Abstract
Polyphenols and antioxidant activity of skins from California almonds subjected to roasting, pasteurisation, and storage were determined by LC-MS quantification, total phenols (TP), and ferric reducing antioxidant power (FRAP). Pasteurisation did not significantly change TP, FRAP, or flavonoids and phenolic acids (FP). Roasted almonds had 26% less TP and 34% less FRAP than raw, but equivalent FP (n = 12). Storing almonds at 4 and 23 °C for 15 mo resulted in gradual increases in FP, up to 177% and 200%, respectively (n = 13). At 4 °C and 15 mo, polyphenols increased 18-fold for p-hydroxybenzoic acid, whilst others were 45–200% higher compared to baseline values. Isorhamnetin-3-O-rutinoside accounted for 48% of the increase in FP. After 15 mo, FRAP and TP increased to 200% and 190% of initial values. Accelerated ageing of whole almonds increased FP content by 10% after 3 days, but TP and FRAP values were not significantly different from baseline to day 10. Thus, in almond skins, roasting decreases TP and FRAP but not FP, whilst storage for up to 15 mo doubles FP.
Keywords: Almonds, Polyphenols, Flavonoids, Antioxidant, Storage, Accelerated ageing
1. Introduction
Tree nuts are a healthful component of the diet (Alasalvar & Shahidi, 2009). Regular consumption of mixed nuts has been associated with reduced risk of coronary disease (Fraser, Sabate, Beeson, & Strahan, 1992). Almonds are the most popular tree nut worldwide, with more than 1.6 billion lbs produced in California in 2009 (Almond Board of California, 2009). Clinical studies have demonstrated that almond consumption improves biomarkers for cardiovascular disease, antioxidant status of smokers, and insulin profiles of hyperlipidemic subjects (Estruch, Martinez-Gonzalez, & Corella, 2006; Jenkins et al., 2008; Li et al., 2007). These benefits can be largely attributed to the nutrient profile of almonds, particularly their content of α-tocopherol, oleic acids, phytosterols, and phenolics (Chen & Blumberg, 2008).
Almond phenolics are comprised of proanthocyanidins, phenolic acids, flavan-3-ols, flavanones, flavonols, isoflavones, lignans, and anthocyanin (Gu et al., 2004; Harnly et al., 2006; Milbury, Chen, Dolnikowski & Blumberg, 2006; Prodanov et al., 2008; Sang, Lapsley, Jeong, Lachance, Ho & Rosen, 2002). Almond phenolics possess in vitro antioxidant activity and are bioavailable to plasma (Chen & Blumberg, 2008; Chen, Milbury, Lapsley, & Blumberg, 2005). Further, the phenolic compounds derived from colonic microflora cleavage of almond polyphenols are bioavailable in humans (Urpi-Sarda et al., 2009). Extracts of almond polyphenols have been reported to act synergistically with vitamin E to enhance LDL resistance against Cu2+-induced oxidation (Chen, Milbury, Chung, & Blumberg, 2007; Chen et al., 2005).
The information on the impact of pre- and post-harvest conditions on almond polyphenol content and profiles are critical to better understand their potential contributions to health promotion and prevention. We have reported that the variation of flavonoid and phenolic acid content in different California almond cultivars was 2.7-fold over a 3-year period (Bolling, Dolnikowski, Blumberg, & Chen, 2010). Polyphenols have been characterised from a variety of almond products including whole almonds, blanched almond skins, blanch water, and roasted skins (Garrido, Monagas, Gomez-Cordoves, & Bartolomé, 2008; Milbury et al., 2006; Monagas et al., 2009). However, the degree to which almond polyphenol content and profiles change in raw almonds during roasting, pasteurisation, and storage has not been previously characterised.
It is hypothesised that the polyphenol content and antioxidant activity of almond skins would decrease when almonds were subjected to roasting, pasteurisation, and storage. We prioritized measuring flavonoids and phenolic acids in almond skins because there is substantial evidence that these compounds are bioavailable and bioactive (Chen & Blumberg, 2008; Chen et al., 2005). Previously, an LC-MS method was developed for a routine quantification of 18 almond flavonoids and phenolic acids (FP) (Bolling, Dolnikowski, Blumberg, & Chen, 2009). In this study, we quantified FP by LC-MS, total phenols (TP), and the ferric reducing antioxidant power (FRAP) assay in skins of raw, roasted, and pasteurised almonds, along with raw almonds stored for up to 15 mo. These measures were only assessed in almond skins because they contain the majority of almond polyphenols (Milbury et al., 2006). An accelerated ageing protocol was used to further investigate the effect of storage on almond skin FP.
