Abstract
Objective
To assess whether a high-salt diet, as measured by urinary sodium concentration, is associated with faster conversion from clinically isolated syndrome (CIS) to multiple sclerosis (MS) and MS activity and disability.
Methods
BENEFIT was a randomized clinical trial comparing early versus delayed interferon beta-1b treatment in 465 patients with a CIS. Each patient provided a median of 14 (IQR: 13 to 16) spot urine samples throughout the 5-year follow-up. We estimated 24-hour urine sodium excretion level at each time point using the Tanaka equations, and assessed whether sodium levels estimated from the cumulative average of the repeated measures were associated with clinical (conversion to MS, EDSS) and magnetic resonance imaging (MRI) outcomes.
Results
Average 24-hour urine sodium levels were not associated with conversion to clinically-definite MS over the 5-year follow-up (hazard ratio [HR]=0.91; 95% CI: 0.67-1.24 per 1g increase in estimated daily sodium intake); nor were they associated with clinical or MRI outcomes (new active lesions after 6 months HR: 1.05; 95% CI 0.97-1.13; relative change in T2 lesion volume: -0.11; 95% CI -0.25-0.04; change in EDSS: -0.01; 95% CI: -0.09-0.08; relapse rate HR: 0.78; 95% CI: 0.56-1.07). Results were similar in categorical analyses using quintiles.
Interpretation
Our results, based on multiple assessments of urine sodium excretion over 5 years and standardized clinical and MRI follow-up, suggest that salt intake does not influence MS disease course or activity.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disorder that likely results from a combination of genetic and environmental factors.1–3 Recently, several in vivo and in vitro studies have suggested that salt (NaCl, sodium chloride) could potentially influence MS disease activity and progression, possibly through modulation of T-cell differentiation.4,5 Further, mice fed a high-sodium diet developed a more aggressive course of experimental autoimmune encephalomyelitis (EAE), the animal model of MS.4,5
Two studies, both of pediatric MS, did not find an association between self-reported dietary sodium intake with risk of MS6 or with relapse rate in MS patients.7 Another study in 70 relapsing-remitting (RR) MS patients who were followed for 2 years found that higher levels of urinary sodium excretion, a marker of dietary sodium intake, were associated with higher relapse rates and lesion volume.8 However, the study was small and relied on a single spot urine sample, which is a poor predictor of average sodium intake. Further, there were few relapse events, and only a cross-sectional assessment of sodium and radiographic outcomes.
We assessed longitudinally whether sodium intake, estimated from sodium excretion in multiple (median 14) urinary samples per subject, is associated with early MS disease activity and progression among participants in the Betaferon/Betaseron in Newly Emerging Multiple Sclerosis for Initial Treatment (BENEFIT) clinical trial, a well-characterized cohort of over 400 patients who were randomized to receive either interferon beta-1b or placebo soon after experiencing a clinically isolated syndrome (CIS).
Methods
Study Population
The BENEFIT study was a clinical trial (NCT00185211) of 468 CIS patients who were randomized to receive either interferon beta-1b (IFNb-1b) or placebo within 60 days of the CIS, and were followed for conversion to MS (both clinically definite MS9 [CDMS] and McDonald MS10 [MDMS]). The full details of the trial and results have been previously described.11 Following conversion to CDMS or after 24 months, patients in the placebo group began treatment with interferon-beta 1b and patients were followed for an additional 3 years for a total of 5 years of follow-up. Expanded Disability Status Scale (EDSS)12 was assessed and MRI imaging done at baseline, then every 3 months through month 12, and then at months 18, 24, 36, 48, and 60. EDSS scores were also assessed at months 30, 42, and 54. Relapses were confirmed as they occurred. At each clinic visit (baseline, every 3 months through month 12, and then every 6 months thereafter), patients provided casual (‘spot') urine samples that were received by the central laboratory within 3 days of collection, and stored at −20°C. Samples were first thawed for assessment of urinary sodium and creatinine for this study.
All participants in BENEFIT provided written informed consent and this study was approved by the Office of Human Subjects Administration at the Harvard T.H. Chan School of Public Health and included only deidentified data and urine samples.
