Abstract
Glioblastoma (GBM) is a highly aggressive brain cancer with limited treatments and poor patient survival. GBM tumors are heterogeneous containing a complex mixture of dividing cells, differentiated cells, and cancer stem cells. It is unclear, however, how these different cell populations contribute to tumor growth or whether they exhibit differential responses to chemotherapy. Here we set out to address these questions using a zebrafish xenograft transplant model (Welker et al., 2016). We found that a small population of differentiated vimentin positive tumor cells, but a majority of Sox2 positive putative cancer stem cells, were dividing during tumor growth. We also observed co-expression of Sox2 and GFAP, another suggested marker of glioma cancer stem cells, indicating that the putative cancer stem cells in GBM9 tumors expressed both of these markers. To determine how these different tumor cell populations responded to chemotherapy, we treated animals with temozolomide and assessed these cell populations immediately after treatment and 5 and 10 days after treatment cessation. As expected we found a significant decrease in dividing cells after treatment. We also found a significant decrease in vimentin positive cells, but not in Sox2 or GFAP positive cells. However, the Sox2 positive cells significantly increased 5 days after TMZ treatment. These data support that putative glioma cancer stem cells are more resistant to TMZ treatment and may contribute to tumor regrowth after chemotherapy.
Keywords: zebrafish, temozolomide, Ki67, Sox2, GFAP, vimentin
Introduction
Glioblastoma (GBM) is an aggressive form of brain cancer with a poor prognosis in patients. Treatment options for GBM are limited, but include surgical resection followed by radiation and chemotherapy with temozolomide (TMZ) (Ostrom et al., 2014). According to the American Brain Tumor Association (ABTA), the median survival for patients with combined chemotherapeutic and radiation therapy is 14–18 months with only 10% of patients surviving beyond 5 years (Stupp et al., 2009). The poor prognosis of patients with GBM, combined with the lack of efficacious therapy options, clearly demonstrates a need for a better understanding of this disease to produce better clinical outcomes.
A major area of interest in GBM research is defining the cell types that contribute both to tumor growth and to tumor recurrence after chemotherapy. Understanding this would elucidate pathways and lineages of interest, leading to better diagnostic and therapeutic options. Cancer stem cells have been shown to be an important tumor cell type although many aspects of how they function in tumors is debated (Vermeulen et al., 2008; Jordan, 2009; Lathia et al., 2015; Nassar and Blanpain, 2016). Chen and colleagues (Chen et al., 2012) discovered, that a small population of neural stem cells propagates tumor growth in a genetic mouse model of GBM. The authors went on to show that ablating these cells decreased tumor growth after TMZ exposure, thus supporting that these cancer stem cells are responsible for tumor recurrence (Chen et al., 2012). Another study focused on Sox2, a transcription factor present in normal and cancer stem cells, that is prevalent in GBM tumors (Hemmati et al., 2003; Schmitz et al., 2007; Gangemi et al., 2009). When Sox2 was decreased in human GBM cells they stopped proliferating and were no longer tumorigenic when transplanted into immunodeficient mice (Gangemi et al., 2009). These data stress the importance of cancer stem cells for tumor recurrence and of Sox2 as a critical cancer stem cell protein in GBM.
Although it has been shown that tumors are comprised of different cell populations, it is unclear how different tumor cell populations contribute to tumor growth and response to chemotherapy. We had generated and standardized a xenograft paradigm using human GBM9 neurospheres transplanted into the embryonic zebrafish brain and showed that these cells formed heterogeneous tumors leading to lethality (Welker et al., 2016). These tumors were comprised of different cell populations including vimentin positive cells and Sox2 positive cells suggesting a mixture of differentiated cells and putative glioma cancer stem cells (Gangemi et al., 2009; Chen et al., 2012; Song et al., 2016). In this study we set out to define which GBM tumor cell populations were dividing and contributing to tumor growth both before and after chemotherapy treatment. Our findings show differential cell division in the different tumor populations and support a role for putative cancer stem cells in resistance to and tumor regrowth after chemotherapy.
Methods
Cell Culture
Cell culture was performed as in Welker et al., 2016 (Welker et al., 2016). Briefly, GFP labeled GBM9 neurospheres (Giannini et al., 2005; Estrada-Bernal et al., 2011; Williams et al., 2011) were obtained and kept in neurobasal media supplemented with B-27, GlutaMax, EGF and FGF.
Zebrafish lines
Zebrafish were maintained at 28°C unless otherwise noted. AB×Tupfel long fin (ABLF) animals are referred to as wild type. casper mutants (roy; nacre; (White et al., 2008) were obtained from Dr. Leonard Zon’s laboratory at Children’s Hospital Boston. All animals were kept in accordance with The Ohio State University Institutional Animal Care and Use Committee protocols. For all experiments, animals were obtained from group crosses.
Transplants
Transplants were performed as in Welker et al., 2016 (Welker et al., 2016). Briefly, when GBM9 neurospheres reached a size of ~1 mm they were dissociated using TrypLE (Gibco), counted and resuspended in HBSS (Gibco) within 15 minutes of transplantation. Cells (50–75 cells) were transplanted in the vicinity of the midbrain hindbrain boundary of 36 hours post fertilization (hpf) embryos.
Cryostat Sections
Casper animals transplanted with GBM9 cells were fixed at 5 or 10 dpt with 4% paraformaldehyde (PFA; Sigma-Aldrich) in PBS (Sigma-Aldrich) at 4°C. Animals were fixed for a minimum of 24 hours. The animals were then transferred into 30% sucrose in PBS (Thermo-Fisher Scientific, Waltham, MA, USA) at 4°C overnight. Next, animals were placed in individual silicon molds filled with OCT compound (Sakura Finetek, Torrance, CA, USA) and were frozen at −80°C for 15 minutes. Frozen animals were cut into 20 μm sections using a cryostat machine. Sections were deposited onto Super Frost Plus slides (Thermo-Fisher Scientific). Sections were cut in the transverse plane, comparable to the coronal sections used in mouse and human brains. Slides were stored at 4°C overnight and subsequently used for histological staining.
