Abstract
Antibiotics are by far the most common medications prescribed for children. Recent epidemiological data suggests an association between early antibiotic use and disease phenotypes in adulthood. Antibiotic use during infancy induces imbalances in gut microbiota, called dysbiosis. The gut microbiome’s responses to antibiotics and its potential link to disease development are especially complex to study in the changing infant gut. Here, we synthesize current knowledge linking antibiotics, dysbiosis, and disease and propose a framework for studying antibiotic-related dysbiosis in children. We recommend future studies into the microbiome-mediated effects of antibiotics focused on four types of dysbiosis: loss of keystone taxa, loss of diversity, shifts in metabolic capacity, and blooms of pathogens. Establishment of a large and diverse baseline cohort to define healthy infant microbiome development is essential to advancing diagnosis, interpretation, and eventual treatment of pediatric dysbiosis. This approach will also help provide evidence-based recommendations for antibiotic usage in infancy.
Introduction
Antibiotics are by far the most common prescription drugs given to children (Chai et al., 2012). Epidemiological studies have identified associations between antibiotic usage in early infancy and occurrence of diseases such as obesity, diabetes, and asthma in later life. Longitudinal studies of antibiotic usage have demonstrated profound short- and long-term effects of antibiotics on the diversity and composition of the gut microbiota. Finally, a large and growing number of studies implicate a causal role for microbiome imbalance (dysbiosis) in numerous diseases (Biedermann and Rogler, 2015). Understanding the short- and long-term effects of early life antibiotic use on the diversity and composition of the gut microbiota is critical in identifying the risks associated with the emerging prescription trends. However, the existing literature is limited in directly implicating microbial dysbiosis as the link between childhood antibiotics and development of disease in later life.
In this review, we synthesize numerous complementary sources, including microecological studies linking antibiotics and dysbiosis, mechanistic studies linking specific types of dysbiosis to specific disease outcomes, and reviews of epidemiological studies supporting antibiotics and increased disease risk. By this approach, we have identified four major types of antibiotics-related dysbiosis, and we have presented a framework for discussing and measuring pediatric dysbiosis in the context of several major diseases. Our analyses indicate substantial existing evidence for a number of causal mechanisms by which the microbiome mediates antibiotic-related disease risk.
Overuse of Antibiotics
The vast majority of antibiotic use occurs in the outpatient setting, where up to a third of prescriptions are unnecessary. In 2010, children received 74.5 million outpatient antibiotic prescriptions—one for every child in the US—accounting for one fourth of all medications for children (Hicks et al., 2013). Numerous studies have demonstrated that antibiotics are often prescribed unnecessarily (Gonzales et al., 2001; McCaig et al., 2003; Nash et al., 2002), with estimates as high as 50% (Kronman et al., 2014). Nearly 30% of children receive an antibiotic prescription during an outpatient primary care visit (McCaig et al., 2003), most often inappropriately, for viral upper respiratory tract infections (Gonzales et al., 2001; Nash et al., 2002; Nyquist et al., 1998). Overuse of broad-spectrum antibiotics for conditions responsive to narrow-spectrum agents has been dramatically increasing (Hersh et al., 2013). Even after adjusting for differences in patient age, comorbidities, and sociodemographic factors, children with the same infections can receive vastly different rates of antibiotic prescriptions depending upon the practice or clinician visited (Fierro et al., 2014; Gerber et al., 2014). This phenomenon also seems to be universal: per capita antibiotic prescribing rates vary widely across US states (Hicks et al., 2013) and European countries (Goossens et al., 2005) without reasonable cause for geographic differences in bacterial infection rates.
In addition to the gut-microbiome-mediated effects as discussed in detail below, inappropriate prescribing of antibiotics can lead to both drug-related adverse effects and the promotion of antibiotic resistance. More than 140,000 emergency department (ED) visits occur annually in the US for antimicrobial-related adverse effects, comprising almost 20% of all ED visits for drug-related adverse effects (Shehab et al., 2008). In addition to this direct patient harm, antibiotic use has been associated with the emergence of antimicrobial resistance, identified by the World Health Organization (WHO) as “one of the three greatest threats to human health.” Importantly, a recent study found that the prevalence of antibiotic resistance genes in the infant gut microbiome increases with age, and infants born via C-section harbored a larger proportion of antibiotic resistance genes (Bäckhed et al., 2015). Infections with resistant bacteria increase morbidity and mortality, and greatly increase the cost of medical care; the Institute of Medicine estimated that, in 2010, roughly $20 billion was spent on the treatment of antibiotic-resistant infections. Knowledge of these facts, however, has done little to curb antimicrobial use. Improving our awareness of the long-term implications of both necessary and unnecessary antibiotic exposure is important to better inform the risk/benefit ratio for antibiotic prescribing and to improve child health.
Normal Host-Microbiome Development
Gastrointestinal Development
Gastrointestinal (GI) development occurs throughout embryonic life, and its basic structure is first formed by the end of the first gestational trimester (Montgomery et al., 1999). Tight junctions are present by 10 weeks of gestation, and intestinal villi are formed by weeks 12–19 (Maheshwari and Zemlin, 2009; Montgomery et al., 1999). Postnatally, an abrupt shift in exposure from amniotic fluid to first foods occurs in the GI tract. This induces many changes along the GI tract, including a change in pH of the stomach. For example, some reports state the pH of the stomach is initially in the range of 6 to 8 (Avery et al., 1966), likely due to buffering by the amniotic fluid, which decreases to that of an adult (pH 1.5–2.5) within the first hours following birth (Lebenthal and Lebenthal, 1999; Ménard, 2004). However, due to the consumption of milk, and its buffering capabilities, the pH of the infant stomach often returns to a high level of 7–7.6 (Hibberd et al., 1982). The higher pH of the stomach early in life has a meaningful impact, including a higher absorption rate of nutrients and a diminished digestive capacity compared to later in life, which may support transit of ingested bacteria to colonize the lower GI tract. Throughout postnatal development, the infant GI tract also increases in size in both longitude and in diameter and loses most of its early-stage porosity within days post-birth due to milk-borne growth factors and hormones that stimulate growth and development (Cummins and Thompson, 2002).
Development of the GI-associated lymphoid tissue (GALT), including mesenteric lymph nodes, Peyer’s patches, and lymphocytes in the lamina propria is complete in full-term infants at birth (Forchielli and Walker, 2005). For example, goblet cells, responsible for mucin production, are functional by 12 weeks of gestation (Montgomery et al., 1999), as are paneth cells, which can secrete defensins and lysozymes by gestational weeks 13 and 20, respectively (Louis and Lin, 2009; Maheshwari and Zemlin, 2009; Rumbo and Schiffrin, 2005). Although full-term infants are born with fully developed digestive tracts, exogenous stimulation through exposure to dietary antigens, hormones, growth factors, and bacteria is required to elicit proper function throughout life (Forchielli and Walker, 2005).
