Abstract
Purpose
Image guided radiotherapy (IGRT) is designed to ensure accurate and precise targeting, but whether improved clinical outcomes result is unknown.
Materials and Methods
A retrospective comparison of locally advanced lung cancer patients treated with and without IGRT from 2001-2012 was conducted. Median local failure-free survival (LFFS), regional, locoregional failure-free survival (LRFFS), distant failure-free survival (DFFS), progression-free survival, and overall survival (OS) were estimated. Univariate and multivariate models assessed the association between patient and treatment-related covariates and local failure.
Results
169 patients were treated with definitive radiotherapy and concurrent chemotherapy with a median follow-up of 48 months in the IGRT cohort and 96 months in the non-IGRT cohort. IGRT was utilized in 36% (62 patients) of patients. OS was similar between cohorts (2-year OS, 47% vs. 49%, p=0.63). The IGRT cohort had improved two year LFFS (80% vs. 64%, p=0.013) and LRFS (75% and 62%, p=0.04). Univariate analysis revealed IGRT and treatment year improved LFFS while group stage, dose, and PET/CT planning had no impact. IGRT remained significant in the multivariate model with an adjusted hazard ratio (aHR) of 0.40 (p=0.01). DFFS (58% vs. 59%, p=0.67) did not differ significantly.
Conclusion
IGRT with daily CBCT confers an improvement in the therapeutic ratio relative to patients treated without this technology.
Introduction
Successive improvements in radiation treatment technology such as image guided radiotherapy (IGRT) have led to improvements in the therapeutic ratio. IGRT is defined as imaging in the treatment room with positional adjustments for geometric deviations from the planned position. Conventional MV portal imaging is limited to verification of bony anatomy, while IGRT visualizes soft tissue structures and bony anatomy. IGRT can be performed with either gantry mounted MV or kV ConeBeam CT (CBCT) or room mounted kV systems for tracking during treatment, video or surface imaging to determine positioning error, or with ultrasound imaging. 1 While MV portal imaging and IGRT both allow for position adjustment, IGRT allows for easier, more frequent positioning changes with improved accuracy leading to a theoretical therapeutic advantage.
Improvements in radiotherapy delivery techniques and delivery are numerous and include higher conformality with multi-beam arrangements, more advanced planning techniques such as intensity modulated radiotherapy (IMRT) and conformal radiotherapy (3DCRT), improved dosimetric modeling using tissue inhomogeneity corrections, improved target delineation using PET/CT planning, and 4D-CT imaging, and finally more rigorous patient positioning using respiratory motion management. In addition to these advancements, the potential for IGRT with CBCT to improve tumor targeting while reducing dose to normal tissues such as the esophagus, heart, and normal lung tissue has real implications for radiotherapy's therapeutic ratio. Unfortunately, the adoption of these technologies and their impact on clinical outcomes are poorly defined.
The impact of IGRT is well-recognized for some cancers.2,3 In head-and-neck cancer, a prospective study by Den et al used daily CBCT to characterize and correct interfraction setup error. Using these data, the investigators examined the CTV to PTV expansions necessary and found that the PTV could be reduced by about 2-3 mm when using daily CBCT.3 For medically inoperable, early stage lung cancer, IGRT has facilitated reduced treatment margins and precision delivery of hypofractionated radiotherapy. 4,5
Locally advanced NSCLC patients have a higher propensity for distant failures, which makes studying local and regional control challenging. There is a paucity of evidence supporting the improvement of outcomes with IGRT in NSCLC in these patients. A retrospective study by Shumway et al examined pathologic control rates of patients with stage IIIA/IIIB NSCLC treated preoperatively with and without 4D-CT and IGRT. 6 Only ten patients of the 53 were treated with 4DCT/IGRT, however these patients had higher rates of nodal down-staging and pathologic complete response following resection.
Locally advanced lung cancer target volumes often closely approximate organs at risk. The use of reduced treatment margins is attractive to reduce off-target toxicity. The authors hypothesized that treatment planning based on individualized tumor motion with four-dimensional CT imaging, followed by daily IGRT with daily kV CBCT may allow more accurate tumor targeting with resultant improved local control and reduced side effects compared to weekly two-dimensional MV portal imaging based on bony landmarks.7-10 We sought to test this hypothesis by comparing kV CBCT and weekly MV portal imaging with regards to local control and treatment related toxicity in locally advanced lung cancer in patients who received concurrent chemotherapy.
