+ migDC subsets slightly as compared to OVA i.d. group (fig. 2g and h), although this change is not statistically significant. The PW laser adjuvant did not significantly alter CD11b+ or CD103+ within cDCs subsets (fig. 2i – j).
In our previous findings describing the CW NIR laser adjuvant, NIR laser illumination of the skin resulted in increased ccl2 and ccr2 gene expression in skin and an increase in the number of MHC class II+ CD11c+ DCs within the dLN 6 h after skin illumination with the laser (4). This led us to evaluate the recruitment of inflammatory monocytes and monocyte derived DC (16, 37, 38) by the NIR laser adjuvant. The CW NIR laser adjuvant increased the number of CD11b+Ly6C+ monocytes in skin-dLN compared to the OVA i.d. only control group (P = 0.0435, fig. 2k and l), while the PW NIR laser induced a statistically marginal increase in this population.
Together, these results suggest that the CW NIR laser adjuvant modulates Lang−CD11b− migDCs and induces recruitment of CD11b+Ly6C+ monocytes, while the PW NIR laser adjuvant shows marginal effect on these DC populations.
The NIR laser adjuvant activates antigen-bearing migDC population within the skin-dLN
In order to further dissect the functional alteration of each DC subset induced by the NIR laser adjuvant, we analyzed the numerical and functional changes in antigen-bearing (OVA+) cells in skin-dLN 24 h after laser treatment and i.d. injection of fluorescently labeled OVA.
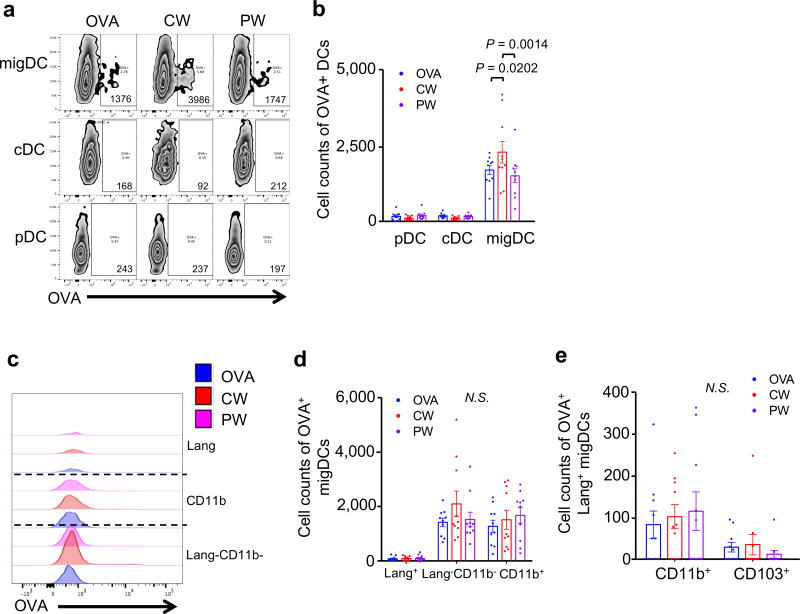
The CW NIR laser induced a significant increase of the number of OVA+ migDC in comparison to the no laser OVA i.d. only control group (P = 0.0202, fig. 3a and b). Across all the migDC subsets, the NIR CW laser preferentially increased OVA uptake by Lang−CD11b− migDCs and the number of OVA+ Lang−CD11b− migDCs, although this numerical increase is not statistically significant (fig. 3c and d). Furthermore, the CW NIR laser induced a marginal increase of the number of OVA+Lang+CD11b+migDC subset (fig. 3e) in comparison to the no laser OVA i.d. only control group.
Figure 3. The effect of NIR laser adjuvant on antigen-bearing DCs within the skin draining lymph nodes.
(a) Representative gates of pDC, cDC, and migDC bearing OVA antigen, number indicates cell count of positive gate. (b) Cell counts of OVA+ pDC, cDC, and migDC population within skin-dLN. (c) Representative histograms of OVA+migDCs. (d) Cell counts of OVA+migDC subpopulation within skin-dLN. (e) Cell counts of OVA+Lang+migDC subpopulation within skin-dLN. (b, d, e) Data were analyzed with two-way ANOVA followed by the Tukey's honestly significant difference (HSD) tests. Experimental and control groups: (a–j) n = 15, 16, 16, 17 for no vaccine, OVA i.d., OVA i.d. + CW 1064 nm, OVA i.d. + PW 1064 nm, respectively. Data are derived from three independent experiments.
On the other hand, mice treated with PW NIR laser adjuvant exhibited more OVA+ cells, including CD11b+, Lang+CD11b+ migDC subsets, while less OVA+ cells in Lang+CD103+ migDC subset, as compared to the no laser OVA i.d. only control group (fig. 3c and d), although these changes were not statistically significant.
In short, these results are consistent with the view that the CW NIR laser adjuvant preferentially modulates Lang−CD11b− migDCs, while the PW NIR laser adjuvant possibly modulates Lang+ and CD11b+ migDCs.
The CW NIR laser adjuvant augments migration of skin-resident migDC to the skin-dLN
Skin-resident migDCs constantly migrate into the skin-dLN in normal and inflammatory settings, orchestrating a wide array of adaptive immune responses (39). Our data consistently show that the NIR laser adjuvant selectively modulates migDCs in the skin-dLN. These findings led us to assess the effect of NIR laser adjuvant on migration of migDC subsets in skin. To this end, we used an established cutaneous FITC-painting assay to quantitate DC migration from skin to the skin-dLN (30). We applied FITC solution to the flank skin of mice prior to the NIR laser treatment followed by i.d. injection of OVA. We then assessed the migration of migDC subsets into the skin-dLN at 24 and 48 h after vaccination.