Microbiome Development
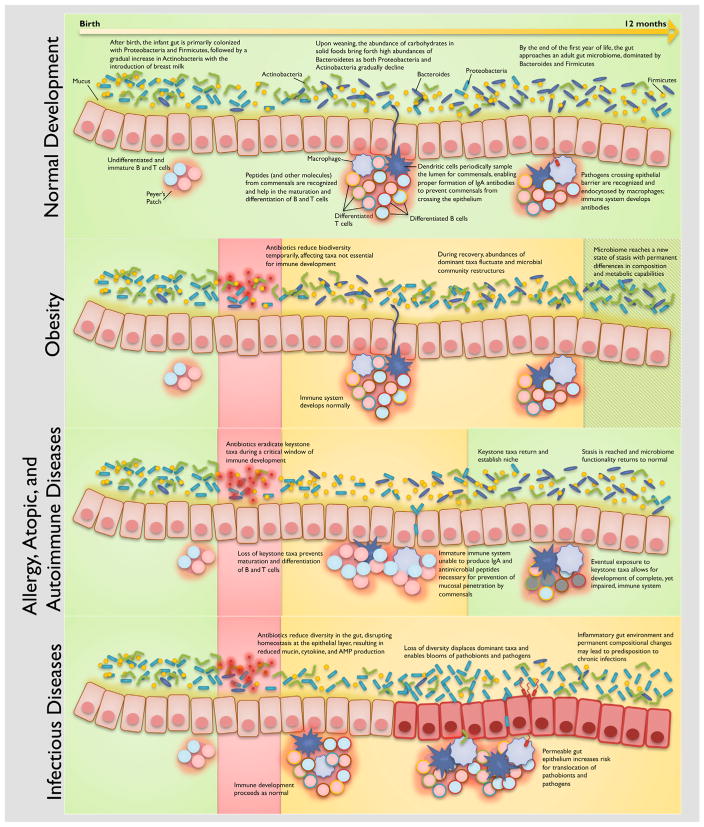
Although the GI tract of a healthy infant is generally considered to be sterile before birth, recent work suggests that initial colonization may take place in-utero (Aagaard et al., 2014; Funkhouser and Bordenstein, 2013; Matamoros et al., 2013). Hours after birth, microorganisms from the mother’s vaginal, fecal, and/or skin microbiome and the environment are important colonizers of the infant gut (Penders et al., 2006), with actual contributions depending on mode of delivery. Several other factors including prematurity, infant diet (breast milk or formula), hygiene, and use of antibiotics will ultimately impact the composition of the infant gut microbiome. Despite a seemingly chaotic colonization with large swings in composition over time, gut microbiome development is governed by Darwinian dynamics: microbes best adapted for the changing conditions of the gut will be most likely to survive. We can see this clearly throughout the first few weeks of life, as the colonization of facultative aerobes reduces the availability of oxygen, which then permit the growth of strict anaerobes (Bezirtzoglou, 1997). As illustrated in Figure 1, we can also see compositional changes in response to diet and host development throughout the first year of life. In the United States, the infant gut is initially colonized with Proteobacteria and Firmicutes, followed by a gradual increase in Actinobacteria (potentially due to the introduction of breast milk) (Sela et al., 2008). By 6 months of age, Bacteroidetes dominate while Proteobacteria and Actinobacteria gradually decline, which may be attributed to the abundance of carbohydrates in solid foods that coincides with weaning (Koenig et al., 2011; Vaishampayan et al., 2010). By the end of the first year of life, the infant gut is dominated by bacteria from the phyla Bacteroides and Firmicutes (Figure 1). The healthy infant gut continues with dramatic compositional changes throughout the first 2 years of life before becoming indistinguishable from an adult gut microbiome at age three (Yatsunenko et al., 2012).
Figure 1. Framework for Host-Microbiome Development in Health, Dysbiosis, and Disease.
Disease classes are associated with cascading dysbiosis types, with important dependencies on the course of host-microbiome development. Note that disease classes and dysbiosis types are not necessarily mutually exclusive. The proposed mechanisms presented are supported by extensive evidence in the literature, both from mechanistic studies and from epidemiological surveys. Due to the very large number of references, the citations represented in this figure can be found in Table 1.
Important Host-Microbiome Interactions
Maturation of the intestinal immune system is contingent on parallel development of the gut microbiome (Figure 1); germ-free animals have been found with significant immunological defects in the GALT (Macpherson and Harris, 2004) as well as improper development of Peyer’s patches and mesenteric lymph nodes (Round and Mazmanian, 2009). Peyer’s patches and the mesenteric lymph nodes develop prenatally, and isolated lymphoid follicles develop postnatally, but all of these tissues require interaction with key members of the gut microbiome in order to ensure proper differentiation and specification and complete development of adaptive immunity (Cherrier and Eberl, 2012; Maynard et al., 2012). The immune system must maintain an anti-inflammatory state (Tsuji and Kosaka, 2008) in the gut, especially during exposure to the considerable number of innocuous antigens from commensals, hormones, and food.