Histology and Immunohistochemistry
All staining was performed on 20 μm tissue sections. Primary antibodies were diluted in either 3% or 5% bovine serum albumin in PBS and incubated at 4°C overnight. Secondary antibodies were diluted in 1% Triton in 1XPBS and incubated at room temperature for 2 hours. n = 5 animals per group.
Ki67
Following cryosection, slides were initially outlined with a Dako Pen (Dako, Carpinteria, CA, USA). Slides were washed 3 times in PBS, 10 minutes per wash for a total of 30 minutes and then permeabilized with 0.5% Triton (Thermo-Fisher Scientific) for 10 minutes. Slides were returned to a 10 minutes PBS wash before antigen retrieval. For antigen retrieval, slides were placed in two, 7 minute rounds of boiling PBS for a total of 14 minutes. Following retrieval, slides were washed in fresh PBS. Slides were blocked for one hour in 3% or 5% bovine serum albumin in PBS (Jackson ImmunoResearch, West Grove, PA, USA) and were then stained with anti-Ki67 (D3B5) rabbit antibody at a 1/100 concentration (Cell Signaling, Danvers, MA, USA; 91295) overnight at 4°C. The following day, slides were washed in three, 30 minute rounds of PBS for a total of 90 minutes. Slides were permeabilized with 0.1% Triton for 10 minutes before being switched into secondary antibody (Life Technologies, Carlsbad, CA, USA) Alexa-Fluor 594 goat anti-rabbit IgG at 1:300 concentration for 2 hours at room temperature. Finally, slides were washed with 3 rounds of PBS, 20 minutes each round, for a total of 60 minutes and then mounted in Fluoromount with 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich) and coverslipped (Thermo-Fisher Scientific).
Vimentin
Procedure was the same as Ki67 except without antigen retrieval. However, in double labeling combinations with nuclear proteins, antigen retrieval was performed as an inevitable part of the procedure. The primary antibody was monoclonal vimentin antibody at a 1:200 concentration (mouse clone V9; Dako; M0725). The secondary antibodies used were Alexa-Fluor 594 rabbit anti-mouse IgG at a 1:200 concentration. (Life Technologies) or Alexa-Fluor 405 goat anti-mouse IgG (Life Technologies) at a 1:200 concentration. The Fluor 594 secondary was used for the single labeling experiments. The secondary antibody used for double labeling experiments was dependent on the wavelength of the other antibody in the experiment.
Sox2
Procedure was the same as Ki67 with antigen retrieval. The primary antibody used was polyclonal rabbit anti-Sox2 antibody at a 1:50 concentration (Abcam, Cambridge, MA, USA; ab97959). The secondary antibody used for the single labeling with DAPI was Alexa-Fluor 594 goat anti-rabbit IgG at a 1:200 concentration, as above. The secondary antibody used for the double labeling was Alexa-Fluor 405 goat anti-rabbit IgG (Life Technologies) at a 1:200 concentration.
Glial Fibrillary Acidic Protein (GFAP)
Procedure was the same as Ki67 except without antigen retrieval. However, in double labeling combinations with nuclear proteins, antigen retrieval was performed as an inevitable part of the procedure. The primary antibodies used were polyclonal rabbit anti-glial fibrillary acidic protein at a 1:200 concentration (GFAP, Dako; Z0334) or monoclonal mouse anti-glial fibrillary acidic protein at a 1:100 concentration (GFAP, DAKO; M0761). The primary antibody used was dependent on the animal origin of the other antibody in the double label. The secondary antibodies used were Alexa-Fluor 594 goat anti-rabbit IgG at a 1:200 concentration, as above, and Alexa-Fluor 405 goat anti-mouse IgG at a 1:200 concentration. The secondary antibody used was dependent on the animal origin of the primary antibody.
Proliferating Cell Nuclear Antigen (PCNA)
Procedure was the same as Ki67 with antigen retrieval. The primary antibody used was proliferating cell nuclear antigen at a 1:100 concentration. (PCNA, DAKO; M0879) and the secondary antibody used was Alexa-Fluor 594 goat anti-mouse IgG at a 1:200 concentration.
Imaging
Imaging of immunohistochemistry and staining analysis was performed on the Andor spinning disk confocal microscope (Andor, Oxford, UK;). 0.4 μm Z-stacks were created from individual 20 μm sections and were observed through three filter channels: 405 (blue; immunohistochemistry), 488 (green; tumor cells), and 594 (red; immunohistochemistry). Cell count and staining analysis was performed on imaged Z-stacks. The total number of GBM9 cells was counted in each of the 5 animals per group, identifiable by green fluorescent expression, followed by the number of cells expressing each individual histological label. Finally, the number of cells expressing both histological labels was evaluated (in either the red or blue channel). To create the triple labeled images, the Z-stacks were compressed in all three filter channels.
Statistics
Numbers are given as mean ± standard deviation. Significance was determined by two-tailed, independent T-test with 8 degrees of freedom. T-test for unequal variances was used when variance were deemed unequal (degrees of freedom <8). Bars in figures report the average cell counts and error bars represent standard deviation for each immunohistochemical group. n = 5 independent animals per group.