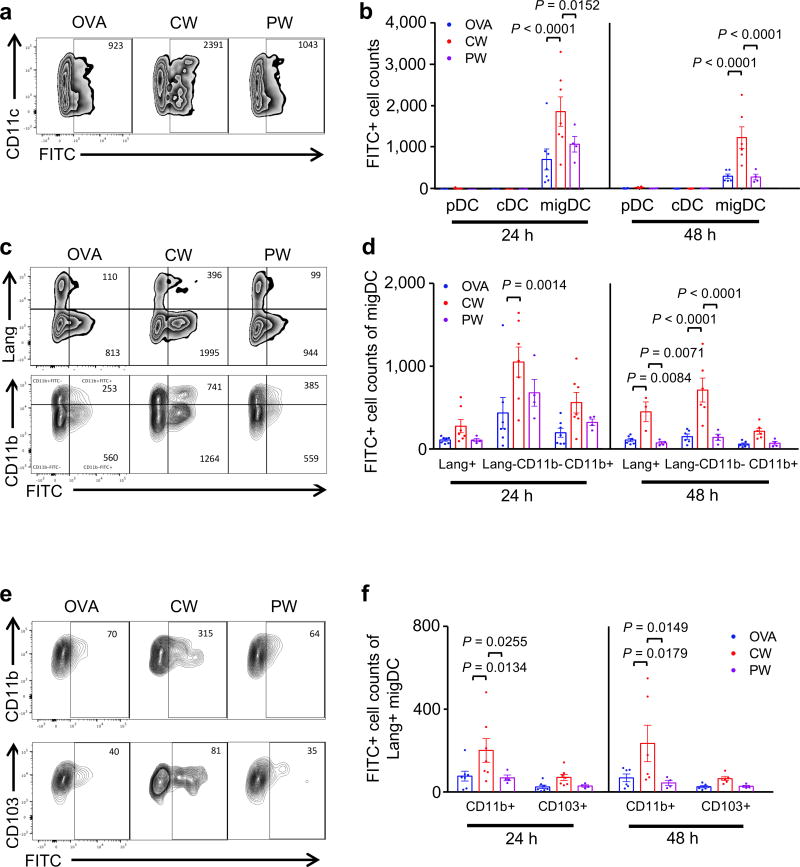
The CW NIR laser adjuvant increased the number of FITC+ migDC populations compared to the no laser-OVA i.d. only control group (24 and 48 h, P < 0.0001), while near background numbers of FITC+ cDCs or pDCs were detected at these time points (fig. 4a and b). Amongst all the DC subsets in skin, the NIR CW laser preferentially facilitated migration of Lang−CD11b− (24 h, P = 0.0014; 48 h, P < 0.0001) and Lang+ (48 h, P = 0.0084) migDC subsets, as compared to no laser OVA i.d. only control group (fig. 4c and d). Further analysis revealed that the CW NIR laser marginally increases Lang+CD103+ and significantly increases Lang+CD11b+ DCs compared to no laser OVA i.d. only control group (24 h: P = 0.0134; 48 h, P = 0.0178, fig. 4e and f).
Figure 4. The effect of NIR laser on emigration of migDC subsets.
Mice were shaved, depilated, and painted with 1% FITC solution on the flank skin 4 hours before vaccination with 40 µg of OVA with or without NIR laser treatment. At the times indicated, single-cell suspensions from skin-dLN were labeled and analyzed based on surface markers and FITC fluorescence by flow cytometry. (a) Representative gates of FITC+ migDC emigrating into the skin-dLN after FITC painting. (b) Cell counts of in the skin-dLN after FITC painting. (c) Representative gates of migDC subsets. (d) Cell counts of migDC subpopulation within skin-dLN after FITC painting. (e) Representative gates of Lang+migDC subsets. (f) Cell counts of Lang+migDC subpopulation within skin-dLN after FITC painting. (b, e, f) Two-way ANOVA followed by the Tukey's HSD tests. Experimental and control groups: n = 6–7, 6–7, 4 for vaccine i.d., vaccine i.d. + CW 1064 nm, vaccine i.d. + PW 1064 nm, respectively. Data are derived from three independent experiments.
The PW laser adjuvant did not significantly alter the number of FITC+ DCs within migDCs subsets in LN compared to no laser OVA i.d. only control group (fig. 4a–f).
Since the CW NIR laser adjuvant most efficiently augments the arrival of FITC+ migDCs to the skin-dLN and the corresponding immune responses to intradermal vaccination (fig. 1), these results indicate that the adjuvant effect of the NIR laser is predominantly mediated by migrational responses of migDC subsets in skin.
The effect of the CW NIR laser adjuvant is mediated by coordination between Lang+ and CD11b−Lang− migDC subsets
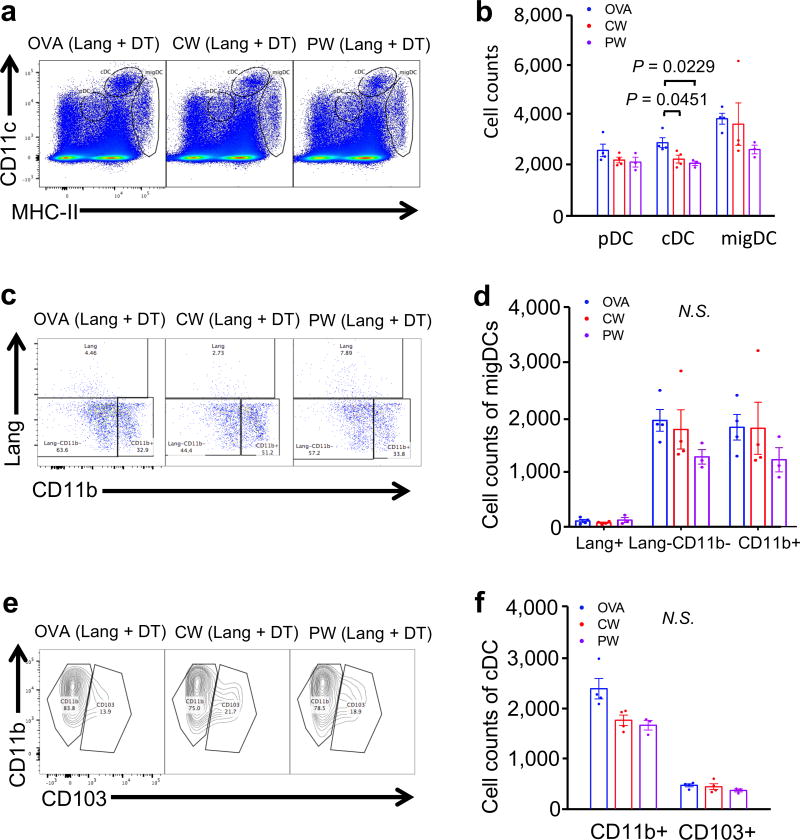
Our data suggest that Lang+ migDC subsets play a pivotal role in the adjuvant effect of the NIR lasers. In order to assess this, we used a genetic mouse model expressing diphtheria toxin (DT) receptor (DTR) under the control of the langerin promoter (Lang-DTR) (28). Lang-DTR mice were injected with diphtheria toxin (DT) 24 h before the OVA immunization. We confirmed that single DT injection efficiently ablated Lang+ migDC up to 48 h after DT administration, while lymphoid tissue-resident cDC were relatively intact (fig. S3a and b), consistent with published observations (28).