2. Materials and methods
2.1. Chemicals
Quercetin-3-O-rutinoside, quercetin-3-O-galactoside, isorhamnetin, kampferol-3-O-rutinoside, kaempferol-3-O-glucoside, isorhamnetin-3-O-glucoside, isorhamnetin-3-O-rutinoside, and naringenin-7-O-glucoside were purchased from Extrasynthese (Genay, France). Naringenin, quercetin, eriodictyol, and daidzein were ordered from Indofine (Belle Mead, NJ). (+)-Catechin, protocatechuic acid, (−)-epicatechin, kaempferol, formic acid, and acetic acid were from Sigma–Aldrich (St. Louis, MO). Methanol was HPLC grade from Fisher Scientific (Fair Lawn, NJ). Water was ultrapure grade.
2.2. Pasteurisation and roasting
Raw, pasteurised, and roasted California almonds were generously provided by the Almond Board of California in 2008. Pasteurised samples were from individual samples of matched raw Butte, Carmel, and Nonpareil almond cultivars (n = 3). Almonds were pasteurised by fumigation with propylene oxide (PPO) or by proprietary short-time (<1 min) high-temperature steam treatment methods (S1 and S2). Matched raw and roasted samples were from Butte, Carmel, and Nonpareil almond cultivars harvested in 2006 (4 samples each, n = 12). Almonds were dry roasted at 295 °F for 14 min.
2.3. Storage experiments
For a 15mo storage study, 4 aliquots of 100 g raw almonds were placed in polypropylene bags. For each time point, the 13 samples analysed represented 7 almond cultivars from the 2007 harvest. Nonpareil, Carmel, Butte had n = 3, whilst Monterey, Fritz, Sonora, Mission had n = 1 samples. Almonds were stored in darkness at 4 and 23 °C at ambient humidity (~30%). At 0, 3, 9, and 15 mo, one almond sample bag was taken for analysis. Almonds were blanched in liquid nitrogen as described below, extracted immediately, and analysed within one week of collection.
Accelerated ageing was used to further examine the effect of storage on almond polyphenols. Whole PPO and roasted almonds (Carmel, Nonpareil, and Butte varieties) and pulverised liquid nitrogen blanched skins (n = 6) were placed in a 40 °C incubator at >95% humidity. PPO treated and roasted almonds were selected for accelerated ageing analysis since they are the most commonly commercially available almonds. Samples were collected at 0, 3, 7, and 10 d for analysis. Masses of pulverised almond skin aliquots were pre-determined to control for moisture accumulation. Whole almonds were immediately blanched in liquid nitrogen, skins peeled, pulverised, and stored at −80 °C until analysis.
2.4. Method of extraction
Almond skins were removed and extracted according to Bolling et al. (2009) and Mandalari et al. (2010). Briefly, almond skins were removed by blanching 3 times in liquid nitrogen, peeled, and then the skins were pulverised under liquid nitrogen to a powder form. Almond powder was steeped twice in 3.5% acetic acid, 50% methanol in water at 4 °C over 20 h (Chen et al., 2005). Aliquots of combined extracts were dried under nitrogen gas and stored at −20 °C in darkness until analysis.
2.5. LC-MS analysis of polyphenols
LC-MS quantification of 16 flavonoids and 2 phenolic acids was performed by our previously published method (Bolling et al., 2009). Briefly, dried almond skin extract was reconstituted in 5% methanol and 1% formic acid in water with 0.1 mM daidzein as an internal standard. An Agilent 1100 MSD quadrupole with electrospray ionisation (ESI) was equipped with a 250 × 4.60 mm Synergi 4μ MAX-RP 80A column (Phenomenex, Torrance, CA) and set to a constant temperature of 25 °C. The polyphenols were eluted by an increasing gradient of 1% formic acid and 100% methanol at a flow rate of 0.2 ml/min, whilst Mass Selective Detector (MSD) signals were acquired in selected ion monitoring mode as negative ions.
A standard solution consisting of (+)-catechin (CA), (−)-epicatechin (EC), daidzein, eriodictyol (E), p-hydroxybenzoic acid (pHBA), isorhamnetin (Iso), isorhamnetin-3-O-glucoside (Iso3Glu), isorhamnetin-3-O-rutinoside (Iso3R), kaempferol (K), kaempferol-3-O-glucoside (K3Glu), kaempferol-3-O-rutinoside (K3R), naringenin (N), naringenin-7-O-glucoside (N7Glu), protocatechuic acid (PCA), quercetin (Q), quercetin-3-galactoside (Q3Gal), and rutin (Q3R), were serially diluted with 1% aqueous formic acid. Dihydrokaempferol (diOHK) was quantified on the basis of eridictyol equivalents and kaempferol-3-O-galactoside (K3Gal) on the basis of kaempferol-3-O-glucoside equivalents. Quercetin-3-O-galactoside coeluted with quercetin-3-O-glucoside. Results are expressed as μg polyphenol/g almond skin. Polyphenol content on a whole almond basis, in μg/100 g almonds, is reported in Supplementary Information to facilitate inclusion in nutrient databases. Routine intra- and inter-day assay coefficients of variation (CV) were 2.4% and 6.8%, respectively.
