Abstract
In recent years with the threat of pandemic influenza and other public health needs, alternative vaccination methods other than intramuscular immunization have received great attention. The skin and mucosal surfaces are attractive sites probably because of both non-invasive access to the vaccine delivery and unique immunological responses. Intradermal vaccines using a microinjection system (BD Soluvia) and intranasal vaccines (FluMist) are licensed. As a new vaccination method, solid microneedles have been developed using a simple device that may be suitable for self-administration. Because coated micorneedle influenza vaccines are administered in the solid state, developing formulations maintaining the stability of influenza vaccines is an important issue to be considered. Marketable microneedle devices and clinical trials remain to be developed. Other alternative mucosal routes such as oral and intranasal delivery systems are also attractive for inducing cross protective mucosal immunity but effective non-live mucosal vaccines remain to be developed.
Keywords: influenza vaccine, microneedles, skin vaccination, mucosal immunization
Introduction
Both vaccination and antiviral therapy are used to prevent and to treat influenza infection [1]. Nonetheless, vaccination is the most cost-effective public health prevention against infectious diseases [2,3]. The delivery of vaccines and drugs using needles and syringes is a common practice in worldwide. However, there are some concerns about the use of needles and syringes. Each year, the unsafe use of injections is estimated to cause approximately 1.6 million deaths, which include needle-stick injuries, work accidents, and an overwhelming number of infections with blood-borne pathogens such as hepatitis B virus, hepatitis C virus, and HIV due to the improper re-use of needles and syringes [4,5]. Another issue is the needle phobia and the discomfort suffered by children and adults [6,7].
Skin has unique immunological properties (Fig. 1). Stratum corneum is a physical barrier of the skin (Fig. 1). The epidermis is the skin outer layer with immunological functions and its thickness varies in a range of approximately 50 – 200 μm (Fig.1) [8]. The epidermal cell types include melanocyes, Langerhans cells (LCs; a subtype of antigen presenting dendritic cells) in addition to the keratinocytes (a kind of epithelial cells) the major cell types in the epidermal layer. The outer dermis contains resident hematopoietic-derived cells, including mast cells, dermal dendritic cells (DCs) and macrophages as well as LCs. The dermis also provides the major site for leukocyte extravasation from the blood to the skin [9]. Therefore, skin might provide an attractive site for vaccine delivery [10]. The skin routes for immunization include intradermal, epidermal, and transcutaneous delivery of vaccines. The intradermal route refers to antigen delivery into the dermis via a syringe and needle, or a microinjection system. The term epidermal mostly reflects the delivery of vaccines into the epidermal and outer dermal layers via arrays of microneedles in the form of a patch or using gene-gun technology. Transcutaneous or transdermal vaccination means the topical application of antigen onto the skin [11–13].
Fig. 1.
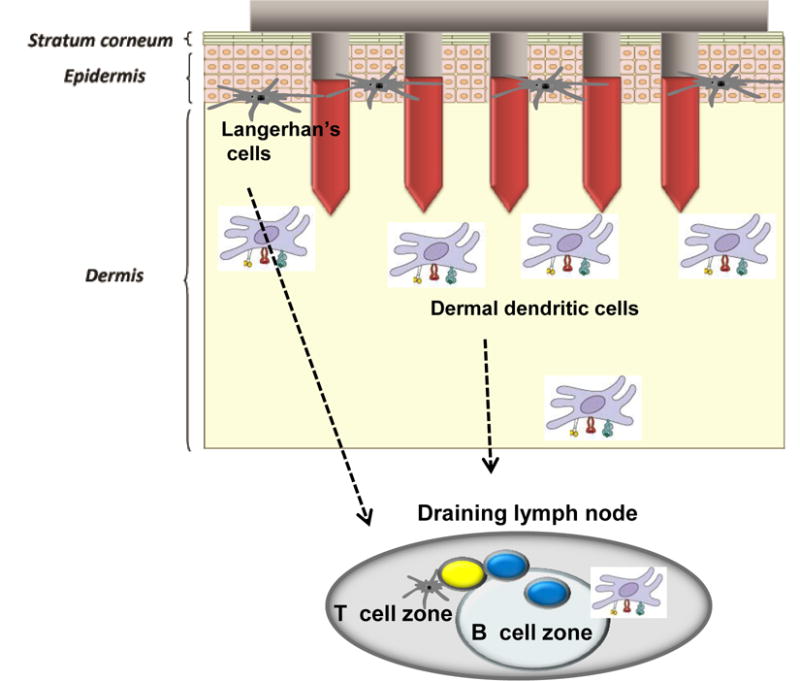
A diagram of skin layers and microneedle immunization targets. Microneedles with a size of approximately 700 μm in length and 150–160 μm in width are designed to deliver antigens to the epidermal and dermal layers of skin. Microneedle immunization can target epidermal Langerhans cells and dermal dendritic cells (DCs) both resident and recruited from the blood. After capture of antigens, these DCs migrate to the draining lymph nodes and trigger T- and B–cell activation.
As an alternative to intramuscular injection, the intradermal vaccines and the live cold adapted vaccines have been licensed and are widely used in humans [14,15]. The fact that these new routes of licensed influenza vaccines provides further support and rationales for developing new vaccination methods targeting to skin and mucosal sites. With briefly overviewing and comparing these newly licensed vaccines in a liquid form, this review covers recent studies with solid microneedles with a size of approximately 700 μm in length and 150–160 μm in width (Fig. 2) delivering influenza vaccines as an additional alternative to intramuscular delivery and their associated vaccine stability issues due to a phase change from liquid formulation to a dried solid state of vaccines. Also, we discuss mucosal delivery of influenza vaccines and their potential avenues in broadening cross-protective immunity.
Fig 2.
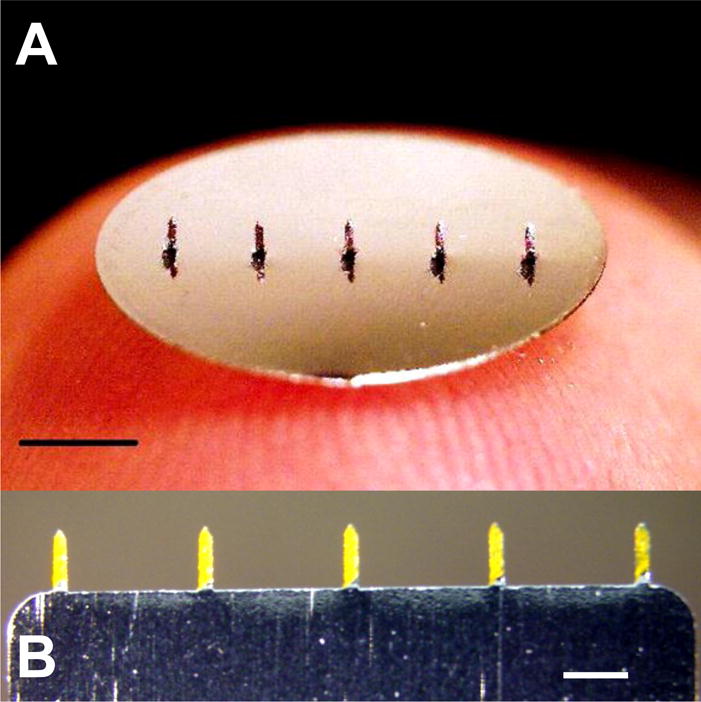
Two different types of microneedles arrays coated with fluorescence. (A) Patch type (scale bar=2mm) coated with sulforhodamine and (B) row-type coated with fluorescein (scale bar=700μm)
Skin vaccination using microneedles
Concept of the skin delivery using various forms of microneedles
The initial designs of microneedles are arrays of microneedles that protrude several hundred microns in sizes (Fig. 2). Coated or dissolvable microneedles, or microneedles alone can be used to either to pierce or to make microscopic holes in the skin’s outer layer, straturm corneum [16,17]. Compounds delivered to the skin using microneedles include bovine serum albumin (as a model protein antigen), oligonucleotides or plasmid DNA vaccines, latex particles of viral dimensions, recombinant bacterial proteins and live attenuated virus vaccines [18–22].
Microneedles are fabricated to create micron-scale needles [23,24]. Solid micorneedle vaccines were demonstrated to be used for coating with protein antigens in a dry formulation [25,26]. These coated vaccines can dissolve from microneedles within the skin on a time scale of seconds or minutes (Fig. 3). This concept of solid microneedle vaccines was applied to various model compounds including proteins such as ovalbumin [24–28].
Fig 3.