The interactions of diverse cell types are necessary to carry out the complex functions of the immune system (Adkins et al., 2004); we highlight several immune cell types with important dependencies on the gut microbiome. Dendritic cells (DCs), one of the most important types of antigen-presenting cells, sample the lumen and are responsible for orchestrating inflammatory or tolerogenic responses. To help the immune system carry out appropriate responses, DCs can suppress or induce the activation of antigen-specific T cells and have the unique ability to differentiate naive T cells into effector or regulatory T cells to target specific antigens (Lanzavecchia and Sallusto, 2001; Macatonia et al., 1995). T helper cells are critical in processing presented antigens into specific cytokines that provide direction for other immune cells and to eventually generate an immunological response. Members of the gut microbiome have been found to differentiate Th17 cells, a class of T helper cells, which secrete IL-17 to produce defensins (Kao et al., 2004) and recruit neutrophils (Aujla et al., 2007) to fight infections at mucosal surfaces (Atarashi et al., 2008; Ivanov et al., 2009). Pro-inflammatory Th17 cells must maintain balance with anti-inflammatory regulatory T cells, particularly for the prevention of autoimmune disorders. Certain Clostridia strains have been found to help with expansion and differentiation of regulatory T cells (Atarashi et al., 2013) and have a direct role in reducing intestinal epithelial permeability by stimulating innate lymphoid cell and T cell production of cytokine IL-22 (Stefka et al., 2014). Innate lymphoid cells help induce pro-inflammatory responses and serve as the main source of IL-22 (Sawa et al., 2010); this cytokine is important for inducing mucus production from goblet cells, stimulating the production of antibacterial proteins, protecting cells from damage, and regulating cell differentiation (Sabat et al., 2014). A number of studies found that microbial signals modulate the amount of IL-22 produced by innate lymphoid cells (Sanos et al., 2009; Sawa et al., 2010; Sonnenberg et al., 2012; Stefka et al., 2014), suggesting the importance of the gut microbiome in host defense mechanisms against infectious and inflammatory diseases (Rutz et al., 2013). Furthermore, Bifidobacterium longum has been found to assist in the maturation of DCs in Peyer’s patches and the development of T cells in the thymus (Dong et al., 2010), and specific microbial signals have been deemed necessary for proper education of regulatory T cells and invariant natural killer T (iNKT) cells (Hansen et al., 2012; Olszak et al., 2012), which are a subset of T cells capable of quickly inducing an abundance of cytokines that can stimulate or suppress a variety of immune responses. Additional important microbe-host interactions and mechanisms will be presented later in the context of our proposed framework. Considering how critical the various immune cells and their intricate signaling networks are for supporting immune health, disruptions hindering their development may have lasting deleterious effects.
Other Major Influences on Microbiome Development
Diet plays a large role in the colonization of the modern infant GI tract due to the vast compositional differences between human milk and infant formula. The most notable difference in the microbiome of breastfed versus formula fed infants is the predominance of Bifidobacteria and Lactobacilli in breastfed infants, while formula-fed infants harbor more Enterococci and Enterobacteria (Palmer et al., 2007). There are also easily detected differences in total community membership between breastfed and formula-fed infants when looking at twin cohorts (Yatsunenko et al., 2012). Human milk is able to modulate bacterial colonization in the infant gut with distinct components not found in formulas: the human milk microbiome, factors that stimulate bacterial growth (prebiotics), and factors that prevent bacterial growth (antimicrobials). The human milk microbiome consists primarily of Proteobacteria and Firmicutes (Landau, 1974) and has of a core group of taxa found in most human milk samples that include Staphylococcus, Streptococcus, Serratia, Pseudomonas, Corynebacterium, Ralstonia, Propioni-bacterium, Sphingomonas, and Bradyrhizobiaceae (Hunt et al., 2011). The human milk microbiome also changes over time, and is dependent on the mother’s weight (Cabrera-Rubio et al., 2012). For example, Weissella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus are predominant in milk immediately after giving birth, and milk from obese mothers is less diverse than that of non-obese mothers (Cabrera-Rubio et al., 2012). These ingested bacteria provide a constant source of community members to help colonize the GI tract. Milk-borne prebiotics that modulate the bacteria present in the GI tract include human milk oligosaccharides (HMOs), which are sugars produced solely for consumption by microbes. These include the “original” HMO, bifidus factor (Landau, 1974), which stimulates Bifidobacterium bifidum and hundreds of other sugars (all within a family of unconjugated glycans containing lactose at the reducing end) that primarily promote the growth of Bifidobacterium longum subsp. infantis (Bode, 2012). Antimicrobials in human milk that also influence the microbes within the GI tract include secretory immunoglobulin A (SIgA), which provides antigen-specific protection against microbes that the mother has already encountered (Rogier et al., 2014), and innate immune proteins, such as lactoferrin and lysozyme, that harbor bactericidal activity (Arnold et al., 1980). Milk obtained from mothers of preterm infants had highest concentrations of cytokines and immunoglobulins immediately after giving birth, further supporting the importance of breast milk consumption in early life (Moles et al., 2015).
Mode of delivery has an impact on the microbiome of infants, as the total microbiome (skin, oral mucosa, nasopharyngeal aspirate, and meconium) of vaginally delivered infants resembles the maternal vaginal and intestinal microbiome, while infants delivered by cesarean section have total microbiomes resembling the maternal skin microbiome (Dominguez-Bello et al., 2010). Specifically, the microbiomes of vaginally delivered infants consist mostly of Lactobacillus, Prevotella, Atopobium, or Sneathia spp, whereas the microbiome of cesarean section delivered infants contain Staphylococcus spp (Dominguez-Bello et al., 2010) and less Bifidobacterium (Biasucci et al., 2010).
Frameworks for Studying Pediatric Dysbiosis and Related Disease
The mechanisms and health consequences of pediatric dysbiosis are complex and multifactorial and are further complicated when also considering infant development (gut microbiome, immune system, and their interactions). Using a systems approach, we consider five interdependent frameworks for understanding dysbiosis that focus on different aspects of the mechanisms that lead to disease. We discuss these conceptual frameworks in terms of their relative merits for clarity, potential for organization, and ability to express multi-factorial disease pathways. We restrict our hypotheses to those that assume long-term health effects of one or more short discrete courses of antibiotics, since that is by far the most common type of antibiotic exposure in human children (Gevers et al., 2014). Each perspective has strengths in its ability to generalize certain aspects of pediatric dysbiosis. In general, we find a combination of the dysbiosis-centric and disease-centric perspectives to be the most useful for discussing disease mechanisms.
A Dysbiosis-Centric View
The gut microbiome is in constant flux; the community composition continuously adapts to environmental exposures and host developmental changes (Caporaso et al., 2011; Human Microbiome Project Consortium, 2012). This adaptability is essential for maintaining gut homeostasis, but drastic changes, such as those induced by antibiotics, can potentially lead to negative health consequences. Pediatric dysbiosis can be characterized by these drastic changes in the microbial community, discussed here as four distinct types. Since broad-spectrum antibiotics are designed to eradicate multiple bacterial taxa, the gut microbiome may be impacted by (1) an unintended loss of keystone taxa that are critical for maintaining homeostasis or proper host development (e.g., immune system) or (2) an overall loss of biodiversity, which can have inherent health risks on its own (e.g., the hygiene hypothesis) and can also lead to other dysbiosis types (Figure 1). Taxa that have been eradicated from their niches leave vacancies to be filled by (3) blooms of pathogens and pathobionts. Even if the infant gut microbiome can recover from these dysbiotic states to arrive at some form of homeostasis, improper or partial recovery can result in a (4) shift in functional capability: for example, becoming more efficient at extracting energy (Figure 1). These dysbiosis types sometimes overlap, further adding to the complexity of the system and the challenge of building a unified conceptual framework for pediatric dysbiosis research. Viewing pediatric dysbiosis from the perspective of different dysbiosis types is particularly important for understanding how small changes to the relatively simple infant gut can manifest as larger repercussions during adulthood. Such a dysbiosis-type framework is crucial for understanding community dynamics within the gut microbiome but is limited in its ability to easily address several factors such as the age of the infant, the overlap and transition between dysbiosis types, the many-to-many relationship between dysbiosis types and disease phenotypes, and the parallel development of the immune system.