MRI
MRI imaging procedures and processing used in BENEFIT have been previously described in full.13 Briefly, T2- and T1-weighted images (following administration of 0.1 mmol/kg of gadolinium–diethylenetriaminepentaacetic acid) were analyzed centrally at the Image Analysis Centre at the VU University Medical Center in Amsterdam where the number of new lesions (including active lesions) and lesion volume were determined. Brain volumes were quantified using the structural image evaluation using normalization of atrophy cross-sectional (SIENAX) algorithm.14
Sodium intake assessment
As 80-90% of ingested sodium is excreted through the urine,15 we used urinary sodium as a marker of dietary sodium intake. Because sodium excretion varies throughout the day,16 assessment of urinary sodium from 24-hour urine collection is considered the gold standard. However, as 24-hour urine samples are not typically collected in large studies, several methods to estimate 24-hour urinary sodium excretion from a single urine sample have been developed. For this study (and to be consistent with previous research,8 we used the Tanaka equations15 in our primary analyses. Though originally developed in a Japanese population, these equations have been extended to other populations.8 The full equation includes an individual's urinary sodium, urinary creatinine, age, height and weight as described below:
For this study, patients had a median of 14 urine samples collected (interquartile range [IQR]: 13 to 16 samples) between baseline and month 60, from which sodium and creatinine were measured.
Urinary sodium and creatinine levels were quantified using a Roche/Hitachi Modular System (Roche Diagnostics, IN, USA) and patient height, weight and age were documented as a part of the existing clinical trial. In the initial laboratory assessment, estimated urinary creatinine levels were considerably lower than in previous global population-based studies.16 Therefore, we reassessed creatinine levels in a subset of samples (n=96) and found the levels obtained in our initial assessments were likely underestimated. However, the initial and re-assessment of creatinine were highly correlated (Spearman correlation =0.87; Supplemental Figure 1 A and B), suggesting that while our estimates of absolute creatinine in the larger cohort may be underestimated, the relative ranking within the population is likely unaffected. We created “corrected” absolute creatinine value in the full study sample using the following calibration equation developed from the regressing the initial estimates on the re-assessed creatinine in the subset of 96 samples with both measurements:
These corrected estimates were then used to estimate 24-hour urinary sodium using the Tanaka equation shown above. Intra-patient variability (characterized by intra-class coefficients [ICC]) in estimates of 24-hour sodium excretion was 0.38 (95% CI: 0.34, 0.41) over follow-up. However, the ICC of the average of alternating samples (e.g. the average of samples 1, 3, 5, 7, 9, […] vs. the average of samples 2, 4, 6, 8, 10, […]) was substantially higher at 0.74 (95% CI: 0.70, 0.78), a result which is consistent with the well-known high day-to-day variability of sodium intake,17,18 and demonstrates that average long term sodium excretion can be reliably estimated with multiple spot urine samples. Use of a large number of urine samples collected over a long period of time from each patient also minimizes the error due changes in factors that affect sodium balance, such as use of diuretics and other drugs. Although a steady-state in which sodium intake is approximately equal to excretion is usually reached within weeks,19,20 a single urine sample could fall in a period of positive or negative sodium balance and thus misrepresent the long term intake.
Statistical analysis
We treated estimated 24-hour urine sodium excretion as a time-dependent variable using at each time point the average of all previous values, and characterized the exposure in three a priori defined variables: 1) as continuous (per g/day), 2) dichotomized on the median (<3.7 vs > 3.7 g/day), and 3) as quintiles based on the overall distribution. For our primary analyses, we started follow-up at month 6, as baseline MRI measurements were obtained within 60 days of a clinical event and they could have been affected by underlying inflammatory processes. We analyzed time to conversion to MDMS and CDMS using Cox proportional hazards models and relapse rate using an Andersen-Gill model to allow for repeated events. Other outcomes including cumulative number of new active lesions (CNAL), T2 lesion volume, brain volume and EDSS scores were analyzed using generalized estimating equations. We modeled the number of CNAL as a count variable and the other outcomes as continuous variables. We conducted further analyses also using deciles and splines to explore the dose response. We adjusted each model for age, sex, randomization status (IFNb-1b vs. placebo), the type of CIS (monofocal vs multifocal), baseline T2 lesion score (the logarithm of the number of T2 lesions), body mass index (weight in kg / height in m2), and 25(OH)D status.
