Abstract
IMPORTANCE
A growing proportion of US smokers now smoke fewer than 10 cigarettes per day (CPD), and that proportion will likely rise in the future. The health effects of smoking only a few CPD over one’s lifetime are less understood than are the effects of heavier smoking, although many smokers believe that their level is modest.
OBJECTIVE
To evaluate the associations of long-term smoking of fewer than 1 or 1 to 10 CPD (low intensity) with all-cause and cause-specific mortality compared with never smoking cigarettes.
DESIGN, SETTING, AND PARTICIPANTS
Prospective cohort study of 290 215 adults in the National Institutes of Health–AARP (formerly known as the American Association of Retired Persons) Diet and Health Study who were aged 59 to 82 years in calendar years 2004–2005 (baseline). Data were gathered with a questionnaire assessing lifetime cigarette smoking history. Hazard ratios (HRs) and 95% CIs were determined for all-cause mortality and cause-specific mortality through the end of 2011. Hazard ratios and 95% CIs were estimated using Cox proportional hazards regression models using age as the underlying time metric and adjusted for sex, race/ethnicity, educational level, physical activity, and alcohol intake. Data analysis was conducted from December 15, 2015, to September 30, 2016.
EXPOSURES
Current and historical smoking intensity during 9 previous age periods (from <15 years to ≥70 years) over the lifetime assessed on the 2004–2005 questionnaire.
MAIN OUTCOMES AND MEASURES
All-cause and cause-specific mortality among current, former, and never smokers.
RESULTS
Of the 290 215 cohort participants who completed the 2004–2005 questionnaire, 168 140 were men (57.9%); the mean (SD) age was 71 (5.3) years (range, 59–82 years). Most people who smoked fewer than 1 or 1 to 10 CPD at baseline reported smoking substantially higher numbers of CPD earlier in their lives. Nevertheless, 159 (9.1%) and 1493 (22.5%) of these individuals reported consistently smoking fewer than 1 or 1 to 10 CPD in each age period that they smoked, respectively. Relative to never smokers, consistent smokers of fewer than 1 CPD (HR, 1.64; 95% CI, 1.07–2.51) and 1 to 10 CPD (HR, 1.87; 95% CI, 1.64–2.13) had a higher all-cause mortality risk. Associations were similar in women and men for all-cause mortality and were observed across a range of smoking-related causes of death, with an especially strong association with lung cancer (HR, 9.12; 95% CI, 2.92–28.47, and HR, 11.61; 95% CI, 8.25–16.35 for <1 and 1–10 CPD, respectively). Former smokers who had consistently smoked fewer than 1 or 1 to 10 CPD had progressively lower risks with younger age at cessation. For example, the HRs for consistent smokers of fewer than 1 and 1 to 10 CPD who quit at 50 years or older were 1.44 (95% CI, 1.12–1.85) and 1.42 (95% CI, 1.27–1.59), respectively.
CONCLUSIONS AND RELEVANCE
This study provides evidence that individuals who smoke fewer than 1 or 1 to 10 CPD over their lifetime have higher mortality risks than never smokers and would benefit from cessation. These results provide further evidence that there is no risk-free level of exposure to tobacco smoke.
Tobacco smoking poses a major public health challenge around the world and has been estimated to cause 5 million deaths per year globally.1 Increasing awareness of the harms of cigarette smoking and concerted tobacco control policies (eg, smoking ban laws) have led to substantial decreases in smoking prevalence in the United States and in many other countries.2 In addition, the percentage of daily smokers who smoked fewer than 10 cigarettes per day (CPD) increased from 16% to 27% from 2005 to 2014, and the proportion who did not smoke every day increased from 19% to 23%.3 Low intensity smoking (eg, ≤10 CPD) was traditionally considered a transient smoking reduction practice among individuals who were trying to quit.4 However, studies indicate that many low-intensity smokers maintain these smoking patterns for many years over their lifetime.5,6 Such a smoking pattern has historically been more common among racial and ethnic minorities who nevertheless have high rates of smoking-related morbidity and mortality.7–9
Duration of smoking, independent of numbers of CPD, is the most critical determinant of disease risk10–13; however, there are few data on the health effects of long-term, low-intensity smoking. There is a common perception, particularly among young people, that such a level of use is safe14; thus, these smokers may substantially underestimate their smoking related health risks. In the previous studies of cigarette smoking and health outcomes, current low-intensity cigarette smoking was associated with an increased risk of incident lung cancer and head and neck cancer15,16 and higher risk of overall mortality and deaths from cardiovascular disease and overall cancer than nonsmoking.17–21 However, these studies have typically examined smoking intensity at a single point in time rather than over the lifetime. Emerging evidence indicates that a substantial proportion of low-intensity smokers smoked a higher number of CPD earlier in their lives,22,23 indicating the importance of examining lifetime smoking history. To date, relatively few epidemiologic studies have investigated the disease risks of long-term, low-intensity smoking.33
To add the needed evidence, we used histories of cigarette smoking as reported on the third questionnaire of the National Institutes of Health (NIH)–AARP (formerly known as the American Association of Retired Persons) Diet and Health Study cohort. We examined the association between long-term smoking of fewer than 1 or 1 to 10 CPD (low intensity) with all-cause and cause-specific mortality among current and former smokers. We also examined the mortality risks of participants who reported smoking fewer than 1 or 1 to 10 CPD at baseline but different amounts, mostly higher, earlier in their lives.