Interestingly, when the numbers of Lang+ DC were reduced, the CW NIR laser adjuvant did not induce an increase in the migDC (fig. 5a and b) or CD11b−Lang− migDC population (fig. 5c and d), which was observed in WT mice (fig. 2a and b; fig 2e and f). In mice lacking Lang+ cells, treatment with the CW NIR laser adjuvant did not induce a significant change in cDC subsets (fig. 5e and f). Mice lacking Lang+ cells treated with the PW NIR laser adjuvant induced a slight decrease of the number of Lang−CD11b− and CD11b+ migDCs (fig. 5c and d) and no other DC populations changed as compared to OVA i.d. group (fig. 5a–f).
Figure 5. Depletion of Lang+ DCs removes CW laser induced population changes.
Lang−DTR/GFP mice were treated with diphtheria toxin 1 day prior to four intradermal injections of 10 µg of A488-labeled ovalbumin (OVA) with or without laser adjuvant illumination. DCs in skin-dLN were processed and stained for multi-parameter flow cytometry 24 hours after intradermal vaccination with 40 µg Alexa Fluor-488-labeled OVA with or without one minute CW or PW 1064 nm NIR laser treatment. (a) Representative gates of plasmacytoid DCs (pDC), classical lymphoid tissue-resident DCs (cDCs), and migratory DCs (migDC); numbers indicate percent of total lymphocytes. (b) Cell counts. (c) Representative gates of migDC subsets, numbers indicate percent parent. (f) Cell counts of migDC subpopulation within skin-dLN. (i) Representative gates of cDC subsets, number indicating percent parent. (j) Cell counts of cDC subpopulation within skin-dLN. (f, j) Data were analyzed with two-way ANOVA followed by the Tukey's honestly significant difference (HSD) tests. Experimental and control groups: (a–j) n = 4, 4, 3 for OVA i.d., OVA i.d. + CW 1064 nm, OVA i.d. + PW 1064 nm, respectively.
Deletion of specific DC subsets alters the impact of the NIR laser adjuvant on humoral and cellular responses to influenza vaccination
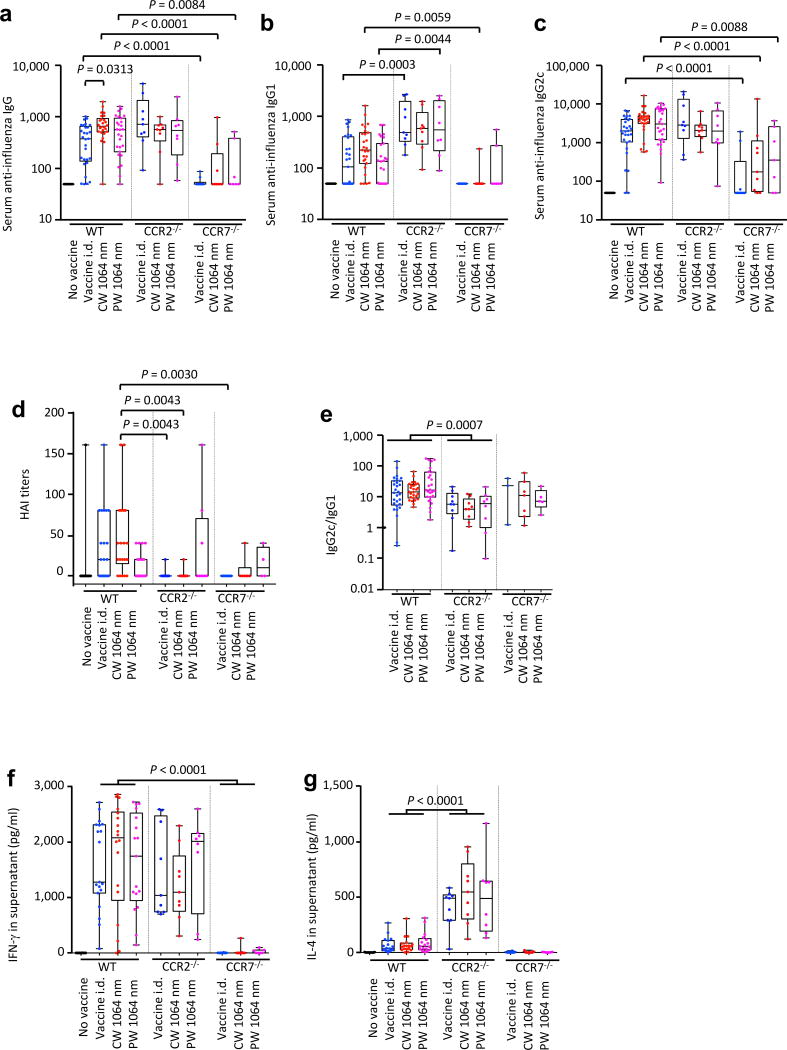
Our data indicate that the NIR laser adjuvant modulates migDC in skin and induces recruitment of CD11b+Ly6C+ monocytes. In our previous findings describing the CW NIR laser adjuvant, NIR laser illumination on the skin resulted in increased ccr7, ccl2, and ccr2 gene expression and an increase in number of MHC class II+ CD11c+ DCs within the dLN 6 h after skin illumination with the laser (4). To probe the contribution of specific DC subsets to the efficacy of the NIR laser adjuvant, we took advantage of genetic mouse models in which we can manipulate specific DC subsets in the context of an established model of influenza vaccination. To this end, we evaluated the response of CCR2 deficient (CCR2−/−) mice to test the contribution of CCR2+ cells to NIR laser adjuvant response. CCR2 knockout mice (CCR2−/−) model was used to test the contribution of inflammatory monocytes and monocyte derived DC (16, 37, 38) to the NIR laser adjuvant effect. CCR7 deficient (CCR7−/−) mice were also included as a control in these experiments. CCR7 loss entirely inhibits migDC trafficking and has been reported to delay antibody production by B cells (40–42).