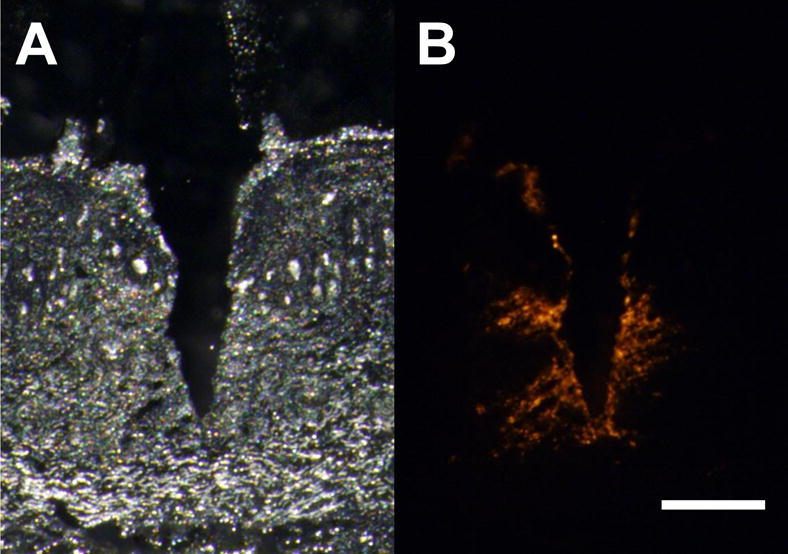
Histology images of insertion and delivery of fluorescence by coated microneedle into guinea pig skin (A) white light and (B) fluorescence images (scale bar=200μm)
In contrast to solid coated microneedles, dissolvable polymer microneedles with encapsulated drugs or vaccines also have been developed. Dissolvable microneedles composed of a biocompatible and mechanically robust material have been fabricated from polymers such as polylactic-co-glycolic acid, dissolving sugar, or polysaccharide [29–31]. Dissolving microneedles in the skin leaves behind no sharp and hazardous waste [29]. This concept of dissolvable polymer microneedles has been demonstrated for the delivery of insulin and other model compounds [29,31,32]. In a recent study, a concept of dissolving polymer microneedles was demonstrated to deliver encapsulated inactivated influenza virus to the skin of mice [32]. Immunized mice with dissolving microneedle influenza vaccines showed effective lung viral control and cellular recall responses after challenge, suggesting that dissolving microneedles can provide a simpler and safer vaccination method [32].
Intradermal influenza vaccination using hollow microneedles
Different from solid microneedles mentioned above, hollow microneedles are composed of a hollow fluid conduit through the body of the individual needle, a vaccine reservoir, and fluid delivery system such as a syringe, pump, or other pressure generating device. One or more hollow needles that are used to flow a liquid formulation into the skin are generally in a range of 1.0–1.5 mm in length (30 – 34 gauge) [33,34]. Intradermal delivery using hollow microneedles has been demonstrated for a variety of compounds and vaccines in animals and in humans [21,22,35]. Intradermal influenza vaccines were first licensed (Table 1) and marketed under various trade names through the commercially available hollow microneedle system, BD Soluvia™ (Becton Dickinson, Franklin Lakes, NJ) [14,36]. This licensed intradermal influenza vaccine is based on a single 1.5 mm stainless steel microneedle in a glass prefilled syringe with a self-deploying safety shield after delivery, which was reported in several studies [8,37,38].
Table 1.
Intradermal influenza vaccines and microneedle influenza vaccines
| Intradermal vaccines | Microneedle vaccines | |
|---|---|---|
| Target tissues | Dermis | Epidermis and dermis |
| Size | 1.5 mm (BD Soluvia) | approximately 700 μm |
| Formulation | Liquid vaccine | Dried solid vaccine |
| Devices | Microinjection systems (or hollowneedles, needles and syringes) | Microneedles (arrays of steel or dissolving biopolymers) |
| Clinical studies | Yes | No reports |
| License | Yes | No (early preclinical stage) |
| Doses1 | 9 μg HA in adults (15 μg HA in elderly) | 0.4 – 10 μg total vaccine proteins in mice |
| Stabilizing agent | – | Trehalose sugar |
| Stable storage | Cold-chain | Cold to room temperature |
| Immunogenicity | Comparable or higher | Comparable or higher |
| Protection | – | Comparable or higher |
HA: Hemagglutinin protein; the whole influenza virus was reported to contain 28 – 29% of total viral proteins [110]. Thus, the HA dose in microneedle vaccines in mouse studies is estimated to be 0.12 – 2.9 μg HA.
The microinjection system using seasonal trivalent influenza vaccine at a lower dose in adult volunteers induced humoral immune responses (hemagglutination inhibition titers, HAI) comparable to those by the standard intramuscular route. That is, 9 μg or 15 μg of intradermal dose of trivalent seasonal influenza vaccines was comparably immunogenic to the 15 μg standard intramuscular dose for all strains [14,39–41]. In the elderly volunteers, intradermal immunization with 15 μg of trivalent inactivated vaccine using the BD microinjection system is likely to be more immunogenic compared to the intramuscular delivery route [39,42–44] or non-inferior to conventional intramuscular vaccination [45]. In a multicenter, randomized study including 1107 healthy volunteers over 60 years of age, intradermal trivalent inactivated influenza vaccines containing 15 or 21 μg of hemagglutinin per strain were administered using a microinjection system and the strain-specific hemagglutination inhibition geometric mean titers were determined in comparison with the standard intramuscular immunization [42]. Seroprotection and seroconversion rates were significantly higher in each intradermal vaccine [42]. Although the mechanisms in enhancing the immunogenicity of intradermal vaccines are not well understood, dermal dendritic cells might be involved in stimulating cellular immune responses in the elderly [46]. Also, in 112 healthy young children aged 3 to 18 years, intradermal influenza vaccination at one fifth of a standard dose was reported to elicit comparable immunogenicity to full-dose intramuscular vaccination [47].
In a study of skin thickness (epidermis-dermis) to have an insight into the potential site of intradermal vaccine delivery, it was reported that the suprascapular had 2.54 mm, the deltoid 2.02 mm, the waist 1.91 mm of mean skin thickness [48]. Therefore, microneedles with 1.5 mm length would be assumed to deliver the antigen into the dermal layer. The skin sites of deltoid or suprascapular are to be appropriate in the body [48]. The microneedle injection system appears to be easy to perform, reliable and consistent in administering the given dose [37,49]. The systemic reactogenicity (such as muscle pain) of intradermal route is significantly less but minor transient reactions (redness) at the injection sites are more frequently observed [50–53]. The acceptability of intradermal route using a microinjection system is high up to over 95% with satisfaction, most responding that the new intradermal vaccination was less painful and quickly administered [14]. It is thus expected that intradermal vaccines will help increase seasonal influenza vaccination rates in adults. Therefore, clinical trials provide sufficient evidence that delivering vaccines to the dermal layer of skin can be a promising approach for vaccination alternative to intramuscular delivery.
Influenza vaccination in the skin of mice using solid microneedles
Intradermal injection of influenza vaccines using needles and syringes or microinjection systems has been widely demonstrated in humans [14,51,54–57]. However, its general application may have some limitations due to discomfort associated with needle sticks (in cases of using hypodermic needles and syringes) and/or cost of microinjection devices. As a new influenza vaccination, solid microneedles coated with inactivated influenza vaccines have been demonstrated in mice [58]. In this study, an array of 5 microneedles with 750 μm in length (Fig. 2) was coated with 3.3 μg total protein of inactivated H1N1 influenza virus vaccines at the vaccine concentration of 5 mg vaccine protein per ml. Using 3 sets of 5-needle array, a total of 10 μg of influenza vaccines was successfully delivered to the mouse skin (Fig. 3, [59]). Microneedle vaccinated mice showed comparable vaccine specific antibody responses and protection as intramuscular vaccination [58]. A similarly comparable immunogenicity was observed in another microneedle skin vaccination with 3 to 10 μg inactivated H3N2 influenza virus vaccine as intramuscular immunization [60]. Relatively high doses of influenza vaccines and multiple arrays of microneedles were required probably due to the un-stabilized dry microneedle formulation [58,60].
Stable microneedle formulations were developed to improve the efficacy of microneedle influenza vaccines [59,61]. A trehalose (disaccharide) known to stabilize biomolecules during drying [62] was included in the microneedle coating formulation as a stabilizer (Table 1). As a result, microneedles coated with as low as 0.4 μg of inactivated influenza (A/PR8, H1N1) virus vaccine induced protective immunity, which was superior to the intramuscular immunization with the same vaccine [59,61]. These findings suggest that vaccination in the skin of mice using a microneedle patch improves protective immunity and simplify the delivery of influenza vaccines. This approach has been proved to be applicable to other influenza vaccines such as influenza H1N1 and H5N1 virus-like particle vaccines [63–67] and 2009 H1N1 inactivated influenza virus vaccines [68].
