Abstract
Cry6Aa1 is a Bacillus thuringiensis (Bt) toxin active against nematodes and corn rootworm insects. Its 3D molecular structure, which has been recently elucidated, is unique among those known for other Bt toxins. Typical three-domain Bt toxins permeabilize receptor-free planar lipid bilayers (PLBs) by forming pores at doses in the 1–50 μg/ml range. Solubilization and proteolytic activation are necessary steps for PLB permeabilization. In contrast to other Bt toxins, Cry6Aa1 formed pores in receptor-free bilayers at doses as low as 200 pg/ml in a wide range of pH (5.5–9.5) and without the need of protease treatment. When Cry6Aa1 was preincubated with Western corn rootworm (WCRW) midgut juice or trypsin, 100 fg/ml of the toxin was sufficient to form pores in PLBs. The overall biophysical properties of the pores were similar for all three forms of the toxin (native, midgut juice- and trypsin-treated), with conductances ranging from 28 to 689 pS, except for their ionic selectivity, which was slightly cationic for the native and midgut juice-treated Cry6Aa1, whereas dual selectivity (to cations or anions) was observed for the pores formed by the trypsin-treated toxin. Enrichment of PLBs with WCRW midgut brush-border membrane material resulted in a 2000-fold reduction of the amount of native Cry6Aa1 required to form pores and affected the biophysical properties of both the native and trypsin-treated forms of the toxin. These results indicate that, although Cry6Aa1 forms pores, the molecular determinants of its mode of action are significantly different from those reported for other Bt toxins.
Keywords: Bacillus, bacterial toxin, insect, membrane, permeability, midgut brush-border membrane, planar lipid bilayers, pore-forming toxin
Introduction
The Gram-positive bacterium Bacillus thuringiensis (Bt)2 forms crystal-like parasporal inclusions during sporulation (1, 2), which often comprise insecticidal proteins (3). Bt insecticides have a long history of successful use (4, 5) against pests, in agriculture (6) and forestry (7), and disease vectors (8). Since 1996, various transgenic crops that express Bt toxins have been grown over a rapidly increasing area (9).
Known Bt toxins belong in majority to the Cry (crystal) family of proteins, whose most extensively studied members are insecticidal. Because of their importance and long use in pest management programs, the elucidation of their mode of action has been the object of considerable work (reviewed in Refs. 10–13). In broad general terms, the Bt mode of action can be described as follows: crystal proteins are first ingested as protoxins, which are solubilized and proteolytically converted to smaller polypeptides in the insect midgut. These activated toxins then bind to specific receptors at the surface of midgut epithelial cells, allowing them to insert and form pores in the cell membrane (14, 15). The presence of such pores interferes with cell physiology by abolishing transmembrane ionic gradients and may lead to colloid-osmotic lysis of the cells by allowing a massive influx of solutes from the midgut lumen (16), resulting in extensive damage to the midgut epithelium and death of the intoxicated larvae. However, many details of this scheme remain unresolved, including whether, when, and how oligomerization of the Bt protein takes place, as critically analyzed in our recent review (17).
Alterations in any one of the steps mentioned above can allow the insect to become resistant to the toxin (18–22). A clear and detailed knowledge of the susceptibility of the toxin to intestinal proteases, interaction with membrane receptors, and pore-forming ability, and of the consequences of the resulting membrane permeabilization on cellular physiology, including intracellular signaling and putative cell defense mechanisms (23), is therefore crucial for understanding and managing insect resistance development.
Bt-corn hybrids have been planted since 1996. They were initially designed to control lepidopteran larvae of corn borers, stalk borers, fall armyworms and other important pests. These corn varieties express one or more Cry toxins, conferring them insect resistance traits, often stacked with herbicide-tolerance traits. The multiple toxin approach (pyramiding) of hybrids expressing different Bt toxins targeted to the same insects is useful for managing insect resistance (24). The introduction in corn of Vip3A, a toxin produced during the vegetative growing phase of Bt (25, 26), has increased the spectrum of lepidopteran targets controlled by Bt-corn (24).
Several coleopteran insects are also major pests of corn worldwide. Damage from corn rootworms (the Western corn rootworm Diabrotica virgifera virgifera LeConte (WCRW) and the Northern corn rootworm Diabrotica barbieri Smith & Lawrence) accounts for over $1 billion in losses in North America annually (27). Single Bt toxin traits for corn rootworm protection were introduced in the United States in 2003 (Cry3Bb Bt-corn), 2005 (binary Cry34Ab1/Cry35Ab1 Bt-corn), and 2007 (mCry3Aa Bt-corn). However, recent reports of resistance to Cry3 toxins have highlighted the urgent need to develop new strategies to delay resistance development, based on the use of toxins with different modes of action to be deployed in trait pyramids (24, 28, 29). Furthermore, the high-dose/refuge strategy may not be as efficient as predicted for recent hybrid corns, due to a lower level of expression of the toxins in the plant and inappropriate refuge size (30). Finally, northward advancement of the limit of WCRW habitat as a result of climate change may result in more severe outbreaks in northern United States and Canada (31, 32).
Cry6Aa1 is a Bt nematocidal toxin (33–35), which is also highly active against corn rootworms (36–38). Its interaction at the cellular and molecular levels with target insects has received limited attention until now. Recently, the high-resolution, 3D structures of this 475-amino acid protein have been elucidated for its native (39) and trypsinized (39, 40) forms. The structure of this protein is completely different from those previously reported for three-domain Bt toxins (41–48), a Bt protoxin (49), β-sheet-rich, aerolysin-like Bt toxins (50, 51), and a Bt crystal protein for which no toxicity has yet been identified (52). On the other hand, the Cry6Aa structure is very similar to that of Escherichia coli hemolysin E (HlyE, SheA, ClyA) (53) and other hemolysins (Nhe, HBL) produced by Bacillus cereus (54, 55).
In this work, the molecular mode of action of Cry6Aa1, in its native and protease-treated forms, was investigated with the planar lipid bilayer electrophysiology technique that was successfully used to demonstrate pore formation by several other Bt toxins (56–60). The results show for the first time that Cry6Aa1 is a pore-forming toxin. It forms pores at very low doses and in a wide range of pH. Moreover, the native Cry6Aa1 does not need proteolytic processing to form pores. Finally, the pore properties are altered by the presence of WCRW midgut brush-border material.
Results
Biochemistry and bioassays
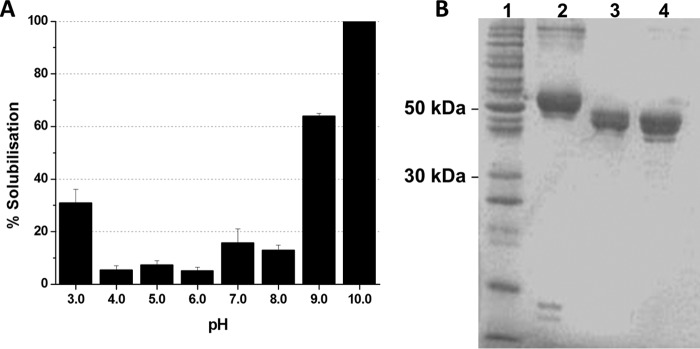
Solubilization is the initial step in the classical mode of action of Cry toxins. The solubilization efficiency of Cry6Aa1 crystals was assessed between pH 3.0 and 10.0 (Fig. 1A). The protein was 100% soluble at pH 10.0. At pH 3.0, 7.0, 8.0, and 9.0, the crystal solubilization efficiency of the toxin was reduced, ranging between 15 and 25%. Solubilization was the lowest between pH 4.0 and 6.0.
Figure 1.
Biochemistry of Cry6Aa1. A, solubilization of native Cry6Aa1 inclusion bodies. Results are mean ± S.E. for three separate experiments. B, SDS-PAGE of purified Cry6Aa1. The samples were boiled for 5 min before being loaded onto a 12% acrylamide gel (2 μg of protein per well). Lane 1: molecular weight markers. Lane 2: Native Cry6Aa1. Lane 3: Cry6Aa1 WCR. Lane 4: Cry6Aa1 TT.
In agreement with previously published data (38), the native Cry6Aa1 produced in Pseudomonas fluorescens runs as a 55-kDa protein on SDS-PAGE (Fig. 1B) with a band corresponding to a 110-kDa protein, suggesting the presence of dimers. Treatment with WCRW midgut juice produced a 45-kDa protein (Cry6Aa1 WCR). The trypsin-treated toxin (Cry6Aa1 TT) migrated as a major 45-kDa protein with a second, faint band corresponding to a slightly smaller polypeptide.