The CCR2−/− mice produced generally higher anti-influenza IgG, IgG1, and IgG2c antibody titers than wild-type (WT) mice, with a statistically significant increase is observed in anti-influenza IgG1 (WT vs CCR2−/− in i.d. only group: P = 0.0003, in PW NIR laser group: P = 0.0044, fig. 6a–c). The CW NIR laser adjuvant induced an increase in anti-influenza IgG and IgG2c compared to i.d. only group in WT mice (fig. 1a–c), but removal of CCR2+ cells abolished the adjuvant effect of the CW NIR laser to increase anti-influenza IgG and IgG2c responses (fig. 6a–c). Moreover, this general increase in binding antibody did not lead to a productive neutralizing antibody response as HAI titers in CCR2−/− mice were significantly lower than those in WT mice (WT vs CCR2−/− in i.d. only group: P = 0.0043, WT vs CCR2−/− in CW NIR laser group: P = 0.0043, fig. 6d). The mean values of IgG2c:IgG1 ratio in CCR2−/− mice showed a significant decrease compared to those of WT mice (WT vs CCR2−/−: P = 0.0007, fig. 6e). In agreement with the heightened TH2 response we observed that the ablation of CCR2+ cells strikingly increases total influenza IL-4 responses, while maintaining the same IFN-γ responses (IL-4 of WT vs CCR2−/−: P < 0.0001, fig. 6f and g). It appears that in the absence of CCR2+ cells intradermal influenza vaccination produces a heavily TH2-skewed response, and a balanced response against influenza vaccination requires CCR2+ cells for a productive neutralizing antibody response. These results are consistent with the published finding that CCR2+ inflammatory DCs have the ability to activate CD4+ T cells and drive their polarization toward TH1 immune responses (38).
Figure 6. Effect of the laser adjuvant on anti-influenza immune responses in CCR2−/− or CCR7−/− mice.
(a–e) Influenza-specific humoral responses in post-challenge (4 days after challenge). C57BL/6 WT, CCR2−/−, or CCR7−/− mice were vaccinated with 1 µg of inactivated influenza virus (A/PR/8/34) with or without laser illumination and challenged intranasally with live homologous virus 4 weeks after vaccination. Titer of influenza-specific serum IgG subclass was determined by ELISA. (a) IgG, (b) IgG1 and (c) IgG2c titers. (d) HAI titers. (e) IgG2c/IgG1 ratio. All experiments were repeated 3 times and pooled to show results. (f–g) Systemic T-cell responses were measured 4 days after challenge by re-stimulating 1×106 splenocytes with inactivated influenza vaccine antigen for 60 hours. Levels of (f) IFN-γ and (g) IL-4 in splenocyte culture supernatants are shown. Data were analyzed with two-way ANOVA followed by the Tukey's HSD tests. WT data from Fig. 1 are shown for comparison. See the Material and Methods section for strategy used for statistical analysis. Experimental and control groups: (a–c, e) n = 30, 29, 26, 27, 9, 9, 8, 10, 9, 7, (d) 14, 22, 22, 22, 9, 9, 8, 7, 6, 4 (f–g) n = 20, 19, 18, 17, 9, 9, 8, 10, 9, 7 for no vaccine in WT, vaccine i.d. in WT, vaccine i.d. + CW 1064 nm in WT, vaccine i.d. + PW 1064 nm in WT, vaccine i.d. in CCR2−/−, vaccine i.d. + CW 1064 nm in CCR2−/−, vaccine i.d. + PW 1064 nm in CCR2−/−, vaccine i.d. in CCR7−/−, vaccine i.d. + CW 1064 nm in CCR7−/−, vaccine i.d. + PW 1064 nm in CCR7−/−groups, respectively.
As predicted, mice lacking CCR7 dependent migration and receiving an i.d. influenza vaccine showed a significant reduction of anti-influenza IgG (WT vs CCR7−/− in i.d. only group: P < 0.0001, WT vs CCR7−/− in CW NIR laser group: P < 0.0001, WT vs CCR7−/− in PW NIR laser group: P = 0.0084, fig. 6a), IgG1 (WT vs CCR7−/− in CW NIR laser group: P = 0.0059, fig. 6b), and IgG2c (WT vs CCR7−/− in i.d. only group: P < 0.0001, WT vs CCR7−/− in CW NIR laser group: P < 0.0001, WT vs CCR7−/− in PW NIR laser group: P = 0.0088, fig. 6c). CCR7−/− mice generally showed a similar IgG2c:IgG1 ratio compared to WT mice (fig. 6e). Consistent with the significant reduction of anti-influenza antibody response, HAI titers in CCR7−/− mice were significantly lower than those in WT mice (WT vs CCR7−/− in i.d. only group: P = 0.0030, fig. 6d). In addition, IFN-γ influenza-specific splenocyte responses were significantly reduced as compared to WT mice (P < 0.0001, fig. 6f). CCR7-deficient mice have been reported to lack primary B and T cell responses, showing severely delayed kinetics of the antibody response (42). However, in previously published reports the humoral response to vaccination eventually reaches a similar level to that of WT mice over time. Here CCR7−/− mice failed to mount significant humoral and cell-mediated immune responses to intradermal vaccination 5 weeks after the primary vaccination, which may further support dependence on migratory cells, including migDC. These data build on those provided above by the FITC painting which consistently show that the effect of the NIR laser is mediated by migrational responses of migDC subsets in skin (fig. 4). Taken together these data support the view that the NIR laser adjuvant modulates the migDC in coordination with CCR2+ inflammatory monocytes in the context of intradermal influenza vaccination.