Methods
Study Design and Population
The NIH-AARP Diet and Health Study, a prospective cohort study, has been described previously.25 From 1995 through 1996, an initial questionnaire regarding demographics, anthropometrics, lifestyle, diet, family history of cancer, and medical history was mailed to 3.5 million members of AARP, aged 50 to 71 years, who resided in 6 states (California, Florida, Pennsylvania, New Jersey, North Carolina, and Louisiana) or in 2 metropolitan areas (Atlanta, Georgia, and Detroit, Michigan). Among 617 119 respondents, 566 398 men and women successfully completed the questionnaire and constituted the cohort. The 2004–2005 questionnaire was sent by mail to a total of 502 682 participants who were believed to be alive; of these, 313 363 cohort participants (62.3%) completed the follow-up questionnaire that assessed lifetime cigarette smoking history and other factors (eg, lifestyle and medical history). This questionnaire served as study baseline for the present analysis. We excluded proxies (n = 14 072), participants who died before the start of follow-up (n = 4), and those with incomplete cigarette smoking information (n = 9072), resulting in 290 205 individuals (92.6%) in our analytic cohort (eFigure in the Supplement). The study was approved by the Special Studies Institutional Review Board of the National Cancer Institute. A letter that accompanied the baseline questionnaire informed participants that they indicated their consent to participate in the study by completing and returning the questionnaire. The participants did not receive financial compensation.
Assessment of Cigarette Smoking and Covariates
In addition to asking whether there was ever and current cigarette smoking (yes or no), the 2004–2005 follow-up questionnaire asked the participants to recall their average number of CPD during 3 age periods (<15, 15–19, 20–24, 25–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years). The participants chose 1 category that best described CPD (0, <1, 1–10, 11–20, 21–30, 31–40, 40–60, and >60 CPD) for each age range. Using this information, we determined age at smoking initiation and age at cessation for former smokers.
Reported numbers of CPD were collapsed into 6 categories (0, <1, 1–10, 11–20, 21–30, and >30 CPD) for data analysis. To assess changes in smoking intensity over the lifetime, we considered smoking from age 20 years or age period of initiation, whichever was later, until the participant’s age at baseline, because the number of CPD can vary substantially before 20 years. We excluded current smokers who reported initiating cigarette smoking at 60 years or later (n = 524) and individuals with missing data at 1 or more age periods (1956 current and 7421 former smokers) from our analysis of lifetime smoking intensity. Smokers were further stratified by whether they reported consistent categories of CPD across each age period. Former smokers who had consistently smoked fewer than 1 or 1 to 10 CPD were also stratified by age when they quit (<20, 20–29, 30–39, 40–49, and ≥50 years).
We examined the concordance of the lifetime smoking information provided on the 2004–2005 follow-up questionnaire with responses on contemporaneous smoking on the 1995–1996 questionnaire, considering fewer than 1 and 11 to 10 CPD as a single category owing to a lack of assessment of fewer than 1 CPD on the 1995–1996 questionnaire. Of the 1567 who reported lifelong smoking as 10 or fewer CPD on the 2004–2005 questionnaire, 1154 (73.6%) reported smoking 10 or fewer CPD, 383 (24.4%) reported smoking 11 to 20 CPD, and 30 (1.9%) reported smoking more than 20 CPD at their age in 1995–1996.
The 2004–2005 questionnaire additionally assessed body mass index (using self-reported height and weight), physical activity, perceived general health, and history of certain health conditions. Sex, race/ethnicity, highest achieved educational level, alcohol use, and ever regular use of pipes or cigars for 1 year or more were assessed via the 1995–1996 questionnaire.
Cohort Follow-up and Outcomes
Study participants were followed up from the date when their 2004–2005 questionnaire was returned and scanned until death or December 31, 2011, whichever came first. Mortality data, including date and cause of death, were obtained by linkage with the National Death Index (http://www.cdc.gov/nchs/ndi/index.htm). Mortality outcomes were defined using International Classification of Diseases, Ninth Edition (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), codes as following: all cancer (ICD-9 140–208, 238.6; ICD-10 C00-C97), lung cancer (ICD-9 162.2–162.9; ICD-10 C34), cardiovascular disease (heart diseases, hypertension without heart disease, cerebrovascular diseases, atherosclerosis, aortic aneurysm and dissection, and other diseases of arteries, arterioles, and capillaries: ICD-9 390–398, 401–404, 410–438, 440–441; ICD-10 I00–I13, I20–51, I160–171), and respiratory disease (pneumonia, influenza, chronic obstructive pulmonary disease, and allied conditions: ICD-9 480–487, 490–496; ICD-10 J09–J18, J40–J47).
Statistical Analysis
Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs, using age as the underlying time metric and adjusting for sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other [Asian, Pacific Islander, and American Indian or Alaskan native], and unknown), educational level (<high school, completion of high school, post–high school training, some college, completion of college, and unknown), alcohol intake, and physical activity level. Never cigarette smokers were used as the reference group. We did not adjust the analysis for body mass index and perceived general health because both factors are related to mortality and can be affected by cigarette smoking. We also examined all-cause mortality associations in men and women separately. Statistical significance was defined as P < .05, and all tests were 2-sided. Data analysis was conducted from December 15, 2015, to September 30, 2016. All analytic tests were performed using SAS, version 9.3 (SAS Institute Inc).
Results
Most of the 290 215 cohort participants (168 140 [57.9%] men and 122 075 [42.1%] women) who completed the 2004–2005 questionnaire (baseline) were in their 60s (126 575 [43.6%]) or 70s (160 352 [55.3%]), with the mean (SD) age of 71 (5.3) years (range, 59–82 years). Of these participants, there were 22 337 current smokers (7.7%), 156 405 former smokers (53.9%), and 111 473 never smokers (38.4%) at baseline. Of 19 857 current smokers with complete information on smoking intensity at different ages, 1341 (6.8%) reported smoking fewer than 1 CPD and 6036 (30.4%) smoked 1 to 10 CPD at baseline; however, most of these participants reported higher numbers of CPD earlier in their lives. Among the fewer than 1 or 1 to 10 CPD smokers at baseline, only 184 (13.7%) and 1922 (31.8%) reported smoking a maximum amount of fewer than 1 or 1 to 10 CPD during their lifetime, respectively (eTable 1 in the Supplement). Most people who smoked fewer than 1 or 1 to 10 CPD at baseline reported smoking substantially higher numbers of cigarettes earlier in their lives. Nevertheless, 159 (9.1%) and 1493 (22.5%) of these individuals reported consistently smoking fewer than 1 or 1 to 10 CPD in each age period that they smoked, respectively.