The native Cry6Aa1, Cry6Aa1 WCR and Cry6Aa1 TT were bioassayed on WCRW larvae at a single dose of 100 μg/cm2. Complete (100%) growth inhibition was observed in response to the three toxins, compared with 81–95% for the binary toxin Cry34Ab1/Cry35Ab1. Mortality after 5 days in response to native Cry6Aa1 was 100%, but only 69–82% for Cry6Aa1 WCR and 81–82% for Cry6Aa1 TT.
Different protease treatments of the toxin affect its biophysical properties
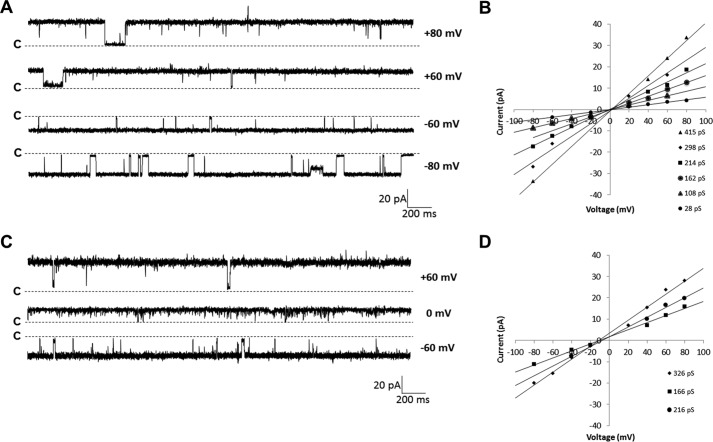
Because Cry6Aa1 naturally exerts its effect in the insect midgut environment, PLB experiments were first performed with Cry6Aa1 WCR (Fig. 2A). The toxin was most likely to insert into the bilayer and display channel activity at positive voltages. It usually became silent at voltages more negative than −60 mV. The minimal dose for clear protein insertion and channel activity was 100 fg/ml, a dose at which the current jumps were well resolved and the success rate of pore formation was close to 100%. The pores usually stayed open for long periods of time, from several hundreds of milliseconds to seconds, with short closures of 250 ms or less. At least five distinct conductance levels could be identified in individual experiments (Fig. 2B), suggesting that either conducting substates were activated or different oligomer species were present in the membrane, or both. The current-voltage relationships were linear, indicating that the channels were essentially ohmic. In three similar experiments, the conductance was between 28 and 459 pS under symmetrical (150 mm KCl) conditions at pH 5.5, the physiological pH of the target insect midgut. The pores were slightly cation-selective, as shown by the leftward shift of the current-voltage relationships obtained under 450:150 mm KCl (cis:trans) conditions (Fig. 2, C and D). The fact that, under these conditions, a positive current was detected in the absence of an applied voltage demonstrates that potassium ions diffuse through the pores faster than chloride ions. The relative permeability of potassium ions over chloride ions was close to 2.6 (Table 1). However, due to the complexity of the recordings with various current jump sizes within the same experiment, further biophysical analysis of channel activity, such as kinetic and opening probability studies, was extremely difficult to perform.
Figure 2.
Cry6Aa1 WCR forms pores in planar lipid bilayers. A, representative single channel current traces of Cry6Aa1 WCR at various holding voltages. 100 fg/ml of protein was added in the cis side compartment of the PLB setup and channel activity was recorded after a few minutes under symmetrical (150 mm KCl) conditions at pH 5.5. The traces were filtered at 1 kHz. B, typical current-voltage relationship graph (IV curves) constructed from data of one Cry6Aa1 WCR experiment under symmetrical conditions. The conductances ranged from 28 to 459 pS. C, representative single channel current traces of Cry6Aa1 WCR at ±60 and 0 mV under asymmetrical (450:150 mm KCl cis:trans) conditions at pH 5.5. D, IV curves of one single type of channel observed in each of three experiments conducted under asymmetrical (450:150 mm KCl cis:trans) conditions. The reversal potential was −11.39 mV ± 0.80 (n = 3). In A and C, the letters c and the dotted lines show the closed state of all channels.
Table 1.
Biophysical properties of the native Cry6Aa1 toxin, Cry6Aa1 TT and Cry6Aa1 WCR
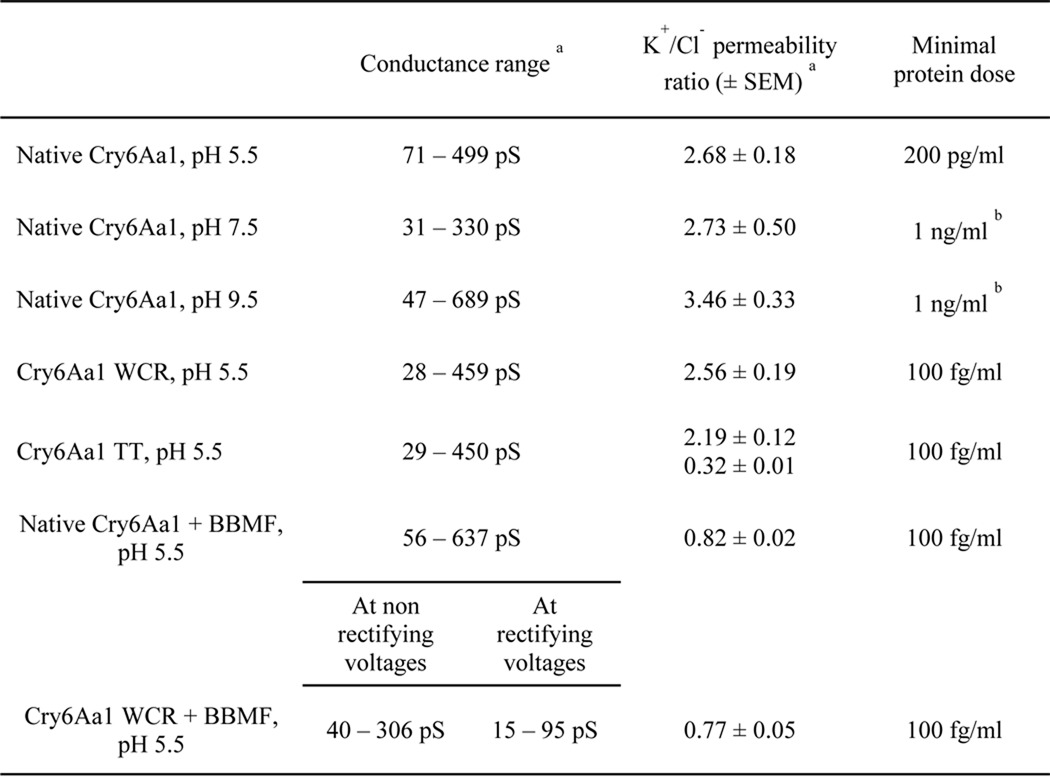
a n = 3 or more.
b Not tested below this dose.
Cry6Aa1 was able to form pores in PLBs without any prior protease treatment (Fig. 3A, two upper traces). However, insertion was less efficient, as a minimum of 200 pg/ml of toxin was required to observe channel activity, a dose 2000 times higher than that used for Cry6Aa1 WCR under the same experimental conditions. The conductance of the pores formed by native Cry6Aa1 ranged from 71 to 499 pS in three similar experiments under symmetrical conditions at pH 5.5 (IV curves not shown). The pores were slightly cationic at that pH and under asymmetrical conditions. The potassium to chloride ion permeability ratio was around 2.7 (Table 1).
Figure 3.
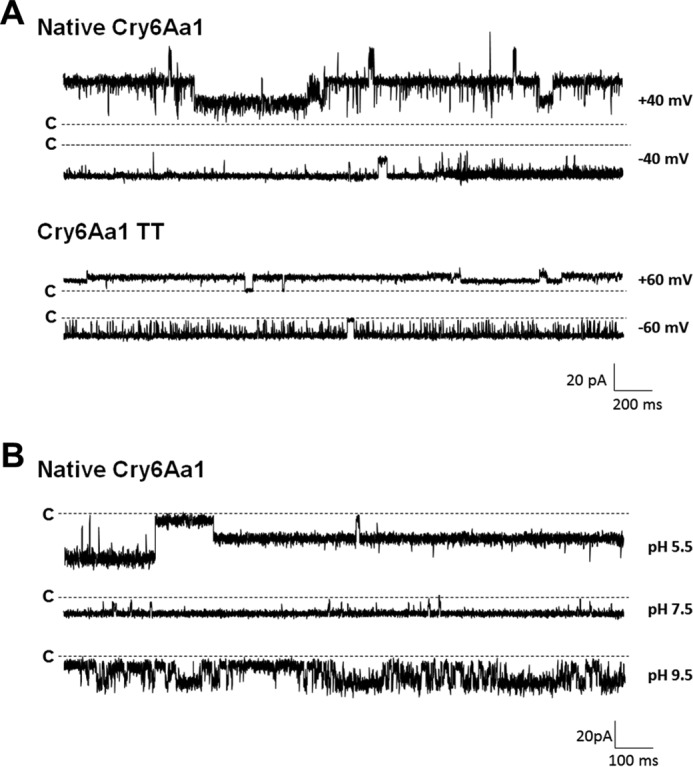
Both native Cry6Aa1 and Cry6Aa1 TT form pores in PLBs. A, representative traces of channel currents recorded under symmetrical (150 mm KCl) conditions at pH 5.5 at ±40 mV for the native Cry6Aa1 toxin (two upper traces) and at ±60 mV for Cry6Aa1 TT (two lower traces). Traces were filtered at 1 kHz. B, representative current traces of Cry6Aa1 at pH 5.5, 7.5, and 9.5. The holding voltage was −60 mV. The trace at pH 5.5 was unfiltered. The traces at pH 7.5 and 9.5 were filtered at 1 kHz. The letters c and the dotted lines indicate the closed state of all channels.