We conducted several sensitivity analyses for each set of outcomes. First, we fit models using the average of all estimated 24-hour urinary sodium levels and assessed the association of non-time varying estimated 24-hour sodium with MS outcomes (assuming variation in estimated 24-hour is random). Next, we fit models using the average of estimated 24-hour urinary sodium during the first year (to relax the assumption that MS outcomes do not influence urinary sodium) and assessed its association with MS outcomes. Finally, we assessed the within person change in estimated 24-hour urinary levels and its association with MS outcomes. Further, we also conducted secondary analyses estimating 24-hour urinary sodium excretion using other similarly validated methods including using the ratio of sodium to creatinine21 or adjustment equations developed by Kawasaki et al,22 which, similar to the Tanaka equations, were developed in a Japanese population but have also similarly been extended to Western populations.23 They include adjustments for age, height, weight, casual urinary sodium and creatinine but are sex-specific. We also conducted sensitivity analyses restricting to casual urine samples collected in the midmorning (10:00am-12:00pm) as some studies have indicated these samples have a higher correlation with 24-hour urinary sodium levels.24
Results
Patient Characteristics and Estimated 24-hr Sodium Levels
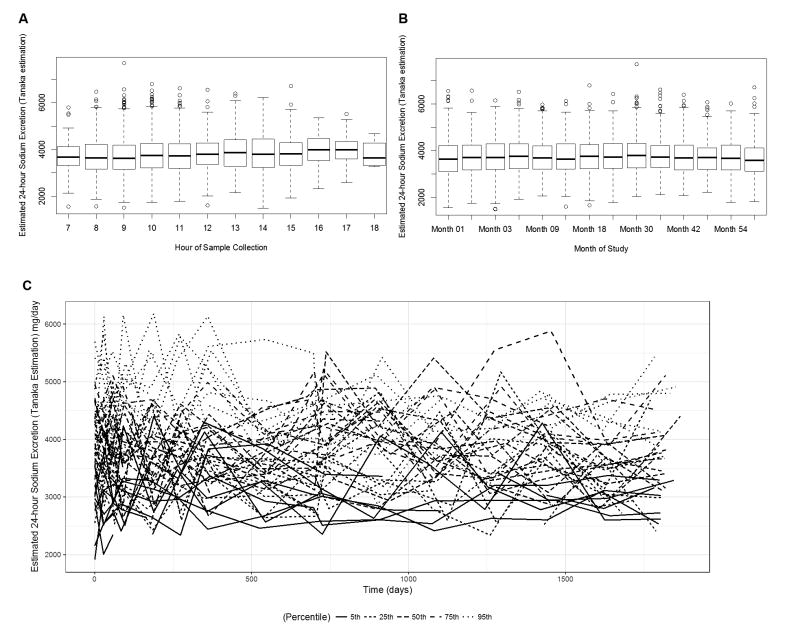
The median 24-hour urinary sodium excretion was 3.7 g/day, across all time points. Patients with higher 24-hour urinary sodium levels at month 6 were more likely to be male and had a higher BMI, and fewer T2 lesions on baseline MRI (Table 1). Other patient characteristics including age, 25-hydroxyvitamin D level, CIS onset type, region of residence in Europe or Canada, and centralized brain volume did not appear to vary across estimated 24-hour urine sodium levels. In addition, overall estimated 24-hour urinary sodium was relatively consistent across time of day of sample collection (Figure 1A) and over the course of follow-up (Figure 1B), but did display expected random day-to-day variation (Figure 1C).