Methods and Materials
Patients
Patients with Stage IIB to IIIB (AJCC 7th Edition) NSCLC who were treated between January 2001 and September 2012 with concurrent chemotherapy and external beam radiotherapy with curative intent were included in this analysis. Patient and treatment characteristics were collected from the electronic medical record with Institutional Review Board approval.
Treatment
IGRT using daily kV CBCT was systematically applied to all lung cancer patients beginning in February 2009 with matching between planning CT and daily CBCT based on soft tissue and bony/anatomical landmarks. Prior to IGRT, patients were imaged with weekly MV portal images; these patients served as the comparative cohort.
Patients in both cohorts were treated with either three-dimensional 3DCRT or IMRT. A prescription goal of ≥ 95% of the treatment dose was prescribed volumetrically to the PTV. Tissue heterogeneity corrections were included in treatment planning system in 2006. Starting in 2007, 4D-CT scans were completed on all patients to define the internal target volume (ITV) with a 5mm expansion to PTV. Prior to 4D-CT, the gross tumor volume (GTV) was defined on free-breathing CT with a uniform 1cm expansion to generate the planning target volume (PTV). The reduction of the size of the PTV expansion in the 4D-CT era is due to the increased accuracy of target delineation using 4D-CT as compared tumor volume definitions using free-breathing CT. A clinical target volume was not utilized in treatment planning. Positron emission tomography (PET/CT) imaging for target delineation was used by image registration and fusion to the planning CT scan when available.
Additional patient selection information, treatment planning techniques, and follow up schedules are described in Supplementary Materials.
Follow up
In general, patients were evaluated clinically and underwent chest CT at 6-8 weeks following treatment, every 3-4 months for the first 2 years, 6 month intervals from years 3 to 5 and then annually thereafter. Radiographic and clinical information was reviewed to score the initial and subsequent failures as local, regional, locoregional or distant. Locoregional failure was defined as either local, regional, or concurrent local and regional relapses. Radiographic response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1. Toxicity was graded retrospectively according to Common Terminology Criteria for Adverse Effects version 4.0.
Statistical Analysis
Failure-free survival for local (LFFS), regional (RFFS), locoregional (LRFFS), and distant (DFFS) disease, progression-free survival (PFS), and overall survival (OS) were estimated using the Kaplan Meier method. Strata were compared with the log-rank statistic. The completion date of EBRT was used as time zero. The analysis was also repeated for time-to-event outcomes censoring patients after the initial site of failure for a pattern of failure analysis. The IGRT and non-IGRT cohorts were assessed for differences in patient (age, sex, race), disease (stage grouping, T and N stage, histology), and treatment (dose, fraction size, 4D-CT planning, PET-based planning, chemotherapy use, agent and schedule) related factors among the IGRT and non-IGRT cohort using a 2-sided t-test, chi-square, or Fisher's exact test as appropriate.
Univariate analyses (UVA) evaluated the association of patient, disease and treatment related covariates on LFFS after assessing for proportional hazards assumptions. Pre-specified clinically relevant and covariates which were statistically significant at the p=0.2 level were considered for inclusion in the multivariate analysis (MVA). All covariates were tested for proportional hazards assumptions and confirmed to be without interactions with other covariates in the model. All statistical measures were performed in SAS software (v9.2; SAS Institute, Cary, NC).
Results
Patients
A total of 169 patients were analyzed including 62 (36%) treated with IGRT. Table 1 describes the patient, disease and tumor related characteristics across the IGRT and non-IGRT cohorts. Median age of patients was 64 years (range 36-87) and did not differ significantly between cohorts (p=0.45). There was an increased proportion of patients with T4 tumors (38 vs. 27%, p=0.15), and performance status of zero to one in the non-IGRT cohort (p<.0001). Squamous cell carcinoma was the most common histology.
Table 1. Patient and Treatment Characteristics.