Results
Identification of a subpopulation of dividing, vimentin-positive GBM9 tumor cells
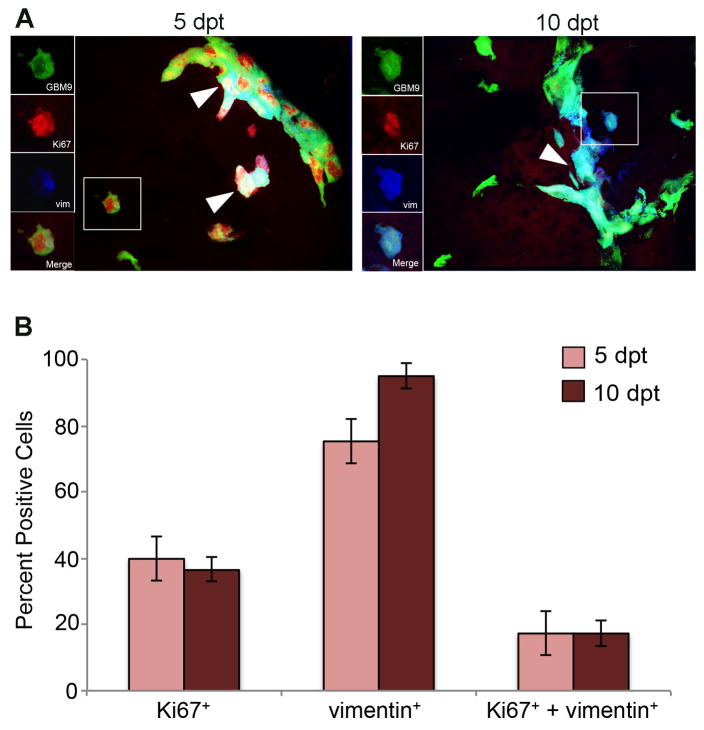
To determine which tumor cells were contributing to tumor growth, we first examined the relationship between differentiated and dividing cells using the markers vimentin (differentiation) and Ki67 (proliferation). Although the “go or grow” hypothesis predicts a separation between proliferative cells and those expressing the mesenchymal, differentiation marker vimentin our previous single labeling data indicated that there may be cells that were expressing markers of both proliferation and differentiation (Welker et al., 2016). In the single labeling experiments, we found that 40% of tumor cells were expressing Ki67 at 5 dpt. At the same time point, 75% of tumor cells were positive for vimentin (Welker et al., 2016) suggesting an overlap in these populations. To test this directly, we performed double-labeling with Ki67 and vimentin to further characterize overlapping cell populations within tumors.
We found that 40 ± 5% of GBM9 tumor cells were positive for Ki67 at 5 dpt indicating that these were dividing cells (Fig. 1). Simultaneously, 75 ± 16% of tumor cells were expressing vimentin, indicating that these were differentiated populations. These numbers were similar to the values we obtained previously in single labels (Welker et al., 2016). Analysis of the double labels revealed that ~17% of tumor cells expressed both vimentin and Ki67 at 5 (17 ± 7%; Fig. 1B, dark red bars) and 10 dpt (17 ± 5%; Fig. 1B, light red bars). Between 5 and 10 dpt there was an increase in vimentin positive cells (but this did not reach significance), but not an increase in double-labeled cells. This indicates that at 10 dpt fewer vimentin positive cells were dividing consistent with differentiated cells. These data confirm that GBM9 tumors contain a significant number of cells that do not exclusively display characteristics of dividing or differentiated cells, but instead concurrently expressed markers of each. Thus, although proliferation or differentiation states may dictate cell behavior, they are not entirely exclusive in these tumors and GBM cancer cells may display a mixed phenotype expressing characteristics of both cell states.
Figure 1. Identification of a subpopulation of dividing, vimentin-positive GBM9 tumor cells.
(A) 20 μm sections of zebrafish with GBM9 tumors (green) were analyzed at 5 and 10 dpt. Sections were labeled with anti-Ki67 (red) and anti-vimentin (blue). Triple labeled cells are white (white arrow heads). The inset to the left of the image shows each of the channels separated. (B) Antibody labeled sections were counted for the number of cells expressing Ki67, vimentin or both Ki67 and vimentin. Light red bars represent 5 dpt and dark red bars represent 10 dpt (mean ± SD). n = 5 animals per group. *p=0.05.