2.6. Total phenols and ferric reducing antioxidant power (FRAP)
TP of almond skin extracts were determined by the method of Singleton, Orthofer, and Lamuela-Raventos (1999) with results expressed as mg gallic acid equivalents (GAE). Routine intra- and inter- day assay CVs were 1.6% and 4.9%, respectively. The FRAP assay was based on the method of Benzie and Strain (1996). Dried almond skin extracts were reconstituted in methanol and incubated at ambient temperature with the FRAP reagent. After 1 h incubation, absorbance was measured at 593 nm. FRAP values were expressed as μmol Trolox Equivalents (TE). Routine intra- and inter-day assay CVs were 0.7% and 4.2%, respectively.
2.7. Statistical analysis
For all experiments, samples were analysed in duplicate. Statistical significance was determined by ANOVA for pasteurised samples. Roasted and raw almond samples, initial baseline and subsequent time points for the storage study and accelerated ageing were analysed using the 2-tailed paired t-test relative to controls.
3. Results and discussion
3.1. General
Previously, we found the flavonoid and phenolic acid (FP) content of raw almonds was concentrated in their skins (Milbury et al., 2006). Thus, hot-water blanched almonds lose the majority of FP content when the skins are removed. Other processing methods are likely to affect almond skin polyphenols, but have not been well-studied. For example, almonds are pasteurised by PPO or steam treatment. Almonds may also be roasted or stored at length prior to consumption. The effects of these processing methods on almond polyphenols and antioxidant activity are not well characterised. Therefore, the objective of this study was to examine samples subjected to typical industrial processes and storage conditions.
3.2. Pasteurisation methods
In the US, almonds are required to be pasteurised to achieve a 4-log reduction in Salmonella bacteria (7 CFR Part 981). Commercial pasteurisation methods include inactivation of microorganisms either by fumigation with polypropylene oxide (PPO) or steam treatment. Both fumigation and steam treatments did not alter FP, TP, and FRAP values as compared to their raw controls (Table 1, Supplemental Table 1). The magnitudes of decrease in FRAP and TP values appeared larger and more varied than the decrease in FP. However, these changes did not reach significance in our study, P = 0.193 and 0.165 for FP and TP, respectively, likely due to the small sample size (n = 3). A larger study is warranted to investigate the impact of pasteurisation on these measures in almonds. Irradiation has also been proposed as an almond pasteurisation method. Teets, Minardi, Sundararaman, Hughey, and Were (2009) found irradiation at 10 and 20 kGy doses increased the recovery of almond flavonoids and phenolic acids by 4% and 11%, respectively, possibly because tightly bound phenolics became more extractable after the high energy treatment. However, this irradiation treatment is not practical for whole almonds because a 3 kGy dose leads to formation of volatiles from lipid peroxidation and consequent deterioration of taste (Mexis, Badeka, Chouliara, Riganakos, & Kontominas, 2009). Therefore, irradiation may not be a viable approach to increasing accessibility of phenolics from whole almonds during digestion and absorption.
Table 1.
The effect of pasteurisation and roasting on almond skin FP, TP, and FRAP values.
| Samples | FP μg/g skin | P-Valueb | Total phenols mg GAE/g skin | P-Value | FRAP μmol TE/g skin | P-Value |
|---|---|---|---|---|---|---|
| Raw | 1809 ± 117 (100) a | 0.193 | 27.6 ± 13.8 (100) | 0.165 | 210 ± 102 (100) | 0.295 |
| PPO | 1583 ± 110 (88) | 16.7 ± 3.6 (61) | 114 ± 43 (55) | |||
| S1 | 1725 ± 57 (96) | 19.5 ± 11.4 (71) | 144 ± 83 (69) | |||
| S2 | 1594 ± 245 (88) | 17.6 ± 11.4 (64) | 95.4 ± 64 (45) | |||
| Raw | 1557 ± 183 (100) | 0.777 | 25.1 ± 9.4 (100) | 0.048 | 179 ± 71(100) | 0.017 |
| Roasted | 1537 ± 146 (99) | 18.5 ± 5.5 (74) | 119 ± 40 (66) |
Numbers in parenthesis indicate% of raw samples. Abbreviations: PPO – propylene oxide, S1 – steam treatment 1, S2 – steam treatment 2.
Significance was assessed by ANOVA for pasteurised samples (n = 3) and by a paired t-test for roasted samples (n = 12). Data is mean ± SD.
3.3. Roasting
Data regarding the quantitative changes to almond skin polyphenols after roasting is lacking. This information is critical for accurate estimation of the contribution of roasted almonds to dietary polyphenol intake since most commercial almonds are roasted. We found roasted almond skins had equivalent FP to raw almond skins (Table 1, Supplemental Table 2). Garrido et al. (2008) found unblanched roasted almond skins contained 500 μg/g polyphenols including proanthocyanidins (PACs), which is 67% less than our value. Although total FP was equivalent in raw and roasted almonds skins, roasted almonds had 215% more Q, 80% more K, 60% more PCA, and 36% more pHBA. Other polyphenols were not significantly different between raw and roasted samples.