A Disease-Centric View
In the context of different aspects of host development and specific taxa affected, the previously described pediatric dysbiosis types can give rise to a variety of health consequences. Deconstruction of the health outcome with a top-down approach is another framework for understanding dysbiosis. In this disease-centered framework, health outcomes are generalized by disease class and then further characterized by specific mechanisms and interactions with subsystems of the framework (host immune system, gut microbiome, host development, etc.) (Figure 1). For example, obesity-related pediatric dysbiosis in the context of this framework begins with antibiotic treatment at any time point during the first 2 years of life. Biodiversity is depleted during treatment but rebounds after treatment ends, inducing large changes in taxonomic composition. In the case of obesity, these compositional changes also result in functional changes affecting metabolism; the microbiome becomes more efficient at extracting energy from multiple sources and hence predisposes the host to obesity (Turnbaugh et al., 2006). Antibiotic exposure at a younger age exacerbates predisposition to disease (Cox et al., 2014), and compounded disturbances may lead to unanticipated consequences (Paine et al., 1998). Other disease classes may include allergies and atopic diseases, auto-immune disorders, diabetes, and infectious disease. This framework encapsulates major interdependencies within each disease class while accounting for temporal factors. The main shortcoming of the disease-centric view of pediatric dysbiosis is that it does not easily allow synthesis of common mechanisms across diseases.
An Age-Centric View
Dysbiosis can resolve with complete recovery and minimal impact to host health or can have drastic unintended consequences depending on the stage of host development. Development of the microbiome and the host immune system can be categorized conveniently, although approximately, into four general stages: (1) 0 to 6 months, (2) 6 to 12 months, (3) 12 to 24 months, and (4) 24 months and older. The infant is most vulnerable to developing immunological defects during Stage 1, when adaptive immunity interaction with keystone taxa is most critical (van der Velden et al., 2001; Prescott et al., 1999; Rautava et al., 2004). By Stage 4, the gut microbiome establishes a new-formed stasis as it reaches maturity, carrying forth any existing functional shifts that could predispose the host to future diseases. Although the vulnerabilities of each stage of development are important considerations for understanding dysbiosis, considering segregated stages hinders characterization of mechanisms that span multiple stages.
A Response-Centric View
The gut microbiome transitions through several stages in response to a course of antibiotics: pre-treatment, during treatment, recovery, and long-term stasis (Figure 2A). Dysbiosis types that emerge during treatment include loss of keystone taxa and short-term metabolic shifts, both of which would be compounded with multiple courses of antibiotics. Immediately after the antibiotic course, the gut microbiome begins to recover, but not without several potential complications. The loss of diversity imposed by antibiotics may allow for blooms of pathogens and pathobionts, the adaptive immune system may be underdeveloped and keystone taxa may still not have recovered (further delaying immune development), and metabolic shifts may begin to take place. Eventually the gut microbiome reaches a form of stasis, which may be different from its pre-treatment stage (Figure 2). At this stage, permanent metabolic shifts may have been established, a loss of biodiversity accompanied by a bloom of pathobionts may persist, and the host may be predisposed to an increased risk of infectious disease. Although the dynamics of the community structure in response to antibiotics are useful for identifying short-term vulnerabilities, mechanisms of dysbiosis typically start during one stage (e.g., treatment stage) and end in another (e.g., recovery stage), making this framework difficult and confusing to work with.
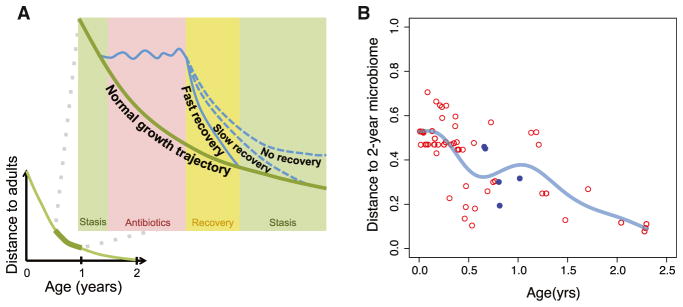
Figure 2. Trajectories for Infant Recovery after Antibiotic Exposure.
(A) Infant gut microbiomes develop rapidly and experience large changes during infancy before becoming indistinguishable from adult microbiomes by age 2. Dysbiosis in infants can displace (no recovery) or delay (slow recovery) development on the normal growth trajectory.
(B) Samples were obtained from a single infant over time (Koenig et al., 2011;; and microbiome distance (Bray-Curtis) to self at 2 years old was plotted over time. Fecal samples collected immediately after antibiotics are denoted in blue. A smoothing spline (in light blue) reveals a noticeable change in trajectory of development after use of antibiotics, mirroring the deviation in trajectory predicted in Figure 2A.
A Recovery-Centric View
Although adult gut microbiomes experience day-to-day changes, they are relatively stable when compared to infant gut microbiomes, which are characterized by large swings in taxonomic composition, especially throughout the first year of life. Regardless of the seemingly random shifts, there exists a clear trajectory of healthy development in the infant gut microbiome when assessing biodiversity and relative abundances of specific taxa (Figure 2). This framework defines dysbiosis in terms of how the microbiome recovers back to this trajectory: fast recovery, slow recovery, or incomplete recovery (Figure 2A). During fast recovery, there may be a short-term loss of diversity but keystone taxa are preserved and the gut microbiome quickly rebounds back to normal with little impact to the host. With a slow recovery, there may be loss of keystone taxa during a critical time for interaction with the immune system, therefore causing a delay in immune development. Biodiversity may be low and it may take some time before keystone taxa can reestablish and interact with the immune system before getting back on the normal trajectory. The host is most vulnerable to infectious disease during this prolonged state of recovery, with both an immature immune system and a low-diversity microbiome. Despite eventually recovering and reestablishing a healthy gut microbiome, the adaptive immune system may have developed antibodies against commensals during the long recovery period, predisposing the host to autoimmune diseases. During an incomplete recovery, the compositional changes are so drastic that the gut microbiome reaches a completely new form of stasis, placing it on a trajectory completely different than expected (Figure 2A). These changes are accompanied by functional and metabolic shifts in the gut microbiome and come with disease risks of their own. The types of recovery in this framework are not mutually exclusive, since it is possible that either a fast or slow recovery rate may lead to an incomplete recovery. This framework also does not address how a recovery type may be dependent on a specific development stage, as considered by the age-centric view.