On the other hand, Cry6Aa1 TT was able to form pores in PLBs at the same dose (100 fg/ml) as Cry6Aa1 WCR (Fig. 3A, two lower traces). The conductance of the pores ranged from 29 to 450 pS under symmetrical conditions at pH 5.5. Smaller amplitude current jumps (corresponding to lower values of conductance) were more often seen, suggesting that the Cry6Aa1 TT protein channels were preferentially staying in their lower conducting states or substates (not shown). Depending on the experiment, the ionic selectivity of Cry6Aa1 TT was either slightly cationic or slightly anionic, with potassium to chloride ion permeability ratios of 2.2 and 0.3, respectively (Table 1). Native Cry6Aa1 and Cry6Aa1 WCR remained active for hours. On the other hand, whereas Cry6Aa1 TT displayed well resolved current jumps, it usually became silent after 5 min, suggesting a less stable partition of this protein into the membrane. In summary, protease treatment of the native Cry6Aa1 toxin affected the amount of protein needed for pore formation and the biophysical properties of the pores, but such treatment was not necessary for efficient pore formation by Cry6Aa1.
Effect of pH on the properties of Cry6Aa1
To explore the possibility that the intestinal pH of target organisms may play a significant role in the specificity of Cry6Aa1, pore formation experiments were conducted at pH 5.5, 7.5, and 9.5 using the native form of Cry6Aa1. The toxin was able to form pores at all three pH values, although the current jumps were better resolved at pH 5.5 and 7.5 than at pH 9.5 (Fig. 3B). Table 1 shows the conductance range of the Cry6Aa1 pore at acidic, almost neutral and alkaline pH values. The cumulative frequency distributions of the toxin conductance (in 50-pS bins) were constructed for each pH (Fig. 4). The conductance distribution of Cry6Aa1 at pH 9.5 was significantly different from those at pH 5.5 and 7.5 (Mann-Whitney test at 5% significance level). The pores were slightly selective to cations at all three pH values. However, as shown in Table 1, the potassium to chloride ion permeability ratio was higher at alkaline pH (3.5 at pH 9.5, compared with 2.7 at pH 5.5 and 7.5), but the differences were not statistically significant (Student's two samples t test at 5% significance level). This shows that the toxin had a very similar behavior at pH 5.5 and 7.5, but, at pH 9.5, the environment was somewhat less favorable to insertion and stable channel activity. These results indicate that other factors play a role in the target specificity of Cry6Aa1 besides pH.
Figure 4.
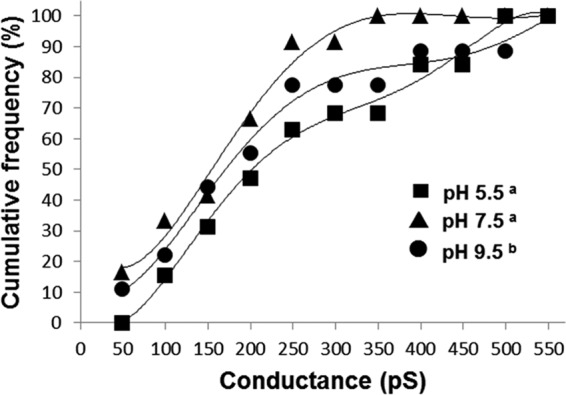
Cumulative frequency distributions of Cry6Aa1 conductances at pH 5.5, 7.5, and 9.5. Conductance values were obtained from IV curves derived from at least 3 experiments under symmetrical conditions and at each pH. They were grouped in 50-pS bins and their cumulative frequency of occurrence was plotted versus conductance. Data were fitted with 5th order polynomials. Legend symbols with different superscripts indicate a significant difference between the corresponding frequency curves (Mann-Whitney test at 5% significance level).
Effect of PLB enrichment with WCRW midgut brush-border membrane material
Binding to receptors on the midgut brush-border membrane is a critical step of the classical mode of action of Bt toxins (17). So far, no receptor of Cry6Aa toxins has been identified in WCRW larvae (38). To test the role of putative receptors or other docking molecules of Cry6Aa1 on the formation and properties of the pores of the toxin, experiments were conducted at pH 5.5 using Cry6Aa1, either in its native form or treated with WCRW gut juice, in PLBs in which WCRW midgut brush-border membrane fractions (BBMF) had been reconstituted.
Cry3Aa is a Bt toxin that is active against the WCRW and that binds to a yet unidentified receptor in the midgut of this insect (38). It was used to ascertain that PLB enrichment with BBMF was achieved. Chymotrypsin-activated Cry3Aa triggered channel activity in enriched PLBs at doses 200 times lower (data not shown) than what was required to form pores in BBMF-free PLBs (1–5 μg/ml), a toxin dose reduction comparable with those observed in earlier PLB studies on the interaction of Cry1 toxins with Manduca sexta (Lepidoptera) (57) and Lymantria dispar (Lepidoptera) (59) midgut receptors.
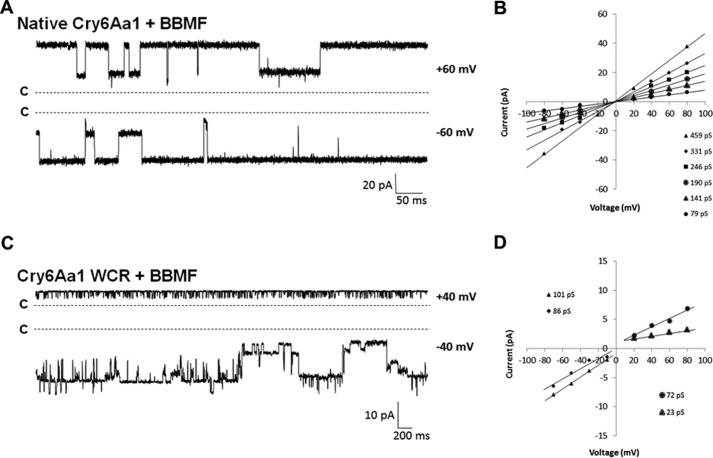
The native Cry6Aa1 inserted itself in a very stable way in the enriched PLB at a minimal dose of 100 fg/ml, 2000-fold less than what was required in BBMF-free bilayers (Fig. 5A). Current jumps were well resolved and several conductance levels could be measured in each experiment (Fig. 5B). Conductance ranged from 56 to 637 pS (Table 1), which included slightly larger values than those measured in the absence of midgut material. A more striking difference was observed at the level of pore selectivity. From slightly cationic in the absence of BBMF in PLBs, the pores became very slightly anionic in enriched bilayers, with the K+/Cl− permeability ratio shifting from 2.7 to 0.8 (Table 1).
Figure 5.
Cry6Aa1 and Cry6Aa1 WCR form pores in PLBs enriched with WCRW midgut BBMFs. A, representative current traces of the native Cry6Aa1 at ±60 mV in BBMF-rich PLBs under symmetrical (150 mm KCl) conditions at pH 5.5. B, typical IV curves constructed from recordings obtained in one typical experiment. C, representative current traces of Cry6Aa1 WCR at ±40 mV in BBMF-rich PLBs under symmetrical (150 mm KCl) conditions at pH 5.5. The current records were filtered at 100 Hz. D, IV curves derived from a typical Cry6Aa1 WCR experiment conducted under the same conditions as in C. Note that the current scales in C and D are not the same. In A and C, the letters c and the dotted lines show the closed state of all channels.