Roasted skins had 26% less TP and 34% less FRAP than raw skins (P ≤ 0.05). Since roasting decreased TP but not FP, the phenolics lost may be PACs. Both raw and roasted almonds have Type A- and B-PACs consisting of (epi)afzelechin, (epi)catechin, and (epi)gallocatechin oligomers (Monagas et al., 2009; Prodanov et al., 2008). PACs are also the most abundant polyphenols in almonds, with 10-fold greater content than FP (Gu et al., 2004; Harnly et al., 2006; Milbury et al., 2006). In pistachios, roasting at 320°F for 40 min decreased PACs by 90% as measured by an acidhydrolysis method, and antioxidant activity, using the total antioxidant activity assay, by 60% of raw samples (Gentile et al., 2007). Thus, whilst it is plausible that almond skin PACs are lost upon roasting, further work is necessary to characterise this loss of phenols and antioxidant capacity.
The impact of roasting on antioxidant activity in nuts and nut skins is inconsistent. Decreased antioxidant activity in roasted almonds and other nuts was reported by Açar, Gökmen, Pellegrini, and Fogliano (2009). Roasting almonds for 302 °F for up to 60 min led to a small (11–26%) but insignificant decrease in whole almond antioxidant activity, using the Trolox Equivalent Antioxidant Capacity (TEAC) method (Açar et al., 2009). The same study found roasting decreased peanut, walnut, hazelnut, and pistachio TEAC values. Because this study used pulverised whole nuts to measure antioxidant activity, the contribution of the skins or the testing of pulverised rather than whole nuts to this decrease is unknown.
Similar to the results of the present study, hydrophilic-ORAC (HORAC) antioxidant activity of peanut skins and defatted peanut flours decreased with increasing roast intensity (Davis, Dean, Price, & Sanders, 2010). However, the hydrophilic and lipophilic ORAC (LORAC) antioxidant activity of some varieties of whole peanuts increased upon roasting (Davis et al., 2010; Talcott, Passeretti, Duncan, & Gorbet, 2005) but did not change flavonoids or TP in others (Chukwumah, Walker, Bogler, & Verghese, 2007). Roasted, defatted peanut flours also had increased L-ORAC values (Davis et al., 2010). Talcott and others (2005) attributed the increased antioxidant activity of whole nuts to increased extractability of phenolic acids in roasted peanuts. Further, increased antioxidant capacity of roasted nuts may also be due to formation of antioxidative Maillard browning products. For example, longer roast times lessened TEAC loss in walnuts, pistachios, almonds, and hazelnuts, and increased cashew TEAC by 60% (Açar et al., 2009). Roasted almonds packaged with almond Maillard browning volatiles had reduced lipid peroxidation compared to those packaged with air (Severini, Gomes, De Pilli, Romani, & Massini, 2000). Thus, Maillard browning products in roasted nuts could contribute to antioxidant activity. Taken together, these data suggest that antioxidant activity of whole nuts may increase upon roasting due to Maillard reaction products and increased phenolic extractability, but antioxidant activity in nut skins may decrease from thermal degradation of antioxidants.
Despite decreased antioxidant activity relative to raw, roasted almond skins may still be considered a good source of dietary FP. The value of “total antioxidant capacity” assays for assessing the ultimate impact on in vivo antioxidant status has yet to be determined, though some suggest there is little direct relationship (Sies, 2007). Almond skins may be removed by a proprietary roasting procedure and then isolated as an industrial by-product for ingredient or supplement use (Garrido et al., 2008). The flavonoid content of almond skins (1.5 mg/g) is similar to blueberry (2.2 mg/g), parsley (2.4 mg/g), and red onion (0.5 mg/g) (USDA, 2007).
3.4. Long-term storage
Previous studies indicate long-term storage decreases the polyphenol content and antioxidant activity of foods. For example, storage of peanuts at 20 and 35 °C for up to 4 mo had 35% less TP than at 0 mo (Talcott et al., 2005). Similarly, green tea catechins decreased 42% after storage at 20 °C for 4 mo (Friedman, Levin, Lee, & Kozukue, 2009). Raw almonds remain edible after storage up to 24 mo at 20 °C in darkness, but lose 83–90% of vitamin E content (Zacheo, Cappello, Gallo, Santino, & Cappello, 2000). Removing almond skins, and exposure to increased temperature, light, and oxygen decrease shelf-life of almonds and promote lipid oxidation and formation of off-flavours (Mexis & Kontominas, 2010; Severini, Pilli, Baiano, & Gomes, 2003).