Immunological responses after influenza vaccination using solid microneedles
A mouse model provides a valuable tool in understanding the detailed host immune responses after delivering vaccines to the skin, which is difficult in humans. With the development of solid microneedle influenza vaccines, we could investigate host immune responses in detail after microneedle vaccination in the skin of mice. Influenza vaccine-coated solid microneedle delivery to the skin of mice could induce virus neutralizing antibody and hemagglutination inhibition (HAI) responses at comparable levels (or higher levels than) to intramuscular route [59,61,65,67]. Enhanced lung virus clearance and less inflammatory cytokine were observed in mice that received microneedle vaccination after challenge infection [59,61] indicating improved protective efficacy of microneedle vaccination. In addition, microneedle vaccination showed significantly higher levels of virus specific recall IgG antibody responses after challenge [59,61], suggesting rapid host anamnestic immune responses in response to the exposure to challenge virus. Whereas, intramuscular immunization showed decreases in levels of virus-specific antibodies at this early time post challenge infection, which might be related with delayed virus clearance.
Regarding cellular immune responses, microneedle vaccination induced higher levels of IFN-γ and IL-4 cytokine-secreting splenocytes compared to intramuscular immunization upon major histocompatibility complex (MHC) II peptide stimulation [59,61], indicating enhanced MHC II-associated CD4+ T helper cell responses, which might be especially important to provide protection in the elderly [69]. Finally, microneedle vaccination in the skin was demonstrated to increase trafficking of dendritic cells to regional lymph nodes, which plays a role in contributing to improved protective immunity [70]. Thus, microneedle vaccination would provide an excellent research tool in studying detailed immune responses after delivery of vaccine antigens to the skin.
Stability of solid microneedle vaccines and their immunogenicity
We found that the simple process of microneedle coating and drying significantly decreased the stability of the influenza microneedle vaccines, as indicated by the loss of hemagglutination activity, probably due to the phase change from liquid to solid formulation [61,66]. Un-stabilized vaccine yielded much weaker protective immune responses. Immunization using un-stabilized vaccine induced IgG1 antibody as a dominant isotype whereas stabilized vaccines maintaining hemagglutination activity shifted the pattern of antibodies to IgG2a antibodies implicating the T helper type 1 (Th1) immune responses [61,66]. Also, much higher dose (10 μg) of unstabilized microneedle vaccines was required to induce protection [58,60]. Importantly, the addition of trehalose to the microneedle coating formulation was required to retain hemagglutination activity after microneedle coating of influenza vaccines. Microneedle vaccines of a low dose of 0.4 μg inactivated virus with a trehalose stabilizer in the coating formulation conferred significantly enhanced protection [59,61], indicating a positive correlation between the stabilization of coated influenza vaccines and the efficacy of protection. However, it was found that trehalose sugar did not have an adjuvant effect on enhancing immunogenicity [61]. Intact influenza virus vaccines (not exposed to microneedle coating) with and without the addition of trehalose showed similar antibody responses after intramuscular immunization [61], indicating a critical role of trehalose as a stabilizing agent in retaining hemagglutination activity of influenza vaccines during coating microneedles and drying process but not as an immune adjuvant.
Influenza vaccines provide a useful system utilizing an easy assay of hemagglutination activity to investigate the effects of vaccine integrity and stability on its immune responses after skin vaccination. Solid microneedle vaccine studies of stabilized and un-stabilized influenza vaccines provide evidence that the functional integrity of hemagglutinin in the influenza vaccine may have a significant impact on the types and qualities of host protective immune responses to influenza vaccination [61,66]. Thus, it is speculated that retaining the receptor-binding functional activity of hemaglutinin protein antigens in influenza vaccines is important for the effective induction of protective immune responses. Some lymphoid dendritic cells are more likely to induce Th1 type immune responses whereas non-receptor mediated uptake of vaccines via macrophage cells may induce Th2 type responses affecting the pattern of antibody isotypes [71]. Among the range of immunologic data, the recall immune responses with microneedle vaccination to the skin were significantly stronger than intramuscular immunization [59,61,66]. After taking up transdermally delivered antigens, skin-derived dendritic cells are known to migrate to the systemic and mucosal compartments [72–74], which might be involved in rapid recall immune responses after microneedle vaccination in the skin. Therefore, detailed immunologic study provides deeper explanations for potential improved protective efficacies by vaccine delivery to the skin. This correlation between the functional integrity of hemagglutinin and protective immunity against influenza is further supported by other influenza vaccines such as H1N1 and H5N1 influenza virus-like particle vaccines [63–67,75].
Coating formulations and stability of solid microneedle vaccines
The stability of microneedle vaccines seems to be related to the composition of coating solution that is composed of 1% carboxymethylcellulose (CMC) sodium salt, detergent Lutrol F-68 NF, and with or without 15% (w/v) D-(+)-trehalose dihydrate stabilizer [59,76,77]. It would be informative to better understand the possible roles of microneedle coating formulation. Sugar combinations of trehalose, mannitol, dextran, and arginine glutamate were developed as a stabilizing formulation for a dry powder form of influenza vaccines [78]. Some carbohydrate compounds such as trehalose, sucrose, glucose, inulin, and dextran were shown to prevent damages caused from drying or freezing of biomolecules [62]. Among the different carbohydrate stabilizers tested, trehalose was found to be the most effective one for stabilizing influenza microneedle vaccines [76]. Addition of trehalose retained 50–80% hemagglutination activity for all three major strains of seasonal influenza A H1N1 and H3N2, and influenza B viruses [76]. In case of a spray freeze drying process, HEPES buffered saline provided a good stability [79].
Drying influenza vaccines in the coating solution caused more damage to hemagglutination activity than drying in phosphate buffered saline indicating the potential effects of components in microneedle coating solution [76]. In the absence of trehalose, hemagglutination activity of vaccines almost disappeared independent of CMC concentrations [64,77]. Although removing CMC from the coating formulation allowed us to retain 100% hemagglutination activity after drying the vaccines in the presence of trehalose, CMC was nonetheless required to produce thick coatings onto microneedles. Without CMC, the mass of virus coated on microneedles was significantly reduced by more than an order of magnitude [64]. The presence of detergent Lutrol F-68 NF (0.5%) that is also needed for effective coating onto metal microneedles did not show significant effects on stability of microneedle vaccines [64]. Therefore, trehalose and CMC are important excipients in the coating solution for microneedle vaccine formulations.
Long-term stability of solid microneedle vaccines
Cold-chain delivery and storage is required during influenza vaccination campaign since temperature is an important factor for determining the efficacy of influenza vaccines. In liquid formulation of influenza vaccines, storage at 4°C is needed for maintaining the stability of vaccines as measured by hemagglutination activity and storage at 25°C resulted in a significant loss in vaccine activity [64]. In contrast, microneedle vaccines showed a similar pattern of stability kinetics at 4°C and 25°C [64]. Within a day storage of influenza microneedle vaccines, there were approximately 30% loss in hemagglutination activity at both 4°C and 25°C and 40% loss at 37°C. After 7 days of storage, a low flat point of approximately 30% activity was maintained up to 28 days monitored at 25°C storage. Most importantly, the 100% protective immunity was observed with microneedle vaccines stored at 25°C for 28 days [64]. Therefore, solid microneedle vaccines can be developed as an alternative to cold-chain influenza vaccines (Table 1).
Epidermal Powder Immunization
As a delivery of vaccines epidermally in the skin, the helium-powered PowderJect device was developed and used to deliver powdered influenza vaccines [80–82]. A powder form of influenza vaccines can be prepared by using different methods such as freeze drying, spray-freeze drying, spray drying, vacuum drying, and supercritical fluid drying [62]. A simple method is air-drying as described for epidermal powder immunization [80,82]. Briefly, vaccine, adjuvant, and trehalose solution are combined, incubated overnight at 4°C with shaking, and plated in a glass petri dish. The vaccine mixture is dried overnight in a desiccator purged with nitrogen gas. The dried solid is collected, ground with a pestle and mortar, and sieved using stainless steel sieves. Dry powders of particulate vaccines are loaded into a trilaminate cassette and used to immunize mice. The helium-powered PowderJect device for delivering powdered vaccines is a reusable model with a 15 cm length composed of an ‘actuation’ button, a helium gas chamber, a vaccine cassette, and a nozzle [80]. The stainless steel gas chamber is filled with helium gas. Once the device is activated, the helium gas is released to rupture the membranes of the trilaminate cassette containing powdered vaccines. The vaccine powders are accelerated to a high speed so that the particles perforate the stratum corneum and land in the epidermis. Epidermal powder immunization was demonstrated to induce cytotoxic T cells, antibody responses in serum and mucosal sites, and protective immunity against influenza viruses in a mouse model [80–82]. The delivery methods of dry influenza vaccine formulations via epidermal powder immunization are successfully tested in phase I clinical trials [83]. However, the complexity of preparation of vaccines and injection device seems to be one of limiting factors for application to humans in general.