Table 1. Baseline characteristics by quintile of estimated 24-hour urinary sodium excretion at 6-months in BENEFIT participants.
| Quintiles of Estimated 24-hr Sodium Excretion | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| No. of patients | 85 | 86 | 85 | 86 | 85 |
| Mean 24-hr sodium excretion* (SD), g | 2.77 (0.03) | 3.32 (0.02) | 3.76 (0.01) | 4.19 (0.01) | 4.94 (0.05) |
| Mean raw sodium concentration (SD), mmol/L | 73.7 (4.4) | 97.4 (5.1) | 122.5 (4.4) | 146.5 (5.2) | 186.8 (5.1) |
| Mean raw creatinine concentration (SD), mg/dL | 76.6 (6.6) | 64.9 (5.0) | 57.3 (3.8) | 53.8 (3.1) | 48.4 (2.4) |
| Mean calibrated creatinine concentration* (SD), mg/dL | 125.9 (7.6) | 112.3 (5.8) | 103.5 (4.5) | 99.4 (3.6) | 93.1 (2.8) |
| Estimated 24-hr sodium excretion using Kawasaki Equations (SD), g | 3.87 (0.07) | 4.51 (0.08) | 4.76 (0.09) | 5.08 (0.10) | 5.89 (0.11) |
| Median quintile value of estimated 24-hr sodium excretion using Kawasaki Equations (IQR), g | 1 (1-2) | 2 (2-4) | 3 (2-4) | 4 (2-5) | 4 (4-5) |
| Ratio of Sodium excretion to creatinine excretion (SD) | 0.61 (0.02) | 0.89 (0.02) | 1.22 (0.03) | 1.5 (0.04) | 2.06 (0.05) |
| Median quintile value of Ratio of Sodium excretion to creatinine excretion (IQR) | 1 (1-2) | 2 (2-2) | 3 (3-4) | 4 (3-5) | 5 (4-5) |
| Mean age at recruitment (SD), years | 30.6 (0.8) | 31.5 (0.8) | 31.2 (0.8) | 31.1 (0.7) | 29.4 (0.8) |
| Female, N (%) | 70 (82) | 66 (77) | 62 (73) | 57 (66) | 48 (56) |
| Randomized to INFB-1b, N (%) | 52 (61) | 51 (59) | 54 (64) | 52 (60) | 58 (68) |
| Monofocal onset, N (%) | 47 (55) | 49 (57) | 40 (47) | 46 (53) | 43 (51) |
| Median no. of T2 Lesions at baseline median (IQR) | 17 (7-39) | 15 (6-34) | 20 (9-40) | 17.5 (8-38) | 13 (6-31) |
| Mean centralized brain volume at baseline median (SD), mm3 | 1049 (5) | 1052 (5) | 1050 (5) | 1062 (5) | 1058 (5) |
| Body mass index (SD), kg/m2 | 22.6 (0.4) | 23.6 (0.4) | 23.9 (0.5) | 24.6 (0.5) | 26.2 (0.5) |
| 25(OH)D levels, nmol/mL | 51.8 (2.2) | 49.2 (1.5) | 51.8 (2.1) | 48.7 (1.6) | 47.7 (2.1) |
| Steroids at first clinical event, N (%) | 65 (76) | 56 (65) | 61 (72) | 60 (70) | 57 (67) |
| Region of residence, N (%) | |||||
| Canada | 3 (4) | 2 (2) | 7 (8) | 9 (10) | 4 (5) |
| Southern Europe | 14 (16) | 11 (13) | 11 (13) | 12 (14) | 15 (18) |
| Central Europe | 57 (67) | 63 (73) | 57 (67) | 59 (68) | 58 (68) |
| Scandinavia | 11 (13) | 10 (12) | 10 (12) | 6 (7) | 8 (9) |
Calibrated creatinine concentration denotes raw values transformed using 1.16 × (original lab values) + 36.86;
Figure 1.
Estimated 24-hour urinary sodium excretion using the Tanaka et. al equations by hour of sample collection (A) and by study visit (B). (C) Spaghetti plots of individual variability over follow-up in estimated 24-hour sodium excretion from selected study patients. Selected patients plotted are those having a patient-specific median excretion (across follow-up) at the 5th, 25th, 50th, 75th and 95th percentiles of the distribution of patient-specific overall median values.