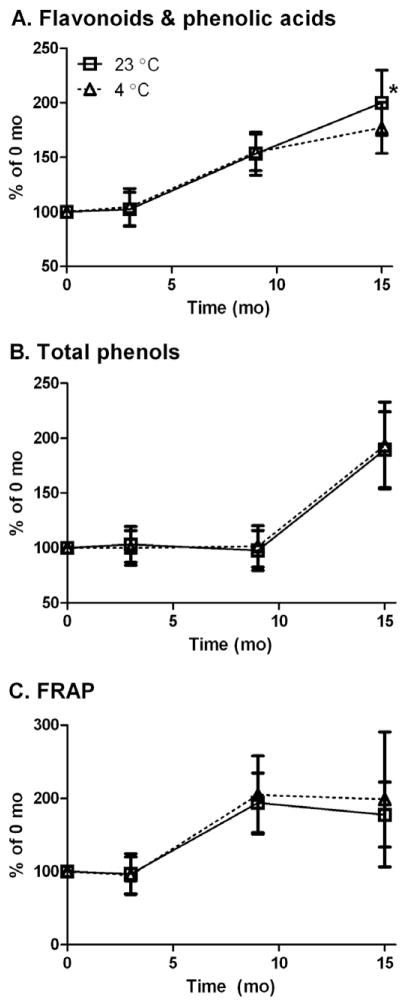
Contrary to our hypothesis that storage would decrease almond skin FP, almonds stored at 4 and 23 °C had increased FP, TP, and FRAP values (Fig. 1). TP remained constant up to 9 mo, and then increased nearly 2-fold at 15 mo. FRAP values remained constant at 3 mo, but increased 2-fold at 9 mo. Almond skin polyphenols increased linearly from 3 mo at 4 and 23 °C with R2 values of 0.95 and 0.99, respectively. Almond skins had equivalent FP at both temperatures through 9 mo, but at 15 mo, the 4 °C almonds had 23% less FP than those held at 23 °C. In contrast, the storage temperature of almonds did not affect TP and FRAP values.
Fig 1.
Storage of raw almonds in darkness at 4 and 23 °C for 15 mo increased flavonoids and phenolic acids determined by LC-MS (A), Total Phenols (B), and FRAP (C) of almond skins. Data is mean ± SEM, n = 13, representing 7 almond varieties. *Significantly differ amongst temperatures, P ≤ 0.05.
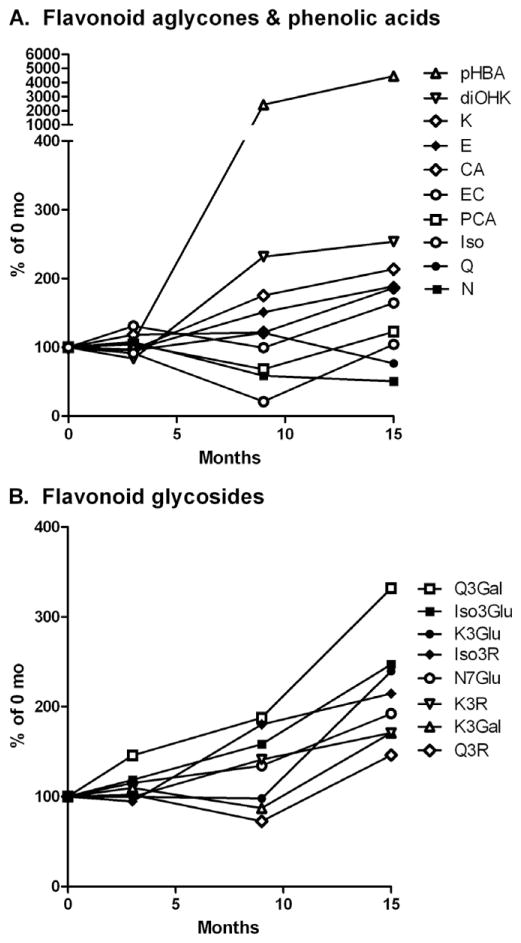
Storage affected individual FP to different degrees (Supplemental Table 3). p-Hydroxybenzoic acid (pHBA) increased the greatest magnitude in almond skins, by 20-fold at 9 mo and 40-fold at 15 mo and 23 °C (Fig. 2). This change accounted for 11% of the increased FP mass at 23 °C (Table 2). Isorhamnetin-3-rutinoside (Iso3R), the most abundant flavonoid in almond skins, increased by 90% at 15 mo, representing 48% of the increased FP. Amongst flavonoids, quercetin-3-galactoside increased the greatest percentage, by 310% at 15 mo. The magnitude of changes for other FP at 15 mo were in the range of 50–250% of their baseline values. At 23 °C, naringenin was the only FP found to be significantly decreased by 52% at 15 mo as compared to baseline. Further, storage at 4 °C had a similar effect on magnitude of naringenin reduction. Isorhamnetin, procatechuic acid (PCA), kaempferol-3-galactoside, and quercetin-3-rutinoside were also decreased by 2–23% at 9 mo, but increased to their original content or more at 15 mo and 23 °C (Supplementary Information).
Fig 2.
