Introduction
Prostate cancer is a major global healthcare issue. Last year, in the United States alone, there were over 180,000 new diagnoses of prostate cancer and more than 26,000 deaths from the disease, making it the second leading cause of cancer death in men. Screening, diagnosis, and management of prostate cancer have undergone profound changes in recent years. One major criticism of historical screening programs has been the resulting overdiagnosis of low-risk cancers, which can lead to unnecessary treatment and decreased quality of life. As a result, screening with prostate-specific antigen (PSA) is recommended less frequently by physicians. However, a solution to the problem of overtreatment of patients with low-risk tumors is the use of active surveillance.[1] In this article, we will focus on the role of active surveillance for patients with low-risk disease and how multiparametric MRI (mpMRI) has impacted decision making for entering and monitoring patients on active surveillance.
Who Should Receive Active Surveillance in Prostate Cancer?
The active surveillance decision-making process begins with a prostate biopsy, for which there are two main triggers: elevated PSA level and/or a palpable lesion on digital rectal examination. The current standard of care is to obtain a 12-core biopsy under transrectal ultrasound (TRUS) guidance, in which two samples are obtained from the apex, the middle, and the base of the prostate on two sides (six samples per side). Each sample is interpreted by a pathologist using the Gleason scoring system, which includes the predominant histologic Gleason grade and a secondary score (both typically rated as 3, 4, or 5), so that in practice the system ranges from 3+3 to 5+5. More recently, the World Health Organization and the International Society of Urological Pathology (ISUP) adopted a new scoring system using a 5-point scale with a 3+3 tumor corresponding to grade 1, and a 3+4 tumor to grade 2, and so on.[2] This was done partly to assuage patient fears raised by the conventional Gleason scoring system in which the lowest number is 6 on a 10-point scale.[3] There is widespread agreement that the risk of death from prostate cancer from 3+3 tumors is negligible. One exception, however, is a large-volume lesion, which raises concern that the biopsy undersampled the lesion, and that the gland could potentially harbor a higher-grade lesion. There is less agreement about whether 3+4 tumors are potentially amenable to active surveillance, although there is considerable evidence that there is a very low risk of death from Gleason 3+4 cancers when the amount of grade 4 disease is minimal.[4] This can be assessed by the percentage of the core that is Gleason 4—a Gleason 3+4 tumor with minimal percentage of Gleason 4 may still be considered as favorable risk. Hopefully, in the future, molecular pathology will be able to provide a more refined risk assessment of Gleason 4 lesions.
Patients who harbor low-volume 3+3 tumors or 3+4 tumors with only a small percentage of grade 4 are eligible for active surveillance. The use of active surveillance in the United States has increased in recent years, with over 40% of low-risk tumors managed in this manner, and even higher rates for men over 75 years of age.[1] Active surveillance is different from watchful waiting, which is usually reserved for elderly men with reduced life expectancy. In watchful waiting, the physician will not perform serial tests such as biopsies because there is no curative intent, so treatment is only given for symptomatic progression. In contrast, active surveillance infers that the patient is followed with a schedule of serial PSA tests and biopsies, with the latter meant to detect patients who convert from a low-grade to an intermediate- or high-grade tumor over time.
Implementing Active Surveillance
Actual active surveillance regimens vary greatly across institutions.[5] At the National Cancer Institute, patients generally receive PSA screening at 6-month intervals and repeat biopsies are performed at 1- to 2-year intervals. At New York University Medical Center, PSA is performed at 3- to 6-month intervals, with MRI and/or biopsy repeated at 1- to 2-year intervals.
Multiple studies have shown that carefully selected patients who remain on active surveillance have a very low risk of dying from prostate cancer over the course of 10 to 15 years, and in that respect, active surveillance has proven to be a very successful management strategy that optimizes quality of life while minimizing prostate cancer–specific death.[6] However, there are two commonly observed problems with active surveillance: 1) approximately 20% to 50% of men initially thought to be good candidates for active surveillance prove to have higher-grade disease on the first or second repeat biopsy[7,8] and convert to active treatment; and 2) surveillance fatigue, which is caused by patient fear and uncertainty about disease status and/or the desire to avoid repeated biopsies can occur. In some cases, men who undergo repeat biopsies develop infections that may require hospitalization. The more biopsy sessions that occur, the greater the cumulative likelihood of a serious post-biopsy infection.[9] Thus, adherence to active surveillance may be problematic, and some men will opt for an active treatment even though their disease status is unchanged. Nonetheless, in properly selected men studied over a long period of time, active surveillance is a safe and appealing approach that spares or delays unnecessary radical treatments without leading to an increase in disease-specific mortality.[6] However, it is clear that if men could be more accurately selected for active surveillance and feel confident about that decision, active surveillance could be an even better management option than it is currently.
The Role of mpMRI
There is growing acceptance that mpMRI of the prostate can help solve both dilemmas experienced by patients on active surveillance. Performed before a patient is accepted onto an active surveillance protocol, mpMRI can identify lesions missed by the standard TRUS biopsy or can more properly characterize cancers detected at TRUS biopsy (Figures 1 and 2).[7,8] Therefore, when a man comes to one of our centers to be managed with active surveillance based on PSA and TRUS biopsy results, an mpMRI is often performed if this has not already been done.