Table 1 compares demographic, lifestyle, and dietary factors, and medical history among never smokers, current smokers, and former smokers who smoked fewer than 1 or 1 to 10 CPD at baseline. Lifelong consistent smokers of fewer than 1 or 1 to 10 CPD tended to start smoking at a somewhat older age than did inconsistent smokers of the same intensity (participants who smoked these amounts at baseline but had smoked different amounts earlier in their lives). A higher proportion of consistent smokers of fewer than 1 or 1 to 10 CPD were non Hispanic black or Hispanic and had lower educational levels, less alcohol use, and less history of myocardial infarction or emphysema. A higher percentage of consistent smokers of fewer than 1 CPD reported having ever regularly used pipes or cigars (18.2%) compared with consistent smokers of 1 to 10 CPD (9.9%).
Table 1.
Demographic and Lifestyle Factors by Lifetime Smoking Statusa
| Consistent or Inconsistent CPD Over Lifetime | CPD
|
||||||
|---|---|---|---|---|---|---|---|
| Never (n = 111 473) | <1
|
1–10
|
|||||
| Current
|
Former Consistent (n = 5803) | Current
|
Former Consistent (n = 20 319) | ||||
| Inconsistent (n = 1182) | Consistent (n = 159) | Inconsistent (n = 4543) | Consistent (n = 1493) | ||||
| Age started smoking cigarettes, No. (%), yb | |||||||
|
| |||||||
| <15 | 36 (22.6) | 36 (22.6) | 679 (11.7) | 982 (21.6) | 196 (13.1) | 1153 (5.7) | |
|
| |||||||
| 15–19 | 46 (28.9) | 46 (28.9) | 2010 (34.6) | 2003 (44.1) | 569 (38.1) | 8508 (41.9) | |
|
| |||||||
| 20–24 | 40 (25.2) | 40 (25.2) | 1777 (30.6) | 1233 (27.1) | 432 (28.9) | 7676 (37.8) | |
|
| |||||||
| 25–29 | 16 (10.1) | 16 (10.1) | 560 (9.7) | 200 (4.4) | 124 (8.3) | 1596 (7.9) | |
|
| |||||||
| ≥30 | 21 (13.2) | 21 (13.2) | 777 (12.0) | 125 (2.8) | 172 (11.5) | 1386 (6.6) | |
|
| |||||||
| Ever regular use of pipe or cigar, No. (%)%b | 9980 (9.0) | 262 (22.2) | 29 (18.2) | 1304 (22.5) | 724 (15.9) | 147 (9.9) | 3865 (19.0) |
|
| |||||||
| Age at start of follow-up, median (IQR), yc | 70.9 (66.3–75.1) | 69.1 (65.5–73.7) | 68.1 (64.9–73.2) | 70.8 (66.0–75.0) | 69.3 (65.5–74.1) | 70.3 (65.9–74.9) | 71.5 (66.7–75.4) |
|
| |||||||
| Male, No. (%)b, | 54 691 (49.1) | 655 (55.4) | 90 (56.6) | 3468 (59.8) | 2236 (49.2) | 469 (31.4) | 9828 (48.4) |
|
| |||||||
| Race/ethnicity, No. (%)b | |||||||
|
| |||||||
| White (non-Hispanic) | 102 232 (91.7) | 1052 (89.0) | 132 (83.0) | 5202 (89.6) | 4141 (91.2) | 1200 (80.4) | 18 174 (89.4) |
|
| |||||||
| Black (non-Hispanic) | 3858 (3.5) | 77 (6.5) | 14 (8.8) | 276 (4.8) | 234 (5.1) | 206 (13.8) | 1033 (5.1) |
|
| |||||||
| Hispanic | 2102 (1.9) | 20 (1.7) | 5 (3.1) | 150 (2.6) | 65 (1.4) | 32 (2.1) | 492 (2.4) |
|
| |||||||
| Asian, Pacific Islander, Native American or Alaskan native | 2176 (2.0) | 17 (2.81.4) | 5 (3.1) | 104 (1.8) | 58 (2.31.3) | 28 (1.9) | 361 (1.8) |
|
| |||||||
| Educational level, No. (%)b | |||||||
|
| |||||||
| ≤HS | 23 612 (21.2) | 232 (19.6) | 52 (32.7) | 892 (15.4) | 1032 (22.7) | 445 (29.8) | 4344 (21.4) |
|
| |||||||
| Post-HS training or some college | 31 627 (28.4) | 419 (35.5) | 45 (28.3) | 1606 (27.7) | 1754 (38.6) | 533 (35.7) | 6475 (31.9) |
|
| |||||||
| ≥College | 53 877 (48.3) | 501 (42.4) | 55 (34.6) | 3176 (54.7) | 1648 (36.3) | 461 (30.9) | 8963 (44.1) |
|
| |||||||
| BMI, median (IQR)c | 26.1 (23.6–29.3) | 26.4 (23.7–29.6) | 27.2 (23.6–29.7) | 26.1 (23.6–29.3) | 25.3 (22.7–28.5) | 25.1 (22.3–28.1) | 26.0 (23.5–29.3) |
|
| |||||||
| Alcohol intake, median (IQR), g/db | 13.0 (0–76.5) | 59.0 (12.4–200.8) | 40.1 (2.5–170.7) | 30.9 (4.6–123.6) | 37.9 (5.9–160.8) | 25.3 (2.1–132.5) | 29.1 (4.1–120.7) |
|
| |||||||
| Physical activity, median (IQR), MET-h/wkc | 15.0 (4.3–36.6) | 14.4 (4.3–36.6) | 11.0 (2.4–32.5) | 16.7 (4.3–37.6) | 10.8 (2.2–28.7) | 10.8 (2.2–27.0) | 17.8 (4.3–39.0) |
|
| |||||||
| Self-reported fair ot poor health status, No. (%) | 11 399 (10.2) | 179 (15.1) | 23 (14.5) | 573 (9.9) | 824 (18.1) | 174 (11.7) | 2201 (10.8) |
|
| |||||||
| Comorbidity, No. (%)d | |||||||
|
| |||||||
| MI | 15 388 (13.8) | 211 (17.9) | 18 (11.3) | 886 (15.3) | 868 (19.1) | 160 (10.7) | 2907 (14.3) |
|
| |||||||
| Stroke | 3251 (2.9) | 53 (4.5) | 5 (3.1) | 191 (3.3) | 198 (4.4) | 62 (4.2) | 605 (3.0) |
|
| |||||||
| Emphysema | 4352 (3.9) | 147 (12.4) | 7 (4.4) | 236 (4.1) | 891 (19.6) | 120 (8.0) | 1018 (5.0) |
|
| |||||||
| Cancer | 28 030 (25.2) | 287 (24.3) | 38 (23.9) | 1506 (26.0) | 1200 (26.4) | 337 (22.6) | 5300 (26.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HS, high school; IQR, interquartile range; MI, myocardial infarction.