The effect of WCRW midgut material on Cry6Aa1 WCR pores was different. Whereas the insertion efficiency of the protein, i.e. the minimal dose required to form pores, remained equal to 100 fg/ml in both BBMF-rich and BBMF-free PLBs, apparent rectification of the currents was observed (Fig. 5C). As shown in Fig. 5D, the range of conductances measured at positive holding voltages was larger than at negative ones in this particular experiment. However, the opposite situation was observed in other experiments, i.e. conductances at negative voltages being larger than those at positive voltages, suggesting that midgut juice-treated Cry6Aa1 inserted itself into PLBs in more than one way. In any case, the conductances at non-rectifying voltages ranged between 40 and 306 pS, whereas those at rectifying voltages were comprised between 15 and 95 pS (Table 1). Just like the native Cry6Aa1, Cry6Aa1 WCR pores were slightly anionic, with a potassium to chloride permeability ratio of 0.8 (Table 1). To make sure that the Cry6Aa1 proteins were not further processed by intrinsic proteases in BBMF-enriched PLBs, control experiments in the presence of a protease inhibitor mixture were performed that confirmed that pore formation and biophysical properties were similar to those described above (data not shown).
These results suggest that the midgut protease digestion step of Cry6Aa1 is not critical for pore formation in PLBs. However, it cannot be excluded that it may affect the molecular recognition process of the toxin and in turn influence its pore formation ability.
Discussion
This study clearly shows, for the first time, that Cry6Aa1, a Bt toxin that is active against nematodes and coleopteran pests, is a pore-forming toxin. Previous studies hypothesized that Cry6Aa is a pore-forming toxin based on protein structural similarities to hemolysin E (39) and other ClyA-type pore forming toxins (40). However, attempts to characterize the mode of action of this toxin were limited to the demonstration that exposure of the nematode Caenorhabditis elegans to Cry6Aa allows its midgut epithelial cells to be labeled by propidium iodide, a well known viability probe (39, 61). Although this result indicates that the toxin acts at the level of these cells and compromises the integrity of their plasma membranes, it does not provide any information on the mechanism by which the cells are damaged. It may be taken as an indication that a necrosis-like mechanism is operating (61), but it does not allow one to conclude, nor to exclude, that cell death is induced by, or somehow involves, pore formation. Furthermore, Huang et al. (40) whose Cry6Aa structure determination only deals with the trypsinized form of the protein, did not conduct any experiments to demonstrate pore formation by the processed toxin (or, obviously, by its full-length form), although they stated in the title of their article that Cry6Aa was a pore-forming toxin. In fact, the present study provides the first direct and indisputable demonstration of pore formation and channel activity by this toxin in pure phospholipid PLBs and in BBMF-enriched PLBs, as well as the first description of the biophysical properties of the channel. Moreover, the native, unprocessed Cry6Aa1 protein displayed channel activity in both types of membranes, a finding that was never reported for other Bt protoxins. Furthermore, pore formation by Cry6Aa1, in its native or protease-processed forms, took place at unprecedented low protein concentrations, several orders of magnitude lower than those used for any other Bt toxin. Finally, it was established that the ability of Cry6Aa1 to form pores was affected by proteases, pH, and midgut brush-border membrane material, which are major components of the insect intestinal environment to which the toxin is exposed in vivo.
Our results on the pH dependence of the solubilization of Cry6Aa1 indicated that pH 10 was the optimal pH at which the native toxin was solubilized. This very alkaline environment is quite different from that of the midgut of WCRW, the Cry6Aa1 susceptible insect, which was reported to be acidic (pH 5.75) (62). Likewise, the intestinal pH of nematodes, such as C. elegans, which are also targeted by Cry6A toxins, has been found to range between 4.4 and 6.3 (63, 64). It should be noted, however, that pH measurements are extremely difficult to conduct in tiny organisms like nematodes and coleoptera. Actually, the documented pH of WCRW was measured in homogenized midgut juice collected from larvae of this insect (62). There is also a pH gradient along the midgut of coleopteran larvae, as measured in Tenebrio molitor and Morimus funereus midguts in which the pH of the anterior midguts was around 5.5, whereas it was close to 8.5 in their posterior part (65, 66). Therefore, it cannot be excluded that such gradients also exist in the WCRW midgut. Furthermore, both radial and longitudinal pH gradients are probably present, as was demonstrated, using 31P nuclear magnetic resonance microscopy, in the midgut of the lepidopteran insect Spodoptera litura (67). On the other hand, the pH dependence of Cry6Aa1 solubility is comparable with that of Cry3A, another Bt toxin that targets coleopteran pests (68). The question of how these toxins become active in the coleopteran midgut, despite the presence of a slightly acidic pH, is still far from having been resolved even though this paradox has been around for almost 25 years for Cry3Aa. Solubilization in the insect midgut apparently involves a more complex set of factors that are not reconstituted in experiments in which the crystals are simply resuspended and incubated in a buffer solution in vitro.
Bioassays of Cry6Aa1 on WCRW larvae showed that the dose of Cry6Aa1 needed to inhibit larval growth or kill the insects was similar to that reported for Cry3Aa1 and Cry34Ab1/Cry35Ab1 (38). It was therefore very surprising to find out that the toxin dose required for pore formation in PLBs was several orders of magnitude lower, even under experimental conditions designed to mimic the in vivo environment in the WCRW midguts (pH, proteolytic processing of the toxin and presence of apical membrane material). The origin of this huge dose difference remains to be investigated.
It is considered that the most biologically relevant form of the Cry6Aa1 proteins investigated in the present study is Cry6Aa1 WCR, the target insect midgut juice-treated Cry6Aa1. This protein formed pores in PLBs displaying very well resolved current jumps. The conductance of the pores was smaller than that reported for other toxins active against coleoptera. The binary toxin Cry34Ab1/Cry35Ab1, which is also active against WCRW, formed pores in PLBs, whose conductance ranged between 310 and 920 pS under the same experimental conditions as those used here (60). Likewise, the conductance of Cry3A pores formed in PLBs under identical experimental conditions was 505 pS (58). On the other hand, the conductance of Cry6Aa1 WCR is similar to that of lepidopteran toxins, such as Cry1Aa, tested in PLBs under identical experimental conditions, whose principal conductance was 450 pS (42), and larger than those of Cry1B and Cry1C, whose principal conductances were 350 and 90 pS, respectively (57).
As mentioned before, the largest difference with any other protease-treated Bt toxins tested so far was at the level of the dose used to observe channel activity. Usually, the dose needed for Cry toxins to efficiently partition into PLBs was in the order of μg/ml (see for example, Refs. 56 and 60). In the case of Cry6Aa1, a dose as low as 100 fg/ml was sufficient for pore formation. Such a dose is also smaller by 3–4 orders of magnitude than those used for the structurally similar bacterial toxins hemolysin E to form pores in lipid bilayers (69, 70).
The sequence of steps involved in the classical mode of action of Bt toxins includes solubilization and protease activation in the gut of target insects (17). However, it was demonstrated in the present study that Cry6Aa1, a Bt toxin whose 3D structure at atomic resolution is entirely different from those of any other known Bt toxin (39, 40), displayed channel activity in PLBs at extremely low doses, without protease processing and over a wide pH range, conditions under which pore formation by other Bt toxins was never reported. The biophysical properties of the pores formed by Cry6Aa1 were not much affected by pH, unlike other Bt toxins that target coleopteran insects such as Cry3Aa, which precipitates and is not able to form pores at acidic pH3 or Cry34Ab1/Cry35Ab1, which was not able to efficiently form pores at alkaline pH (60), as well as Bt toxins that are active against lepidopteran pests, like Cry1C, which has been shown to display different pore properties, such as ionic selectivity, depending on pH (56). Trypsin or WCRW midgut juice treatment provided proteins with identical molecular weights, even though trypsin is not a major protease in WCRW midgut juice (62). Furthermore, the products of such protease treatment, Cry6Aa1 TT and Cry6Aa1 WCR, formed pores at a similar dose and with similar conductances, but different ion selectivities. Cry6Aa1 WCR pores were slightly cationic, whereas those made by Cry6Aa1 TT displayed dual selectivity, sometimes to anions and at other times to cations, depending on the experiment. Such unusual behavior was never observed with other Bt toxins. It may be indicative of two different membrane insertion and oligomerization modes of Cry6Aa1 TT. More work is needed to fully understand the structure-function relationships involved in the role of pH and protease processing on Cry6Aa1 pore formation and properties. The recent publication of the 3D structures of the native and the trypsin-treated toxins should provide the necessary information at the molecular level to undertake structure-function studies, in particular in the light of the discovery that these two structures are very similar to the α-helix-rich molecules of other bacterial pore-forming toxins (39, 40). Among these toxins, hemolysin E from E. coli has been studied quite extensively. The structures of both its native and membrane-inserted forms have been determined (53, 71) and the conformational changes that lead to its oligomerization and membrane insertion during pore formation have been analyzed in considerable detail (71–74). Clearly, much remains to be done before the structure of the pores formed by Cry6Aa, or any other Bt toxin, is elucidated with a similar level of detail.