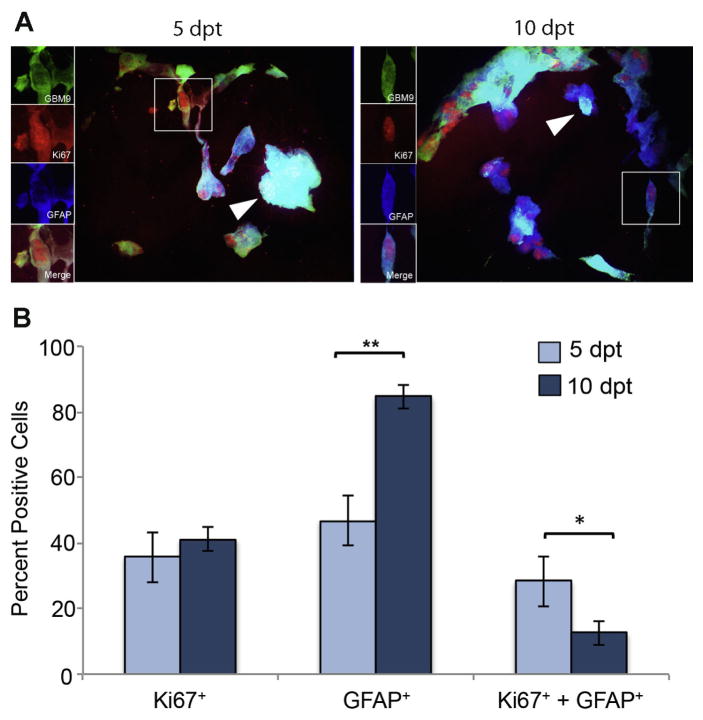
The population of dividing GFAP-positive GBM9 tumor cells decreases during tumor growth
We next addressed the relationship between proliferation and differentiation as measured by Ki67 and GFAP. GFAP is known to be a protein expressed in mature glia, such as astrocytes or ependymal cells, making it a marker for differentiation. However, evidence also supports that progenitor cells, like radial glia, and cancer stem cells can express GFAP as well (Doetsch et al., 1999; Kriegstein and Alvarez-Buylla, 2009; Chen et al., 2012). To better understand the cells expressing GFAP in GBM, we performed concurrent immunohistochemistry for Ki67 and GFAP. At 5 dpt, 36 ± 9 % of tumors expressed Ki67. This number was not statistically different at 10 dpt (41 ± 3%). At 5 dpt, 47 ± 5% of cells were positive for GFAP expression and this number increased significantly to 84 ± 3% at 10 dpt (p<0.0001). These values were consistent with those previously observed (Welker et al., 2016). At 5 dpt, 28 ± 8% of tumor cells were positive for both Ki67 and GFAP (Fig. 2B, dark blue bars). This population decreased significantly to 12 ± 4% by 10 dpt (p<0.01; Fig. 2B, light blue bars). If we just looked at the GFAP positive cells, 63% were Ki67 positive at 5 dpt and only 15% were Ki67 positive at 10 dpt. These data show that earlier in tumor formation more GFAP positive cells are dividing than later during tumor formation. Although the number of cells expressing GFAP increased significantly between 5 and 10 dpt, the population of those cells undergoing cell division decreased during this time. These data support that GFAP positive cells contribute more to tumor growth at early phases of tumor development and this contribution decreases over time.
Figure 2. Identification of a subpopulation of dividing, GFAP-positive GBM9 tumor cells.
(A) 20 μm sections of zebrafish with GBM9 tumors (green) were analyzed at 5 and 10 dpt. Sections were labeled with anti-Ki67 (red) and anti-GFAP (blue). Triple labeled cells are white (white arrow heads). The inset to the left of the image shows each of the channels separated. (B) Antibody labeled sections were counted for the number of cells expressing Ki67, GFAP or both Ki67 and GFAP. Light blue bars represent 5 dpt and dark blue bars represent 10 dpt (mean ± SD). n = 5 animals per group. **p<0.0001; *p<0.01.
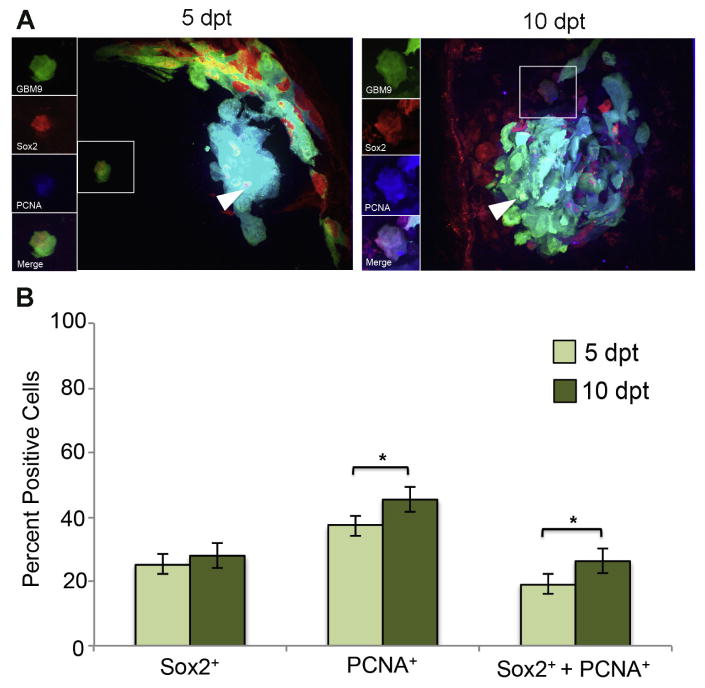
The population of dividing Sox2-positive GBM9 tumor cells increases during tumor growth
We had previously shown that GBM9 tumors in zebrafish contained Sox2 positive cells consistent with a cancer stem cell population (Welker et al., 2016). Due to their perceived role in tumor initiation and propagation, we hypothesized that these putative cancer stem cells would be dividing in vivo. For this analysis we switched from Ki67 to PCNA due to anti-Ki67 antibody incompatibility with the anti-Sox2 antibody. At 5 dpt, we found that 25 ± 6% of GBM9 tumor cells were positive for Sox2 expression (Fig. 3B, dark green bars). At the same time point, we observed that 37 ± 5% of tumor cells were PCNA positive; a value similar to what was found using Ki67. At 10 dpt, the number of tumor cells expressing PCNA and the number of tumor cells expressing Sox2 increased to 45 ± 2% and 28 ± 4%, respectively (Fig. 3B, light green bars). 19 ± 3% of tumor cells were simultaneously expressing both Sox2 and PCNA at 5 pt. At 10 dpt, this number increased significantly to 26 ± 4% (p<0.05). If we looked only at Sox2 positive cells, 51% were expressing PCNA at 5 dpt and 58% at 10 dpt. We conclude from this analysis that the Sox2 positive cells within the tumor were making a significant contribution to tumor cell growth consistent with their designation as putative cancer stem cells.
Figure 3. Sox2 expressing GBM9 cells show increased cell division during tumor growth.
(A) 20 μm sections of zebrafish with GBM9 tumors (green) were analyzed at 5 and 10 dpt. Sections were stained with anti-PCNA (red) and anti-Sox2 (blue). Triple labeled cells are white (white arrow heads). The inset to the left of the image shows each of the channels separated. (B) Antibody labeled sections were counted for the number of cells expressing PCNA, Sox2 or both PCNA and Sox2. Light green bars represent 5 dpt and dark green bars represent 10 dpt (mean ± SD). n = 5 animals per group. *p<0.05.