Conversion to Definite MS
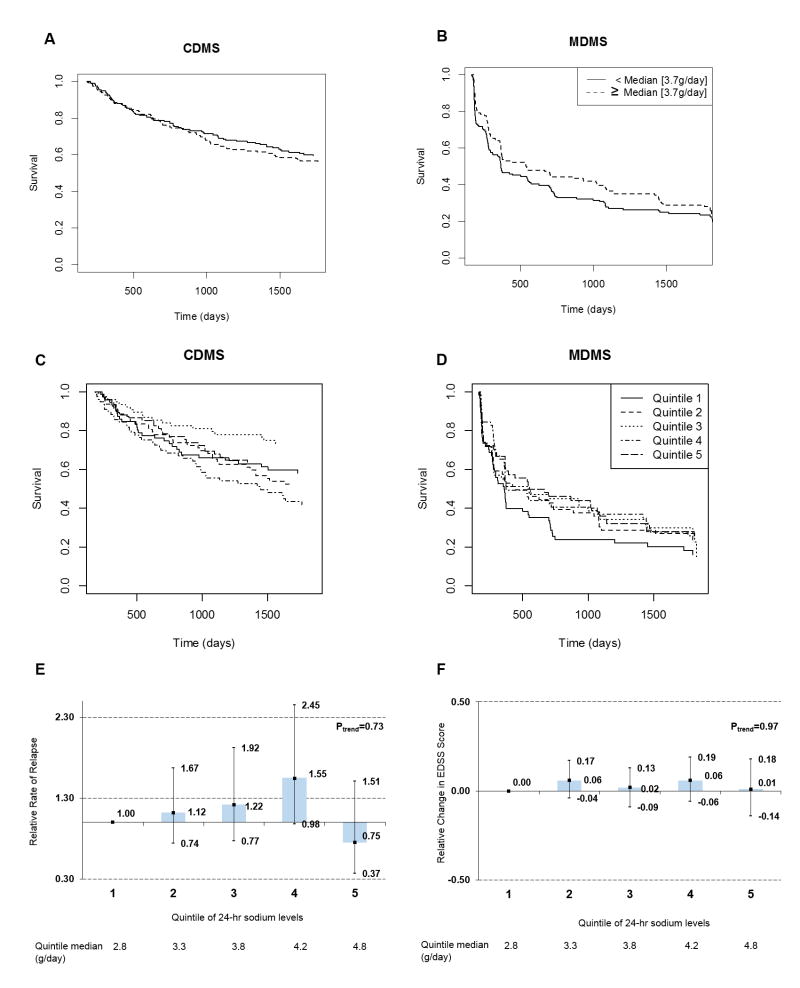
Starting follow-up at 6-months, 217 (75.0%) patients converted to MDMS and 150 (39%) patients converted to CDMS over the 5 years. We did not observe any association between higher levels (above the median or using quintiles) of estimated 24-hour sodium excretion levels and faster conversion rates after 6 months (median analyses: for CDMS: P=0.50 for MDMS: P=0.12; Figure 2A and 2B; quintile analyses: for CDMS: P=0.88 for MDMS: P=0.10; Figure 2C and 2D). Similarly, in multivariate analyses, we did not observe any association in continuous (per 1g increase in estimated 24-hour urine sodium level: for CDMS: HR=0.91, 95% CI: 0.67 to 1.24, P=0.55; for MDMS: HR=0.83, 95% CI: 0.66 to 1.05, P=0.13) or quintile analyses (Table 2). We observed similar results in analyses starting follow-up at month 12. In analyses using splines, we also did not detect any association between estimated 24-hour urine sodium levels and CIS to MS conversion (CDMS: P for any association=0.58; MDMS: P for any association: 0.39).
Figure 2.
Relation between estimated 24-hour urinary sodium excretion using the Tanaka equations and MS outcomes from months 6 through 60 for sodium intake dichotomized at the median (A, B) and quintile categories (C, D). (A) Median analyses: Conversion to CDMS (P=0.50), (B) Median analyses: conversion to MDMS (P=0.12), (C) Quintile analyses: Conversion to CDMS (P=0.88), (D) Quintile analyses: conversion to MDMS (P=0.10) , (E) relapse rate , (F) change in EDSS. Estimates are adjusted for age, sex, randomization status (IFNB-1b, placebo), CIS onset type (multifocal, monofocal), 25-hydroxyvitamin D status, and body mass index.