The molecular recognition step of the mode of action of Bt has been difficult to study for coleopteran active toxins due to the lack of information on the proteins that may constitute their putative receptors. In this study, target insect midgut BBMFs were successfully reconstituted in PLBs and it was shown that both the native Cry6Aa1 and the protease-treated Cry6Aa1 WCR toxins interacted with the apical membrane of the WCRW midgut, although in different ways. For pore formation in the presence of midgut membrane material, a 2000-fold reduction in the dose of the native Cry6Aa1 toxin was observed, down to the same dose as that of Cry6Aa1 WCR, whereas the Cry6Aa1 WCR dose remained the same with or without midgut material. Such an effect of midgut BBMFs on the dose required for Bt pores has been reported for Cry1Aa (59) and Cry1Ac (57), for which a significant, but not as large as here, dose reduction was observed. Moreover, the conductance of the Cry6Aa1 WCR pores was also affected by the presence of WCRW midgut apical membrane fractions, showing some kind of current rectification. Such current rectifying effect and change in the biophysical characteristics of the pores was shown for Cry1Ac toxins when M. sexta midgut brush-border aminopeptidase N, which acts as a receptor to a range of Bt toxins, was reconstituted in PLBs (57). Finally, the pore selectivity of both native Cry6Aa1 and Cry6Aa1 WCR changed from cationic to anionic in BBMF-enriched PLBs. This has never been observed before with other Bt toxins and may be related to different modes of oligomerization that will expose differently charged residues in the aqueous part of the pores, thus affecting their selectivity.
It is generally accepted that pH, specific proteases, and receptors in the midgut of target insects are major determinants of Bt Cry toxin specificity. However, in this PLB study of Cry6Aa1 under various pH, proteolytic processing, and BBMF exposure conditions, it was demonstrated that, although these factors affected pore formation and properties to some extent, the toxin was actually extremely efficient in vitro under any of the experimental conditions that were used. The doses required for pore formation in PLBs, as well as the specificity of the toxin to particular nematodes and coleopteran insects, suggest that Cry6Aa1 does not necessarily have the same mode of action as other Cry toxins.
Experimental procedures
Chemicals
The lipids used in the planar lipid bilayer experiments were 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE). Liposomes were prepared with l-α-phosphatidylcholine (PC) from egg yolk. All the lipids were purchased from Avanti Polar Lipids (Alabaster, AL). Analytical grade salts, MES, HEPES, Tris, CHES, CAPS, EGTA, and n-decane were purchased from Sigma (Oakville, Ontario, Canada). Additionally, a protease inhibitor mixture (P8340) containing inhibitors of each major class of proteases, N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK)-treated trypsin and N-p-tosyl-l-lysine chloromethyl ketone (TLCK)-treated α-chymotrypsin (both from bovine pancreas), was also purchased from Sigma.
Full-length Cry6Aa1 expression and purification
The Cry6Aa1 protein was expressed in P. fluorescens and the inclusion bodies were purified using the methods described previously (75). Approximately 100 mg of Cry6Aa1 inclusion bodies were thawed at 4 °C and centrifuged at 31,000 × g for 20 min at 4 °C. The pellet was mixed with 20 ml of 0.1 m CAPS/KOH (pH 10) and incubated at room temperature with rocking to extract the target protein.
The solubilized Cry6Aa1 was purified at room temperature by ion-exchange chromatography on a 5-ml HiTrap Q HP column with an AKTA protein purifier (GE Healthcare Life Sciences). The toxin extracts were diluted in 20 mm CAPS/KOH (pH 10) and filtered by a vacuum driven filter with a 0.45-μm pore diameter low protein-binding membrane. The samples were injected at 3 ml/min on a column pre-equilibrated with buffer A (50 mm CAPS/KOH, pH 10). The column was washed with ∼25 ml of buffer A and the protein was eluted using a linear gradient of 0–100% buffer B (50 mm CAPS, pH 10 + 1 m NaCl) over 40 column volumes. The fractions containing the purified target protein were identified by SDS-PAGE analysis. Peak fractions containing the target protein were pooled and concentrated using a centrifugal filter device with a 30-kDa molecular mass cut off. Quantification of target bands was done by comparing densitometric values of the bands against bovine serum albumin standard samples run on the same gel to generate a standard curve using the ImageQuant software package (GE Healthcare Life Sciences).
Trypsin processing of Cry6Aa1
Approximately 100 mg of Cry6Aa inclusion bodies were thawed at 4 °C and centrifuged at 23,000 × g for 25 min at 4 °C. The pellet was mixed with 10 ml of digestion buffer (0.1 m CAPS/KOH buffer, pH 10) and 6.6 mg of TPCK-treated trypsin from bovine pancreas was added to the sample to attain a Cry6Aa1:trypsin ratio of 15:1 (w/w). The reaction tube was incubated overnight at room temperature (∼16 h) with gentle rocking. After digestion, the mixture was centrifuged at 31,000 × g for 25 min at 4 °C. The supernatant was saved for ion-exchange chromatography purification. The buffer of the purified toxin fractions was finally exchanged by dialysis against 4 liters of 20 mm CAPS/KOH, pH 10.
Midgut juice collection and extraction
Approximately 150 third-instar WCRW larvae were obtained from Crop Characteristics (Farmington, MN) and shipped with corn roots. Using a scalpel, both the anterior and posterior ends of the larvae were removed. The gut was pulled out using forceps and stored on ice in 0.15 m NaCl and 8.5% sucrose. The tissue was homogenized on ice using a glass tissue homogenizer and the insoluble material was removed by centrifugation in a microcentrifuge at 7,500 × g for 15 min at 4 °C. The supernatants were quantified for total protein concentration using a Bradford assay (Fisher, Hampton, NH) and then stored in small aliquots at −80 °C until further use.
WCRW midgut juice processing of Cry6Aa1
Full-length Cry6Aa1 (10 mg) purified as described above was mixed with 1 mg of total protein of WCRW gut juice in 10 ml of 0.2 m sodium citrate at pH 6.0 and 4 mm EDTA. After 3 h of incubation at room temperature, aliquots were removed and analyzed by SDS-PAGE to ensure completion of the digestion. The reaction was stopped by the addition of a mixture of protease inhibitors, and the mixture was dialyzed overnight against 20 mm CAPS/KOH (pH 10) before ion-exchange chromatography purification. Fractions containing target protein were pooled and concentrated using a Millipore 30-kDa molecular mass cutoff membrane ultrafiltration device.
Cry3Aa preparation
Cry3Aa toxin inclusion bodies were prepared as described previously using the P. fluorescens heterologous expression system (75). Inclusion bodies were solubilized in 20 ml of 0.1 m CAPS (pH 10) at room temperature for 2 h. The solubilized protein was centrifuged for 20 min at 31,000 × g at 4 °C. The technique used for activation of the protoxin was modified from that described by Carroll et al. (76). The solubilized protoxin (10 mg in 2 ml) was mixed with 10 mg of TLCK-treated chymotrypsin in 9 ml of 25 mm Tris (pH 8.5). The sample was incubated for 16 h at 37 °C. The chymotrypsin-treated Cry3A was purified by ion exchange chromatography as described above, except that the pH of buffers A and B was increased to 11. Fractions containing the target protein were identified by SDS-PAGE analysis, pooled, concentrated to ∼2 ml with Amicon spin concentrators (30-kDa molecular mass cutoff) and further purified by chromatography on a Superdex 75 column (∼180 ml bed volume) at 1 ml/min.
Solubilization experiments
The solutions used to evaluate the solubility of native Cry6Aa1 contained 150 mm KCl and 50 mm of sodium citrate (pH 3, 4, and 5), MES/KOH (pH 6), HEPES/KOH (pH 7), Tris/HCl (pH 8), CHES/KOH (pH 9), or CAPS/KOH (pH 10). The inclusion bodies were incubated for 2 h at room temperature. The suspension was then centrifuged at 100,000 × g for 1 h and the protein content of the supernatant was estimated with the Bradford assay.
Bioassays
The toxicity of the purified native, midgut juice- and trypsin-treated Cry6Aa1 proteins was assayed at 100 μg/cm2 by diet surface contamination as described previously (38). Sixteen insects were used for each condition.