Current Evidence for Pediatric Dysbiosis-Associated Disease Mechanisms
In considering several alternative lenses through which to discuss and organize pediatric dysbiosis, we have decided to use a combination of the dysbiosis-centric and disease-centric perspectives for summarizing and synthesizing existing knowledge about potential disease mechanisms (Table 1). This combined framework allows us to map multiple causes to the same disease while keeping track of different developmental and treatment stages that underlie the various known or proposed mechanisms. Although the causal pathway between dysbiosis and disease can take many forms, we present four important disease classes in major contributing dysbiosis types.
Table 1.
References Synthesizing Mechanistic and Epidemiological Evidence Linking Antibiotics, Changes in the Gut Microbiome, and Disease
Obesity
Evidence for antibiotics-induced obesity is primarily characterized by shifts in functional capability, or more specifically, long-lasting metabolic shifts that result from incomplete recovery back to the normal trajectory. Recent work found that mice given sub-therapeutic levels of antibiotics after weaning exhibited increased adiposity, large taxonomic changes in their gut microbiomes, and increased levels of short-chain fatty acids (SFCAs) as well as counts of bacterial genes involved in SFCA metabolism (Cho et al., 2012). These mice also had lower caloric output in their fecal pellets despite dietary intake similar to controls, suggesting their gut microbiota developed the ability to extract increased energy from indigestible components (Cho et al., 2012). Furthermore, low-dose antibiotics started even earlier in life (prior to weaning) resulted in mice showing a more pronounced increase of adiposity, and induced adipogenesis synergistically with a high-fat diet; fecal transplantation into germ-free mice lead to increased fat mass relative to transplantation from mice without antibiotics, implicating the gut microbiome in a causal role on the pathway to obesity (Cox et al., 2014). Some epidemiological studies further substantiate the long-lasting effects of early exposures, finding that antibiotic exposures among infants younger than 6 months are significantly associated with increased BMI later on in life, although in general these findings are somewhat mixed and warrant follow-up in a prospective study (Ajslev et al., 2011; Bailey et al., 2014; Trasande et al., 2013). The 0–6 month window is a time of rapid host and microbiome development and therefore also likely represents a period when the microbiome may be most susceptible to adopting long-term changes. Additional studies, especially with human subjects, are necessary to understand how antibiotic exposures during various developmental windows can alter the gut microbiome and, in turn, host metabolism.
Allergy and Atopic Disorders
A considerable number of epidemiological studies link early antibiotic exposures, especially multiple courses, to atopic diseases later in life (Droste et al., 2000; Johnson et al., 2005; McKeever et al., 2002; Ong et al., 2014). As mentioned previously, normal development of the immune system is dependent on key members of the gut microbiome for the development of regulatory components of the immune system as well as maintaining homeostasis at the gut epithelium. Allergic and atopic disorders are primarily caused by impaired components of the adaptive immune system that rely largely on the gut microbiome (Fujimura and Lynch 2015): for example, B cell maturity (Lundell et al., 2014) and regulatory T cell differentiation and expansion (Atarashi et al., 2013). Distinct compositions of infant gut microbiomes have been associated with the development of atopic diseases later in life (Abrahamsson et al., 2012; Atarashi et al., 2013; Bisgaard et al., 2011; Björkstén et al., 2001), and therefore it is conceivable that early exposure to antibiotics, especially broad-spectrum antibiotics, could be responsible for shaping the gut microbiome with predisposition toward allergy and atopic diseases. We hypothesize that two dysbiosis types may be responsible for allergy and atopic diseases: loss of keystone taxa and blooms of pathogens and pathobionts. Evidence for loss of keystone taxa has been established in mouse studies, where antibiotic exposure led to changes in the gut microbiome, which eventually impacted the immune system. Reductions in regulatory T cell counts (Russell et al., 2012) and increases in serum IgE concentrations and basophil-associated TH2 cell responses (Hill et al., 2012) were observed with onset of the allergic disease phenotype. These observations agree with previous studies reporting that an overabundance of IgE and the cytokine IL-4, produced by TH2 cells, are associated with allergies (Haas et al., 1999). Another study found that antibiotics given to neonatal mice reduced the abundance of Clostridia and as a result induced food allergies; Clostridia colonization is important for stimulating IL-22 production to prevent food antigens from crossing the gut epithelium (Stefka et al., 2014). Microbial taxa considered important for immune development may differ from one developmental stage to the next, therefore warranting further investigation into the importance of timing of antibiotic exposure in atopic disease. Although some antibiotic exposures may only create short-term dysbiosis and eventually allow the microbiome to recover, if the period of dysbiosis coincides with critical developmental time points, there is potential for long-term impact on immune health. Several studies have indicated the first 6 months of life as the most critical for immune development (van der Velden et al., 2001; Prescott et al., 1999; Rautava et al., 2004), suggesting the importance of host-microbiome interactions during this time. Germ-free mice have been shown to develop immune defenses against allergic asthma if colonized as neonates, but not if colonized in adulthood (Olszak et al., 2012). Similarly, Helicobacter pylori colonization in neonatal mice stomachs provided increased protection against asthma, compared to adult colonization (Arnold et al., 2011). Furthermore, Russell et al. (2012) induced asthma in mice with early antibiotic exposure but failed to reproduce the same phenotype with antibiotic exposure in adult mice. These studies suggest that antibiotic exposure during this critical window of development may have the most pronounced and long-lasting consequences. In addition to the loss of keystone taxa, antibiotic exposure commonly results in an immediate reduction of biodiversity that may allow for unusual blooms of rare members of the gut microbiome. In a recent study, antibiotic usage in infancy selected for “illness-associated” bacteria and was linked to asthma development later in life (Teo et al., 2015). Blooms of certain strains of Clostridia, despite the importance of this class of bacteria in immune development, may actually contribute to atopic disease (Penders et al., 2013). Additionally, severe dysbiosis in a developing neonatal gut may allow for bacterial translocation of commensals and hence the development of systemic antibodies against these otherwise innocuous microbes. As seen in Crohn’s disease (Adams et al., 2008), it is highly plausible that inappropriate immune responses against commensals could also lead to hypersensitivity to common antigens, eventually leading to allergy and atopic diseases.