Table 2. Adjusted hazard of conversion to definite MS by quintiles of estimated 24-hour sodium excretion*--months 6 to 60--BENEFIT.
| Quintile of 24-hour sodium excretion | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ptrend | |
| CDMS | ||||||
| No. of conversions / No. of patients | 28/70 | 34/89 | 21/59 | 43/83 | 24/79 | |
| HR** (95% CI) | 1.00 [ref] | 1.10 (0.66-1.83) | 0.75 (0.42-1.33) | 1.60 (0.96-2.65) | 0.75 (0.41-1.37) | 0.77 |
| MDMS | ||||||
| No. of conversions / No. of patients | 46/62 | 50/63 | 44/61 | 39/50 | 38/55 | |
| HR** (95% CI) | 1.00 [ref] | 1.13 (0.75-1.70) | 1.07 (0.70-1.63) | 0.83 (0.53-1.30) | 0.84 (0.51-1.37) | 0.25 |
Estimated using the Tanaka et al equations.
Adjusted for age, sex, randomization status (IFNB-1b, placebo), CIS onset type (multifocal, monofocal), 25-hydroxyvitamin D status, and body mass index.
Relapse and EDSS
We did not observe any association between estimated 24-hour urine sodium excretion and relapse rate from month 6 to month 60 (Figure 2E; per 1g increase in estimated 24-hour urine sodium excretion: RR: 0.86, 95% CI: 0.0.71-1.05, P=0.14). Results were similar when considering estimated 24-hr sodium excretion and time to first relapse after conversion to definite MS (per 1g increase in estimated 24-hour urine sodium excretion: RR: 0.88, 95% CI 0.72 to 1.07, p=0.19). Similarly, we did not observe any associations between estimated 24-hr sodium excretion and confirmed changes in EDSS score from month 6 through month 60 (Figure 2F; per 1g increase in estimated 24-hr sodium excretion: β=-0.01., 95% CI −0.09 to 0.08, p = 0.90)
MRI
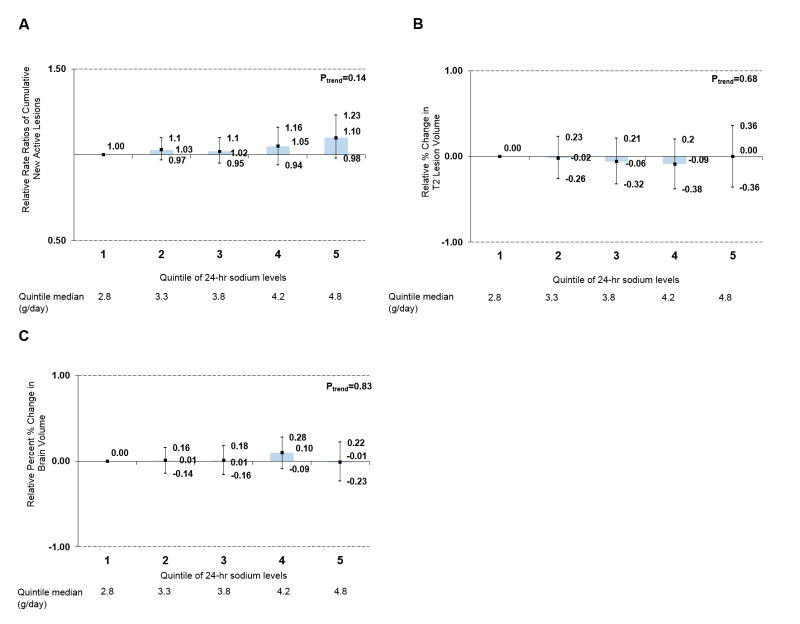
Estimated 24-hour urine sodium excretion was not associated with MRI activity. Levels were not associated with developing new lesions from month 6 to month 60 (per 1g increase in estimated 24-hour urine sodium excretion: RR of CNAL=1.05, 95% CI 0.97 to 1.13, p=0.24 Figure 3A). Similarly, it was not associated with percent changes in T2 lesion volume (Figure 3B) or percent change in brain volume (Figure 3C) month 6 to month 60 in continuous or categorical models (per 1g increase: change in T2 lesion volume: -0.11; 95% CI -0.25-0.04; P=0.17).