Planar lipid bilayers
Membranes were formed with a mixture of POPE:POPC 1:1 (w/w) at a final concentration of 20 mg/ml in n-decane. Pasteur pipettes that were previously pulled and sealed on a Bunsen burner were used to paint a bilayer across a 250-μm aperture in a Delrin membrane separating two 1-ml chambers (cis and trans). Disposable chamber-membrane systems were used to prevent contamination from one experiment to the next. The aperture was pretreated with 0.5 μl of the lipid mixture dissolved in n-decane. The lipids were dried under N2 for 10 min before use.
Experiments were conducted in a solution composed of 150 mm KCl, 1 mm CaCl2, and 10 mm of MES/KOH (pH 5.5), HEPES/KOH (pH 7.5), or Tris/HCl (pH 9.5). For asymmetrical conditions experiments, the KCl concentration was raised to 450 mm, on the cis side of the membrane, using a stock solution of 3 m KCl, 1 mm CaCl2, and 10 mm MES/KOH (pH 5.5), HEPES/KOH (pH 7.5), or Tris/HCl (pH 9.5).
The lipid bilayer quality was assessed by measuring the membrane capacitance, whose value was optimally around 180 pF. Before injection of the proteins, the membrane current was monitored for 30 min with holding voltages ranging from −150 to +150 mV to make sure that there was no contaminating pore-forming material in the bilayer system. Magnetic stirrers were used in both cis and trans compartments. All experiments were performed at room temperature. The proteins to be tested were added to the cis chamber to reach final concentrations that ranged from 100 fg/ml to 1 μg/ml.
The electrical connections between the experimental chambers and the recording instrumentation were made with Ag/AgCl electrodes and agar salt bridges (2 m KCl, 1 mm EGTA, and 2% agar). Currents were recorded with an Axopatch-1D patch clamp amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA), filtered at 10 kHz, digitized at a 50 kHz sampling frequency (Digidata 1440, Molecular Devices). They were processed and analyzed using pClamp 10.5 software (Axon Instruments).
Holding voltages were applied to the membrane from +80 to −80 mV in 20-mV steps and for variable durations. For each applied voltage, the amplitudes of the current jumps were measured, grouped, and averaged. Current-voltage (IV) plots were then constructed and the data points were fitted with linear curves whose slopes provided the conductances of the pores. Reversal potentials were obtained from IV relationships of experiments conducted under asymmetrical ionic conditions. Potassium over chloride permeability ratios were calculated from the reversal potential given by the horizontal axis intercept, with the Goldman-Hodgkin-Katz equation (77).
Brush-border membrane fragment fusion in PLBs
Previously isolated WCRW larvae midguts were used to prepare BBMFs as described elsewhere (78). The BBMFs were aliquoted, stored at −80 °C in 10 mm HEPES/KOH (pH 7.5), and used within the next five months. Compared with the initial crude homogenate, the BBMF were enriched 8.4-fold, as indicated by aminopeptidase N activity (79). BBMF-enriched liposomes were prepared as described elsewhere (80), with some modifications. Egg yolk PC was used as the base lipid. It was dried under N2 and hydrated in 150 mm KCl, 1 mm EGTA, and 10 mm HEPES/KOH (pH 7.5) at a final BBMF proteins:lipid ratio of 1:60 (w/w). The BBMF-enriched liposomes were mechanically fused to the PLBs (59) from the cis side using a Pasteur pipette with a very small round tip. The membrane current was monitored for 30 min before toxin addition. No contaminating or BBMF intrinsic channel activity could be observed during these control periods.
Data presentation
All experiments were performed at least three times. The cumulative frequency distribution of pore conductances was obtained by grouping the conductances in 50-pS bins and the Mann-Whitney test for independent variables was used to evaluate the significance at p < 0.05 of the difference between the data obtained under each experimental condition.
Author contributions
V. C., T. H., D. M., K. N., V. V., and J. L. S. conceived experiments; V. C. and X. X. prepared the experimental materials and S. G. performed protein analysis; S. Y. T. conducted WCRW bioassay experiments; E. F. conducted the solubilization and PLB experiments, with the help of V. L. and L. P., and wrote the first draft of the manuscript; the subsequent versions were discussed and written by V. C., K. N., V. V., and J. L. S.
Acknowledgment
We thank Ted Letherer for supporting D. virgifera virgifera bioassays.
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Collaborative Research and Development grant (CRDPJ 44052-12 to J. L. S.), in partnership with Dow AgroSciences Canada Inc., a Université de Montréal graduate student scholarship (to E. F.), and an NSERC undergraduate student research award (to V. L.). E. F., V. L., L. P., V. V., and J. L. S. declare that they have no conflicts of interest with the contents of this article. S. G., V. C., D. M., S. Y. T., and K. N. are employed by Dow AgroSciences, LLC. T. H. and X. X. are former employees of that company.
E. Fortea, V. Vachon, and J.-L. Schwartz, unpublished data.
- Bt
- Bacillus thuringiensis
- PLB
- planar lipid bilayer
- WCRW
- Western corn rootworm
- Cry6Aa1 WCR
- WCRW midgut juice-treated Cry6Aa1
- Cry6Aa1 TT
- trypsin-treated Cry6Aa1
- BBMF
- brush-border membrane fraction
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPE
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- PC
- l-α-phosphatidylcholine
- CHES
- 2-(cyclohexylamino)ethanesulfonic acid
- CAPS
- 3-(cyclohexylamino)-1-propanesulfonic acid
- TPCK
- N-p-tosyl-l-phenylalanine chloromethyl ketone
- TLCK
- N-p-tosyl-l-lysine chloromethyl ketone.
References
- 1. Bulla L. A. Jr., Bechtel D. B., Kramer K. J., Shethna Y. I., Aronson A. I., and Fitz-James P. C. (1980) Ultrastructure, physiology, and biochemistry of Bacillus thuringiensis. Crit. Rev. Microbiol. 8, 147–204 [DOI] [PubMed] [Google Scholar]
- 2. Aronson A. (2002) Sporulation and δ-endotoxin synthesis by Bacillus thuringiensis. Cell Mol. Life Sci. 59, 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Frankenhuyzen K. (2009) Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101, 1–16 [DOI] [PubMed] [Google Scholar]
- 4. Federici B. A. (2005) Insecticidal bacteria: an overwhelming success for invertebrate pathology. J. Invertebr. Pathol. 89, 30–38 [DOI] [PubMed] [Google Scholar]
- 5. Bravo A., Likitvivatanavong S., Gill S. S., and Soberón M. (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanchis V., and Bourguet D. (2008) Bacillus thuringiensis: applications in agriculture and insect resistance management: a review. Agron. Sustain. Dev. 28, 11–20 [Google Scholar]
- 7. van Frankenhuyzen K. (2000) Application of Bacillus thuringiensis in forestry. in Entomopathogenic Bacteria: from Laboratory to Field Application (Charles J. F., Delécluse A., and Nielsen-Leroux C., eds) pp. 371–376, Kluwer Associate Publishing, Norwell, MA [Google Scholar]
- 8. Becker N. (2000) Bacterial control of vector-mosquitoes and blackflies. in Entomopathogenic Bacteria: from Laboratory to Field Application (Charles J. F., Delécluse A., and Nielsen-Leroux C., eds) pp. 383–396, Kluwer Associate Publishing, Norwell, MA [Google Scholar]
- 9. James C. (2015) 20th Anniversary (1996 to 2015) of the Global Commercialization of Biotech Crops and Biotech Crop Highlights in 2015. ISAAA Brief No. 51, ISAAA, Ithaca, NY [Google Scholar]
- 10. Rajamohan F., Lee M. K., and Dean D. H. (1998) Bacillus thuringiensis insecticidal proteins: molecular mode of action. Prog. Nucleic Acid Res. Mol. Biol. 60, 1–27 [DOI] [PubMed] [Google Scholar]
- 11. Schnepf E., Crickmore N., Van Rie J., Lereclus D., Baum J., Feitelson J., Zeigler D. R., and Dean D. H. (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aronson A. I., and Shai Y. (2001) Why Bacillus thuringiensis insecticidal toxins are so effective: unique features of their mode of action. FEMS Microbiol. Lett. 195, 1–8 [DOI] [PubMed] [Google Scholar]