Mucosal Immunization
Many infectious agents enter the body through mucosal surfaces [84]. Mucosal immune responses are being recognized to be important for providing effective protection against pathogens entering mucosal surfaces [85]. Local immune responses at mucosal sites are most efficiently induced by the administration of vaccines onto mucosal surfaces through oral, nasal, sublingual, rectal or vaginal routes. Several mucosal vaccines have been approved for human use. Licensed mucosal vaccines include oral vaccines against poliovirus [86], Salmonella typhi [87], V. cholera [88], and rotavirus [89], and a nasal spray vaccine against influenza virus [90]. Due to the repeated outbreaks of pandemic influenza viruses and the threat of many mucosal pathogens, the research of testing mucosal vaccines is now increasing with new information on the mucosal immune system.
Epithelial cells are layered on the mucosal surfaces of the respiratory, gastrointestinal, and urogenital tracts exposed to the outside (Fig. 4). Mucosal tissues are sites of intense immunological activity, where dispersed lymphoid and antigen-presenting cells such as dendritic cells (DCs) are present (Fig. 4) [85]. More antibody producing cells are estimated to be present in the intestinal mucosa than in the spleen and lymph nodes [85]. Epithelial cells detect and uptake microbial and/or vaccine components through non-specific endocytosis or pattern recognition receptors such as Toll-like receptors [91,92]. Upon encountering microorganisms or vaccine antigens, together with intraepithelial lymphocytes and underlying dendritic cells and macrophage cells, cytokines and chemokines are produced to trigger innate, nonspecific defenses and to promote adaptive immune responses (Fig. 4) [91,92].
Fig. 4.
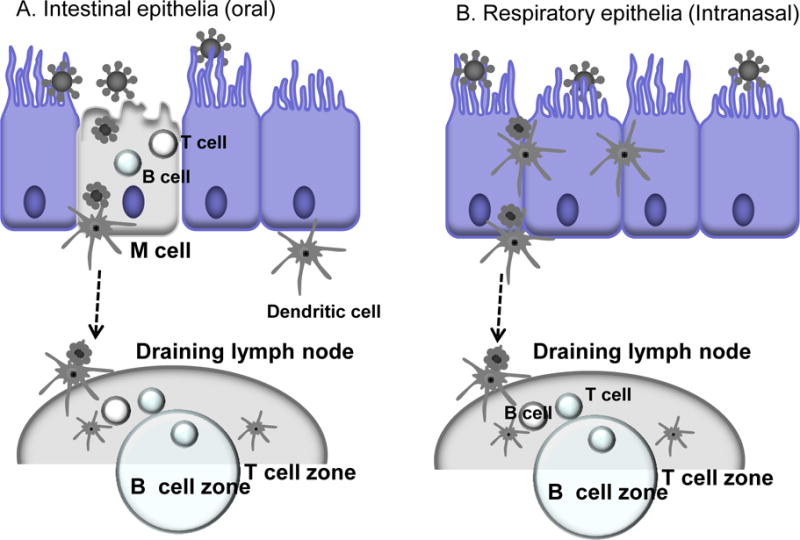
A hypothetical diagram for antigen sampling at mucosal sites. Mucosal organized lymphoid tissues are important in inducing immune responses after vaccination via mucosal routes [85]. A) Intestinal epithelia: The follicle-associated epithelium contains microfold (M) cells that capture and deliver antigens across the epithelial barrier. The dendritic cells residing at the subepithelium take over antigens from M cells and traffic to the draining lymph node for the induction of mucosal (and systemic) immune responses. B) Respiratory epithelia: Dendritic cells (DC) in the mucosal subepithelial layer are assumed to be mainly involved in sampling most antigens at mucosal sites immediately under epithelia by extending dendrites. DCs from mucosal surfaces travel to the nearest draining lymph node to present antigens to the adaptive immune system.
Oral influenza vaccination
Oral delivery is considered a convenient route for administration of vaccines probably because of its easy acceptability and administration [93]. The most effective oral vaccines are live attenuated poliovirus [86], and live attenuated Salmonella typhi [87]. M cells are specialized for endocytosis and rapid transepithelial transport of intact antigens into intraepithelial pockets that contain B and T cells and occasional dendritic cells (Fig. 4). Microparticles that are up to 1 μm in diameter and accessible to M cells are taken up most efficiently (Fig. 4) [94]. Oral immunization of humans with influenza vaccine powder forms was investigated several decades ago. In previous clinical studies, it was demonstrated that ingestion of inactivated influenza virus vaccine powders in enteric-coated capsule formulations stimulated local synthesis of secretory IgA antibody in human nasal and saliva secretions [95–97]. Similarly, the emulsion-inactivated vaccine showed high immunological activity inducing reliable increases in the levels of secretory IgA specific to influenza A and B viruses [98]. However, systemic serum antibody responses were low or not detected in subjects received oral vaccination [95]. Systemic immune responses are needed to meet regulatory criteria for vaccine immunogenicity. In oral vaccine studies using animal models, oral vaccination was shown to induce both systemic and mucosal immune responses as well as protection [99–105]. The efficacy of non-live mucosal vaccines via oral delivery can be significantly increased by incorporating into enteric-coated gelatin capsules and copolymer microparticles, liposomes, or proteosomes [106], which are attractive targets for M cells. However, there might be a gap in translating results from mouse studies to humans particularly for oral vaccination since serum antibodies were not significantly induced in clinical studies after oral vaccination [107]. Nonetheless, cross-protection against mucosal infection by a variety of respiratory viruses such as influenza might better correlate with the level of mucosal immune responses including secretory IgA antibody in a mouse model [108–111]. Recently, oral vaccination of mice with 25 μg of whole inactivated influenza virus vaccines induced significant levels of serum and mucosal IgG and IgA antibodies cross-reactive to homologous and heterologous virus as well as cross protection [112]. In addition, recall immune responses were observed in orally vaccinated mice upon challenge infection [112]. Therefore, the oral method of influenza vaccination could be superior in providing cross protection if effective serum antibody responses are elicited along with mucosal immune responses in humans.
Intranasal influenza vaccination
Immunologically, intranasal influenza vaccination is another attractive route as a needle-free vaccine delivery method (Fig. 4). Nasal vaccine delivery offers potential advantages in providing cross protection. The nasal epithelium contains follicular associated lymphoid tissues that are effective in inducing mucosal immune responses. These cells include B cells that produce IgA antibodies at mucosal sites of entry for many respiratory pathogens including influenza. Inactivated influenza vaccines when intranasally delivered to mice have been shown to induce IgG and IgA antibody responses in serum and mucosal sites, which are important for conferring enhanced cross protection [108–111,113,114]. Mucosal IgA antibodies were shown to be effective in neutralizing viruses and in inhibiting hemagglutination of red blood cells by influenza virus [115]. Mice that were intranasally immunized with influenza vaccines were found to protect against influenza viruses that are antigenically different [110,114]. Also, antibodies induced by intranasal immunization were cross-reactive and the ability of the vaccines to produce cross-reactive antibodies showed correlation with the efficacy of cross protection [108,116]. Comparable systemic and mucosal immune responses were demonstrated to be induced after intranasal immunization of mice with suspended whole inactivated virus or virus-like particle vaccines [108,110,111,117].
An early study demonstrated that aerosolized inactivated vaccine provided protection against illness in man after intranasal immunization [118]. However, inactivated and split influenza vaccines are likely to be less immunogenic via the intranasal route compared to the intramuscular delivery, thus requiring the use of mucosal adjuvants. Therefore, various mucosal adjuvants are being developed and tested. Mostly using mouse models, potential mucosal adjuvants for intranasal vaccine delivery that have been tested include detoxified lipopolysaccharide [119], TLR-9 agonist [17], immunostimulatory complexes, MF59 emulsion [120], TLR3 agonists [121], chitosan [9], and bacterial outer membrane complex [122]. Bacterial toxins, such as cholera toxin (CT) and the closely analogous heat-labile enterotoxin (LT) and their derivatives, have been used as potential mucosal adjuvants in experimental models and clinical trials to enhance the immunogenicity of mucosal vaccines [110,123–125]. However, in humans, some side effects including facial paralysis (Bell’s Palsy) and an adverse event of facial nerve disorder when given intranasally were reported to be associated with intranasal influenza vaccination with an inactivated virosomal vaccine formulated bacterial toxin [126–128].