Includes individuals with complete information on smoking frequency at different age stages (20–24, 25–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years), including those who reported not smoking at 1 or more age stages but reported smoking at a later age stage. Excludes those who started smoking at 60 years or older.
Assessed in the initial cohort questionnaire in 1995–1996.
Assessed in the follow-up questionnaire in 2004–2005.
Self-reported ever diagnosis before the follow-up survey in 2004.
During a mean (SD) follow-up of 6.6 (1.3) years, 37 331 participants died. As expected, there was a dose-dependent association between reported number of CPD at baseline with all-cause mortality as well as with deaths from examined smoking-related outcomes (Table 2). Even those who reported smoking fewer than 1 CPD (HR, 1.99; 95% CI, 1.76–2.25) or 1 to 10 CPD (HR, 2.60; 95% CI, 2.45–2.75) had an increased risk of all-cause mortality. Relative to never smokers, lifelong consistent smokers of fewer than 1 and 1 to 10 CPD were at increased risk of all-cause mortality (HR, 1.64; 95% CI, 1.07–2.51, and HR, 1.87; 95% CI, 1.64–2.13, respectively) (Table 3). However, these risks were lower than the risks in those who reported fewer than 1 or 1 to 10 CPD smoking at baseline but reported smoking more or less CPD in earlier age periods (HR, 2.05; 95% CI, 1.77–2.37, and HR, 2.87; 95% CI, 2.69–3.06, respectively). Positive associations were observed for all examined smoking-related causes of death among consistent smokers of 1 to 10 CPD with stronger associations for lung cancer (HR, 11.61; 95% CI, 8.25–16.35) and respiratory disease (HR, 6.00; 95% CI, 4.05–8.89). With 24 deaths occurring among the 159 consistent smokers of fewer than 1 CPD, our statistical power for individual causes of death were low. Nevertheless, we observed an association with lung cancer (HR, 9.12; 95% CI, 2.92–28.47) and cardiovascular disease (HR, 2.78; 95% CI, 1.49–5.18), although no association was noted for overall cancer mortality.
Table 2.
Smoking Status With All-Cause and Cause-Specific Mortality in the NIH-AARP Diet and Health Study Cohorta
| Characteristic | No. | All Cause (n = 37 331)
|
All Cancer (n = 13 762)
|
Lung Cancer (n = 3801)
|
Cardiovascular Disease (n = 9496)
|
Respiratory Disease (n = 3139)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | ||
| Smoking status and CPD at baseline (2004–2005 questionnaire) | |||||||||||
|
| |||||||||||
| Never | 111 473 | 9821 (9) | 1 [Reference] | 3468 | 1 [Reference] | 253 | 1 [Reference] | 2631 | 1 [Reference] | 324 | 1 [Reference] |
|
| |||||||||||
| <1 | 1754 | 266 (15) | 1.99 (1.76–2.25) | 92 | 1.91 (1.55–2.35) | 37 | 10.73 (7.59–15.15) | 63 | 1.71 (1.33–2.21) | 26 | 6.38 (4.27–9.51) |
|
| |||||||||||
| 1–10 | 6627 | 1360 (21) | 2.60 (2.45–2.75) | 522 | 2.83 (2.58–3.11) | 253 | 18.38 (15.42–22.91) | 299 | 2.13 (1.89–2.40) | 197 | 11.04 (9.23–13.19) |
|
| |||||||||||
| 11–20 | 7721 | 1722 (22) | 2.96 (2.81–3.11) | 713 | 3.44 (3.17–3.74) | 374 | 24.05 (20.46–28.26) | 402 | 2.61 (2.34–2.90) | 261 | 12.76 (10.81–15.05) |
|
| |||||||||||
| 21–30 | 3329 | 900 (27) | 3.57 (3.33–3.82) | 390 | 4.40 (3.96–4.89) | 228 | 34.54 (28.83–41.39) | 195 | 2.89 (2.50–3.35) | 136 | 15.52 (12.67–19.01) |
|
| |||||||||||
| >30 | 2906 | 854 (29) | 3.91 (3.65–4.21) | 370 | 4.80 (4.30–5.35) | 212 | 36.83 (30.61–44.33) | 179 | 3.05 (2.62–3.55) | 151 | 20.17 (16.57–24.56) |
|
| |||||||||||
| Smoking status at baseline, stratified by earlier status in lifetimeb | |||||||||||
|
| |||||||||||
| Never | 111 473 | 9821 | 1 [Reference] | 3468 | 1 [Reference] | 253 | 1 [Reference] | 2631 | 1 [Reference] | 324 | 1 [Reference] |
|
| |||||||||||
| Consistent CPD throughout lifetime | |||||||||||
|
| |||||||||||
| <1 | 159 | 21 (13) | 1.64 (1.07–2.51) | 4 | 0.90 (0.34–2.39) | 3 | 9.12 (2.92–28.47) | 10 | 2.78 (1.49–5.18) | 0 | NDc |
|
| |||||||||||
| 1–10 | 1493 | 232 (16) | 1.87 (1.64–2.13) | 91 | 2.10 (1.71–2.59) | 38 | 11.61 (8.25–16.35) | 50 | 1.50 (1.13–1.99) | 27 | 6.00 (4.05–8.89) |
|
| |||||||||||
| 11–20 | 2727 | 603 (22) | 2.91 (2.68–3.16) | 263 | 3.57 (3.14–4.05) | 151 | 27.35 (22.32–33.52) | 139 | 2.50 (2.10–2.97) | 88 | 12.24 (9.64–15.55) |
|
| |||||||||||
| 21–30 | 675 | 192 (28) | 3.73 (3.23–4.31) | 91 | 4.96 (4.02–6.12) | 52 | 39.84 (29.52–53.76) | 39 | 2.77 (2.02–3.80) | 27 | 16.18 (10.84–24.15) |
|
| |||||||||||
| >30 | 455 | 145 (32) | 4.20 (3.56–4.96) | 65 | 5.37 (4.19–6.88) | 46 | 52.64 (38.25–72.45) | 30 | 3.16 (2.20–4.53) | 27 | 25.10 (16.91–37.25) |