The NIR laser adjuvant depends on the function of Lang+ DC and inflammatory DCs
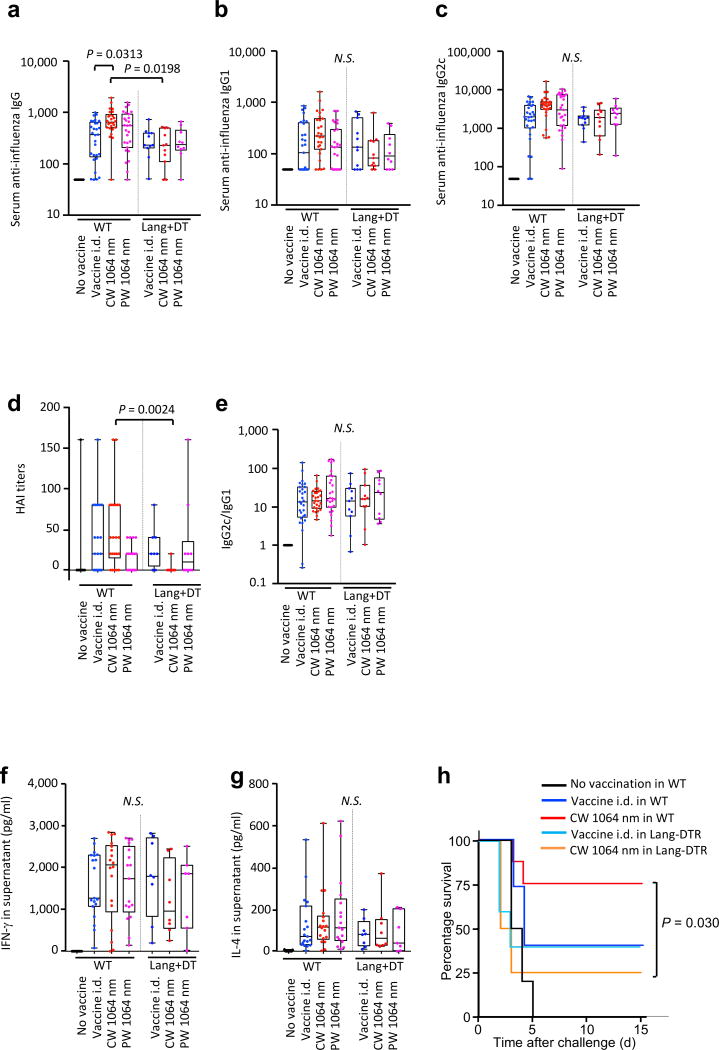
To assess the functional contribution of each DC subset, we used a genetic mouse model expressing a diphtheria toxin (DT) receptor (DTR) under the control of the langerin promoter (Lang-DTR). Lang-DTR/GFP mice injected with DT were used to evaluate the immune function of Langerhans cells (LCs) and Lang+ dermal DCs (28, 43–45).
Depletion of Lang+ cells via DT injection 24 h before the vaccination abolished the effect of the CW NIR laser adjuvants on anti-influenza IgG and IgG2c responses (fig. 7a–c) and notably decreased IgG response (WT vs Lang/DTR + DT in the CW NIR laser group: P = 0.0313, fig. 7a). Depletion of Lang+ cells consistently and significantly decreased HAI titers in the CW NIR laser group (WT vs Lang/DTR + DT in CW NIR laser group: P = 0.0024), but the response in the PW NIR laser group was not affected (fig. 7d). The IgG2:IgG1 ratio was not significantly changed in any group (fig. 7e). Depletion of Lang+ cells resulted in a notable decrease in IFN-γ response in CW NIR laser group (fig. 7f and g), although the decrease was not statistically significant.
Figure 7. Effect of the laser adjuvant on anti-influenza immune responses in Lang+ cell-depleted mice.
(a–e) Influenza-specific humoral responses in post-challenge (4 days after challenge). Lang-DTR/GFP mice were treated with diphtheria toxin 1 day prior to vaccination with 1 µg of inactivated influenza virus (A/PR/8/34) with or without laser illumination and challenged intranasally with live homologous virus 4 weeks after vaccination. Titer of influenza-specific serum IgG subclass was determined by ELISA. (a) IgG, (b) IgG1 and (c) IgG2c titers. (d) HAI titers. (e) IgG2c/IgG1 ratio. All experiments were repeated 3 times and pooled to show results. (f–g) Systemic T-cell responses were measured 4 days after challenge by re-stimulating 1×106 splenocytes with inactivated influenza vaccine antigen for 60 hours. Levels of (f) IFN-γ and (g) IL-4 in splenocyte culture supernatants are shown. Data were analyzed with two-way ANOVA followed by the Tukey's honestly significant difference (HSD) tests. WT data from Fig. 1 are shown for comparison. See the Material and Methods section for strategy used for statistical analysis. Experimental and control groups: (a–c, e) n = 30, 29, 26, 27, 11, 11, 10, (d) n = 14, 22, 22, 22, 8, 11, 10, (f–g) n = 20, 19, 18, 17, 8, 8, 7 for no vaccine in WT, vaccine i.d. in WT, vaccine i.d. + CW 1064 nm in WT, vaccine i.d. + PW 1064 nm in WT, vaccine i.d. in Lang/DTR + DT, vaccine i.d. + CW 1064 nm in Lang/DTR + DT, vaccine i.d. + PW 1064 nm in Lang/DTR + DT groups, respectively. (h) Kaplan-Meier survival plots of influenza-vaccinated mice for 15 days following lethal challenge. Data were analyzed with Gehan-Breslow-Wilcoxon test. EID50, the 50% egg infectious dose. Experimental and control groups: n = 10, 15, 8, 5, 4 for no vaccine in WT, vaccine i.d. in WT, vaccine i.d. + CW 1064 nm in WT, vaccine i.d. in Lang/DTR + DT, vaccine i.d. + CW 1064 nm in Lang/DTR + DT, respectively.