Figure 3.
Relation between estimated 24-hour urinary sodium excretion using the Tanaka equations and MRI outcomes from months 6 through 60. (A) relative rate of CNAL, (B) change in T2 lesion volume, (C) change in brain volume. Estimates are adjusted for age, sex, randomization status (IFNB-1b, placebo), CIS onset type (multifocal, monofocal), 25-hydroxyvitamin D status, and body mass index.
We conducted several sensitivity analyses that similarly did not indicate any association between estimated 24-hour urine sodium excretion and MS outcomes. First, we did not observe any association between non-time-varying estimated 24-hour urine sodium excretion (a metric that minimizes random variation in estimated excretion) and any MS outcome. Next, we did not observe any association between the average estimated 24-hour urine sodium excretion during the first year and subsequent MS outcomes. Finally, we did not observe any association between within-person changes in estimated 24-hour urine sodium excretion and MS outcomes. All of the above analyses were also conducted separately by randomized treatment group (placebo or interferon beta-1b) and no associations between estimated 24-hour urine sodium excretion and any of the outcomes were observed. In addition, we repeated all analyses using the Kawasaki estimates for 24-hour urine sodium levels and the ratio between sodium to creatinine. As with the Tanaka estimate, we did not observe any associations between each additional sodium metric and MS outcomes.
Discussion
In this large, prospective study among patients with CIS and including a median of 14 urine sodium measurements per patient, estimated 24-hour urine sodium levels were not associated with conversion to definite MS (both CDMS and MDMS) or with MS activity or early progression as indicated by both clinical and MRI outcomes over the 5 years of follow-up. One previous study was conducted among a population of 70 RRMS patients in Argentina and found that high levels of urinary sodium excretion (>4.8 g/day) were associated with an increased relapse rate and correlated with cross-sectional MRI activity.8 In this study, the long term average sodium intake -- the hypothesized cause of MS exacerbations -- was estimated from a single spot urine sample, which is a very poor proxy for long term sodium excretion or intake. The error (i.e. the difference between intake estimated from the single spot urine and the true long term intake) is due mostly to random day-to-day within person variations in sodium intake, and is thus unlikely to correlate with MS outcomes. As a result of such error, any hypothetical true association between actual sodium intake and MS outcomes would be strongly attenuated towards the null. Thus, the strong association reported by Farez et al. would imply an even stronger association for true intake. The results of our study with a larger sample size, multiple measurements and diverse set of MS outcomes demonstrate that such strong association does not exist in the European and Canadian populations in BENEFIT. Thus, the association reported in the Argentine study was either a chance occurrence or reflects an unknown mechanism unique to the population included in that study.
Our study is consistent with findings from a previous investigation of pediatric MS patients7 that found no association between self-reported dietary sodium intake and time until relapse. However, in that study sodium intake was estimated using a food frequency questionnaire, which may be unreliable and underestimate intake of sodium.25,26 Further, the questionnaire was administered only at baseline and could thus not account for changes in diet during the follow-up. For these reasons, it cannot be excluded that the null result was due to inadequate assessment of sodium intake.
Although the results of these epidemiological studies appear to be in conflict with EAE studies indicating an earlier and more aggressive disease course associated with high salt intake4,5 and in vitro observations that sodium chloride induces Th-17 generation4,5 and IFNγ secretion,27 and causes a loss of Treg function,27 changes that are expected to adversely affect the course of MS, the clinical relevance of these observation is uncertain. Among other considerations, it is uncertain whether high salt intake in humans can lead to sodium concentrations in the relevant fluid compartments that are comparable to those used in the in vitro experiments.
Our study has several strengths including its longitudinal design, nearly-uniform treatment with INFB-1b, large sample size of nearly 500 patients with CIS, and systematic collection of both clinical and MRI data. Further, the per-patient number of samples (median of 14) in our study is considerably larger than in the previous report where one measure of 24-hour sodium excretion per patient was used.8 Using formulas derived by Beaton et al.17 to calculate the number of samples needed to estimate a person's true 24-hour sodium levels with a specified degree of error, and assuming a coefficient of variation of 16% (as estimated in our samples), we estimate that 14 samples is sufficient to estimate 24-hour sodium levels to within 4% of their true mean 95% of the time.