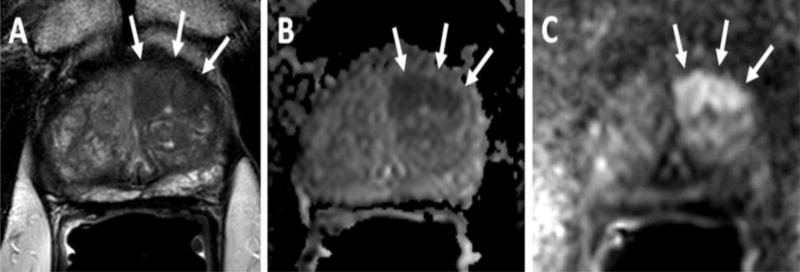
Figure 1.
63-year old male with a serum PSA=5.95 with Gleason 3+3 (<5% core involvement) in the left apical PZ. Axial T2W MRI (A) shows an ill-defined lesion in the left apical anterior transition zone (arrows), ADC map (B) and b2000 DW MRI (C) confirms the lesion (arrows). TRUS/MRI fusion guided biopsy revealed Gleason 4+3 (50% core involvement) within this lesion. Patient`s clinical decision changed to radical prostatectomy from active surveillance after mpMRI and TRUS/MRI guided biopsy. (Figure courtesy of Baris Turkbey, MD, National Cancer Institute)
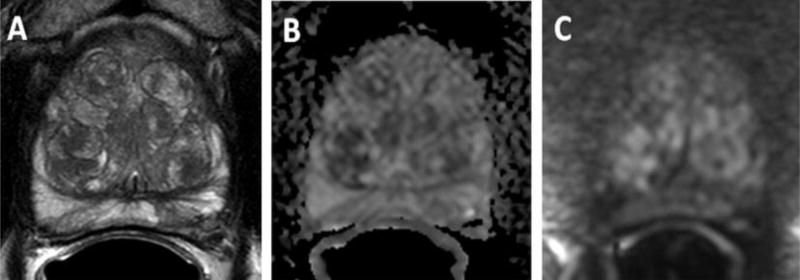
Figure 2.
66-year-old male with a serum PSA=10.8ng/ml. Pre-MRI biopsy revealed Gleason 3+3 prostate cancer (2% core involvement) in left apical PZ. mpMRI revealed no focal lesions but extensive BPH changes. Axial T2W MRI (A), ADC map (B) and b2000 DW MRI (C) shows no focal lesion. MRI excluded presence of a clinically significant lesion in this patient and patient proceeded to active surveillance. (Figure courtesy of Baris Turkbey, MD, National Cancer Institute)
The mpMRI must be carefully acquired according to guidelines set forth in the Prostate Imaging Reporting and Data System (PI-RADS) version 2, and these are beyond the scope of this article.[10] However, both the acquisition and the interpretation should be PI-RADS version 2–compliant. A PI-RADS score of 3 is considered sufficient to warrant an MRI-guided biopsy.
Because a standard TRUS-guided biopsy predominantly samples the posterior peripheral zone, the rest of the gland is undersampled. Moreover, since TRUS-guided biopsies are really blind samples of the prostate, tumors in the posterior peripheral zone may be incompletely sampled or their size greatly underestimated. Therefore, before placing a patient on active surveillance, we perform an MRI to identify any lesions that were potentially missed or undersampled. If a lesion is judged to be PI-RADS score 3 or above, it is biopsied using an MRI-TRUS fusion device, several of which are commercially available. These systems process the MRI scans so that only the prostate gland is present on the image (known as segmentation) and the MRI lesions are marked on the segmented prostate. This is sent electronically to the ultrasound biopsy suite, where the patient undergoes a three-dimensional TRUS of the prostate, which is then fused electronically to the MRI using software included in the fusion device. Once fusion has occurred, a tracking method is used, so that when the TRUS probe is moved, the MRI also moves in the same way. Thus, the lesion, discovered by MRI, can be biopsied under ultrasound using MRI-TRUS fusion technology. In the case of active surveillance candidates, approximately 20% to 30% of patients who were initially considered good candidates for active surveillance are directed toward active treatments such as surgery or radiation as a consequence of finding additional lesions or resampling known lesions with MRI guidance (Figure 1).[7,8]
For patients in whom the MRI is negative or reveals nothing more than was discovered by TRUS biopsy, active surveillance is an excellent choice (Figure 2). Thus, an initial MRI followed by MRI-TRUS–guided biopsy has become routine in our institutions to identify patients who are ideal candidates for active surveillance. This provides greater assurance to the clinician and patient that the proper management has been selected. In the United Kingdom, MRI prior to initiation of active surveillance is already a standard practice guideline.