In contrast to killed and split vaccines, live attenuated influenza vaccines FluMist™ (MedImmune) [15] and Nasovac™ (Serum Institute of India, Ltd., Pune, India) [129] are licensed for intranasal delivery in humans. Humans in clinical trials receiving nasal live attenuated influenza virus vaccines showed cross reactive hemagglutination inhibition and cross protection against variants not present in the vaccine [130,131]. The efficacy data of Nasovac are not sufficiently available yet although there are no serious side effects [129]. FluMist vaccine is generally well tolerated with runny nose/nasal congestion being the most common adverse events mild to moderate in severity. Compared with intramuscular trivalent influenza vaccines, FluMist is likely to increase the incidence of medically defined wheezing in vaccine naïve children aged less than 24 months [132] and is associated with higher frequency of hospitalization in young children aged 6 to 11 months [49]. Thus, FluMist is not approved for young children of less than 2 years old. The meta-analysis of intranasal live attenuated influenza vaccine in children aged 2–17 years demonstrated that live attenuated influenza vaccine showed higher efficacy after 2 doses in year 1 and revaccination in year 2, when compared with inactivated influenza vaccine [133]. Based on results of meta-analysis of clinical studies comparing the efficacies of intranasal live vaccines and inactivated subunit influenza vaccines in all aged groups, both vaccines were similarly efficacious in preventing culture confirmed influenza illness [134]. However, their compartmental responses were different as reporting that the live virus vaccine was lower in inducing serum hemagglutination inhibiting antibodies but higher in levels of local IgA antibodies compared to inactivated virus vaccines [134]. The seroconversion efficacy of FluMist is diminished in adults or in the elderly populations who have pre-existing immunity [135]. Intramuscular trivalent influenza vaccines are recommended in the elderly and the population at high risk of influenza-related complications [136]. Therefore, the choice between the two vaccines needs to be made by weighing the advantage of the attractive intranasal administration of live vaccines against concerns about the risks of using infectious influenza virus at large scale as well as potential gene reassortment with new influenza strains. FluMist seasonal influenza vaccine is one of most successful intranasal influenza vaccine, and effective and well tolerated in children, adolescents, and some adult populations.
In clinical studies with intranasal vaccine delivery, influenza split vaccines adjuvanted with bacterial outer membrane proteins (proteasome) were administered to nostrils of humans using a metered-dose nasal spray pump delivering the vaccine in aerosolized droplets of mean particle size of between 40–50 μM [137]. Individuals who received intranasal proteasome influenza vaccines consistently induced significant increases in serum antibodies specific to influenza as measured by hemagglutination inhibition titers and mucosal IgA antibodies against different strains of influenza virus [138]. The proteasome influenza vaccine delivered intranasally induced significant increases in serum antibody titers as well as mucosal IgA antibodies in healthy adults 18–45 years of age, and proteasome adjuvant formulation was needed to achieve this response [137]. The efficacy of a trivalent intranasal proteosome influenza formulation was determined in an experimental human challenge study using a live influenza virus infection homologous to the vaccine strain. The two dose regimes of intranasal proteosome vaccines were 100% protective against febrile illness while the single dose showed 65% efficacy as determined with the laboratory confirmation of influenza virus [137]. Development of safe and effective non-live influenza nasal vaccines would be significant since non-live vaccines present no risk of transmission of live virus after vaccination.
Pulmonary vaccination
Lungs contain local antigen-presenting cells such as macrophage and dendritic cells as well as brochoalveolar lymphoid tissues [139,140]. Pulmonary vaccination was shown to induce both systemic and local IgG/IgA immunity [141]. Aerosol vaccination with inactivated influenza virus droplets ranging in size from 1 μm to 100 μm was assessed in clinical studies [118,142]. After prime-boost immunizations of aerosolized vaccines in humans, substantial levels of cross-reactive mucosal antibodies and satisfactory protection were reported [118,142]. In a comparative study, the efficacy of aerosol administration was relatively lower in protection rates than subcutaneous vaccination in healthy volunteers [143].
In preclinical studies, a small amount of vaccine could induce substantial levels of protective immunity when delivered to the deep lungs in combination with an immune complex adjuvant, and long-term and immune memory responses were demonstrated to be induced by adjuvanted influenza pulmonary vaccination [144–146]. Importantly, pulmonary delivery was shown to be more effective than intranasal immunization, from a study of different respiratory sites of antigen deposition [147]. Consistent with this study, we found that the suspended vaccine volumes used for intranasal immunization significantly influenced the levels of IgG and IgA antibodies detected in sera after intranasal delivery. Use of 50 μl volume of 1 μg of influenza virus-like particle vaccine induced more than 10 fold higher levels of serum IgG antibodies than those from the use of 10 fold less volume (5 μl) of the same amount (1 ug) of influenza virus-like particle vaccine (unpublished data). It is assumed that use of higher volumes of vaccine solution will make it more delivered to the lower respiratory tract and deep into the lungs. It was reported that aerosol infection of mice induced more pathogenicity than intranasal delivery of the same virus [148]. Also, live attenuated virus vaccine that replicates in the lungs was shown to be more immunogenic and protective immunity in mice [149]. Therefore, it might be possible that intranasal immunization with 50 μl of liquid vaccines in mice make substantial amounts of vaccines available targeted to the lower respiratory tracts and lungs as well as to the nasal cavity. This would provide the rationale explanations for the induction of effective systemic and mucosal immunity and cross protection after intranasal immunization with 50 μl of non-replicating influenza vaccines in a mouse model [108–111,114,150].
In summary, despite some promising clinical trials and successful pre-clinical studies, no pulmonary influenza vaccines are available. Effective inhaler devices/systems with reliable reproducibility need to be developed. Also, additional data should be obtained to substantiate the safety of pulmonary vaccine delivery.
Expert commentary & five-year view
Compared to mucosal routes such as oral or intranasal immunization [112,150], a small dose of microneedle vaccines is expected to be immunogenic and to provide protective immunity (Table 1). In a mouse model, solid microneedles were used successfully to deliver various forms of influenza vaccines including soluble hemagglutinin protein antigens [151], inactivated whole influenza viruses [58–60], and virus-like particle vaccines [66,67]. Microneedle vaccines could induce comparable or superior protective immune responses in mice, which are similar to the findings reported from clinical studies of intradermal influenza vaccines (Table 1). Intradermal delivery of influenza vaccines reported promising results with older people or infants less than 1 year old [39,42–44,52], suggesting that skin vaccination could be a promising alternative site to intramuscular route. In addition, solid microneedle vaccines may be developed as a patch-based platform enabling self-administration possible by patient themselves. The simple and small size of microneedle systems would facilitate the storage and distribution to the central locations or even to individual households by the postal service. The solid microneedle vaccines stored for a month at 25°C provided good protection [77], indicating a possible alternative to cold-chain current influenza vaccines. However, there is a significant gap in developing marketable application devices for implementing solid microneedle vaccination.
Despite some promising results with solid microneedle vaccination using mouse models, solid coated microneedle influenza vaccines and their efficacies should be tested and assessed in more relevant animals (pig, guinea pig and monkey models) that are considered to have similar skin physiology as humans. Most importantly, efficacy of solid microneedle vaccination should be tested in human clinical studies. We still do not well understand the protective immune correlates and mechanisms of immune responses after delivering vaccines to the skin. Also, the T cell immune responses would be improved by vaccination in the skin as implicated by intradermal delivery of influenza vaccines to the elderly [42]. Similar results of improved cellular immune responses were obtained with microneedle vaccination in the skin of mice [59,61,67]. However, the capability of microneedle vaccination to induce mucosal immune responses still remains to be investigated. As well, cross-reactive immune responses and cross protection after microneedle vaccinations in the skin are to be carried out in future. More importantly, the stability of microneedle vaccines in dry formulation is a difficult problem to be resolved and needs continued studies. Therefore, substantial limitations exist in regarding the development of coating and manufacturing microneedles in an amenable way for commercialization and mass vaccination. Theoretically a patch form of solid microneedle vaccines might be possible but there is a long way for commercialization of solid microneedles and for their practical applications.
Mucosal immunization is another attractive strategy. Its major rationale is that many pathogens enter the body via mucosal surfaces. In terms of convenience of vaccination and acceptability to humans, oral vaccination is an attractive route. The challenge is how we can develop effective oral influenza vaccines. Vaccines that are administered orally are diluted in mucosal secretions, degraded or destabilized in the acidic stomach condition, attacked by enzymes, and excluded by mucosal barriers. So, relatively 10 to 100 fold large doses of vaccines are needed to induce protective immune responses [112]. M cells particularly adhere to microparticles and actively transport them into Peyer’s patches in intestines. Therefore, ligands targeting M cells, incorporated into microparticles would be a promising approach to increase the efficacy of uptake of oral vaccines in intestines [103,152]. Challenges in developing oral vaccination in humans include the requirement for higher doses of vaccines and lower efficacy in inducing systemic antibody responses [95] in contrast to studies in mice [101,112,153]. Another challenge is to better understand how oral vaccination can induce systemic responses and local mucosal immune responses. In addition to inducing mucosal immune responses, much effort needs to be invested to design and develop effective oral vaccines to enhance systemic immune responses as well by clinical trials.