We next assessed the contribution of Lang+ cells modulated by the NIR laser adjuvant to protection against lethal influenza virus challenge. WT and Lang-DTR mice were vaccinated 24 h after the DT injection with or without the CW NIR laser treatment. Mice were subsequently challenged intra-nasally with homologous live influenza virus and monitored for survival time. Consistent with our previous study (4), the CW NIR laser treatment consistently conferred better protection compared to no laser vaccine i.d. only control group (fig. 7h). In contrast, depletion of Lang+ cells upon vaccination abolished the beneficial effect of the CW NIR laser in Lang-DTR mice (WT vs Lang/DTR in CW NIR laser group: P = 0.030, fig. 7h) with greater weight loss (fig. S4) upon viral challenge. These data support the view that Lang+ cells are necessary for the adjuvant effect of the CW NIR laser.
Thus, our results support the view that Lang+ cells are needed for the CW NIR laser adjuvant effect in the context of intradermal influenza vaccination.
Discussion
In this study, we showed for the first time that the NIR laser adjuvant modulates migDC in the skin and, in the context of intradermal influenza vaccination, adjuvanting effects functionally depend on DC subsets expressing CCR2 and Langerin/CD207. We also found a fundamental difference between the adjuvant effect of CW and PW NIR lasers. The adjuvant effect of the CW NIR laser was shown to be mediated by the coordinated expansion and activation of Lang−CD11b− migDC in the presence of Lang+ migDCs and CCR2+ inflammatory monocytes, while the PW NIR adjuvant shows limited effect on Lang+ and CD11b+ migDCs. Our results show that the CW NIR laser adjuvant ultimately induced a mixed TH1-TH2 response likely as a result of collaboration among multiple DC subpopulations in skin. In the past decades, multiple phenotypically and functionally distinct DC subsets in the skin have been described. Lang+CD103+ and CD11b+ DCs have been demonstrated to promote distinct TH1 and TH2 responses, respectively (39), and CCR2+ inflammatory monocytes to be capable of inducing TH1 or TH2 response (16). The CW NIR laser adjuvant requires CCR2+ inflammatory monocytes, which are likely responsible for induction of a mixed TH1-TH2 response. On the other hand, the expansion of CD11b−Lang− and functional dependence on Lang+ migDC could have contributed to the net difference in productive immunity between the CW and PW NIR laser adjuvant. This double negative CD11b−Lang− migDC subset is less well characterized due to the lack of research tools specifically targeting this subpopulation (39). It has been shown that the CD11b−Lang− migDC subpopulation is a bona fide Fms-like tyrosine kinase 3 ligand dependent DC, which by transcriptome analysis likely branches from a common precursor with Lang−CD11b+ migDCs, capable of cross-presentation ex vivo, transports FITC, and is important to the cutaneous immune environment (34), suggesting its important role in immune protection in the context of vaccination. In accordance, it has been demonstrated to share transcriptional dependence with CD11b- migDCs on KLF4 and to regulate TH2 immunity (46). Further investigation of the significance of CD11b−Lang− migDC subset in skin vaccination and protective immunity in the context of the NIR CW laser adjuvant is warranted.
Our results support the clear advantage of the NIR laser adjuvant over chemical vaccine adjuvants approved for intradermal vaccination. Conventional chemicals or biologics are designed to trigger a “danger signal” to the innate immunity in order to enhance the immune response to vaccine antigens (47, 48). Amplification of innate immunity typically involves persistent inflammatory responses that are intrinsically linked to the reactogenic and toxic effects of adjuvants (31, 49, 50). In contrast to this, we found that the NIR laser adjuvant possesses a unique ability to selectively modulate specific DC populations in skin depending on the laser parameter without inducing apparent inflammation. A broad range of transdermal and intradermal vaccination technologies (51, 52) would benefit from the inclusion of an adjuvant, but use of conventional chemical or biological adjuvants in the confines of the skin results in unacceptable inflammatory responses (53, 54). Consequently only a small number of proposed adjuvants are ideally suited for use in the skin (55, 56). In the present study, both of the representative clinical adjuvants, Alum and AddaVax, (an oil-in-water emulsion adjuvant with a formulation similar to MF59®), induced significant upregulation of influenza-specific IgE in the context of intradermal influenza vaccination (fig 1f), which may lead to an adverse response induced by vaccination. In contrast, the NIR laser adjuvant did not induce an IgE response to intradermal vaccination. Our previous study showed that the NIR laser adjuvant induces selective cellular signaling without inducing apparent tissue inflammation (4, 25). The current study further demonstrates that the NIR laser adjuvant is able to selectively modulate the most versatile and important cell population to augment adaptive immune responses, namely migDC, without inducing adverse IgE responses. These results show distinct mechanisms of action of the NIR laser compared to conventional vaccine adjuvants, highlighting a novel possibility of reproducible control over the immune response by a physical parameter for desirable protection with an intradermal vaccine.