Limitations include that BENEFIT participants were treated nearly uniformly with INFB-1b, and the results of our study may not apply to patients on other therapies. While our results suggest there is no association between sodium intake and MS prognosis, they cannot comment as to whether sodium intake modifies risk of developing MS. In addition, patients in the BENEFIT trial were primarily Caucasian and resided in Europe and Canada, and it is currently unknown as to whether similarly null results would apply to other populations and ethnicities. The large number of samples (median 14 samples) contributed by each patient as well as the high ICC between average alternating samples lends credence to our analysis.
Taken together, these results suggest that high sodium intakes do not play a major role in influencing MS disease course or activity in patients treated with INFB-1b, at least in the early stages of disease.
Acknowledgments
This study was funded by research grants from the National Multiple Sclerosis Society (RG5146A1 PI: Munger; RG4296A4 PI: Ascherio) and by the National Institute of Neurological Disease and Stroke (NS071082 PI: Ascherio)
Footnotes
Author Contributions: KLM and AA contributed to the conception and design of the study; KCF, KLM and AA contributed to the acquisition and analysis of data; all authors contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest: Drs. Fitzgerald, Munger, Edan, Radue, Pohl and Ascherio have nothing to disclose. Drs. Hartung, Freedman, Montalbán, Kappos have nothing to disclose with respect to the submitted work. Dr. Wicklein is a salaried employee of Bayer AG during the study period.
References
- 1.Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Multiple Sclerosis Genetics Consortium et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascherio A. Environmental factors in multiple sclerosis. Expert Rev Neurother. 2013;13:3–9. doi: 10.1586/14737175.2013.865866. [DOI] [PubMed] [Google Scholar]
- 4.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinewietfeld M, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald J, et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult Scler Relat Disord. 2016;6:87–92. doi: 10.1016/j.msard.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nourbakhsh B, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016 doi: 10.1136/jnnp-2016-313410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:26–31. doi: 10.1136/jnnp-2014-307928. [DOI] [PubMed] [Google Scholar]
- 9.Poser CM, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 10.McDonald WI, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 11.Kappos L, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 13.Moraal B, et al. Magnetic resonance imaging predictors of conversion to multiple sclerosis in the BENEFIT study. Arch Neurol. 2009;66:1345–1352. doi: 10.1001/archneurol.2009.243. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 16.Dyer AR, Shipley M, Elliott P. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. I. Estimates of reliability. The INTERSALT Cooperative Research Group. Am J Epidemiol. 1994;139:927–939. doi: 10.1093/oxfordjournals.aje.a117099. [DOI] [PubMed] [Google Scholar]
- 17.Beaton GH, et al. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr. 1979;32:2546–2559. doi: 10.1093/ajcn/32.12.2546. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, et al. Assessment of the association between habitual salt intake and high blood pressure: methodological problems. Am J Epidemiol. 1979;110:219–226. doi: 10.1093/oxfordjournals.aje.a112806. [DOI] [PubMed] [Google Scholar]
- 19.Maronde RF, Milgrom M, Vlachakis ND, Chan L. Response of thiazide-induced hypokalemia to amiloride. JAMA. 1983;249:237–241. [PubMed] [Google Scholar]
- 20.Bock HA, Stein JH. Diuretics and the control of extracellular fluid volume: role of counterregulation. Semin Nephrol. 1988;8:264–272. [PubMed] [Google Scholar]
- 21.Mann SJ, Gerber LM. Estimation of 24-hour sodium excretion from spot urine samples. J Clin Hypertens Greenwich Conn. 2010;12:174–180. doi: 10.1111/j.1751-7176.2009.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell MJ, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 24.Cogswell ME, et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18-39 y. Am J Clin Nutr. 2013;98:1502–1513. doi: 10.3945/ajcn.113.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimm EB, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. 1136. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 26.Day N, McKeown N, Wong M, Welch A, Bingham S. Epidemiological assessment of diet: a comparison of a 7-day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Int J Epidemiol. 2001;30:309–317. doi: 10.1093/ije/30.2.309. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez AL, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 125:4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]