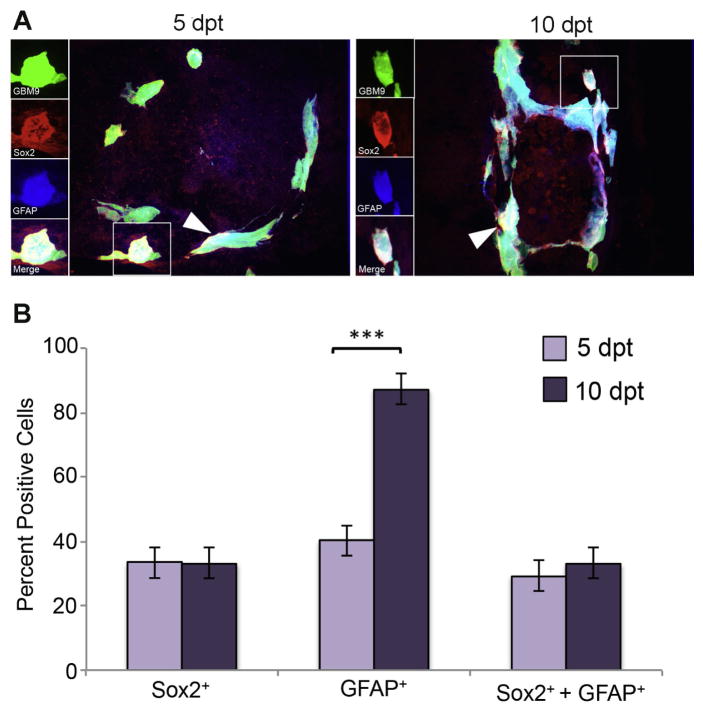
Putative GBM9 cancer stem cells express both Sox2 and GFAP
Studies have also shown that GFAP can be a marker of both stem cells and differentiated cells, therefore we investigated the correlation between Sox2 and GFAP expression in GBM9 tumors.
At 5 dpt, we found that 40 ± 5% of tumor cells were positive for GFAP expression (Fig. 4B, dark purple bars). At the same time point, we observed 33 ± 12% of tumor cells were Sox2 positive. At 10 dpt, the number of tumor cells expressing GFAP increased significantly to 87 ± 7% (p<0.0001) (Fig. 4B, light purple bar) while Sox2 levels stayed about the same. Approximately 29 ± 6% of tumor cells were found to be simultaneously expressing both Sox2 and GFAP at 5 dpt and 33 ± 5% at 10 dpt. Importantly, 87% of the Sox2 positive cells at 5 dpt and 100% of the Sox2 positive cells at 10 dpt were GFAP positive. It was also observed in tissue from human gliomas that there was a substantial overlap between Sox2 and GFAP (Bradshaw et al., 2016). These data support that the putative cancer stem cells in these tumors express both Sox2 and GFAP and that GFAP is expressed by both stem cells and presumably differentiated cells.
Figure 4. Putative GBM9 cancer stem cells express both Sox2 and GFAP.
(A) 20 μm sections of zebrafish with GBM9 tumors (green) were analyzed at 5 and 10 dpt. Sections were stained with anti-Sox2 (red) and anti-GFAP (blue). Triple labeled cells are white (white arrow heads). The inset to the left of the image shows each of the channels separated. (B) Antibody labeled sections were counted for the number of cells expressing GFAP, Sox2 or both GFAP and Sox2. Light purple bars represent 5 dpt and dark purple bars represent 10 dpt (mean ± SD). n = 5 animals per group. ***p<0.0001.
Tumors reoccur post-TMZ treatment in GBM9 transplanted zebrafish
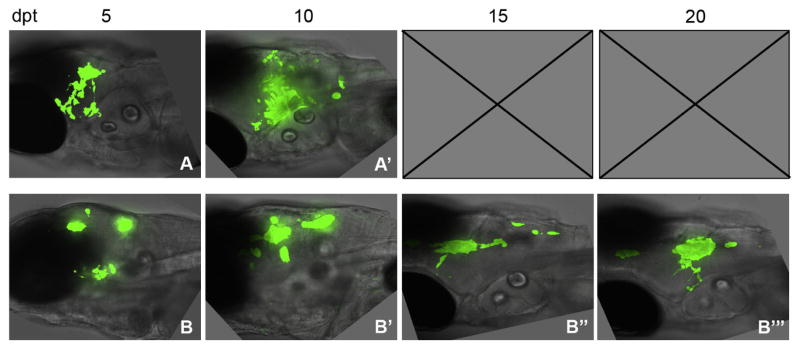
Our data show that tumor cells change over time during tumor growth. Therefore, we next set out to address how the different tumor cell types responded to TMZ. We were interested in two issues. What cells remained after exposure to this chemotherapeutic treatment and if tumors reoccurred, what cells constituted that recurrence. TMZ is the most commonly used chemotherapeutic agent for treating GBM and it has previously been shown that GBM tumors in zebrafish are decreased by TMZ treatment (Geiger et al., 2008; Stupp et al., 2009; Welker et al., 2016). We had shown using GBM9 transplants, that TMZ treatment from 5–10 dpt resulted in a significant decrease in tumor size and a dramatic increase in animal survival (Welker et al., 2016). We next asked whether tumors recurred after treatment. Control un-treated animals transplanted with GBM9 cells exhibited significant tumor growth causing lethality at ~8 dpt (Fig. 5A; (Welker et al., 2016)). Consistent with the previous study, 5 days of TMZ treatment resulted in a decrease in tumor burden as compared to controls (Fig. 5, A-A′ and B-B′). We then removed TMZ and analyzed animals at 15 and 20 dpt and found that tumors did indeed reoccur (Fig. 5, B″-B‴). These data support that GBM9 tumors are decreased by 5-day TMZ treatment, but regrow after cessation of treatment.