Investigators have established that heterogeneous DC populations found in the skin cover a broad array of functions from inducing protection against various pathogens to maintaining peripheral tolerance (39). It is becoming increasingly evident that specialized DC subsets can exert specific functions, but these functions are often defined by the cues the DC receives from their microenvironment (57, 58). Hence, the induction of the immune response should be considered the result of balanced expansion and activation of specialized DC subsets and environmental cues. Since our results show that the CW or PW NIR laser evoked distinct immunological events, each NIR laser adjuvant appears able to provide immunostimulatory cues unique to its laser parameters in skin. Although the precise molecular signaling involved in this process has yet to be explored, our previous and current studies have shown that the NIR laser adjuvant is non-damaging to the tissue and does not induce typical inflammatory responses, but rather selective innate signaling including temporal upregulation of a selective set of chemokines that enhances the activation and recruitment of antigen presenting cells (APCs) in the skin (4). Onikienko et al. identified an important role of release of intracellular Hsp70 into the extracellular space in skin tissue in nanosecond-pulsed high-frequency laser adjuvant, thereby inducing TH1 response via the TLR4 receptor (5, 6, 59). HSPs expressed on the surface of stressed and damaged cells can serve as a type of danger signal and are recognized by APCs through specific receptors, such as TLRs and scavenger receptors resulting in priming T cells (60, 61).
In addition, a thermal mechanism does not appear to mediate the impact of the NIR laser on the immune system. Laser light is absorbed by skin chromophores depending on its wavelength and generates heat in the skin. While the thermal profile of PW 1064 nm laser in skin is equivalent to that of the CW 1064 nm laser (4), yet the current study shows that these lasers evoked quite distinct effects on the adaptive immune response and DC subsets in skin. Therefore, we believe the thermal effect plays a minimal role in the adjuvant effect of the NIR lasers. In this study, we have shown the possible link between cues induced by the CW NIR laser and the enrichment of CD11b−Lang− migDC and functional dependence on Lang+ migDC, while demonstrating that cues induced by the PW NIR laser led to limited modulation of Lang+ and CD11b+ migDCs. The precise relationship between molecular cues and their interactions with specific migDC subpopulations need to be further characterized in future studies for the optimization of the NIR laser adjuvant.
In summary, the NIR laser adjuvant possesses the unique ability to selectively target specific DC subsets in skin and could offer protection by a NIR laser-adjuvanted vaccine depending on its laser parameters in the context of intradermal vaccination. These findings boost efforts to customize the combination of intradermal vaccines with the laser adjuvant for the induction of immune protection from pathogens.
Supplementary Material
Acknowledgments
We thank Dr. Hang Lee, who is a consultant for the Harvard Catalyst Bio-Statistical Consulting Group, for statistical analysis in this work. We also thank Madeline Penson for her excellent technical assistance and Susan Raju Paul and Patrick Reeves (all at Massachusetts General Hospital) for fruitful discussions.
Funding: Research reported in this manuscript was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R41AI114012, R42AI114012, R01AI105131 (S.K.), Kanae Foundation for the Promotion of Medical Science (Y.K.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (Y.K.), Uehara Memorial Foundation (Y.K.), Japan Foundation for Pediatric Research (Y.K.), and the VIC Innovation Fund (S.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
K Morse performed most of the experiments; K Morse, Y Kimizuka, M Chan, M Shibata, Y Shimaoka, S Takeuchi, B Li, Z Yang, A Shigeta, RF Sîrbulescu, S Kashiwagi performed vaccination studies, K Morse, Y Kimizuka, MPK Chan, Y Shimaoka, S Takeuchi, performed immunoassays; K Morse, S Kashiwagi designed most of the experiments; K Morse, C Nirschl, Y Kimizuka, MPK Chan, Y Shimaoka, S Takeuchi, H Chen, RF Sîrbulescu, B Forbes, RYY Tan contributed to the establishment of the animal model and immunoassays; R Bronson, Y Kimizuka, K Tsukada, W Katagiri performed skin damage study; CJ Nirschl, N Anandasabapathy contributed to analysis and experimental design related to dendritic cell study; JJ Locascio performed statistical analysis on antibody responses and advised statistical tests in this study; K Morse, S Kashiwagi designed the study; K Morse, T Brauns, JA Gelfand, MC Poznansky, A Sluder, N Anandasabapathy, S Kashiwagi analyzed the data; K Morse, N Anandasabapathy, MC Poznansky, A Sluder, S Kashiwagi wrote the manuscript.
Potential conflict of interest:
All authors: No reported conflicts.
References
- 1.Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicology letters. 2011;203:97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashiwagi S, Yuan J, Forbes B, Hibert ML, Lee EL, Whicher L, Goudie C, Yang Y, Chen T, Edelblute B, Collette B, Edington L, Trussler J, Nezivar J, Leblanc P, Bronson R, Tsukada K, Suematsu M, Dover J, Brauns T, Gelfand J, Poznansky MC. Near-infrared laser adjuvant for influenza vaccine. PLoS One. 2013;8:e82899. doi: 10.1371/journal.pone.0082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashiwagi S, Brauns T, Gelfand J, Poznansky MC. Laser vaccine adjuvants. History, progress, and potential. Hum Vaccin Immunother. 2014;10:1892–1907. doi: 10.4161/hv.28840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashiwagi S, Brauns T, Poznansky MC. Classification of Laser Vaccine Adjuvants. J Vaccines Vaccin. 2016;7 doi: 10.4172/2157-7560.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, Nussenzweig MC. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 12.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devi KS, Anandasabapathy N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol. 2017;39:137–152. doi: 10.1007/s00281-016-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nirschl CJ, Anandasabapathy N. Duality at the gate: Skin dendritic cells as mediators of vaccine immunity and tolerance. Hum Vaccin Immunother. 2016;12:104–116. doi: 10.1080/21645515.2015.1066050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Schlitzer A, McGovern N, Ginhoux F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin Cell Dev Biol. 2015;41:9–22. doi: 10.1016/j.semcdb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Li B, Wu MX. Effective and lesion-free cutaneous influenza vaccination. Proc Natl Acad Sci U S A. 2015;112:5005–5010. doi: 10.1073/pnas.1500408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Shah D, Chen X, Anderson RR, Wu MX. A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nature communications. 2014;5:4447. doi: 10.1038/ncomms5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terhorst D, Chelbi R, Wohn C, Malosse C, Tamoutounour S, Jorquera A, Bajenoff M, Dalod M, Malissen B, Henri S. Dynamics and Transcriptomics of Skin Dendritic Cells and Macrophages in an Imiquimod-Induced, Biphasic Mouse Model of Psoriasis. J Immunol. 2015;195:4953–4961. doi: 10.4049/jimmunol.1500551. [DOI] [PubMed] [Google Scholar]