| IGRT Cohort | Non IGRT Cohort | ||||
|---|---|---|---|---|---|
| N=62 | N=107 | ||||
| N (%) | N (%) | P-value | |||
| Patient Characteristics | |||||
| Age | 0.45 | ||||
| Median | 63 | 61 | |||
| Range | 38-85 | 36-84 | |||
| ≥70 Years | 14 (22) | 25 (23) | |||
| ECOG | <0.0001 | ||||
| 0-1 | 50 (81) | 98 (92) | |||
| 2 | 12 (19) | 9 (8) | |||
| Race | 0.84 | ||||
| Caucasian | 49 (79) | 86 (80) | |||
| African-American | 13 (21) | 21 (20) | |||
| Sex | 0.40 | ||||
| Male | 45 (73) | 71 (66) | |||
| Female | 17 (27) | 36 (34) | |||
| Group Stage | 0.66 | ||||
| IIB | 1 (2) | 4 (4) | |||
| IIIA | 36 (58) | 57 (53) | |||
| IIIB | 25 (40) | 46 (43) | |||
| T Stage | 0.43 | ||||
| 1 | 10 (16) | 13 (12) | |||
| 2 | 22 (35) | 30 (28) | |||
| 3 | 11 (17) | 22 (21) | |||
| 4 | 17 (27) | 41 (38) | |||
| x | 2 (3) | 1 (1) | |||
| N Stage | 0.25 | ||||
| 0 | 6 (10) | 13 (12) | |||
| 1 | 3 (5) | 11 (10) | |||
| 2 | 36 (58) | 66 (62) | |||
| 3 | 17 (27) | 17 (16) | |||
| Histology | 0.05 | ||||
| Adenocarcinoma | 24 (39) | 28 (26) | |||
| Squamous Cell | 24 (39) | 42 (39) | |||
| Large Cell/Neuroendocrine | 3 (5) | 5 (5) | |||
| NSCLC NOS | 9 (15) | 32 (30) | |||
| Other/Unknown | 2 (3) | 0 (0) | |||
| Treatment Characteristics | |||||
| PET Planned | 62 (100) | 84 (79) | 0.0004 | ||
| 4D CT Planned | 62 (100) | 37 (35) | <0.0001 | ||
| Tissue heterogeneity corrections | 62 (100) | 26 (24) | <0.0001 | ||
| Radiation Dose | 0.06 | ||||
| Median | 70 Gy | 66 Gy | |||
| Range | 56-74 Gy | 45-76 (Gy) | |||
| ≥ 60 Gy | 61 (98) | 101 (94) | 0.42 | ||
| ≥ 70 Gy | 35 (55) | 41 (38) | 0.04 | ||
| Fraction Size | 0.06 | ||||
| Median (Range) | 2 Gy (2-2.7 Gy/Fx) | 2 Gy (1.5-2.7 Gy/Fx) | |||
| Chemotherapy (Any) | 62 (100) | 107 (100) | 1.0 | ||
| Induction † | 10 (16) | 30 (28) | 0.08 | ||
| Concurrent † | 62 (100) | 107 (100) | 1.0 | ||
| Adjuvant † | 8 (13) | 21 (20) | 0.30 | ||
| Chemotherapy Agent† | <0.0001 | ||||
| Carboplatin and Paclitaxel | 48 (77) | 70 (65) | |||
| Carboplatin/Cisplatin and Etoposide | 1 (2) | 5 (4.6) | |||
| Carboplatin and Pemetrexed | 9 (14) | 0 (0) | |||
| Carboplatin and Gemcitabine | 0 (0) | 14 (13) | |||
| Other/Unknown | 4 (6) | 18 (17) | |||
Percentages exclude patients not receiving chemotherapy.
Abbreviations: N=Number, %=Percent, Fx=Fraction, ECOG=Eastern Cooperative Oncology Group
Treatment
Table 1 describes treatment and planning characteristics across cohorts. Among the entire group, median EBRT dose was 66 Gy (45-76 Gy) and fraction size was 2 Gy/fraction (1.5-2.7 Gy/fraction). Radiation dose with and without IGRT was statistically different (median 70 Gy and 66 Gy respectively, p=<0.0001). In the IGRT cohort 99% received ≥60 Gy compared to 91% in the non-IGRT cohort and 61% and 31% received ≥70 Gy in the respective cohorts. All patients were treated with an intention to give ≥60 Gy with a lower amount reflecting early treatment discontinuation. PET/CT was incorporated into treatment planning more often in the IGRT cohort (100% vs. 79%, p<0.0001).
Chemotherapy was similar with respect to use and schedule across cohorts with the exception of induction chemotherapy which was more common in the non-IGRT cohort (10% vs. 28% of patients receiving chemotherapy, p=0.08). Carboplatin and paclitaxel accounted for 70% of patients receiving chemotherapy.