It would seem logical that MRI could also be used in place of repeat biopsies to monitor patients who are on active surveillance. Although this is a very attractive possibility for patients due to the risk and burden associated with multiple biopsies over time, good long-term data are not yet available to support this policy. In our own institutions, MRI is commonly performed on a routine basis (annually in the case of the National Cancer Institute), and changes in the appearance of the MRI can trigger a repeat targeted biopsy. However, stable MRIs are increasingly being used to prolong the interval between biopsies to 2 to 5 years, recognizing the slow growth of prostate cancer, provided serum PSA levels and prostate exams remain stable. Slowly increasing PSA levels can be attributed to benign prostatic hyperplasia, which can be accurately measured on MRI. Unfortunately, MRI is not sensitive for detecting microscopic changes in disease—for example, the change between Gleason 3+3 and Gleason 3+4 (ISUP grade group 1 to 2).[8] Thus, some clinicians still perform routine biopsies approximately every 1 to 3 years notwithstanding a stable MRI. Several large studies are underway to test the value of serial MRI in monitoring patients on active surveillance, and it is hoped that these will provide support for using MRI in place of repeated biopsies when the scan and other clinical features remain stable. In our own experience, the vast majority of active surveillance patients who have an initial qualifying MRI and MRI-TRUS biopsy exhibit minimal or no change in their MRI over many years, making this approach quite promising.
Other Biomarkers for Active Surveillance
A variety of other commercially available serum, urine, and tissue biomarkers have been introduced to help clinicians decide whether to initiate and maintain a patient on active surveillance.[1,11] Their value relative to MRI has not been tested adequately to draw conclusions as to whether these can be used in place of MRI or as an adjunct to MRI.
One of these serum markers is the Prostate Health Index, which combines total, free, and proPSA using a mathematical formula. This test was previously shown to predict changes on biopsy in men on active surveillance, and in the future might be used to monitor patients in conjunction with mpMRI.[12] Several genomic tissue tests including Prolaris, Oncotype DX, and Decipher are also commercially available to help determine aggressiveness beyond the information provided by Gleason score. These may be used to help assess eligibility for active surveillance in borderline cases such as high-volume Gleason 6 or low-volume Gleason 3+4; however, there are no published data on their utility for monitoring during surveillance, and they require tissue from a biopsy.
Conclusion
Active surveillance is an excellent alternative to surgery or radiation in patients with low-risk cancers. However, the current methods of ascertaining whether a patient harbors a low-risk cancer are flawed, and data obtained by PSA or traditional TRUS biopsy do not accurately predict good candidates for active surveillance. MRI- and MRI-TRUS–guided biopsies of the prostate appear to assist in the decision to place a patient on active surveillance by detecting lesions outside the normal biopsy template or by providing more information about a lesion within the potentially undersampled template. Less certain is the role of MRI in delaying or eliminating subsequent biopsies, although it is increasingly being used in this manner, since repeat prostate biopsies are a source of patient noncompliance. The role of other new biomarkers in the decision-making process and their utility compared with MRI remains to be determined. What is most encouraging is that more men can now safely and confidently delay or avoid unnecessary radical surgery for low-risk prostate cancers and retain a high quality of life even with a prostate cancer diagnosis.
KEY POINTS.
Historical screening programs have led to the overdiagnosis of low-risk prostate cancer, resulting in unnecessary treatment and decreased quality of life.
Active surveillance in properly selected men is a safe, appealing approach that spares radical treatment and does not increase disease-specific mortality.
Current methods of identifying low-risk patients are flawed, and can not always accurately predict candidates for active surveillance.
Acknowledgments
Funding: Dr. Loeb is supported by the Louis Feil Charitable Lead Trust and the National Cancer Institute at theNational Institutes of Health (Award Number K07CA178258).
Footnotes
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References
- 1.Tosoian JJ, Carter HB, Lepor A, Loeb S. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol. 2016;13:205–15. doi: 10.1038/nrurol.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 3.Loeb S, Curnyn C, Sedlander E. Perspective of prostate cancer patients on Gleason scores and the new grade groups: initial qualitative study. Eur Urol. 2016 Jun 6; doi: 10.1016/j.eururo.2016.05.039. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diolombi ML, Epstein JI. Metastatic potential to regional lymph nodes with Gleason score ≤ 7, including tertiary pattern 5, at radical prostatectomy. BJU Int. 2016 Aug 6; doi: 10.1111/bju.13623. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol. 2016;13:151–67. doi: 10.1038/nrurol.2015.313. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 7.Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627–36. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Frye TP, George AK, Kilchevsky A, et al. Magnetic resonance imaging-transrectal ultrasound guided fusion biopsy to detect progression in patients with existing lesions on active surveillance for low and intermediate risk prostate cancer. J Urol. 2016 Sep 6; doi: 10.1016/j.juro.2016.08.109. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghesi M, Ahmed H, Nam R, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2016 Aug 16; doi: 10.1016/j.eururo.2016.08.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Weinreb JC, Barenstz JO, Choyke PL, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb S, Bruinsma SM, Nicholson J, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol. 2015;67:619–26. doi: 10.1016/j.eururo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tosoian JJ, Loeb S, Feng Z, et al. Association of [−2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol. 2012;188:1131–6. doi: 10.1016/j.juro.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]