|
| |||||||||||
| Inconsistent CPD between baseline and earlier in the lifetime | |||||||||||
|
| |||||||||||
| <1 | 1182 | 186 (16) | 2.05 (1.77–2.37) | 70 | 2.12 (1.67–2.69) | 28 | 11.92 (8.06–17.62) | 38 | 1.53 (1.11–2.12) | 20 | 7.21 (4.59–11.34) |
|
| |||||||||||
| 1–10 | 4543 | 1001 (22) | 2.87 (2.69–3.06) | 383 | 3.10 (2.78–3.44) | 189 | 20.66 (17.09–24.98) | 223 | 2.38 (2.08–2.73) | 152 | 13.09 (10.78–15.90) |
|
| |||||||||||
| 11–20 | 4401 | 979 (22) | 2.99 (2.80–3.19) | 393 | 3.36 (3.03–3.74) | 198 | 22.62 (18.75–27.29) | 223 | 2.60 (2.27–2.98) | 152 | 13.29 (10.94–16.15) |
|
| |||||||||||
| 21–30 | 2397 | 638 (27) | 3.56 (3.28–3.86) | 273 | 4.34 (3.83–4.91) | 158 | 33.66 (27.54–41.13) | 134 | 2.81 (2.36–3.34) | 101 | 16.15 (12.90–20.22) |
|
| |||||||||||
| >30 | 1825 | 588 (32) | 4.40 (4.04–4.78) | 252 | 5.31 (4.67–6.04) | 144 | 40.69 (33.09–50.04) | 120 | 3.36 (2.79–4.03) | 110 | 23.40 (18.77–29.16) |
Abbreviations: AARP, formerly known as the American Association of Retired Persons: CPD, cigarettes per day: HR, hazard ratio: ND, not determined: NIH, National Institutes of Health.
Adjusted for sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, Pacific Islander, American Indian or Alaskan native, missing), educational level (<high school, high school, post–high school training, some college, college graduate or postgraduate, missing), alcohol intake, and physical activity. Age was used as the underlying time metric.
Includes individuals with complete information on smoking frequency at different age stages (20–24, 25–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years), including those who reported not smoking at 1 or more age stages but reported smoking at a later age stage. Excludes those who started smoking at 60 years or older.
Because no death was observed in this category, a risk estimate was not able to be computed.
Table 3.
Smoking Cessation With All-Cause and Cause-Specific Mortality Among Lifelong Low-Intensity Smokersa,b
| Characteristic | Total, No. | All Cause
|
All Cancer
|
Lung Cancer
|
Cardiovascular Disease
|
Respiratory Disease
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | ||
| Never smoker | 111 473 | 9821 | 1 [Reference] | 3468 | 1 [Reference] | 253 | 1 [Reference] | 2631 | 1 [Reference] | 324 | 1 [Reference] |
|
| |||||||||||
| <1 CPD | |||||||||||
|
| |||||||||||
| Current | 159 | 21 | 1.64 (1.07–2.51) | 4 | 0.90 (0.34–2.39) | 3 | 9.12 (2.92–28.47) | 10 | 2.78 (1.49–5.18) | 0 | NDc |
|
| |||||||||||
| Former (all) | 5803 | 554 | 1.08 (0.99–1.17) | 180 | 1.00 (0.86–1.16) | 14 | 1.11 (0.65–1.89) | 137 | 0.97 (0.82–1.16) | 14 | 0.90 (0.53–1.54) |
|
| |||||||||||
| Age at cessation, yd | |||||||||||
|
| |||||||||||
| ≥50 | 407 | 61 | 1.44 (1.12–1.85) | 17 | 1.18 (0.74–1.91) | 1 | 0.96 (0.14–6.86) | 20 | 1.70 (1.10–2.64) | 0 | NDc |
|
| |||||||||||
| 40–49 | 780 | 85 | 1.23 (0.99–1.53) | 31 | 1.29 (0.91–1.84) | 5 | 2.94 (1.21–7.13) | 20 | 1.09 (0.70–1.69) | 2 | 0.95 (0.24–3.83) |
|
| |||||||||||
| 30–39 | 852 | 79 | 1.05 (0.84–1.31) | 25 | 0.95 (0.64–1.40) | 1 | 0.53 (0.08–3.79) | 26 | 1.24 (0.83–1.83) | 3 | 1.33 (0.43–4.14) |
|
| |||||||||||
| 20–29 | 1071 | 111 | 1.18 (0.98–1.43) | 30 | 0.91 (0.64–1.31) | 2 | 0.86 (0.21–3.45) | 30 | 1.18 (0.83–1.70) | 5 | 1.75 (0.72–4.24) |
|
| |||||||||||
| 1–10 CPD | |||||||||||
|
| |||||||||||
| Current | 1493 | 232 | 1.87 (1.64–2.13) | 91 | 2.10 (1.71–2.59) | 38 | 11.61 (8.25–16.35) | 50 | 1.50 (1.13–1.99) | 27 | 6.00 (4.05–8.89) |
|
| |||||||||||
| Former (all) | 20 319 | 2146 | 1.17 (1.12–1.23) | 759 | 1.18 (1.09–1.28) | 120 | 2.55 (2.05–3.17) | 524 | 1.06 (0.97–1.17) | 128 | 2.13 (1.74–2.62) |
|
| |||||||||||
| Age at cessation, yd | |||||||||||
|
| |||||||||||
| ≥50 | 2057 | 307 | 1.42 (1.27–1.59) | 107 | 1.48 (1.22–1.79) | 28 | 5.08 (3.44–7.51) | 78 | 1.33 (1.06–1.67) | 32 | 4.04 (2.81–5.82) |
|
| |||||||||||
| 40–49 | 3222 | 382 | 1.27 (1.14–1.40) | 134 | 1.29 (1.09–1.54) | 23 | 2.94 (1.92–4.51) | 91 | 1.13 (0.92–1.39) | 31 | 2.96 (2.05–4.29) |
|
| |||||||||||
| 30–39 | 4755 | 504 | 1.14 (1.04–1.25) | 190 | 1.23 (1.06–1.42) | 31 | 2.75 (1.89–4.00) | 118 | 0.99 (0.82–1.19) | 19 | 1.25 (0.77–2.00) |
|
| |||||||||||
| 20–29 | 4430 | 424 | 1.11 (1.00–1.22) | 160 | 1.17 (1.00–1.37) | 19 | 1.94 (1.22–3.09) | 91 | 0.88 (0.71–1.08) | 17 | 1.42 (0.87–2.32) |
Abbreviations: CPD, cigarettes per day: ND, not determined: HR, hazard ratio.