Autoimmune Diseases
Although autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, and multiple sclerosis have a large genetic component, the gut microbiome has recently been found to be a potential major mediator of these diseases (Cani et al., 2008; Giongo et al., 2011; Lee and Mazmanian, 2010; Sellitto et al., 2012; Wen et al., 2008). Regulation of autoimmune responses by the gut microbiome is complex and usually involves direct modulation of adaptive and innate immunity but can also occur indirectly via hormones and parental experiences (Yurkovetskiy et al., 2015). Interestingly, germ-free mice are incapable of developing rheumatoid arthritis and multiple sclerosis (Lee and Mazmanian, 2010; Wu et al., 2010). In support of the hygiene hypothesis in autoimmune disease, one study found that the incidence of diabetes in genetically predisposed non-obese diabetic (NOD) mice doubled when mice were raised in conventional, compared to pathogen-free, breeding environments (Bach, 2002). This suggests that antibiotics could exacerbate the onset of diabetes. Recent work found that the number of courses of antibiotics administered during childhood is associated with risk of juvenile rheumatoid arthritis (Horton et al., 2014) and the risk of inflammatory bowel disease (Hviid et al., 2011). There is also evidence that antibiotics are associated with celiac disease (Mårild et al., 2013). Studies examining the effects of antibiotic exposure on type 1 diabetes have yielded inconsistent results: one study found that antibiotics given to NOD mice during pregnancy modulated type 1 diabetes development in offspring (Tormo-Badia et al., 2014), but other studies found antibiotics to be protective (Brugman et al., 2006; Cani et al., 2008). There is currently limited evidence linking antibiotic exposures to autoimmune disorders, but we hypothesize that the underlying mechanisms are driven by loss of keystone taxa and blooms of pathogens and pathobionts, similar to those of allergy and atopic disorders due to the critical role of the immune system in these diseases.
Infectious Diseases
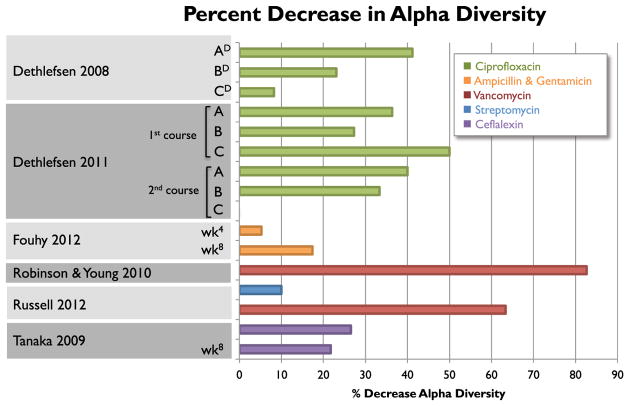
Antibiotics are used to eradicate one or more bacterial taxa; therefore, a temporary reduction in biodiversity is expected. Current studies report a large range of percent losses of biodiversity after antibiotic exposure (Figure 3), suggesting that some subjects may take longer to recover to baseline than others (Figure 2). The recovery period represents a vulnerable time for the host, since not all members of the microbial community are present to suppress blooms of (potential) pathogens and pathobionts, and hence prevent infection. A number of studies support this theory, showing an increased susceptibility to infection after antibiotic exposure (Croswell et al., 2009; Deshmukh et al., 2014; Lawley et al., 2008; Sekirov et al., 2008), with a number of studies highlighting the proliferation of antibiotic-resistant strains (Ayres et al., 2012; Brandl et al., 2008; Buffie et al., 2012; Donskey et al., 2000). Clostridium difficile infection in adults is an archetypical example of how loss of biodiversity enables the bloom of a pathogen in the gut. Necrotizing enterocolitis in pre-term infants has also been linked to antibiotic use prior to onset of disease (Alexander et al., 2011; Cotten et al., 2009), and the gut microbiomes of children about to succumb to necrotizing enterocolitis exhibit decreased biodiversity and blooms of Gammaproteobacteria (Mai et al., 2011; Wang et al., 2009). Although pre-term infants have a distinct set of health risks, this mode of infection can be extended to other disease agents in full-term infants as well (Figure 2B). This need for ecological checks and balances in the gut microbial community extends beyond its bacterial members; antibiotic-induced dysbiosis has been shown to impair innate antiviral immunity against the influenza virus (Abt et al., 2012) as well as enable blooms of opportunistic fungi, such as Candida albicans (Noverr et al., 2004). Longer-duration antibiotic therapy appears to be correlated with length of recovery period (Fouhy et al., 2012), which also increases the risk of infection (Alexander et al., 2011). Identifying when a microbiome is fully recovered will be challenging given the inter-individual deviations of the adult gut microbiome, and will be even more difficult with the highly variable, developing infant gut microbiome. Lawley et al. (2008) found that mice exposed to antibiotics still exhibited increased colonization of Salmonella serovar Typhimurium despite recovery of bacterial counts, suggesting that not only is microbiome recovery challenging to define, but also that current methods for measuring biodiversity may be insufficient for assessing infection risk. Although previous studies have focused on short-term risks for infection, it also is plausible that antibiotic exposure could lead to an incomplete, yet stable and permanent, recovery of the microbiome (Dethlefsen et al., 2008), potentially predisposing the recipient to infectious disease later in life.
Figure 3. Percent Decrease in Gut Microbiome Biodiversity across Studies with Different Antibiotic Exposures.
All fecal samples were collected 1 week after antibiotic course was completed, except where noted by subscripts. The Dethlefsen and Relman (2011) study included three subjects (A, B, and C) who received two courses 6 months apart. DSample taken during antibiotic treatment; 4sample taken 4 weeks after antibiotic completion; 8sample taken 8 weeks after antibiotic completion (Dethlefsen et al., 2008; Fouhy et al., 2012; Robinson and Young, 2010; Russell et al., 2012; Tanaka et al., 2009).