LFFS, RFFS, LRFFS, PFS, and OS
After a median follow-up of 56 months (median: 48, range: 8.6-68.6 months in the IGRT cohort; median: 96, range: 44.5-146.3 months in the non-IGRT cohort), 52 local failures were observed (10 in the IGRT cohort). After accounting for the differing lengths of follow-up, the absolute difference in actuarial LFFS was 16% (80% vs. 64%, p=0.013) favoring the IGRT cohort. IGRT similarly improved both the 2-year rate of RFFS (84% vs. 78%, p=0.21) and of LRFFS (75% vs. 62%, p=0.04). Kaplan Meier estimates for LFFS and LRFFS are depicted in Figure 1. The median OS and PFS for the entire population was 22 months (95% CI 17-24 months) and 19 months (95% CI 11-19 months) and was similar between cohorts (2-year OS 47% vs. 49%, p=0.63) (2 year PFS 43% vs. 45%, p=0.12) (Figure 2). No differences were noted in the 2-year DFFS rate (58% vs. 59%, p=0.63). PFS, RFFS, DFFS, and OS plots are illustrated in Figure 2. Clinical endpoints comparing IGRT and non-IGRT cohorts are illustrated in Table 2.
Figure 1.
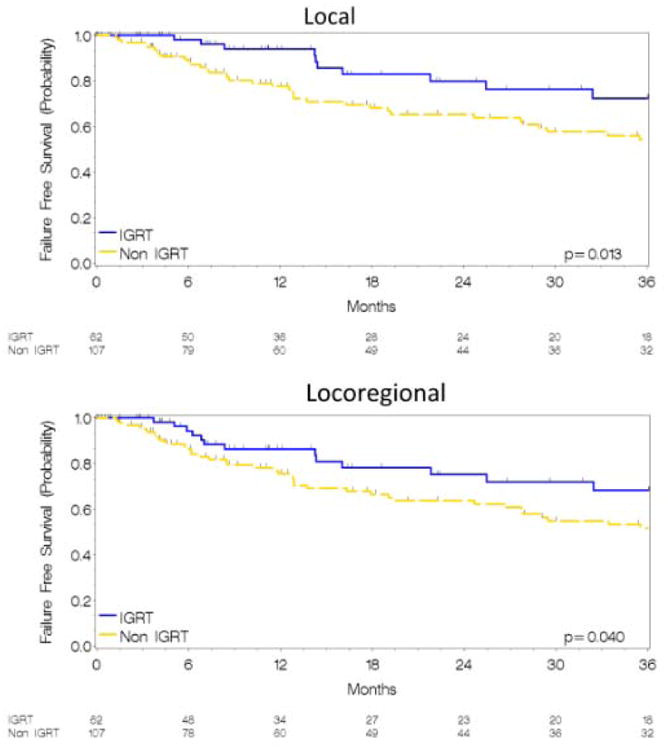
Kaplan Meier estimates for LFFS (Figure 1A) and LRFS (Figure 1B) in IGRT and non-IGRT cohorts.
Figure 2.
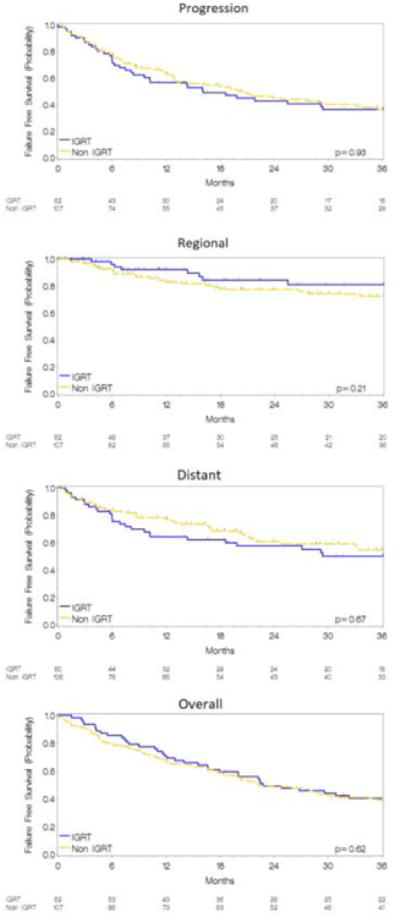
Kaplan Meier estimates for FFS from any progression (Figure 2A), regional (Figure 2B), distant (Figure 2C), and overall survival (Figure 2D) for IGRT and non-IGRT cohorts.
Table 2.
Clinical Outcomes and Patterns of Failure.
| Two Year Failure Free Survival | Crude Patterns of Failure | |||||
|---|---|---|---|---|---|---|
| IGRT | Non-IGRT | IGRT | Non-IGRT | |||
| N=62 | N=107 | N=62 | N=107 | |||
| (%) | (%) | P-value | N (%) | N (%) | P-value | |
| Any Failure | 43 | 45 | 0.12 | 35 (56) | 67 (63) | 0.24 |
| Local | 80 | 64 | 0.013 | 10 (16) | 42 (39) | 0.002 |
| Regional | 84 | 78 | 0.21 | 8 (13) | 24 (22) | 0.13 |
| Locoregional* | 75 | 62 | 0.04 | 13 (21) | 45 (42)† | 0.005 |
| Distant | 58 | 59 | 0.67 | 28 (46) | 49 (46)† | 0.99 |
| Overall Survival | 47 | 49 | 0.63 | |||
In patterns of failure, locoregional reflects simultaneous sites including local, regional, and/or distant.