Includes individuals with complete information on smoking frequency at different age stages (20–24, 25–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years), including those who reported not smoking at 1 or more age stages but reported smoking at a later age stage. Excludes those who started smoking at 60 years or older.
Adjusted for sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, Pacific Islander, American Indian or Alaskan native, missing), educational level (<high school, high school, post–high school training, some college, college graduate or postgraduate, missing), alcohol intake, and physical activity. Age was used as the underlying time metric.
Because no death was observed in this category, a risk estimate was not able to be computed.
Categorized by age at quitting cigarette smoking.
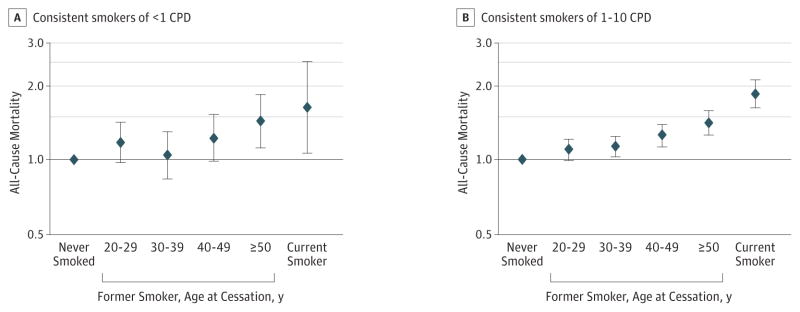
As reported in Table 3, we evaluated mortality risks among former smokers who had consistently smoked fewer than 1 or 1 to 10 CPD but quit before baseline. Compared with never smokers, former consistent smokers of fewer than 3 CPD were at a marginally increased risk of all-cause mortality (HR, 1.08; 95% CI, 0.99–0.17). Risks among former consistent smokers of 1 to 10 CPD were higher for all-cause mortality (HR, 1.17; 95% CI, 1.12–1.23) and mortality from cancer (HR, 1.18; 95% CI, 1.09–1.28), lung cancer (HR, 2.55; 95% CI, 2.05–3.17), and respiratory disease (HR, 2.13; 95% CI, 1.74–2.62) than were never smokers. Former consistent smokers of fewer than 1 and 1 to 10 CPD who quit at an older age were at higher all-cause mortality risk compared with those who quit at a younger age, with HRs of 1.44 (95% CI, 1.12–1.85) and 1.42 (95% CI, 1.27–1.59) for consistent smokers of fewer than 1 and 1 to 10 CPD who quit at 50 years or older, respectively; however, their risks were still lower than were those for current consistent smokers at the same intensity (Figure). The results for all-cause mortality were similar in men and women (eTable 2 in the Supplement). We further restricted our analysis to participants who reported never regularly using pipes or cigars in the initial cohort questionnaire and observed similar results for low-intensity smoking with mortality (eTable 3 in the Supplement).
Figure. Association Between Smoking Status and All-Cause Mortality.
Hazard ratios among lifelong consistent smokers of fewer than 1 cigarette per day (CPD) (A) and (B) 1 to 10 CPD relative to never smokers. Includes individuals with complete information on smoking frequency at different age stages, including those who reported not smoking at 1 or more age stages (20–24, 25–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years) but reported smoking at a later age stage; those who started smoking at 60 years or older were excluded. Data were adjusted for sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, Pacific islander, American Indian or Alaskan native, and unknown), educational level (<high school, high school, post–high school training, some college, college graduate or postgraduate, and unknown), alcohol intake, and physical activity. Age was used as the underlying time metric. Error bars indicate 95% CI.
Discussion
A growing proportion of US cigarette smokers smoke fewer than 10 CPD, but, to our knowledge, few studies have examined the health effects of long-term, low-intensity smoking. In the NIH-AARP cohort of older adults, we found that individuals who reported consistently smoking fewer than 1 or 1 to 10 CPD over their lifetime had higher mortality risks than did never smokers, with still higher risks found among persons who additionally smoked larger numbers of CPD earlier in their lives. Furthermore, mortality risks for consistently smoking fewer than 1 or 1 to 10 CPD were lower among former smokers than current smokers and lower among smokers who quit at a younger age than among those who quit at an older age. Together, these results indicate that long-term smoking even small numbers of CPD has substantial negative health effects and provide further evidence that smoking cessation benefits all smokers, regardless of how few cigarettes they smoke.