Future Directions
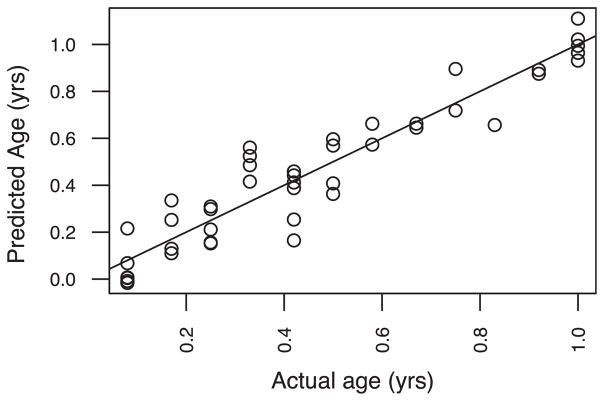
The framework presented here links together the existing epidemiological and mechanistic studies on antibiotics and various gut-mediated disease outcomes. Large, integrated studies designed to focus on short- and long-term impact of antibiotics, both in terms of microbiome composition and disease risk, with careful consideration of the factors presented here, will be critical as we move toward an increased understanding of related disease etiologies. Such studies will enable important applications, such as the development of diagnostic tools to discover complex microbial biomarkers for dysbiosis risk. To demonstrate the potential importance, we used a random forest machine learning model trained on gut microbiome data from a cohort of healthy American infants of varying ages (Knights et al., 2011; Yatsunenko et al., 2012). Given the gut microbiome of a healthy infant, we were able to use our model to accurately predict the infant’s age, what we term the predictive microbiome maturity index (MMI), within 1.3 months (SE) (Figure 4). The MMI has clinical importance as a diagnostics tool: the predictive MMI of a dysbiotic infant gut microbiome is likely very different from the infant’s true age. Subramanian et al. (2014) used a similar predictive model and found that children with severe acute malnutrition had gut microbiomes that were significantly immature compared to healthy children. There is enormous potential for the microbiome field to revolutionize diagnostics and therapeutics, yet published human infant studies have not been designed to infer causality. Establishment of a large and diverse baseline cohort to define healthy development of the infant microbiome in presence and absence of perturbation by caesarian delivery, breast-feeding alternatives, and antibiotic usage is essential to refine our understanding of “normal development” so that pediatric dysbiosis can be identified robustly. Additionally, longitudinal and cross-sectional studies assessing the short-term, mechanistic, and longer-term health impact of antibiotics will be necessary to advance the diagnosis, interpretation, and treatment of pediatric dysbiosis and to provide evidence-based recommendations regarding safe practices for antibiotic usage in infants. In conclusion, the primary goal of continued research in pediatric dysbiosis will be to gain a mechanistic understanding how usage of antibiotics in children may disrupt normal development of the gut microbiota, and at times consequently the immune system, potentially leading to increased risk of diseases like obesity, diabetes, allergies, asthma, and inflammatory bowel disease.
Figure 4. Predicted Microbiome Maturity Index.
The predictive microbiome maturity index (MMI) for a given child is compared to the true age of that child. The MMI was predicting using random forests regression algorithm trained on the microbiome compositions and true ages of all children except for one being predicted. True age was predicted to within ± 1.3 months (SD of the predicted error), demonstrating the feasibility of modeling the maturation of the gut microbiota as a predictable process across individuals. Microbiome samples were obtained from children living in the US (Yatsunenko et al., 2012).
Acknowledgments
We thank Marc Jenkins for insightful discussions during the writing of this manuscript.
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008599. http://dx.doi.org/10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–440. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RJ, Heazlewood SP, Gilshenan KS, O’Brien M, McGuckin MA, Florin THJ. IgG antibodies against common gut bacteria are more diagnostic for Crohn’s disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008;103:386–396. doi: 10.1111/j.1572-0241.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Ajslev TA, Andersen CS, Gamborg M, Sørensen TIA, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011;35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RR, Brewer M, Gauthier JJ. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun. 1980;28:893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Müller A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Nomoto K, Shimizu K, Watanuki M, Tanaka R. Increased resistance of mice to Salmonella enterica serovar Typhimurium infection by synbiotic administration of Bifidobacteria and transgalactosylated oligosaccharides. J Appl Microbiol. 2001;91:985–996. doi: 10.1046/j.1365-2672.2001.01461.x. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery GB, Randolph JG, Weaver T. Gastric acidity in the first day of life. Pediatrics. 1966;37:1005–1007. [PubMed] [Google Scholar]
- Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18:799–806. doi: 10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. this issue. [DOI] [PubMed] [Google Scholar]
- Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063–1069. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- Bezirtzoglou E. The intestinal microflora during the first weeks of life. Anaerobe. 1997;3:173–177. doi: 10.1006/anae.1997.0102. [DOI] [PubMed] [Google Scholar]
- Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86:13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr. 2015;174:151–167. doi: 10.1007/s00431-014-2476-2. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Li N, Bonnelykke K, Chawes BLK, Skov T, Paludan-Müller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S, Klatter FA, Visser JTJ, Wildeboer-Veloo ACM, Harmsen HJM, Rozing J, Bos NA. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96:544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12:R50. doi: 10.1186/gb-2011-12-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130:23–31. doi: 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- Cherrier M, Eberl G. The development of LTi cells. Curr Opin Immunol. 2012;24:178–183. doi: 10.1016/j.coi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, Ambalavanan N, Benjamin DK, Jr NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins AG, Thompson FM. Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut. 2002;51:748–754. doi: 10.1136/gut.51.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O’Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, Yang Y, Wang WP. The role of intestinal bifidobacteria on immune system development in young rats. Early Hum Dev. 2010;86:51–58. doi: 10.1016/j.earlhumdev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000;343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000;30:1547–1553. doi: 10.1046/j.1365-2222.2000.00939.x. [DOI] [PubMed] [Google Scholar]
- Fierro JL, Prasad PA, Localio AR, Grundmeier RW, Wasserman RC, Zaoutis TE, Gerber JS. Variability in the diagnosis and treatment of group a streptococcal pharyngitis by primary care pediatricians. Infect Control Hosp Epidemiol. 2014;35(Suppl 3):S79–S85. doi: 10.1086/677820. [DOI] [PubMed] [Google Scholar]
- Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93(Suppl 1):S41–S48. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- Fouhy F, Guinane CM, Hussey S, Wall R, Ryan CA, Dempsey EM, Murphy B, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012;56:5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592–602. doi: 10.1016/j.chom.2015.04.007. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber JS, Prasad PA, Localio AR. Variation in antibiotic prescribing across a pediatric primary care network. J Ped Infect Dis. 2014 doi: 10.1093/jpids/piu086. Published online October 30, 2014. http://dx.doi.org/10.1093/jpids/piu086. [DOI] [PMC free article] [PubMed]
- Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33:757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- Haas H, Falcone FH, Holland MJ, Schramm G, Haisch K, Gibbs BF, Bufe A, Schlaak M. Early interleukin-4: its role in the switch towards a Th2 response and IgE-mediated allergy. Int Arch Allergy Immunol. 1999;119:86–94. doi: 10.1159/000024182. [DOI] [PubMed] [Google Scholar]