- 13. Bravo A., Gill S. S., and Soberón M. (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49, 423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carroll J., and Ellar D. J. (1993) An analysis of Bacillus thuringiensis δ-endotoxin action on insect midgut membrane permeability using a light-scattering assay. Eur. J. Biochem. 214, 771–778 [DOI] [PubMed] [Google Scholar]
- 15. Kirouac M., Vachon V., Rivest S., Schwartz J. L., and Laprade R. (2003) Analysis of the properties of Bacillus thuringiensis insecticidal toxins using a potential-sensitive fluorescent probe. J. Membr. Biol. 196, 51–59 [DOI] [PubMed] [Google Scholar]
- 16. Knowles B. H., and Ellar D. J. (1987) Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificity. Biochim. Biophys. Acta 924, 509–518 [Google Scholar]
- 17. Vachon V., Laprade R., and Schwartz J. L. (2012) Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J. Invertebr. Pathol. 111, 1–12 [DOI] [PubMed] [Google Scholar]
- 18. McGaughey W. H., and Oppert B. (1998) Mechanism of insect resistance to Bacillus thuringiensis toxins. Isr. J. Entomol. 32, 1–14 [Google Scholar]
- 19. Frutos R., Rang C., and Royer F. (1999) Managing insect resistance to plants producing Bacillus thuringiensis toxins. Crit. Rev. Biotechnol. 19, 227–276 [Google Scholar]
- 20. Ferré J., and Van Rie J. (2002) Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47, 501–533 [DOI] [PubMed] [Google Scholar]
- 21. Pigott C. R., and Ellar D. J. (2007) Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 71, 255–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pardo-López L., Soberón M., and Bravo A. (2013) Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 37, 3–22 [DOI] [PubMed] [Google Scholar]
- 23. Kao C. Y., Los F. C. O., Huffman D. L., Wachi S., Kloft N., Husmann M., Karabrahimi V., Schwartz J. L., Bellier A., Ha C., Sagong Y., Fan H., Ghosh P., Hsieh M., Hsu C. S., Chen L., and Aroian R. V. (2011) Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 7, e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Head G. P., and Greenplate J. (2012) The design and implementation of insect resistance management programs for Bt crops. GM Crops Food 3, 144–153 [DOI] [PubMed] [Google Scholar]
- 25. Estruch J. J., Warren G. W., Mullins M. A., Nye G. J., Craig J. A., and Koziel M. G. (1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. U.S.A. 93, 5389–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milne R., Liu Y., Gauthier D., and van Frankenhuyzen K. (2008) Purification of Vip3Aa from Bacillus thuringiensis HD-1 and its contribution to toxicity of HD-1 to spruce budworm (Choristoneura fumiferana) and gypsy moth (Lymantria dispar) (Lepidoptera). J. Invertebr. Pathol. 99, 166–172 [DOI] [PubMed] [Google Scholar]
- 27. Ma B. L., Meloche F., and Wei L. (2009) Agronomic assessment of Bt trait and seed or soil-applied insecticides on the control of corn rootworm and yield. Field Crops Res. 111, 189–196 [Google Scholar]
- 28. Hibbard B. E., Clark T. L., Ellersieck M. R., Meihls L. N., El Khishen A. A., Kaster V., Steiner H. Y., and Kurtz R. (2010) Mortality of Western corn rootworm larvae on MIR604 transgenic maize roots: field survivorship has no significant impact on survivorship of F1 progeny on MIR604. J. Econ. Entomol. 103, 2187–2196 [DOI] [PubMed] [Google Scholar]
- 29. Gassmann A. J., Petzold-Maxwell J. L., Keweshan R. S., and Dunbar M. W. (2011) Field-evolved resistance to Bt maize by Western corn rootworm. PLoS ONE 6, e22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tabashnik B. E., and Gould F. (2012) Delaying corn rootworm resistance to Bt corn. J. Econ. Entomol. 105, 767–776 [DOI] [PubMed] [Google Scholar]
- 31. Aragón P., Baselga A., and Lobo J. M. (2010) Global estimation of invasion risk zones for the Western corn rootworm Diabrotica virgifera virgifera: integrating distribution models and physiological thresholds to assess climatic favourability. J. Appl. Ecol. 47, 1026–1035 [Google Scholar]
- 32. Aragón P., and Lobo J. M. (2012) Predicted effect of climate change on the invasibility and distribution of the Western corn rootworm. Agric. For. Entomol. 14, 13–18 [Google Scholar]
- 33. Narva K. E., Schwab G. E., Galasan T., and Payne J. M. (August 17, 1993) Gene encoding a nematode-active toxin cloned from a Bacillus thuringiensis isolate. U. S. Patent 5,236,843 [Google Scholar]
- 34. Wei J. Z., Hale K., Carta L., Platzer E., Wong C., Fang S. C., and Aroian R. V. (2003) Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. U.S.A. 100, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo H., Xiong J., Zhou Q., Xia L., and Yu Z. (2013) The effects of Bacillus thuringiensis Cry6A on the survival, growth, reproduction, locomotion, and behavioral response of Caenorhabditis elegans. Appl. Microbiol. Biotechnol. 97, 10135–10142 [DOI] [PubMed] [Google Scholar]
- 36. Narva K. E., Schwab G. E., and Bradfisch G. A. (February 16, 1993) Bacillus thuringiensis gene encoding a coleopteran-active toxin. U. S. Patent 5,186,934 [Google Scholar]
- 37. Bradfisch G. A., Muller-Cohn J., Narva K. E., Fu J. M., and Thompson M. (October 26, 1999) Bacillus thuringiensis isolates, toxins, and genes for controlling certain coleopteran pests. U. S. Patent 5,973,231 [Google Scholar]
- 38. Li H., Olson M., Lin G., Hey T., Tan S. Y., and Narva K. E. (2013) Bacillus thuringiensis Cry34Ab1/Cry35Ab1 interactions with Western corn rootworm midgut membrane binding sites. PLoS ONE 8, e53079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dementiev A., Board J., Sitaram A., Hey T., Kelker M. S., Xu X., Hu Y., Vidal-Quist C., Chikwana V., Griffin S., McCaskill D., Wang N. X., Hung S.-C., Chan M. K., Lee M. M., Hughes J., Wegener A., Aroian R. V., Narva K. E., and Berry C. (2016) The pesticidal Cry6Aa toxin from Bacillus thuringiensis is structurally similar to HlyE-family alpha pore-forming toxins. BMC Biol. 14, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang J., Guan Z., Wan L., Zou T., and Sun M. (2016) Crystal structure of Cry6Aa: a novel nematicidal ClyA-type α-pore-forming toxin from Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 478, 307–313 [DOI] [PubMed] [Google Scholar]
- 41. Li J., Carroll J., and Ellar D. J. (1991) Crystal structure of insecticidal δ-endotoxin from Bacillus thuringiensis at 2.5-Å resolution. Nature 353, 815–821 [DOI] [PubMed] [Google Scholar]
- 42. Grochulski P., Masson L., Borisova S., Pusztai-Carey M., Schwartz J. L., Brousseau R., and Cygler M. (1995) Bacillus thuringiensis CrylA(a) insecticidal toxin: crystal structure and channel formation. J. Mol. Biol. 254, 447–464 [DOI] [PubMed] [Google Scholar]
- 43. Galitsky N., Cody V., Wojtczak A., Ghosh D., Luft J. R., Pangborn W., and English L. (2001) Structure of the insecticidal bacterial δ-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallogr. D Biol. Crystallogr. 57, 1101–1109 [DOI] [PubMed] [Google Scholar]
- 44. Morse R. J., Yamamoto T., and Stroud R. M. (2001) Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 9, 409–417 [DOI] [PubMed] [Google Scholar]
- 45. Boonserm P., Davis P., Ellar D. J., and Li J. (2005) Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 348, 363–382 [DOI] [PubMed] [Google Scholar]
- 46. Boonserm P., Mo M., Angsuthanasombat C., and Lescar J. (2006) Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-Å resolution. J. Bacteriol. 188, 3391–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo S., Ye S., Liu Y., Wei L., Xue J., Wu H., Song F., Zhang J., Wu X., Huang D., and Rao Z. (2009) Crystal structure of Bacillus thuringiensis Cry8Ea1: An insecticidal toxin toxic to underground pests, the larvae of Holotrichia parallela. J. Struct. Biol. 168, 259–266 [DOI] [PubMed] [Google Scholar]