An ideal vaccination at a single site would be to induce protective immune responses in the systemic circulating system as well as at the relevant mucosal sites. In this aspect, nasal/pulmonary delivery of influenza vaccines has particular potential [118,141]. The advantage of nasal route is its superiority in conferring cross protection against antigenically different influenza viruses compared to intramuscular immunization, indicating that nasal immunization might be particularly effective for protection against a breadth of respiratory pathogens [108,110,111,114]. However, most promising results were obtained from mouse studies where intranasal immunization is carried out by liquid drops without using special devices of spray types. Intranasal delivery of inactivated virosome vaccines to humans was shown to be related with some side effects [154,155]. Nonetheless, intranasal route is still a promising one since the successful intranasal vaccines (FluMist, Proteosome influenza vaccine) have been developed and licensed.
Both mucosal and systemic immune effectors are likely to enhance protection against most pathogens. Therefore, taking advantages of intrinsic immunological properties of delivery sites, effective vaccine strategies might be prime-boost combinations that involve mucosal and systemic routes. Mucosal delivery of vaccines might prime the immune system for both systemic and mucosal responses, probably by stimulating the expression of homing receptors by responding lymphocytes [156]. However, it largely remains to be investigated how prime-boost combinations of different vaccine delivery sites may induce protective systemic and mucosal immune responses.
Table 2.
Comparative summary of microneedle and mucosal delivery of influenza vaccines
| Delivery | Route | Formulation | Dose | Comments |
|---|---|---|---|---|
| Hollow microneedles | Intradermal | Liquid | Low/comparable | Licensed |
| Solid microneedles | Skin | Solid/stabilizer | Low/comparable | preclinical |
| Dissolving microneedles | Skin | Solid/stabilizer | Low/comparable | preclinical |
| Epidermal powder | Skin | Powder/stabilizer | Low/comparable | Clinic*/preclinic |
| Oral | Oral | Liquid/microparticles | High/adjuvant | Clinic*/preclinic |
| FluMist sprayer | Nasal | Liquid spray | Low (live vaccine) | Licensed |
| Intranasal | Nasal | Liquid spray | High/adjuvant | Clinic*/preclinic |
| Pulmonary | Lung | Liquid/Aerosol | Moderate/adjuvant | Clinic*/preclinic |
Clinical studies are limited.
Key Issues.
Delivery of vaccines to the skin is possible and would be easy using a patch form of microneedles as a self-administrative device, which might provide improved or comparable protection as intramuscular vaccination.
The stability issues associated with coating of vaccines into solid microneedles remain to be resolved.
Due to differences in skin physiology, additional preclinical studies using more relevant animal models (pigs, monkeys) and clinical studies should be performed before developing commercial microneedle vaccines.
Oral delivery of influenza vaccines is a feasible option but there is a gap in systemic antibody responses reported between mouse animal studies and clinical trials.
The requirement of high vaccine doses and repeated immunizations is a challenging problem in developing effective oral influenza vaccine formulations.
There is a limitation in evaluating and interpreting mouse models for intranasal or pulmonary delivery probably due to the lack of information regarding clear description on sites of antigen deposition (upper and lower respiratory tracts, lungs).
The safety concerns on adjuvants and the availability of reliable inhalation devices for intranasal and pulmonary vaccine delivery should be addressed as future studies.
Acknowledgments
This work was supported in part by NIH/NIAID grants AI087782 (S.M.K.) and AI093772 (S.M.K.), and the Korea Ginseng Society (S.M.K).
References
- 1.Stephenson I, Nicholson KG. Influenza: vaccination and treatment. Eur Respir J. 2001;17(6):1282–1293. doi: 10.1183/09031936.01.00084301. [DOI] [PubMed] [Google Scholar]
- 2.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 3*.Plotkin SA. Vaccines: past, present and future. Nat Med. 2005;11(4 Suppl):S5–11. doi: 10.1038/nm1209. This article is an excellent review of current vaccines available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bulletin of the World Health Organization. 1999;77(10):801–807. [PMC free article] [PubMed] [Google Scholar]
- 5.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 6.Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. The American journal of tropical medicine and hygiene. 2003;68(3):341–344. [PubMed] [Google Scholar]
- 7.Breau LM, McGrath PJ, Craig KD, Santor D, Cassidy KL, Reid GJ. Facial expression of children receiving immunizations: a principal components analysis of the child facial coding system. The Clinical journal of pain. 2001;17(2):178–186. doi: 10.1097/00002508-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26(26):3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4(3):211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie R, Bourgeois AL, Frech SA, et al. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine. 2007;25(18):3684–3691. doi: 10.1016/j.vaccine.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24(35–36):6110–6119. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Glenn GM, Taylor DN, Li X, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6(12):1403–1406. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 14.Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza(R) 9 mug intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Advances in therapy. 2011;28(8):640–649. doi: 10.1007/s12325-011-0042-0. [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004;103(1–2):177–185. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 16*.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. This article reports an extensive review on microneedle devices, types, and safety issues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prausnitz MR, Langer R. Transdermal drug delivery. Nature Biotechnology. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birchall J, Coulman S, Pearton M, et al. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch micro-fabricated micro-needles. Journal of drug targeting. 2005;13(7):415–421. doi: 10.1080/10611860500383705. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Pearton M, Allender C, Brain K, et al. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharmaceutical research. 2008;25(2):407–416. doi: 10.1007/s11095-007-9360-y. [DOI] [PubMed] [Google Scholar]
- 21.Dean CH, Alarcon JB, Waterston AM, et al. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin. 2005;1(3):106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 22.Mikszta JA, Dekker JP, 3rd, Harvey NG, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infection and immunity. 2006;74(12):6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prausnitz MR. Microneedles for transdermal drug delivery. Advanced drug delivery reviews. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Widera G, Johnson J, Kim L, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007a;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharmaceutical research. 2007b;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 27.Pearton M, Kang SM, Song JM, et al. Changes in human Langerhans cells following intradermal injection of influenza virus-like particle vaccines. PLoS One. 2010;5(8):e12410. doi: 10.1371/journal.pone.0012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharmaceutical research. 2006;23(5):1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birchall JC. Microneedle array technology: the time is right but is the science ready? Expert Rev Med Devices. 2006;3(1):1–4. doi: 10.1586/17434440.3.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17(4):187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14(4):375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Atmar RL, Patel SM, Keitel WA. Intanza((R)): a new intradermal vaccine for seasonal influenza. Expert review of vaccines. 2010;9(12):1399–1409. doi: 10.1586/erv.10.134. This is a good review article for licensed influenza intradermal vaccines. [DOI] [PubMed] [Google Scholar]
- 37.Laurent PE, Bonnet S, Alchas P, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25(52):8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Laurent PE, Pettis R, Easterbrook W, Berube J. Evaluating new hypodermic and intradermal injection devices. Med Device Technol. 2006;17(2):16–19. [PubMed] [Google Scholar]
- 39.Duggan ST, Plosker GL. Intanza 15 microg intradermal seasonal influenza vaccine: in older adults (aged >or=60 years) Drugs & aging. 2010;27(7):597–605. doi: 10.2165/11203880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Falsey AR. New emerging technologies and the intradermal route: the novel way to immunize against influenza. Vaccine. 2010;28(Suppl 4):D24–32. doi: 10.1016/j.vaccine.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC medicine. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198(5):650–658. doi: 10.1086/590434. This paper is a good research article demonstrating the immunological superiority of intradermal vaccines in the elderly. [DOI] [PubMed] [Google Scholar]
- 43.Coudeville L, Andre P, Bailleux F, Weber F, Plotkin S. A new approach to estimate vaccine efficacy based on immunogenicity data applied to influenza vaccines administered by the intradermal or intramuscular routes. Human vaccines. 2010;6(10):841–848. doi: 10.4161/hv.6.10.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27(52):7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 45.Van Damme P, Arnou R, Kafeja F, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC infectious diseases. 2010;10:134. doi: 10.1186/1471-2334-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? The Journal of infectious diseases. 2008;198(5):632–634. doi: 10.1086/590435. [DOI] [PubMed] [Google Scholar]
- 47.Chiu SS, Peiris JS, Chan KH, Wong WH, Lau YL. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics. 2007;119(6):1076–1082. doi: 10.1542/peds.2006-3176. [DOI] [PubMed] [Google Scholar]