- Hansen CHF, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, Tlaskalova-Hogenova H, Hansen AK. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh AL, Jackson MA, Hicks LA American Academy of Pediatrics Committee on Infectious Diseases. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132:1146–1154. doi: 10.1542/peds.2013-3260. [DOI] [PubMed] [Google Scholar]
- Hibberd CM, Brooke OG, Carter ND, Haug M, Harzer G. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Arch Dis Child. 1982;57:658–662. doi: 10.1136/adc.57.9.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks LA, Taylor TH, Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, Kambayashi T, Larosa DF, Renner ED, Orange JS, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DB, Scott FI, Haynes K, Putt ME, Rose CD, Lewis JD, Strom BL. Antibiotic exposure and the development of juvenile idiopathic arthritis: a population-based case-control study. 2014;66(11 Suppl) Abstract 929. [Google Scholar]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KM, Foster JA, Forney LJ, Scühtte UME, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE. 2011;6:e21313. doi: 10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CC, Ownby DR, Alford SH, Havstad SL, Williams LK, Zoratti EM, Peterson EL, Joseph CLM. Antibiotic exposure in early infancy and risk for childhood atopy. J Allergy Clin Immunol. 2005;115:1218–1224. doi: 10.1016/j.jaci.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- Knights D, Parfrey LW, Zaneveld J, Lozupone C, Knight R. Human-associated microbial signatures: examining their predictive value. Cell Host Microbe. 2011;10:292–296. doi: 10.1016/j.chom.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronman MP, Zhou C, Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics. 2014;134:e956–e965. doi: 10.1542/peds.2014-0605. [DOI] [PubMed] [Google Scholar]
- Landau W. Human colostral whey M-1 glycoproteins and their L. bifidus var. Penn growth promoting activities. Life Sci. 1974;14:967–976. doi: 10.1016/0024-3205(74)90086-1. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999;23(Suppl):S3–S6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Louis NA, Lin PW. The Intestinal Immune Barrier. Neoreviews. 2009;10:e180–e190. [Google Scholar]
- Lundell AC, Johansen S, Adlerberth I, Wold AE, Hesselmar B, Rudin A. High proportion of CD5+ B cells in infants predicts development of allergic disease. J Immunol. 2014;193:510–518. doi: 10.4049/jimmunol.1302990. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Zemlin M. Ontogeny of the intestinal immune system. Hematology Meeting Reports (formerly Haematologica Reports) 2009;2 [Google Scholar]
- Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårild K, Ye W, Lebwohl B, Green PHR, Blaser MJ, Card T, Ludvigsson JF. Antibiotic exposure and the development of coeliac disease: a nationwide case-control study. BMC Gastroenterol. 2013;13:109. doi: 10.1186/1471-230X-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, de La Cochetiere MF. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21:167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis. 2003;9:432–437. doi: 10.3201/eid0904.020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Smith C, Collins J, Heatlie H, Frischer M, Hubbard R. Early exposure to infections and antibiotics and the incidence of allergic disease: a birth cohort study with the West Midlands General Practice Research Database. J Allergy Clin Immunol. 2002;109:43–50. doi: 10.1067/mai.2002.121016. [DOI] [PubMed] [Google Scholar]
- Ménard D. Functional development of the human gastrointestinal tract: hormone- and growth factor-mediated regulatory mechanisms. Can J Gastroenterol. 2004;18:39–44. doi: 10.1155/2004/640897. [DOI] [PubMed] [Google Scholar]
- Moles L, Manzano S, Fernández L, Montilla A, Corzo N, Ares S, Rodriguez JM, Espinosa-Martos I. Bacteriological, biochemical, and immunological properties of colostrum and mature milk from mothers of extremely preterm infants. J Pediatr Gastroenterol Nutr. 2015;60:120–126. doi: 10.1097/MPG.0000000000000560. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Mulberg AE, Grand RJ. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology. 1999;116:702–731. doi: 10.1016/s0016-5085(99)70193-9. [DOI] [PubMed] [Google Scholar]
- Nash DR, Harman J, Wald ER, Kelleher KJ. Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156:1114–1119. doi: 10.1001/archpedi.156.11.1114. [DOI] [PubMed] [Google Scholar]
- Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279:875–877. doi: 10.1001/jama.279.11.875. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong MS, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol. 2014;112:441–445. doi: 10.1016/j.anai.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Paine RT, Tegner MJ, Johnson EA. Compounded Perturbations Yield Ecological Surprises. Ecosystems. 1998;1:535–545. [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, Hamelmann E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132:601–607. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease—an extended version. J Pediatr Gastroenterol Nutr. 2004;38:378–388. doi: 10.1097/00005176-200404000-00004. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1:279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogier EW, Frantz AL, Bruno MEC, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci USA. 2014;111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbo M, Schiffrin EJ. Ontogeny of intestinal epithelium immune functions: developmental and environmental regulation. Cell Mol Life Sci. 2005;62:1288–1296. doi: 10.1007/s00018-005-5033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76:4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. The genome sequence of Bifidobacterium longum subsp infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105:18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M, Bai G, Serena G, Fricke WF, Sturgeon C, Gajer P, White JR, Koenig SSK, Sakamoto J, Boothe D, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS ONE. 2012;7:e33387. doi: 10.1371/journal.pone.0033387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735–743. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- Shu Q, Lin H, Rutherfurd KJ, Fenwick SG, Prasad J, Gopal PK, Gill HS. Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice. Microbiol Immunol. 2000;44:213–222. doi: 10.1111/j.1348-0421.2000.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Sjogren YM, Jenmalm MC, Böttcher MF, Björkstén B, Sverremark-Ekström E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39:518–526. doi: 10.1111/j.1365-2222.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci USA. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, Shirakawa T, Sonomoto K, Nakayama J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. 2009;56:80–87. doi: 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, et al. The infant nasopharyngeal microbiome impacts the incidence of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015 doi: 10.1016/j.chom.2015.03.008. Published online April 8, 2015. http://dx.doi.org/10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed]
- Tormo-Badia N, Håkansson Å, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80:250–260. doi: 10.1111/sji.12205. [DOI] [PubMed] [Google Scholar]
- Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji NM, Kosaka A. Oral tolerance: intestinal homeostasis and antigen-specific regulatory T cells. Trends Immunol. 2008;29:532–540. doi: 10.1016/j.it.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy. 2001;31:997–1006. doi: 10.1046/j.1365-2222.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L, Pickard JM, Chervonsky AV. Microbiota and Autoimmunity: Venues Less Visited. Cell Host Microbe. 2015;17:548–552. doi: 10.1016/j.chom.2015.04.010. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]