Relatively few studies have focused on the health risks of low-intensity smoking,17–20 and these studies have typically not incorporated information about changes in smoking over the lifetime, such that reported risk estimates are for a mixture of consistent low-intensity smokers and those who additionally smoked higher numbers of CPD earlier in life. Nevertheless, analogous findings to those in our study were observed in a recent case-control consortium study of head and neck cancer.16 In this case-control study, participants who had averaged smoking of fewer than 0 to 3, more than 3 to 5, and more than 5 to 10 CPD over their lifetime had higher odds of head and neck cancer compared with never smokers.
Several lines of evidence support our findings that long-term use of cigarettes at even very low intensity, including fewer than 1 CPD, is associated with disease. For example, regular exposure to second-hand smoke, which exposes bystanders to lower levels of the toxicants contained in mainstream smoke, is causally related to a broad spectrum of health outcomes, including cancer, cardiovascular disease, stroke, and respiratory diseases.26 In a large prospective study conducted in China, HRs for female never smokers with smoking husbands were 1.15 (95% CI, 1.01–1.31) and 1.37 (95% CI, 1.06–1.78) for all-cause and cardiovascular disease–specific mortality, respectively, compared with female never smokers with nonsmoking husbands.27 Furthermore, smoking duration has been found to be a substantially more important determinant of disease risk than CPD, which is consistent with elevated disease risks with long-term, low-intensity smoking, as was observed in the present study.10–13 In a cohort of British physicians, lung cancer risks increased in proportion to smoking duration to the fourth or fifth power,10 but to smoking intensity squared with analogous patterns found in the more recent Cancer Prevention Study II.11 Our results were also internally consistent, with higher risks observed among lifelong low-intensity smokers who continued to smoke at baseline relative to low-intensity smokers who had previously stopped. Together, these results provide strong evidence that long-term exposure to even low levels of cigarette smoke has detrimental effects on health.
Our results have several important implications for tobacco control. First, in support of the 2010 Surgeon General’s Report,28 our results provide further evidence that there is no risk-free level of tobacco smoke exposure. In our study, smokers who reported smoking fewer than 1 CPD were at substantially increased risk of mortality than were never smokers, and we observed substantial benefits of smoking cessation. These data, therefore, provide additional evidence that all smokers, no matter how few cigarettes they smoke per day, should be encouraged and assisted to quit, as recommended by the 2008 Public Health Service–sponsored Clinical Practice Guidelines.29 Second, our results also have implications for dual users or polyusers who may smoke cigarettes at low-intensity levels in combination with other tobacco products, such as other combustible tobacco, smokeless tobacco, and electronic nicotine delivery systems. Future research examining the health effects of low-intensity cigarette smoking should include examination of the use of electronic nicotine delivery systems, given the widespread use and unknown health effects.
Key strengths of our study included our large sample size, prospective design, and information on potentially confounding factors, such as diet and lifestyle. Furthermore, detailed assessment of cigarette smoking intensity over the lifespan allowed us to identify lifelong low-intensity smokers. Our study also had limitations. Despite the large size of our cohort, the numbers of low-intensity smokers were modest. Participants recalled their smoking retrospectively. Nevertheless, a large amount of literature indicates that cigarette smoking is well reported, with strong correlations observed for self-report and measured levels of cotinine in blood or urine, including among low-intensity and nondaily users.30,31 Methodologic studies indicate that smokers reliably recall their lifetime smoking, including when they started smoking and their CPD when they smoked the most.32,33 More recent exposures are generally recalled better than those earlier in life; nevertheless, assessments of smoking have shown good validity for CPD 20 years earlier (κ = 0.63) and fair validity for CPD 32 years earlier (κ = 0.63) in middle-aged adults.34 Concordance of recall after 10 years was also good in the present study (74% concordance among consistent smokers of ≤=10 CPD). Although some participants likely underestimated their past use, the effects of such misclassification are likely modest among long-term smokers, given that smoking duration rather than smoking intensity has been shown to contribute substantially more to diseases such as lung cancer.10–12 Our fewer than 1 CPD category is also quite broad. There is the potential for substantial differences in disease risks across the range of smoking in this category, for example, smoking 25 days per month relative to once a month. Future studies with detailed assessments of fewer than 1 CPD and nondaily smoking are needed.
In addition, participants in our study were mostly in their 60s to 70s and white and reflect the smoking use patterns of a particular set of US birth cohorts. Smokers would have been less likely to have lived long enough to enter our study than never smokers, such that our relative risks for smoking may be underestimated. We see no reason to suspect that the patterns of association observed in our study would not apply to other birth cohorts and populations. Nevertheless, additional studies of lifelong low-intensity smoking are needed, particularly in younger groups as well as racial and ethnic minorities. We also lacked detailed information about use of other tobacco products. It seems likely that lifelong low-intensity cigarette smokers who use additional tobacco products, particularly other combusted tobacco products, have higher disease risks compared with those who exclusively use cigarettes.
Conclusions
Participants who reported consistently smoking fewer than 1 or 1 to 10 CPD over their lifetime were at substantially higher risk of premature mortality from a broad spectrum of smoking related causes of death than were never smokers. Former consistent smokers of fewer than 1 or 1 to 10 CPD had higher mortality risks relative to never smokers but lower risk than those who continued to smoke at these levels. These findings provide further evidence that there is no safe level of cigarette smoking. All smokers should be targeted for smoking cessation, regardless of how few cigarettes they smoke per day. Further studies are needed to examine the health risks of low-intensity cigarette smoking in combination with electronic nicotine delivery systems and other tobacco products.
Supplementary Material
Key Points.
Question
Do people who smoke at low-intensity (ie, <1 or 1–10 cigarettes per day) over their lifetime have increased risk of mortality relative to those who never smoke?
Findings
Among 290 215 older adults of the National Institutes of Health–AARP Diet and Health Study cohort, low-intensity smoking over the lifetime was associated with a significantly higher risk of all-cause mortality, including deaths from lung cancer and cardiovascular disease. Former smokers who had consistently used fewer than 1 or 1 to 10 cigarettes per day but who had quit smoking had progressively lower risks with a younger age at cessation.