Distant disease was a component of failure in 33 of the 58 patients with locoregional failure. Abbreviations: N=Number, %=Percent
Univariate and Multivariate Analyses: LFFS and LRFFS
In the UVA, neither stage grouping nor T stage increased the hazard for local failure. Similarly, neither radiation dose (HR 1.0, p=0.39), Positron emission tomography (PET) based planning (HR 0.80, p=0.55), nor 4D-CT planning (HR 0.89, p=0.68) significantly impacted LFFS. Treatment date influenced LFFS suggesting improvement with more recent treatment, an expected result with the IGRT cohort comprising the final 4 years of treatment (HR 0.89, p=0.01). The use of IGRT resulted in a 52% reduction in the hazard for LF (HR 0.48, p=0.02).
The impact of IGRT on LFFS remained significant in the MVA after adjusting for age, treatment date, stage, dose, and use of concurrent chemotherapy with an adjusted hazard ratio (aHR) of 0..40 (p=0.01). Other variables in the model failed to show significance (Table 3).
Table 3. Univariate and Multivariate Analysis for Local Failure Free Survival.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P-value | aHR | (95% CI) | P-value | |
| Age (year) | 1.00 | (0.98-1.03) | 0.77 | 1.00 | (0.98-1.04) | 0.50 |
| Treatment Date (2001-2012) | 0.89 | (0.81-0.97) | 0.01 | |||
| ECOG | 1.06 | (0.69-1.62) | 0.80 | |||
| Group Stage (IIIB vs. IIB/IIIA) | 1.25 | (0.76-2.05) | 0.37 | 1.35 | (0.81-2.24) | 0.25 |
| T Stage | 1.01 | (0.80-1.28) | 0.92 | |||
| N Stage | 1.28 | (0.91-1.80) | 0.16 | |||
| Histology | 0.96 | (0.76-1.19) | 0.68 | |||
| Radiation Dose (Gy) | 1.00 | (1.00-1.00) | 0.39 | 1.00 | (1.00-1.00) | 0.27 |
| Fraction Size (Gy/fx) | 0.98 | (0.97-1.01) | 0.29 | |||
| IGRT | 0.48 | (0.21-0.85) | 0.02 | 0.40 | (0.20-0.81) | 0.01 |
| PET Planned | 0.80 | (0.38-1.66) | 0.55 | |||
| 4D CT Planned | 0.89 | (0.51-1.54) | 0.68 | |||
| Chemotherapy | ||||||
| Induction* | 1.49 | (0.83-2.67) | ||||
| Adjuvant* | 0.53 | (0.24-1.18) | ||||
Patients receiving induction, concurrent, and adjuvant include patients receiving chemotherapy at more than one time point. Abbreviations: HR=Hazard Ratio, fx=Fraction, aHR=Adjusted Hazard Ratio, ECOG=Eastern Cooperative Oncology Group
Patterns of Failure
Failures were noted in 112 patients, crude failure rates of 56% vs. 63% for IGRT vs. non-IGRT cohorts. Significant differences were noted between first sites of failure. Fewer local first failures occurred in the IGRT cohort compared to the non-IGRT cohort accounting for 9.7% and 22.4% of first failures respectively. More patients in the IGRT group progressed with distant disease as their first site of failure relative to the non-IGRT cohort (37.1% vs. 20.6%, p=0.002). This difference in distant disease was offset with more multiple sites of first failure in the non IGRT cohort (3.2% vs 17.8%, p=0.001). (Table 2)
Toxicity
Toxicities are described in Table 4. Any toxicity did not significantly differ between the IGRT and non-IGRT cohort 92% vs. 89%. Acute toxicity analysis showed the IGRT cohort had fewer toxicities when limited to grade ≥ 3 (10% vs. 24%, p=0.02). Any grade esophagitis was the most common acute toxicity experienced in 79% in the IGRT and 75% in the non-IGRT cohort. Only two patients suffered acute grade 5 toxicity from hypoxic respiratory failure and both were in the non-IGRT group. For late toxicities, any late and late Grade 2 toxicities were less common in the IGRT cohort compared to the non-IGRT cohort.