Figure 5. GBM tumors reoccur after TMZ treatment.
Animals were transplanted with GBM9 cells and treated with TMZ for 5 days from 5 to 10 dpt. Control transplanted animals not treated with TMZ at (A) 5 dpt and (A′) 10 dpt. (B) Zebrafish treated continuously from 5 to 10 dpt with 50 μm TMZ. (B) Zebrafish before TMZ treatment at 5 dpt. (B′) Zebrafish at 10 dpt immediately after 5 days of TMZ treatment. (B″) Zebrafish at 15 dpt, 5 days after the end of treatment. (B‴) Zebrafish at 20 dpt, 10 days after the end of treatment. n = 5 animals per group.
Proliferation is ablated post-TMZ treatment, but Sox2 positive cells rebound as tumors regrow
Using Ki67, vimentin, GFAP, and Sox2 expression we asked what cells constituted post-treatment recurring tumors. Since TMZ is a DNA alkylating agent that targets dividing cells, we hypothesized that Ki67 would be significantly decreased in post-treatment tumors. We fixed and performed immunohistochemistry on animals transplanted with GBM9 cells and treated with 50 μM TMZ from 5–10 dpt. At 10 dpt we saw an almost complete ablation of Ki67 positive cells from 36 ± 9% at 10 dpt without treatment to 6 ± 6% at 10 dpt after 5 days of TMZ treatment (p<0.001; Fig. 6, light red bar). At 15 and 20 dpt (5 and 10 days post treatment, respectively), however, we saw a statistically significant increase in Ki67 positive cells to 19 ± 3 % and 22 ± 7% (p< 0.01; Fig. 6). However, the percentage of Ki67 positive cells on 15 dpt was not statistically different from the percentage on 20 dpt indicating that while there is an increase in Ki67 positive cells, it reached a plateau. Indeed, the percentage of Ki67 positive cells before treatment (~ 37%) and 10 days after treatment (~22%) were statistically different (p=0.02) revealing that the percentage of Ki67 positive cells did not return to pre-treatment levels even 10 days after cessation of TMZ.
Figure 6. Quantification of Ki67 immunohistochemistry pre and post-TMZ treatment.
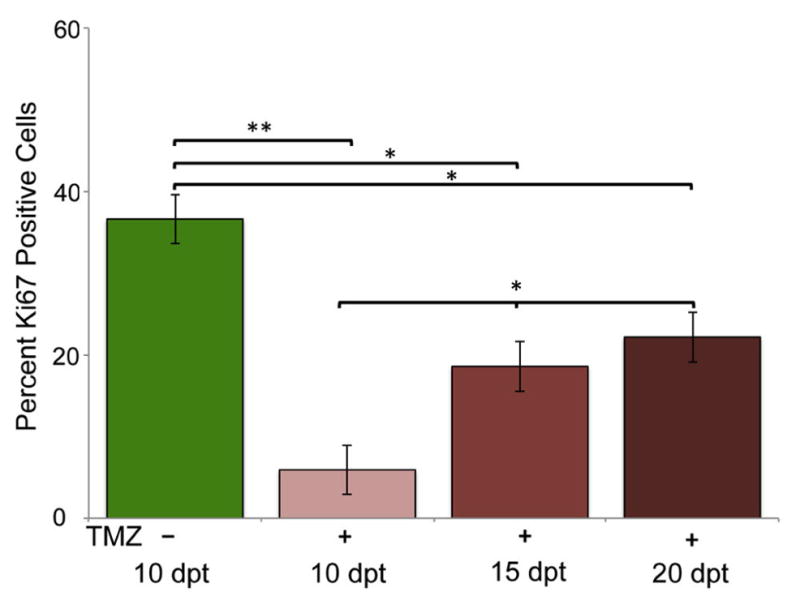
(A) Percentage of Ki67 positive cells in untreated GBM9 transplanted zebrafish 10 dpt (green bar), GBM9 transplanted zebrafish treated with TMZ from 5 to 10 dpt at 10 dpt (light red bars), GBM9 transplanted zebrafish treated with TMZ at 15 dpt (medium red bars), and GBM9 transplanted zebrafish treated with TMZ at 20 dpt (dark red bars)(mean ± SD). n = 5 animals per group.**p<0.001, *p<0.01
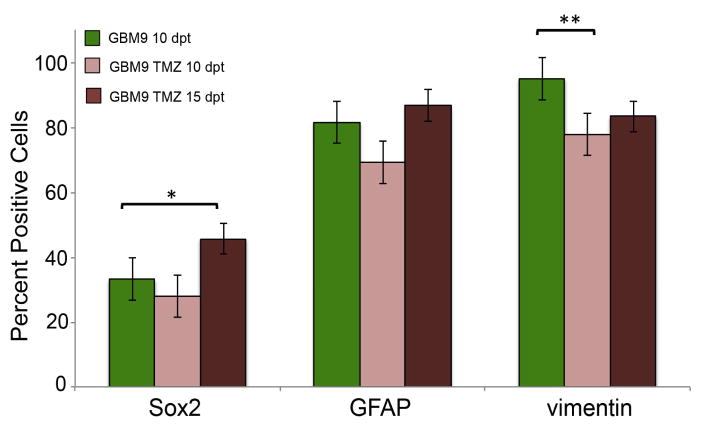
To determine what cell populations remained after treatment, we performed immunohistochemistry for Sox2, GFAP, and vimentin after 5 days of TMZ treatment. All three proteins examined were present, thus we did not see ablation of any single population. However, only the vimentin positive cells were statistically decreased after treatment from 95 ± 3% to 78 ± 6% (p=0.001; Fig. 7, light red bars) as compared to control un-treated GBM9 tumors (Fig. 7, green bars). This is an 18% decrease, which is comparable to the percentage of vimentin+/Ki67+ cells found in tumors at 5 and 10 dpt. Thus, it is possible that this decrease in vimentin positive cells after TMZ treatment is due to loss of those vimentin positive cells that were dividing. In contrast, cells expressing the stem cell marker Sox2 did not show a statistically significant decrease after TMZ treatment suggesting that these cells may be less susceptible to TMZ treatment compared to differentiated cells even though both populations contain dividing cells. GFAP positive tumor cells also did not show a significant decrease after TMZ treatment.