Meaning
This study provides evidence that individuals with lifelong, low-intensity smoking have higher mortality risks than those who never smoked and would benefit from cessation.
Acknowledgments
Funding/Support: This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology & Genetics.
Footnotes
Conflict of Interest Disclosures: Although Dr Reyes-Guzman is a current employee of the US Food and Drug Administration (FDA)/Center for Tobacco Products (CTP), this work was not done as part of her official duties. No other disclosures were reported.
Disclaimer: This publication reflects the views of the authors and should not be construed to reflect the FDA/CTP’s views or policies.
Role of the Funder/Sponsor: The sponsor reviewed and approved final submission but had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Dr Inoue-Choi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and design: Inoue-Choi, Hartge, Caporaso, Freedman.
Acquisition, analysis, or interpretation of data: Inoue-Choi, Liao, Reyes-Guzman, Hartge, Freedman.
Drafting of the manuscript: Inoue-Choi.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Inoue-Choi, Reyes-Guzman, Freedman.
Administrative, technical, or material support: Liao, Hartge, Freedman.
References
- 1.World Health Organization. WHO Grobal Report: Mortality Attributable to Tobacco. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2.Jiang N, Gonzalez M, Ling PM, Glantz SA. Relationship of smokefree laws and alcohol use with light and intermittent smoking and quit attempts among US adults and alcohol users. PLoS One. 2015;10(10):e0137023. doi: 10.1371/journal.pone.0137023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 4.Wortley PM, Husten CG, Trosclair A, Chrismon J, Pederson LL. Nondaily smokers: a descriptive analysis. Nicotine Tob Res. 2003;5(5):755–759. doi: 10.1080/1462220031000158753. [DOI] [PubMed] [Google Scholar]
- 5.Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: an increasingly prevalent pattern. Arch Intern Med. 2009;169(19):1742–1744. doi: 10.1001/archinternmed.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassmiller KM, Warner KE, Mendez D, Levy DT, Romano E. Nondaily smokers: who are they? Am J Public Health. 2003;93(8):1321–1327. doi: 10.2105/ajph.93.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobacco Use Among US Racial/Ethnic Minority Groups. Atlanta, GA: US Dept of Health & Human Services, Centers for Disease Control & Prevention; 1998. [Google Scholar]
- 8.Fagan P, Rigotti NA. Light and intermittent smoking: the road less traveled. Nicotine Tob Res. 2009;11(2):107–110. doi: 10.1093/ntr/ntn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 10.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63(19):6556–6562. [PubMed] [Google Scholar]
- 12.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15(3):517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 13.Lubin JH, Couper D, Lutsey PL, Woodward M, Yatsuya H, Huxley RR. Risk of cardiovascular disease from cumulative cigarette use and the impact of smoking intensity. Epidemiology. 2016;27(3):395–404. doi: 10.1097/EDE.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrock SM, Weitzman M. Adolescents’ perceptions of light and intermittent smoking in the United States. Pediatrics. 2015;135(2):246–254. doi: 10.1542/peds.2014-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garfinkel L, Stellman SD. Smoking and lung cancer in women: findings in a prospective study. Cancer Res. 1988;48(23):6951–6955. [PubMed] [Google Scholar]
- 16.Berthiller J, Straif K, Agudo A, et al. Low frequency of cigarette smoking and the risk of head and neck cancer in the INHANCE consortium pooled analysis. Int J Epidemiol. 2016;45(3):835–845. doi: 10.1093/ije/dyv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosengren A, Wilhelmsen L, Wedel H. Coronary heart disease, cancer and mortality in male middle-aged light smokers. J Intern Med. 1992;231(4):357–362. doi: 10.1111/j.1365-2796.1992.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 18.Luoto R, Uutela A, Puska P. Occasional smoking increases total and cardiovascular mortality among men. Nicotine Tob Res. 2000;2(2):133–139. doi: 10.1080/713688127. [DOI] [PubMed] [Google Scholar]
- 19.Prescott E, Scharling H, Osler M, Schnohr P. Importance of light smoking and inhalation habits on risk of myocardial infarction and all cause mortality: a 22 year follow up of 12 149 men and women in The Copenhagen City Heart Study. J Epidemiol Community Health. 2002;56(9):702–706. doi: 10.1136/jech.56.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjartveit K, Tverdal A. Health consequences of smoking 1–4 cigarettes per day. Tob Control. 2005;14(5):315–320. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med. 1994;154(2):169–175. [PubMed] [Google Scholar]
- 22.Zhu SH, Sun J, Hawkins S, Pierce J, Cummins S. A population study of low-rate smokers: quitting history and instability over time. Health Psychol. 2003;22(3):245–252. doi: 10.1037/0278-6133.22.3.245. [DOI] [PubMed] [Google Scholar]
- 23.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46(2):e31–e37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: a review. Circulation. 2010;121(13):1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 26.The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking & Health; 2014. [Google Scholar]
- 27.Wen W, Shu XO, Gao YT, et al. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. BMJ. 2006;333(7564):376. doi: 10.1136/bmj.38834.522894.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality. [Accessed June 17, 2016];Treating tobacco use and dependence: 2008 update. http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/index.html. Updated June 2015.
- 30.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2001;153(8):807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- 31.Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1264–1272. doi: 10.1158/1055-9965.EPI-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colby SM, Clark MA, Rogers ML, et al. Development and reliability of the lifetime interview on smoking trajectories. Nicotine Tob Res. 2012;14(3):290–298. doi: 10.1093/ntr/ntr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brigham J, Lessov-Schlaggar CN, Javitz HS, McElroy M, Krasnow R, Swan GE. Reliability of adult retrospective recall of lifetime tobacco use. Nicotine Tob Res. 2008;10(2):287–299. doi: 10.1080/14622200701825718. [DOI] [PubMed] [Google Scholar]
- 34.Krall EA, Valadian I, Dwyer JT, Gardner J. Accuracy of recalled smoking data. Am J Public Health. 1989;79(2):200–202. doi: 10.2105/ajph.79.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.