- 48.Laurent A, Mistretta F, Bottigioli D, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine. 2007;25(34):6423–6430. doi: 10.1016/j.vaccine.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 49**.Belshe RB, Newman FK, Wilkins K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25(37–38):6755–6763. doi: 10.1016/j.vaccine.2007.06.066. This paper is an excellent report demonstrating the well-designed dose comparisons of influezna vaccines between intradermal and intramusucular routes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(10):1331–1338. doi: 10.1086/652144. [DOI] [PubMed] [Google Scholar]
- 51.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27(3):454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 52.Sugimura T, Ito Y, Tananari Y, et al. Improved antibody responses in infants less than 1 year old using intradermal influenza vaccination. Vaccine. 2008;26(22):2700–2705. doi: 10.1016/j.vaccine.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25(4):659–663. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 54.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 55.La Montagne JR, Fauci AS. Intradermal influenza vaccination–can less be more? N Engl J Med. 2004;351(22):2330–2332. doi: 10.1056/NEJMe048314. [DOI] [PubMed] [Google Scholar]
- 56.Langley JM. Intradermal injection of reduced-dose influenza vaccine was immunogenic in young adults. ACP J Club. 2005;142(3):68–69. [PubMed] [Google Scholar]
- 57.Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7(8):1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Q, Zarnitsyn VG, Ye L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106(19):7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27(49):6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4(3):e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4(9):e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amorij JP, Huckriede A, Wilschut J, Frijlink HW, Hinrichs WL. Development of Stable Influenza Vaccine Powder Formulations: Challenges and Possibilities. Pharmaceutical research. 2008 doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of Microneedles Coated with Influenza Virus-like Particle Vaccine. AAPS PharmSciTech. 2010;11(3):1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song JM, Kim YC, Barlow PG, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88(2):244–247. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quan FS, Kim YC, Vunnava A, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song JM, Kim YC, Lipatov AS, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010;17(9):1381–1389. doi: 10.1128/CVI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. The Journal of infectious diseases. 2011;204(4):582–591. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. Journal of controlled release : official journal of the Controlled Release Society. 2010;147(3):326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201(2):190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Becker G, Sornasse T, Nabavi N, et al. Immunoglobulin isotype regulation by antigen-presenting cells in vivo. Eur J Immunol. 1994;24(7):1523–1528. doi: 10.1002/eji.1830240710. [DOI] [PubMed] [Google Scholar]
- 72.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. The Journal of clinical investigation. 2004;113(7):998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guebre-Xabier M, Hammond SA, Epperson DE, Yu J, Ellingsworth L, Glenn GM. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J Virol. 2003;77(9):5218–5225. doi: 10.1128/JVI.77.9.5218-5225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hon H, Jacob J. Tracking dendritic cells in vivo: insights into DC biology and function. Immunol Res. 2004;29(1–3):69–80. doi: 10.1385/IR:29:1-3:069. [DOI] [PubMed] [Google Scholar]
- 75.Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus-like particles. Virus research. 2009;143(2):140–146. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharmaceutical research. 2010;28(1):135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maa YF, Ameri M, Shu C, Payne LG, Chen D. Influenza vaccine powder formulation development: spray-freeze-drying and stability evaluation. J Pharm Sci. 2004;93(7):1912–1923. doi: 10.1002/jps.20104. [DOI] [PubMed] [Google Scholar]
- 79.Amorij JP, Meulenaar J, Hinrichs WL, et al. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine. 2007;25(35):6447–6457. doi: 10.1016/j.vaccine.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 80.Chen D, Endres RL, Erickson CA, et al. Epidermal immunization by a needle-free powder delivery technology: immunogenicity of influenza vaccine and protection in mice. Nat Med. 2000;6(10):1187–1190. doi: 10.1038/80538. [DOI] [PubMed] [Google Scholar]
- 81.Chen D, Weis KF, Chu Q, et al. Epidermal powder immunization induces both cytotoxic T-lymphocyte and antibody responses to protein antigens of influenza and hepatitis B viruses. J Virol. 2001;75(23):11630–11640. doi: 10.1128/JVI.75.23.11630-11640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen D, Periwal SB, Larrivee K, et al. Serum and mucosal immune responses to an inactivated influenza virus vaccine induced by epidermal powder immunization. J Virol. 2001;75(17):7956–7965. doi: 10.1128/JVI.75.17.7956-7965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dean HJ, Chen D. Epidermal powder immunization against influenza. Vaccine. 2004;23(5):681–686. doi: 10.1016/j.vaccine.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 84.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nature reviews. Immunology. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 85.Brandtzaeg P, Baekkevold ES, Farstad IN, et al. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunology today. 1999;20(3):141–151. doi: 10.1016/s0167-5699(98)01413-3. [DOI] [PubMed] [Google Scholar]
- 86.Modlin JF, Onorato IM, McBean AM, et al. The humoral immune response to type 1 oral poliovirus vaccine in children previously immunized with enhanced potency inactivated poliovirus vaccine or live oral poliovirus vaccine. Am J Dis Child. 1990;144(4):480–484. doi: 10.1001/archpedi.1990.02150280102022. [DOI] [PubMed] [Google Scholar]
- 87.Levine MM, Hone D, Tacket C, Ferreccio C, Cryz S. Clinical and field trials with attenuated Salmonella typhi as live oral vaccines and as “carrier” vaccines. Res Microbiol. 1990;141(7–8):807–816. doi: 10.1016/0923-2508(90)90114-6. [DOI] [PubMed] [Google Scholar]
- 88.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 2010;8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandran A, Santosham M. RotaTeq: a three-dose oral pentavalent reassortant rotavirus vaccine. Expert review of vaccines. 2008;7(10):1475–1480. doi: 10.1586/14760584.7.10.1475. [DOI] [PubMed] [Google Scholar]
- 90.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338(20):1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 91.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. The Journal of clinical investigation. 1997;100(1):6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. American journal of physiology. Gastrointestinal and liver physiology. 2001;280(4):G710–719. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 93.Fooks AR. Development of oral vaccines for human use. Current opinion in molecular therapeutics. 2000;2(1):80–86. [PubMed] [Google Scholar]
- 94.des Rieux A, Fievez V, Momtaz M, et al. Helodermin-loaded nanoparticles: characterization and transport across an in vitro model of the follicle-associated epithelium. J Control Release. 2007;118(3):294–302. doi: 10.1016/j.jconrel.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 95.Lazzell V, Waldman RH, Rose C, Khakoo R, Jacknowitz A, Howard S. Immunization against influenza in humans using an oral enteric-coated killed virus vaccine. J Biol Stand. 1984;12(3):315–321. doi: 10.1016/s0092-1157(84)80012-8. [DOI] [PubMed] [Google Scholar]
- 96.Bergmann KC, Waldman RH, Tischner H, Pohl WD. Antibody in tears, saliva and nasal secretions following oral immunization of humans with inactivated influenza virus vaccine. Int Arch Allergy Appl Immunol. 1986;80(1):107–109. doi: 10.1159/000234034. [DOI] [PubMed] [Google Scholar]
- 97.Bergmann KC, Waldman RH. Oral immunization with influenza virus: experimental and clinical studies. Curr Top Microbiol Immunol. 1989;146:83–89. doi: 10.1007/978-3-642-74529-4_9. [DOI] [PubMed] [Google Scholar]
- 98.Avtushenko SS, Sorokin EM, Zoschenkova NY, Zacharova NG, Naichin AN. Clinical and immunological characteristics of the emulsion form of inactivated influenza vaccine delivered by oral immunization. Journal of biotechnology. 1996;44(1–3):21–28. doi: 10.1016/0168-1656(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 99.Mann JF, Shakir E, Carter KC, Mullen AB, Alexander J, Ferro VA. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27(27):3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 100.Pang GT, Clancy RL, O’Reilly SE, Cripps AW. A novel particulate influenza vaccine induces long-term and broad-based immunity in mice after oral immunization. J Virol. 1992;66(2):1162–1170. doi: 10.1128/jvi.66.2.1162-1170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takase H, Murakami Y, Endo A, Ikeuchi T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine. 1996;14(17–18):1651–1656. doi: 10.1016/s0264-410x(96)00128-4. [DOI] [PubMed] [Google Scholar]
- 102.Ghazi HO, Potter CW, Smith TL, Jennings R. Comparative antibody responses and protection in mice immunised by oral or parenteral routes with influenza virus subunit antigens in aqueous form or incorporated into ISCOMs. J Med Microbiol. 1995;42(1):53–61. doi: 10.1099/00222615-42-1-53. [DOI] [PubMed] [Google Scholar]
- 103.Lei H, Xu Y, Chen J, Wei X, Lam DM. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology. 2010;407(2):319–324. doi: 10.1016/j.virol.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 104.Van Daal GJ, Beusenberg FD, So KL, et al. Protection against influenza A virus infection in mice by oral immunization with a polyvalent bacterial lysate. Int J Immunopharmacol. 1991;13(7):831–840. doi: 10.1016/0192-0561(91)90034-5. [DOI] [PubMed] [Google Scholar]