Table 4. Toxicity.
| IGRT Cohort | Non IGRT Cohort | ||
|---|---|---|---|
| N=62 | N=107 | ||
| N (%) | N (%) | P-value | |
| Any toxicity | 0.64 | ||
| No | 5 (8) | 11 (10) | |
| Yes | 57 (92) | 96 (90) | |
| Acute | 0.74 | ||
| No | 6 (10) | 12 (11) | |
| Yes | 56 (91) | 95 (89) | |
| Any Acute Grade 2 | 0.57 | ||
| No | 14 (24) | 30 (28) | |
| Yes | 48 (76) | 77 (72) | |
| Any Acute Grade 3 | 0.02 | ||
| No | 56 (90) | 81 (6) | |
| Yes | 6 (10) | 26 (24) | |
| Late | 0.02 | ||
| No | 41 (65) | 88 (82) | |
| Yes | 21 (35) | 19 (18) | |
| Any Late Grade 2 | 0.08 | ||
| No | 46 (75) | 91 (85) | |
| Yes | 16 (25) | 16 (15) | |
| Any Late Grade 3 | 0.30 | ||
| No | 58 (94) | 95 (89) | |
| Yes | 4 (6) | 12 (11) |
Abbreviations: N=Number, %=Percent.
Discussion
Our analysis demonstrates a substantial local control benefit for patients treated with IGRT using daily kV CBCT compared to weekly MV portal imaging. The absolute benefit in the 2-year LFFS with IGRT was 16%. Despite the limitations of a retrospective analysis, the demonstrated improvement with the use of daily IGRT in local control represents a substantial advance in treatment for locally advanced disease. In light of the recent advances in radiation therapy including PET/CT staging and 4D-CT, use of daily IGRT was the only factor in our analysis that improved local and locoregional control. These results may not be generalizable to other forms of IGRT, including room mounted MV systems or video or surface based IGRT.
Group stage, specifically T stage, has been shown to impact rates of locoregional failure and survival;11 however, our analysis failed to show group or T stage impacted local failure (HR 29, p=0.37 and 1.01, p=0.92, respectively). Local failure was experienced in 14 of 58 (24%) T4 tumors compared to 38 of 111 (34%) remaining tumors (HR for LFFS 0.81, p=0.51) illustrating T4 tumors did not influence the impact of IGRT on LFFS.
In this study, dose did not impact LFFS (HR 1.00, p=0.39). For patients treated above 60 Gy, 51 of 162 (31%) failed locally compared to 1 of 7 (14%) less than 60 Gy (p=0.29). Subsequent estimates of 2 yr LFFS were not different (39% vs. 85% for ≥ 60 Gy and <60 Gy respectively, p=0.29). Although median dose with and without IGRT was statistically different (70 Gy and 66 Gy, p=<0.0001), it did not account for IGRT's effect as demonstrated by both the clinical data and subset analysis.
In terms of chemotherapy, randomized data from large cooperative group trials have demonstrated a survival benefit of 5% with the use of concurrent chemoradiation as compared to sequential treatment.12 A meta-analysis illustrated that improved locoregional control led to improved survival.13 We only included patients treated with concurrent chemotherapy in order to make our results more applicable to current practice strategies.
The main limitation to our analysis is the span of time over which treatment was delivered, which reflects a bias towards recent technical advancements in the IGRT cohort other than IGRT itself. We acknowledge that fully examining whether IGRT was solely responsible for improved LFFS or a surrogate for all improvements in treatment technique over the 11 year span is difficult. There were several additional technical advancements that occurred contemporaneously to the implementation of IGRT. These include ENI, tissue heterogeneity corrections in dose calculations, and PET/CT planning. ENI was used in a portion of patients in the non-IGRT cohort, but omission of ENI has failed to show increased failure rates.14,15 While ENI contributes substantially to the toxicity analysis, no data suggests local control is diminished with ENI and would not account for difference in LFFS. 14,15 While PET/CT planning was employed more commonly in the IGRT cohort comparatively (100% vs. 79%, p<0.0001) and has been shown to create smaller tumor derived volumes and change nodal GTV contours, the effect on patterns of failure is unclear.16 In our analysis 70% vs. 63% failed locally in the PET/CT vs. non-PET/CT group with corresponding 2-year LFFS estimates of 73% vs. 58% (p=0.55) with a non-significant impact on the hazard for local failure on UVA (HR 0.80, p=0.55) suggesting the omission of PET/CT did not impact the hazard for LF.