- 48. Hui F., Scheib U., Hu Y., Sommer R. J., Aroian R. V., and Ghosh P. (2012) Structure and glycolipid binding properties of the nematicidal protein Cry5B. Biochemistry 51, 9911–9921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evdokimov A. G., Moshiri F., Sturman E. J., Rydel T. J., Zheng M., Seale J. W., and Franklin S. (2014) Structure of the full-length insecticidal protein Cry1Ac reveals intriguing details of toxin packaging into in vivo formed crystals. Protein Sci. 23, 1491–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akiba T., Abe Y., Kitada S., Kusaka Y., Ito A., Ichimatsu T., Katayama H., Akao T., Higuchi K., Mizuki E., Ohba M., Kanai R., and Harata K. (2009) Crystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cells. J. Mol. Biol. 386, 121–133 [DOI] [PubMed] [Google Scholar]
- 51. Xu C., Chinte U., Chen L., Yao Q., Meng Y., Zhou D., Bi L. J., Rose J., Adang M. J., Wang B. C., Yu Z., and Sun M. (2015) Crystal structure of Cry51Aa1: A potential novel insecticidal aerolysin-type β-pore-forming toxin from Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 462, 184–189 [DOI] [PubMed] [Google Scholar]
- 52. Akiba T., Higuchi K., Mizuki E., Ekino K., Shin T., Ohba M., Kanai R., and Harata K. (2006) Nontoxic crystal protein from Bacillus thuringiensis demonstrates a remarkable structural similarity to pore-forming toxins. Proteins 63, 243–248 [DOI] [PubMed] [Google Scholar]
- 53. Wallace A. J., Stillman T. J., Atkins A., Jamieson S. J., Bullough P. A., Green J., and Artymiuk P. J. (2000) E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100, 265–276 [DOI] [PubMed] [Google Scholar]
- 54. Fagerlund A., Lindbäck T., Storset A. K., Granum P. E., and Hardy S. P. (2008) Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 154, 693–704 [DOI] [PubMed] [Google Scholar]
- 55. Madegowda M., Eswaramoorthy S., Burley S. K., and Swaminathan S. (2008) X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus. Proteins 71, 534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwartz J. L., Garneau L., Savaria D., Masson L., Brousseau R., and Rousseau E. (1993) Lepidopteran-specific crystal toxins from Bacillus thuringiensis form cation- and anion-selective channels in planar lipid bilayers. J. Membr. Biol. 132, 53–62 [DOI] [PubMed] [Google Scholar]
- 57. Schwartz J. L., Lu Y. J., Söhnlein P., Brousseau R., Laprade R., Masson L., and Adang M. J. (1997) Ion channels formed in planar lipid bilayers by Bacillus thuringiensis toxins in the presence of Manduca sexta midgut receptors. FEBS Lett. 412, 270–276 [DOI] [PubMed] [Google Scholar]
- 58. Schwartz J. L., and Laprade R. (2000) Membrane permeabilisation by Bacillus thuringiensis toxins: protein insertion and pore formation. in Entomopathogenic Bacteria: from Laboratory to Field Application (Charles J. F., Delécluse A., and Nielsen-Leroux C., eds) pp. 199–218, Kluwer Associate Publishing, Norwell, MA [Google Scholar]
- 59. Peyronnet O., Vachon V., Schwartz J. L., and Laprade R. (2001) Ion channels induced in planar lipid bilayers by the Bacillus thuringiensis toxin Cry1Aa in the presence of gypsy moth (Lymantria dispar) brush border membrane. J. Membr. Biol. 184, 45–54 [DOI] [PubMed] [Google Scholar]
- 60. Masson L., Schwab G., Mazza A., Brousseau R., Potvin L., and Schwartz J. L. (2004) A novel Bacillus thuringiensis (PS149B1) containing a Cry34Ab1/Cry35Ab1 binary toxin specific for the Western corn rootworm Diabrotica virgifera virgifera LeConte forms ion channels in lipid membranes. Biochemistry 43, 12349–12357 [DOI] [PubMed] [Google Scholar]
- 61. Zhang F., Peng D., Cheng C., Zhou W., Ju S., Wan D., Yu Z., Shi J., Deng Y., Wang F., Ye X., Hu Z., Lin J., Ruan L., and Sun M. (2016) Bacillus thuringiensis crystal protein Cry6Aa triggers Caenorhabditis elegans necrosis pathway mediated by aspartic protease (ASP-1). PLoS Pathog. 12, e1005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaiser-Alexnat R. (2009) Protease activities in the midgut of Western corn rootworm (Diabrotica virgifera virgifera LeConte). J. Invertebr. Pathol. 100, 169–174 [DOI] [PubMed] [Google Scholar]
- 63. Allman E., Johnson D., and Nehrke K. (2009) Loss of the apical V-ATPase a-subunit VHA-6 prevents acidification of the intestinal lumen during a rhythmic behavior in C. elegans. Am. J. Physiol. Cell Physiol. 297, C1071–C1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chauhan V. M., Orsi G., Brown A., Pritchard D. I., and Aylott J. W. (2013) Mapping the pharyngeal and intestinal pH of Caenorhabditis elegans and real-time luminal pH oscillations using extended dynamic range pH-sensitive nanosensors. ACS Nano 7, 5577–5587 [DOI] [PubMed] [Google Scholar]
- 65. Vinokurov K. S., Elpidina E. N., Oppert B., Prabhakar S., Zhuzhikov D. P., Dunaevsky Y. E., and Belozersky M. A. (2006) Diversity of digestive proteinases in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 145, 126–137 [DOI] [PubMed] [Google Scholar]
- 66. Lončar N., Božić N., Nenadović V., Ivanović J., and Vujčić Z. (2009) Characterization of trypsin-like enzymes from the midgut of Morimus funereus (Coleoptera: Cerambycidae) larvae. Arch. Biol. Sci. 61, 713–718 [Google Scholar]
- 67. Skibbe U., Christeller J. T., Callaghan P. T., Eccles C. D., and Laing W. A. (1996) Visualization of pH gradients in the larval midgut of Spodoptera litura using 31P-NMR microscopy. J. Insect Physiol. 42, 777–790 [Google Scholar]
- 68. Koller C. N., Bauer L. S., and Hollingworth R. M. (1992) Characterization of the pH-mediated solubility of Bacillus thuringiensis var. san diego native δ-endotoxin crystals. Biochem. Biophys. Res. Commun. 184, 692–699 [DOI] [PubMed] [Google Scholar]
- 69. Ludwig A., Bauer S., Benz R., Bergmann B., and Goebel W. (1999) Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol. Microbiol. 31, 557–567 [DOI] [PubMed] [Google Scholar]
- 70. Oscarsson J., Mizunoe Y., Li L., Lai X.-H., Wieslander Å, Uhlin B. E. (1999) Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol. Microbiol. 32, 1226–1238 [DOI] [PubMed] [Google Scholar]
- 71. Mueller M., Grauschopf U., Maier T., Glockshuber R., and Ban N. (2009) The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature 459, 726–730 [DOI] [PubMed] [Google Scholar]
- 72. Eifler N., Vetsch M., Gregorini M., Ringler P., Chami M., Philippsen A., Fritz A., Müller S. A., Glockshuber R., Engel A., and Grauschopf U. (2006) Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 25, 2652–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tzokov S. B., Wyborn N. R., Stillman T. J., Jamieson S., Czudnochowski N., Artymiuk P. J., Green J., and Bullough P. A. (2006) Structure of the hemolysin E (HlyE, ClyA, and SheA) channel in its membrane-bound form. J. Biol. Chem. 281, 23042–23049 [DOI] [PubMed] [Google Scholar]
- 74. Vaidyanathan M. S., Sathyanarayana P., Maiti P. K., Visweswariah S. S., and Ayappa K. G. (2014) Lysis dynamics and membrane oligomerization pathways for cytolysin A (ClyA) pore-forming toxin. RSC Adv. 4, 4930–4942 [Google Scholar]
- 75. Tan S. Y., Rangasamy M., Wang H., Vélez A. M., Hasler J., McCaskill D., Xu T., Chen H., Jurzenski J., Kelker M., Xu X., Narva K., and Siegfried B. D. (2016) RNAi induced knockdown of a cadherin-like protein (EF531715) does not affect toxicity of Cry34/35Ab1 or Cry3Aa to Diabrotica virgifera virgifera larvae (Coleoptera: Chrysomelidae). Insect Biochem. Mol. Biol. 75, 117–124 [DOI] [PubMed] [Google Scholar]
- 76. Carroll J., Convents D., Van Damme J., Boets A., Van Rie J., and Ellar D. J. (1997) Intramolecular proteolytic cleavage of Bacillus thuringiensis Cry3A δ-endotoxin may facilitate its coleopteran toxicity. J. Invertebr. Pathol. 70, 41–49 [DOI] [PubMed] [Google Scholar]
- 77. Hille B. (2001) Selective permeability: independence. in Ion Channels of Excitable Membranes, 3rd Ed., pp. 441–470, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 78. Wolfersberger M., Lüthy P., Maurer A., Parenti P., Sacchi V. F., Giordana B., and Hanozet G. M. (1987) Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. A Physiol. 86, 301–308 [Google Scholar]
- 79. George S. G., and Kenny A. J. (1973) Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem. J. 134, 43–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. MacDonald R. C., MacDonald R. I., Menco B. P. M., Takeshita K., Subbarao N. K., and Hu L.-r. (1991) Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1061, 297–303 [DOI] [PubMed] [Google Scholar]