- 25.Kimizuka Y, Callahan JJ, Huang Z, Morse K, Katagiri W, Shigeta A, Bronson R, Takeuchi S, Shimaoka Y, Chan MP, Zeng Y, Li B, Chen H, Tan RY, Dwyer C, Mulley T, Leblanc P, Goudie C, Gelfand J, Tsukada K, Brauns T, Poznansky MC, Bean D, Kashiwagi S. Semiconductor diode laser device adjuvanting intradermal vaccine. Vaccine. 2017;35:2404–2412. doi: 10.1016/j.vaccine.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Kim P, Farinelli B, Doukas A, Yun SH, Gelfand JA, Anderson RR, Wu MX. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS ONE. 2010;5:e13776. doi: 10.1371/journal.pone.0013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Anandasabapathy N, Feder R, Mollah S, Tse SW, Longhi MP, Mehandru S, Matos I, Cheong C, Ruane D, Brane L, Teixeira A, Dobrin J, Mizenina O, Park CG, Meredith M, Clausen BE, Nussenzweig MC, Steinman RM. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med. 2014;211:1875–1891. doi: 10.1084/jem.20131397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, Rost BE, Relyveld E, Siber GR. Adjuvant properties of aluminum and calcium compounds. Pharmaceutical biotechnology. 1995;6:229–248. doi: 10.1007/978-1-4615-1823-5_8. [DOI] [PubMed] [Google Scholar]
- 32.Henri S, Guilliams M, Poulin LF, Tamoutounour S, Ardouin L, Dalod M, Malissen B. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol. 2010;88:366–375. doi: 10.1038/icb.2010.34. [DOI] [PubMed] [Google Scholar]
- 33.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollah SA, Dobrin JS, Feder RE, Tse SW, Matos IG, Cheong C, Steinman RM, Anandasabapathy N. Flt3L dependence helps define an uncharacterized subset of murine cutaneous dendritic cells. J Invest Dermatol. 2014;134:1265–1275. doi: 10.1038/jid.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GH, Chung TJ, Chan DK, Tan KK, Hon TL, Fossum E, Bogen B, Choolani M, Chan JK, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, Ginhoux F. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol. 2011;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 40.Villablanca EJ, Mora JR. A two-step model for Langerhans cell migration to skin-draining LN. Eur J Immunol. 2008;38:2975–2980. doi: 10.1002/eji.200838919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 43.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 44.Clausen BE, Kel JM. Langerhans cells: critical regulators of skin immunity? Immunol Cell Biol. 2010;88:351–360. doi: 10.1038/icb.2010.40. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 46.Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, Wu X, Wong R, Anderson DA, Murphy TL, Pearce EJ, Murphy KM. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015;42:916–928. doi: 10.1016/j.immuni.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 48.Petrovsky N. Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev Vaccines. 2008;7:7–10. doi: 10.1586/14760584.7.1.7. [DOI] [PubMed] [Google Scholar]
- 49.Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18:1217–1225. doi: 10.1177/0961203309345724. [DOI] [PubMed] [Google Scholar]
- 50.Cruz-Tapias P, Agmon-Levin N, Israeli E, Anaya JM, Shoenfeld Y. Autoimmune (auto-inflammatory) syndrome induced by adjuvants (ASIA)--animal models as a proof of concept. Current medicinal chemistry. 2013;20:4030–4036. doi: 10.2174/09298673113209990253. [DOI] [PubMed] [Google Scholar]
- 51.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zehrung D, Jarrahian C, Wales A. Intradermal delivery for vaccine dose sparing: overview of current issues. Vaccine. 2013;31:3392–3395. doi: 10.1016/j.vaccine.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012;31:159–164. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitoriano-Souza J, Moreira N, Teixeira-Carvalho A, Carneiro CM, Siqueira FA, Vieira PM, Giunchetti RC, Moura SA, Fujiwara RT, Melo MN, Reis AB. Cell recruitment and cytokines in skin mice sensitized with the vaccine adjuvants: saponin, incomplete Freund's adjuvant, and monophosphoryl lipid A. PLoS ONE. 2012;7:e40745. doi: 10.1371/journal.pone.0040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu JC, Mathias-Santos C, Greene CJ, King-Lyons ND, Rodrigues JF, Hajishengallis G, Ferreira LC, Connell TD. Intradermal administration of the Type II heat-labile enterotoxins LT-IIb and LT-IIc of enterotoxigenic Escherichia coli enhances humoral and CD8+ T cell immunity to a co-administered antigen. PLoS One. 2014;9:e113978. doi: 10.1371/journal.pone.0113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattsson J, Schon K, Ekman L, Fahlen-Yrlid L, Yrlid U, Lycke NY. Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsalpha in CD11b(+) DCs. Mucosal Immunol. 2015;8:815–827. doi: 10.1038/mi.2014.111. [DOI] [PubMed] [Google Scholar]
- 57.Romani N, Flacher V, Tripp CH, Sparber F, Ebner S, Stoitzner P. Targeting skin dendritic cells to improve intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:113–138. doi: 10.1007/82_2010_118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clausen BE, Stoitzner P. Functional Specialization of Skin Dendritic Cell Subsets in Regulating T Cell Responses. Front Immunol. 2015;6:534. doi: 10.3389/fimmu.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onikienko SB, Zemlyanoy AB, Margulis BA, Guzhova IV, Varlashova MB, Gornostaev VS, Tikhonova NV, Baranov GA, Lesnichiy VV. Diagnostics and correction of the metabolic and immune disorders. Interactions of bacterial endotoxins and lipophilic xenobiotics with receptors associated with innate immunity. Donosologiya (St. Petersburg) 2007;1:32–54. [Google Scholar]
- 60.Segal BH, Wang XY, Dennis CG, Youn R, Repasky EA, Manjili MH, Subjeck JR. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today. 2006;11:534–540. doi: 10.1016/j.drudis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.