Figure 7. Quantification of Sox2, GFAP and vimentin immunohistochemistry pre and post-TMZ treatment.
(A) Percentage positive cells for Sox2, GFAP and vimentin in GBM9 transplanted zebrafish untreated with TMZ at 10 dpt (green bars), GBM9 transplanted zebrafish treated with TMZ from 5 to 10 dpt at 10 dpt (light red bars) and GBM9 transplanted zebrafish treated with TMZ at 15 dpt (dark red bars) (mean ± SD). n = 5 animals per group. *p<0.05, **p<0.001
To determine the composition of regrowing tumors, we examined protein expression 5 days after cessation of treatment. Analysis of vimentin positive cells revealed that the percentage of these cells did not increase after cessation of treatment. However, the population of Sox2 expressing cells showed a significant increase over time after treatment from 33 ± 5% of tumor cells before treatment to 46 ± 8% of cells 5 days after cessation of treatment (p<0.05; Fig. 7). The GFAP positive cells showed this similar trend, but it did not reach statistical significance. These data suggest that these putative cancer stem cells are more refractory after chemotherapeutic treatment and contribute to a greater extent to the regrowing tumor.
Discussion
We had previously standardized human GBM transplants in embryonic zebrafish and showed that GBM9 neurospheres transplanted into the embryonic zebrafish brain formed tumors containing a dynamic population of both differentiated cells and Sox2 expressing putative cancer stem cells (Welker et al., 2016). In this work we set out to define the dividing cell populations in a GBM neurospheres xenograft model over time in vivo, to determine how these populations responded to the main chemotherapeutic agent used in glioblastoma, TMZ, and to establish which of these populations reoccurred and were dividing after TMZ treatment. Importantly, we followed these markers over multiple time points analyzing trends during tumor growth in vivo. Our data support that more differentiated cell populations expressing the intermediate filament protein Vimentin contained a small population of dividing cells, which were affected by TMZ treatment and did not reoccur after treatment in the time frame examined. In contrast, we found that the putative cancer stem cells expressing Sox2 and GFAP were not diminished after TMZ treatment and showed an increase in cell division post treatment. These data support that Sox2 expressing GBM cells are acting consistent with a stem cell population conferring resistance to TMZ and contributing to tumor regrowth after treatment. Importantly, this experimental paradigm can be used to test agents that inhibit these cells and thus could slow tumor growth after TMZ treatment.
Analysis of Ki67 and vimentin revealed that expression of these markers is not entirely exclusive as originally hypothesized. The go or grow theory of cancer suggests that tumor cells exhibit either a motile, mesenchymal “go” phenotype or a stationary, proliferative “grow” phenotype. However, our results demonstrate that there is a population of cells simultaneously expressing markers indicative of both growth (Ki67) and mesenchymal motility (vimentin). Indeed 23 and 18% of vimentin positive cells were dividing at 5 and 10 dpt respectively. These findings attest to non-conformity of cancer cells. As expected, we found that TMZ had a dramatic effect on cell proliferation, as revealed by Ki67 labeling. After cessation of TMZ treatment, the percentage of Ki67 cells increased, but even after 10 days post treatment had not returned to levels seen before treatment. This suggests that certain cell populations may not recover to divide again or reach the same level after TMZ treatment. In support of this hypothesis, we found that the percentage of tumor cells expressing vimentin was statistically decreased after TMZ treatment by a percentage that was consistent with the population of vimentin cells that were dividing. This suggests that tumor cells expressing characteristics of differentiated cells that are also dividing are perhaps more susceptible to chemotherapeutic agents. We also found that over time after cessation of TMZ, vimentin positive cells did not increase over the time frame examined. An interpretation of these data is that this is a more vulnerable tumor cell population and that TMZ treatment has a longer term affect on these cells.
We were also interested in examining the role of putative cancer stem cells in these tumors. The gold standard for proving that a tumor cell is a cancer stem cell is to perform transplants of putative stem cells and show that they can form tumors of the original complexity (Lathia et al., 2015). There is no firm definition of the cancer stem cell population in GBM, however studies support that these cells express Sox2 (deCarvalho et al., 2010; Guo et al., 2011; Chen et al., 2012; Lathia et al., 2015). Sox2 is a transcription factor in embryonic stem cells and is expressed at high levels in GBM tumors and strongly correlates with other GBM cancer stem cell markers such as, Nanog, Oct4, and CD-133 (Hemmati et al., 2003; Guo et al., 2011; Song et al., 2016). A study examining patient glioma tissue found that 40% of Sox2 expressing cells also expressed GFAP (Guo et al., 2011). We found an even higher overlap between these two proteins in these GBM9 tumors transplanted into zebrafish with 100% of Sox2 cells also positive for GFAP by 10 dpt (Fig. 4). This supports that in these tumors it is likely that the population of cancer stem cells expresses both Sox2 and GFAP. We cannot rule out that these cells also express vimentin. By 10 dpt, 95% of cells in the tumor express vimentin and ~30% express Sox2 implying that there must be an overlap between these populations. However, because we cannnot select for Sox2, GFAP or vimentin in live cells, we cannot at present perform single cell transplants and thus cannot definitively conclude that these are cancer stem cells. Future analysis will be required to dissect out the subpopulations of Sox2 cancer stem cells and to define the cancer stem cell population in other glioblastoma cell lines. However, our data support that the Sox2 expressing cells in GBM9 tumors are consistent with cancer stem cells.