Similar to LFFS, IGRT patients saw a 5% improvement in 2 year LRFFS (75% vs. 62%, p=0.04). This benefit was not significant in the multivariate model. No benefit was noted in PFS or OS. These outcomes are not unexpected as distant relapse remains a significant issue and was not affected by use of IGRT. As demonstrated by the meta-analysis, local control translates to OS; thus local control is a relevant outcome as symptoms related to recurrent primary tumor can be problematic and improved control may translate into improved OS.13 Longer follow up and a larger sample size would be required to appropriately evaluate OS.
The incidence of any toxicity and Grade ≥3 acute toxicities were less common in the IGRT cohort and likely reflect the smaller treatment margins and omission of ENI. Although we acknowledge an inability to account for other differences in technique, our analysis demonstrates the benefits of modern treatment techniques with a contribution from IGRT. How much IGRT with CBCT contributed to the improvements is difficult to evaluate, but our analysis did not show inferiority of IGRT in terms of toxicity.
Despite the differences among cohorts, IGRT remained the only significant factor contributing to improved LFFS on MVA, which supports the hypothesis that IGRT improves targeting and localization of the tumor. Prior to IGRT, therapy was limited in its ability to respond to patient and tumor changes during and in between treatments, which likely contributed to suboptimal treatment, resulting in local failure. To our knowledge, this is the first study to show substantial statistical benefit with cone-beam CT based-IGRT among lung cancer patients.
Aside from visualizing the target prior to treatment, IGRT in NSCLC has other potential benefits that are currently being investigated. IGRT can be employed to track tumor volumes throughout treatment, which can have implications for overall survival.17,18 Adaptive radiotherapy, which utilizes IGRT to evaluate throughout therapy with the potential to shrink field sizes, is dependent on IGRT and is aimed towards improving the therapeutic ratio for these patients. These strategies depend on the principle that proper identification of the target improves local control, which is supported by this study.
While modern prospective studies have varied in their incorporation of IGRT (RTOG 0617 did not require IGRT, while CALGB 31102 and RTOG 1106 did), our results indicate that incorporation of these technologies may improve outcomes and should be studied in the prospective setting.
Conclusion
Our analysis demonstrated a substantial 16% improvement in the 2 year LFFS among patients treated with IGRT using daily CBCT relative to those treated with weekly MV portal imaging. After controlling for patient and treatment variables, the impact of IGRT remained significant. The inclusion of IGRT in future trials should be considered carefully. Results of current trials such as CALGB 31102 and RTOG 1106 requiring IGRT will assist in further defining the role of IGRT in locally advanced lung cancer.
Supplementary Material
Appendix 1. Treatment Planning Parameters and Patient Selection
Patient Selection for Study
Locally advanced lung cancer patients treated with and without IGRT from 2001-2012 with concurrent chemoradiation for locally advanced NSCLC were included in the study. A database was created using clinical records for patients treated only at our institution.
Patient Selection for Therapy
Patients were discussed in multidisciplinary tumor boards with surgical oncology, medical oncology, and radiation oncology. The patients were treated with concurrent chemoradiation.
Simulation Process and Target Delineation
Patients were simulated using CT simulation for treatment planning purposes. Normal structures were contoured following the simulation process. Starting in 2007, 4D-CT scans were completed on all patients to define the internal target volume (ITV). For 4D-CT, the envelope of gross tumor motion was delineated by using maximal intensity projection of the 4D-CT and modifying contours by visual verification of the coverage on each phase of the 4D-CT. Prior to 4D-CT, the gross tumor volume (GTV) was defined on free-breathing CT with a uniform 1cm expansion to generate the planning target volume (PTV). Once the ITV technique was used, the PTV expansion was 5 mm. No clinical target volume (CTV) expansions were applied.
Treatment Planning
Patients in both cohorts were treated with either three-dimensional conformal radiotherapy (3DCRT) or intensity-modulated radiotherapy (IMRT). A prescription goal of ≥ 95% of the treatment dose was prescribed volumetrically to the PTV. Patients were treated with 4 to 6 beams with 6 or 10 MV energy using 3D conformal principles. Heterogeneous dose calculations were applied to patients after June of 2006. Elective nodal irradiation (ENI) was used sparingly and not systematically in the non-IGRT cohort. The number of patients treated with ENI could not be accurately quantified given the infrequency of use. No ENI occurred during the IGRT era. PET/CT imaging for target delineation was used by image registration and fusion to the planning CT scan when available. Physics performed quality assurance checks for each plan created prior to delivery of radiation therapy.