Changes to flavonoid aglycone and phenolic acid (A) and flavonoid glycoside (B) content in almond skins upon storage at 23 °C for 15 mo. Abbreviations: CA – catechin, EC – epicatechin, diOHK – dihydroxykaempferol, E – eriodictyol, Iso – isorhamnetin, Iso3Glu – isorhamnetin-3-O-glucoside, Iso3R – isorhamnetin-3-Orutinoside, K – kaempferol, K3Gal – kaempferol-3-O-galactoside, K3Glu – kaempferol-3-O-glucoside, K3R – kaempferol-3-O-rutinoside, N – naringenin, N7Glu – naringenin-7-O-glucoside, PCA – procatechuic acid, pHBA – p-hydroxybenzoic acid, Q – quercetin, Q3Gal – quercetin-3-O-galactoside, Q3R – quercetin-3-O-rutinoside.
Table 2.
Almond skin flavonoid and phenolic acid content and changes in almonds stored at 4 and 23 °C for 15 mo.
| Temperature | Flavonoid or phenolic acid | 0 mo μg/g skin | 15 mo μg/g skin | Difference μg/g skin | P-Value difference |
|---|---|---|---|---|---|
| 4 °C | Iso3R | 639 ± 201 | 1274 ± 365 | 635 ± 205 | 1.18 × 10−5 |
| Iso3Glu | 139 ± 142 | 315 ± 325 | 176 ± 191 | 0.0857 | |
| K3R | 196 ± 155 | 295 ± 218 | 98.8 ± 70.8 | 0.1944 | |
| CA | 184 ± 98 | 278 ± 157 | 93.3 ± 88.5 | 0.0822 | |
| pHBA | 3.97 ± 0.881 | 75.2 ± 25.0 | 71.2 ± 24.7a | 3.01 × 10−10 | |
| diOHK | 49.9 ± 26.6 | 117 ± 52 | 66.7 ± 31.3 | 0.0004 | |
| N7Glu | 35.8 ± 20.4 | 58.9 ± 33.3 | 23.1 ± 17.1 | 0.0431 | |
| EC | 68.6 ± 32.5 | 88.2 ± 50.1 | 19.7 ± 31.7 | 0.2466 | |
| Q3Gal | 6.45 ± 4.71 | 16.0 ± 10.8 | 9.51 ± 6.59 | 0.0078 | |
| K3Glu | 23.2 ± 11.8 | 29.1 ± 15.7 | 5.81 ± 6.39 | 0.2961 | |
| K | 2.40 ± 2.04 | 4.82 ± 4.5 | 2.42 ± 2.54 | 0.0885 | |
| E | 2.75 ± 1.11 | 4.86 ± 1.81 | 2.11 ± 0.98 | 0.0015 | |
| K3Gal | 7.17 ± 6.31 | 8.84 ± 7.60 | 1.67 ± 2.88 | 0.5489 | |
| Q3R | 8.15 ± 3.50 | 8.46 ± 3.12 | 0.310 ± 1.389 | 0.8138 | |
| Q | 3.12 ± 0.95 | 2.17 ± 0.84 | −0.950 ± 0.361 | 0.0124 | |
| Iso | 10.9 ± 18.7 | 8.11 ± 13.9 | −2.80 ± 5.25 | 0.6685 | |
| PCA | 44.6 ± 15.3 | 24.9 ± 9.02 | −19.7 ± 13.5a | 0.0005 | |
| N | 83.4 ± 41.3 | 36.3 ± 14.8 | −47.1 ± 27.7 | 0.0007 | |
| Sum | 1508 ± 411 | 2644 ± 659 | 1135 ± 356a | 2.09 × 10−5 | |
| 23 °C | Iso3R | 639 ± 201 | 1361 ± 407 | 722 ± 240 | 6.56 × 10−6 |
| Iso3Glu | 139 ± 142 | 325 ± 376 | 186 ± 246 | 0.1081 | |
| pHBA | 3.97 ± 0.881 | 170 ± 25 | 166 ± 25a | 4.09 × 10−18 | |
| CA | 184 ± 98 | 338 ± 190 | 154 ± 116 | 0.0157 | |
| K3R | 196 ± 155 | 317 ± 224 | 121 ± 79 | 0.1228 | |
| diOHK | 49.9 ± 26.6 | 124 ± 57 | 73.9 ± 34.9 | 0.0003 | |
| EC | 68.6 ± 32.5 | 103 ± 49 | 34.6 ± 33.1 | 0.0435 | |
| N7Glu | 35.8 ± 20.4 | 65.7 ± 36.4 | 29.9 ± 22.3 | 0.0162 | |
| K3Glu | 23.2 ± 11.8 | 52.1 ± 47.5 | 28.8 ± 46.9 | 0.0446 | |
| Q3Gal | 6.45 ± 4.71 | 19.4 ± 15.5 | 12.9 ± 11.6 | 0.0081 | |
| PCA | 44.6 ± 15.3 | 50.4 ± 8.9 | 5.78 ± 12.32a | 0.2520 | |
| Q3R | 8.15 ± 3.50 | 12.3 ± 10.8 | 4.18 ± 9.24 | 0.1978 | |
| K3Gal | 7.17 ± 6.31 | 10.7 ± 7.0 | 3.51 ± 3.61 | 0.1921 | |
| K | 2.40 ± 2.04 | 5.19 ± 4.56 | 2.79 ± 2.70 | 0.0550 | |
| E | 2.75 ± 1.11 | 5.17 ± 2.01 | 2.42 ± 1.06 | 0.0009 | |
| Q | 3.12 ± 0.95 | 2.43 ± 0.89 | −0.692 ± 0.409 | 0.0669 | |
| Iso | 10.9 ± 18.7 | 9.25 ± 15.6 | −1.65 ± 3.65 | 0.8086 | |
| N | 83.4 ± 41.3 | 39.7 ± 16.8 | −43.7 ± 26.3 | 0.0017 | |
| Sum | 1508 ± 411 | 3010 ± 826 | 1501 ± 516a | 4.69 × 10−6 |
Data is mean ± SD, n = 13. Abbreviations as in Fig. 2.