One piece of evidence supporting this conclusion is that the Sox2 expressing tumor cells were not statistically decreased by TMZ treatment even though they contained a higher percentage of dividing cells. This raises the possibility that these cells are more refractory to TMZ treatment, which is a feature of cancer stem cells including those in GBM (Chen et al., 2012). Interestingly, we found that the percentage of tumor cells expressing Sox2 increased after TMZ treatment beyond the percentage present before TMZ treatment or at the latest time examined in GBM9 transplanted untreated fish (10 dpt), which die around this time precluding analysis at later time points. These data support that the Sox2 population increases after chemotherapeutic treatment and thus is able to contribute to tumor regrowth. This is consistent with reciprocal studies showing that decreasing Sox2 expression in cells from freshly derived GBM patient tumors caused the tumor cells to stop dividing and rendered them non-tumorigenic when transplanted into mice (Gangemi et al., 2009). In sum these data support that cancer stem cells are major contributors to tumor growth and importantly tumor growth after chemotherapeutics and that this zebrafish xenograft model is an excellent system for elucidating tumor cell composition and the role of cancer stem cells in tumor behavior.
The concurrent labeling of PCNA and Sox2 revealed that a majority of these putative cancer stem cells were dividing. Dividing stem cells are thought to be responsible for tumor propagation (Lathia et al., 2015). From 5 to 10 dpt, the percentage of tumor cells that were dividing Sox2 cells increased significantly from 19 to 26%. However, the total number of Sox2 cells did not increase during this same time frame, suggesting that more Sox2 cells were dividing as time went on. Indeed the percentage of dividing cells that were Sox2 positive increased from 51 to 58% from 5–10 dpt. This increase suggests that even at a later time point, tumors contain active populations of putative stem cells that contribute to tumor growth. However, whereas the majority of the Sox2 expressing putative cancer stem cells were dividing, not all were. It is believed that non-dividing or quiescent stem cells may be responsible for tumor recurrence following treatment with therapeutics like TMZ, which specifically target dividing cells (Lathia et al., 2015). After TMZ treatment we found that Sox2 expressing tumor cells were not significantly decreased and their numbers increased after treatment more than any other cell type examined. It may be that the non-dividing Sox2 cells contribute to this rebound and to tumor growth after treatment.
Highlights.
Glioblastoma cells transplanted into the zebrafish brain contain a heterogeneous population of dividing cells.
Tumors contain putative cancer stem cells that express both Sox2 and GFAP.
Tumors decrease in size and show reduced cell division upon treatment with temozolomide TMZ, but reoccur post treatment.
Sox2 positive cells are refractory to TMZ and play a role in tumor reoccurrence post treatment.
Acknowledgments
We thank Drs. Michelle Emond for zebrafish facility help, Balveen Kaur for the GBM9 cells, Paula Monsma for help with the spinning disc, Michael Berberoglu for the PCNA antibody and help with immunohistochemistry and the Beattie, Kaur, Jontes and Puduvalli labs for intellectual discussions. This work was funded by P30NS045758 and NS050414 (C. E. B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bradshaw A, Wickremesekera A, Brasch HD, Chibnall AM, Davis PF, Tan ST, Itinteang T. Cancer Stem Cells in Glioblastoma Multiforme. Front Surg. 2016;3:48. doi: 10.3389/fsurg.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCarvalho AC, Nelson K, Lemke N, Lehman NL, Arbab AS, Kalkanis S, Mikkelsen T. Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo. Stem Cells. 2010;28:181–190. doi: 10.1002/stem.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Estrada-Bernal A, Lawler SE, Nowicki MO, Ray Chaudhury A, Van Brocklyn JR. The role of sphingosine kinase-1 in EGFRvIII-regulated growth and survival of glioblastoma cells. J Neurooncol. 2011;102:353–366. doi: 10.1007/s11060-010-0345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- Geiger GA, Fu W, Kao GD. Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res. 2008;68:3396–3404. doi: 10.1158/0008-5472.CAN-07-6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, Schroeder MA, James CD. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, Zhang Y, Ling EA, Gao J, Hao A. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–775. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell stem cell. 2009;4:203–205. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar D, Blanpain C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu Rev Pathol. 2016;11:47–76. doi: 10.1146/annurev-pathol-012615-044438. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M, Bachmann M, Rieber EP, Weigle B. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer. 2007;96:1293–1301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WS, Yang YP, Huang CS, Lu KH, Liu WH, Wu WW, Lee YY, Lo WL, Lee SD, Chen YW, Huang PI, Chen MT. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc. 2016 doi: 10.1016/j.jcma.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- Welker AM, Jaros BD, Puduvalli VK, Imitola J, Kaur B, Beattie CE. Standardized orthotopic xenografts in zebrafish reveal glioma cell-line-specific characteristics and tumor cell heterogeneity. Dis Model Mech. 2016;9:199–210. doi: 10.1242/dmm.022921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, Zon LI. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell stem cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SP, Nowicki MO, Liu F, Press R, Godlewski J, Abdel-Rasoul M, Kaur B, Fernandez SA, Chiocca EA, Lawler SE. Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 2011;71:5374–5380. doi: 10.1158/0008-5472.CAN-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]