Treatment Delivery
Patients were treated once daily for a duration of 4-6 weeks. IGRT using daily kV CBCT was systematically applied to all lung cancer patients beginning in February 2009. IGRT was implemented with matching between planning CT and daily CBCT based on soft tissue and bony/anatomical landmarks. Prior to IGRT, patients were imaged with weekly MV portal images.
Follow up
In general, patients were evaluated clinically and underwent chest CT at 6-8 weeks following treatment, every 3-4 months for the first 2 years, 6 month intervals from years 3 to 5 and then annually thereafter. Radiographic and clinical information was reviewed to score the initial and subsequent failures as local, regional, or distant. Radiographic response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1.
Treatment Failures
Each local and regional failure was reviewed by the study coordinator and principal investigator. PET/CT or biopsy was used to differentiate radiation related lung changes with recurrence. Date of treatment failure was scored as either the date of the initial scan documenting growth or increased hypermetabolic activity or the date of pathologic evaluation when available.
Toxicity
Acute and late toxicity was graded retrospectively by reviewing clinic notes and records from hospitalizations according to Common Terminology Criteria for Adverse Effects version 4.0.
Footnotes
Conflicts of Interest Notification: There are no conflicts of interests for any of the authors for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korreman SS. Image-guided radiotherapy and motion management in lung cancer. The British journal of radiology. 2015;88(1051):20150100. doi: 10.1259/bjr.20150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupelian PA, Langen KM, Willoughby TR, Zeidan OA, Meeks SL. Image-guided radiotherapy for localized prostate cancer: treating a moving target. Seminars in radiation oncology. 2008;18(1):58–66. doi: 10.1016/j.semradonc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Den RB, Doemer A, Kubicek G, et al. Daily image guidance with cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. International journal of radiation oncology, biology, physics. 2010;76(5):1353–1359. doi: 10.1016/j.ijrobp.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 4.Sonke JJ, Rossi M, Wolthaus J, van Herk M, Damen E, Belderbos J. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. International journal of radiation oncology, biology, physics. 2009;74(2):567–574. doi: 10.1016/j.ijrobp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Guckenberger M, Wilbert J, Krieger T, et al. Four-dimensional treatment planning for stereotactic body radiotherapy. International journal of radiation oncology, biology, physics. 2007;69(1):276–285. doi: 10.1016/j.ijrobp.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 6.Shumway D, Corbin K, Salgia R, et al. Pathologic response rates following definitive dose image-guided chemoradiotherapy and resection for locally advanced non-small cell lung cancer. Lung cancer. 2011;74(3):446–450. doi: 10.1016/j.lungcan.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. International journal of radiation oncology, biology, physics. 2002;53(5):1337–1349. doi: 10.1016/s0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- 8.Jaffray DA, Wong JW. Exploring “target of the day” strategies for a medical linear accelerator with cone-beam-CT scanning capabilities. Paper presented at: XII International Conference on the Use of Computers in Radiation Therapy. 1997:1997. [Google Scholar]
- 9.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3(2):177–186. doi: 10.1097/JTO.0b013e3181622bdd. [DOI] [PubMed] [Google Scholar]
- 10.Silvano G. New radiation techniques for treatment of locally advanced non-small cell lung cancer (NSCLC) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2006;17(2):ii34–35. doi: 10.1093/annonc/mdj918. [DOI] [PubMed] [Google Scholar]
- 11.Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Therapeutic advances in medical oncology. 2011;3(3):127–138. doi: 10.1177/1758834011401951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. Journal of the National Cancer Institute. 2011;103(19):1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 14.Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. American journal of clinical oncology. 2007;30(3):239–244. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 15.Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(35):5557–5561. doi: 10.1200/JCO.2007.13.2191. [DOI] [PubMed] [Google Scholar]
- 16.Greco C, Rosenzweig K, Cascini GL, Tamburrini O. Current status of PET/CT for tumour volume definition in radiotherapy treatment planning for non-small cell lung cancer (NSCLC) Lung cancer. 2007;57(2):125–134. doi: 10.1016/j.lungcan.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Jabbour SK, Kim S, Haider SA, et al. Reduction in Tumor Volume by Cone Beam Computed Tomography Predicts Overall Survival in Non-Small Cell Lung Cancer Treated With Chemoradiation Therapy. International journal of radiation oncology, biology, physics. 2015;92(3):627–633. doi: 10.1016/j.ijrobp.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissonnette JP, Purdie TG, Higgins JA, Li W, Bezjak A. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. International journal of radiation oncology, biology, physics. 2009;73(3):927–934. doi: 10.1016/j.ijrobp.2008.08.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