P ≤ 0.05 for temperature effect, tested using a 2-tailed paired t-test.
Whilst the percentage increase in FP at 15 mo from baseline was less at 4 than 23 °C (75% vs. 100%), only the contents of pHBA and PCA were significantly different between temperatures (Table 2). The magnitude of pHBA increase was less at 4 °C, with an 18-fold increase from initial values. PCA decreased 44% at 4 °C but increased by 13% at 23 °C at 15 mo. Interestingly, our previous study showed that both pHBA and PCA were identified as the two strongest discriminating factors for almond harvest year using multivariate analysis of almond FP content, FRAP, and TP (Bolling et al., 2010). Since the almonds from three harvest years were analysed at the same time, our current data in changes of pHBA and PCA content in almond skin during storage support the emergence of these variables as annual discriminating factors.
Despite differential changes to absolute FP content, the relative FP profiles (individual content/sum) remained essentially unchanged for the majority of compounds (±3%) at both temperatures. At 23 °C, pHBA increased from 0.3% to 6.2% of total polyphenols, and to 3.2% at 4 °C. Iso3R increased from 43% to 49% of FP at 4 °C. Naringenin decreased from 5.5% to 1.3% at 15 mo for both temperatures. Therefore, the almond skins subjected to storage for up to 15 mo have similar FP profiles.
This data demonstrates that the changes in FP content of almond skins during long-term storage reflect a dynamic process, and could have a number of causes. Storage could induce a physical transformation which in turn increases the extractability of FP. For example, storage could affect the physical structure of cellulose or lignin in the skin and make FP more accessible for extraction. Similarly, degradation of proanthocyanidins or covalently bound FP may increase soluble phenolic content in almond skins. Alternatively, polyphenol synthesis may continue after harvest in almond, as has been observed in a few foods. In bambarranut seeds (Voandzeia subterranean Thouars), an edible groundnut, TP increased by 42% after 24 mo storage at 30–35 °C (Sreeramulu, 1983). Similar to the results of the present study, in bambarranuts, PCA decreased 8-fold and pHBA increased 90%. Sreeramulu (1983) reported that the relationship between decreased PCA and increased pHBA content was due to reduced germination viability. Similarly, peanuts undergoing germination had increased TP content (Sobolev, Horn, Potter, Deyrup, & Gloer, 2006). In asparagus, ferulic acid, dihydroferulates, and ferulic dimers and trimers increased nearly 3-fold upon storage at 25 °C, with a 7% smaller increase at 4 °C after 8 d (Jaramillo, Rodríguez, Jiménez, Guillén, Fernández-Bolaños & Heredia, 2007). Therefore, the increase in FP content in almond skins during storage might be a result of ongoing polyphenol synthesis. Since few studies report increases in flavonoids and phenolic acids in foods during storage, future work is warranted to elucidate mechanisms for flavonoid and phenolic acid increases in almonds.
Regardless of the mechanisms involved in the increase of extractable FP from almond skin, this finding highlights the complicated nature of food compositional analysis. Foods are dynamic biological systems and are continuously subjected to physical or chemical influences before and after harvest, and efforts to preserve foods may affect both the content and profile of their bioactive components. Therefore, in light of the present study, efforts to compare polyphenol composition of almonds between years should control for storage length.
Our 15 mo storage data for 2007 almonds is comparable to the FP content of 2006 almonds reported in our previous study, as 2006 almonds were analysed ~15 mo after harvest (Bolling et al., 2010). On an equivalent storage basis, 2007 almonds had 70% more FP than 2006 (P = 0.0001) (Supplemental Table 4). These data contrast to our initial report, where no significant differences were found in FP content between 2006 and 2007 almonds (Bolling et al., 2010). Amongst individual polyphenols, Iso3R content from 2007 was 95% greater than 2006, representing 53% of the overall FP mass difference. pHBA was also 40-fold greater in 2007 compared to 2006 where the majority of FP increased, but epicatechin, quercetin-3-rutinoside, quercetin, naringenin, and isorhamnetin were decreased 17–65%. Therefore, after controlling for storage, differences in almond FP content between annual harvests may be of greater magnitude than previously reported.