- 105.Amorij JP, Westra TA, Hinrichs WL, Huckriede A, Frijlink HW. Towards an oral influenza vaccine: comparison between intragastric and intracolonic delivery of influenza subunit vaccine in a murine model. Vaccine. 2007;26(1):67–76. doi: 10.1016/j.vaccine.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 106.McGhee JR, Yamamoto M, Kang DW, et al. Isotype of anti-SIV responses in infected rhesus macaques and in animals immunized by mucosal routes. AIDS research and human retroviruses. 1992;8(8):1389. doi: 10.1089/aid.1992.8.1389. [DOI] [PubMed] [Google Scholar]
- 107.Waldman RH, Bergmann KC, Stone J, et al. Age-dependent antibody response in mice and humans following oral influenza immunization. Journal of clinical immunology. 1987;7(4):327–332. doi: 10.1007/BF00915555. [DOI] [PubMed] [Google Scholar]
- 108.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003;21(23):3212–3218. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 109.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One. 2011;5(11):e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75(11):5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perrone LA, Ahmad A, Veguilla V, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83(11):5726–5734. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quan FS, Compans RW, Kang SM. Oral vaccination with inactivated influenza vaccine induces cross-protective immunity. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 114.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82(3):1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mazanec MB, Lamm ME, Lyn D, Portner A, Nedrud JG. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992;23(1–2):1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- 116.Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14(4):350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 117.Alsharifi M, Furuya Y, Bowden TR, et al. Intranasal flu vaccine protective against seasonal and H5N1 avian influenza infections. PLoS One. 2009;4(4):e5336. doi: 10.1371/journal.pone.0005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waldman RH, Mann JJ, Small PA., Jr Immunization against influenza. Prevention of illness in man by aerosolized inactivated vaccine. JAMA : the journal of the American Medical Association. 1969;207(3):520–524. doi: 10.1001/jama.207.3.520. [DOI] [PubMed] [Google Scholar]
- 119.Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine. 2000;18(22):2416–2425. doi: 10.1016/s0264-410x(99)00572-1. [DOI] [PubMed] [Google Scholar]
- 120.Boyce TG, Hsu HH, Sannella EC, et al. Safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccines administered intranasally to healthy adults. Vaccine. 2000;19(2–3):217–226. doi: 10.1016/s0264-410x(00)00171-7. [DOI] [PubMed] [Google Scholar]
- 121.Ichinohe T, Watanabe I, Ito S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005;79(5):2910–2919. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pal S, Luke CJ, Barbour AG, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis major outer membrane protein, using the outer surface protein A of Borrelia burgdorferi as an adjuvant, can induce protection against a chlamydial genital challenge. Vaccine. 2003;21(13–14):1455–1465. doi: 10.1016/s0264-410x(02)00680-1. [DOI] [PubMed] [Google Scholar]
- 123.Peppoloni S, Ruggiero P, Contorni M, et al. Mutants of the Escherichia coli heat-labile enterotoxin as safe and strong adjuvants for intranasal delivery of vaccines. Expert Rev Vaccines. 2003;2(2):285–293. doi: 10.1586/14760584.2.2.285. [DOI] [PubMed] [Google Scholar]
- 124.Jertborn M, Nordstrom I, Kilander A, Czerkinsky C, Holmgren J. Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect Immun. 2001;69(6):4125–4128. doi: 10.1128/IAI.69.6.4125-4128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wassen L, Schon K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44(4):408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 126.Gluck R, Mischler R, Durrer P, et al. Safety and immunogenicity of intranasally administered inactivated trivalent virosome-formulated influenza vaccine containing Escherichia coli heat-labile toxin as a mucosal adjuvant. J Infect Dis. 2000;181(3):1129–1132. doi: 10.1086/315337. [DOI] [PubMed] [Google Scholar]
- 127.Gluck R. Review of intranasal influenza vaccine. Adv Drug Deliv Rev. 2001;51(1–3):203–211. doi: 10.1016/s0169-409x(01)00174-0. [DOI] [PubMed] [Google Scholar]
- 128.Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 129.Dhere R, Yeolekar L, Kulkarni P, et al. A pandemic influenza vaccine in India: from strain to sale within 12 months. Vaccine. 2011;29(Suppl 1):A16–21. doi: 10.1016/j.vaccine.2011.04.119. [DOI] [PubMed] [Google Scholar]
- 130.Xie H, Liu TM, Lu X, et al. A live attenuated H1N1 M1 mutant provides broad cross-protection against influenza A viruses, including highly pathogenic A/Vietnam/1203/2004, in mice. The Journal of infectious diseases. 2009;200(12):1874–1883. doi: 10.1086/648405. [DOI] [PubMed] [Google Scholar]
- 131.Suguitan AL, Jr, McAuliffe J, Mills KL, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS medicine. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bergen R, Black S, Shinefield H, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. The Pediatric infectious disease journal. 2004;23(2):138–144. doi: 10.1097/01.inf.0000109392.96411.4f. [DOI] [PubMed] [Google Scholar]
- 133.Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: A meta-analysis of 8 randomized controlled studies. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.11.104. [DOI] [PubMed] [Google Scholar]
- 134.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20(9–10):1340–1353. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 135*.Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71(12):1591–1622. doi: 10.2165/11206860-000000000-00000. This article has a good review on FluMist influenza vaccines. [DOI] [PubMed] [Google Scholar]
- 136.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 137.Treanor J, Nolan C, O’Brien D, et al. Intranasal administration of a proteosome-influenza vaccine is well-tolerated and induces serum and nasal secretion influenza antibodies in healthy human subjects. Vaccine. 2006;24(3):254–262. doi: 10.1016/j.vaccine.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 138.Plante M, Jones T, Allard F, et al. Nasal immunization with subunit proteosome influenza vaccines induces serum HAI, mucosal IgA and protection against influenza challenge. Vaccine. 2001;20(1–2):218–225. doi: 10.1016/s0264-410x(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 139.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 140.Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2001;18(4):692–704. [PubMed] [Google Scholar]
- 141.Holmgren J, Czerkinsky C, Lycke N, Svennerholm AM. Mucosal immunity: implications for vaccine development. Immunobiology. 1992;184(2–3):157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- 142.Waldman RH, Wood SH, Torres EJ, Small PA., Jr Influenza antibody response following aerosal administration of inactivated virus. American journal of epidemiology. 1970;91(6):574–585. [PubMed] [Google Scholar]
- 143.Waldman RH, Bond JO, Levitt LP, et al. An evaluation of influenza immunization: influence of route of administration and vaccine strain. Bulletin of the World Health Organization. 1969;41(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- 144.Wee JL, Scheerlinck JP, Snibson KJ, et al. Pulmonary delivery of ISCOMATRIX influenza vaccine induces both systemic and mucosal immunity with antigen dose sparing. Mucosal immunology. 2008;1(6):489–496. doi: 10.1038/mi.2008.59. [DOI] [PubMed] [Google Scholar]
- 145.Vujanic A, Snibson KJ, Wee JL, et al. Long-term antibody and immune memory response induced by pulmonary delivery of influenza ISCOMATRIXTM vaccine. Clinical and vaccine immunology : CVI. 2011 doi: 10.1128/CVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vujanic A, Wee JL, Snibson KJ, et al. Combined mucosal and systemic immunity following pulmonary delivery of ISCOMATRIX adjuvanted recombinant antigens. Vaccine. 2010;28(14):2593–2597. doi: 10.1016/j.vaccine.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 147.Minne A, Louahed J, Mehauden S, Baras B, Renauld JC, Vanbever R. The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response. Immunology. 2007;122(3):316–325. doi: 10.1111/j.1365-2567.2007.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith JH, Nagy T, Barber J, Brooks P, Tompkins SM, Tripp RA. Aerosol inoculation with a sub-lethal influenza virus leads to exacerbated morbidity and pulmonary disease pathogenesis. Viral Immunol. 2011;24(2):131–142. doi: 10.1089/vim.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pena L, Vincent AL, Ye J, et al. Modifications in the polymerase genes of a swine-like triple-reassortant influenza virus to generate live attenuated vaccines against 2009 pandemic H1N1 viruses. Journal of virology. 2011;85(1):456–469. doi: 10.1128/JVI.01503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. Journal of virology. 2009;83(9):4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weldon WC, Martin MP, Zarnitsyn V, et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clinical and vaccine immunology : CVI. 2011;18(4):647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.D’Souza B, Bhowmik T, Shashidharamurthy R, Oettinger C, Selvaraj P, D’Souza M. Oral microparticulate vaccine for melanoma using M-cell targeting. Journal of drug targeting. 2011 doi: 10.3109/1061186X.2011.622395. [DOI] [PubMed] [Google Scholar]
- 153.Lidbury BA, Grissell TV, Sizer PJ, Pang GT, Clancy R, Cripps AW. Erythrocytes enhance the immunogenicity of oral vaccination with gamma irradiated influenza virus: increasing the dose of irradiation results in a significant diminution of lung IgA response. Vaccine. 1997;15(14):1529–1537. doi: 10.1016/s0264-410x(97)00090-x. [DOI] [PubMed] [Google Scholar]
- 154.Sendi P, Locher R, Bucheli B, Battegay M. Intranasal influenza vaccine in a working population. Clin Infect Dis. 2004;38(7):974–980. doi: 10.1086/386330. [DOI] [PubMed] [Google Scholar]
- 155.Sendi P, Locher R, Bucheli B, Battegay M. The decision to get vaccinated against influenza. Am J Med. 2004;116(12):856–858. doi: 10.1016/j.amjmed.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 156.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10(3–4):313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]