3.5. Accelerated ageing analysis
To further investigate the effect of storage on FP content, we subjected roasted and PPO almonds to accelerated ageing at >95% RH and 40 °C. Zacheo, Cappello, Perrone, and Gnoni (1998) found that high relative humidity (80% RH at 20 °C) increased lipoxygenase activity and malondialdehyde in almonds by 33– 40% after 20 d. Zacheo et al. (2000) report rancidity as a primary determinant of almond shelf life, secondary to mould growth at high humidity. It was hypothesised that lipid peroxidation in the seed induced by the accelerated ageing condition would decrease almond skin FP due to their antioxidant-oxidant interaction. Therefore, both intact almonds and pulverised almonds skins removed by liquid nitrogen blanching were subjected to accelerated ageing.
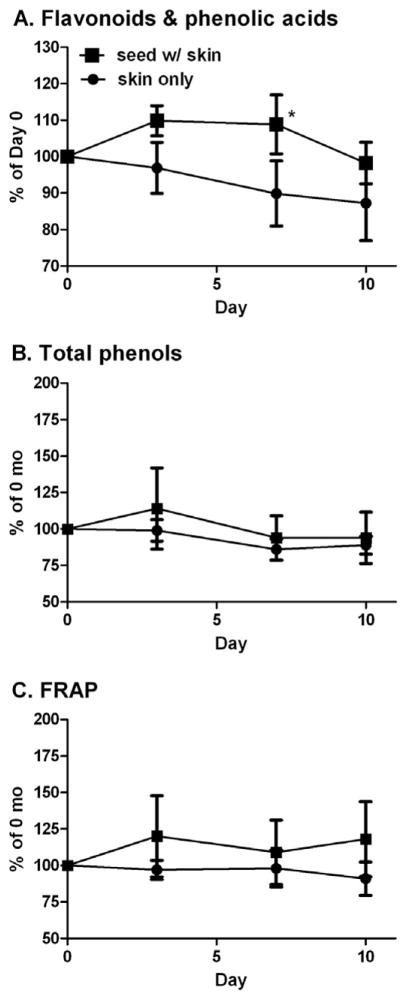
In the present study, almonds quickly became rancid by smell and appearance and had mould growth beyond 10 d. TP and FRAP values of almond skin extracts did not significantly change when subjected to accelerated ageing (Fig. 3). However, similar to almonds stored for 15 mo, the FP content of skins from whole almonds increased by 10% at day 3, was significantly greater than skins alone at day 7, and then decreased to the baseline values at day 10. The FP of skins alone decreased linearly (R = −0.99) from baseline and was 13% less at day 10. Therefore, an intact seed coat is required to observe increases in almond skin FP content when almonds are exposed to high humidity and temperature.
Fig 3.
Flavonoids and phenolic acids determined by LC-MS (A), TP (B), and FRAP (C) in skins from whole almonds or powdered almond skins subjected to accelerated ageing treatment. Data is mean ±SEM, n = 6 of roasted and pasteurised samples of 3 varieties. *Significantly differ amongst days, P ≤ 0.05.
In contrast with long-term storage, changes to individual FP of almond skins during accelerated ageing were small and inconsistent (Table 3). Notably, in whole almonds, naringenin increased by 28%, in contrast to the decline observed in almonds stored for 15 mo. Moreover, pHBA did not significantly increase in whole almonds, but doubled in content in almond skins alone at 7 d. Similar to almonds stored 15 mo, Iso3R increased in whole almonds insignificantly by 9%. Similarly, changes to FP profiles were insignificant. The decreased FP accumulation during accelerated ageing compared to long-term storage may be due to different starting materials, e.g. use of raw vs. roasted/PPO almonds, temperature, humidity, or duration of storage. Thus, tests of accelerated ageing of whole almonds may model the increase in total FP, but to a lesser degree observed than during long-term storage.
Table 3.
Almond skin flavonoid and phenolic acid content of whole almonds or powdered almond skins subjected to accelerated ageing treatment.
| Material | Flavonoid or phenolic acid | Day 0 μg/g skin | Day 7 μg/g skin | Difference μg/g skin | % of Day 0 | P-Value difference |
|---|---|---|---|---|---|---|
| Whole almond seed | Iso3R | 641 ± 118 | 700 ± 166 | 59.6 | 109 | 0.4895 |
| K3R | 208 ± 117 | 242 ± 144 | 33.8 | 116 | 0.6651 | |
| N | 93.2 ± 12.0 | 120 ± 21 | 26.4 | 128 | 0.0251 | |
| N7Glu | 55.0 ± 19.1 | 70.8 ± 24.1 | 15.8 | 129 | 0.2376 | |
| PCA | 26.0 ± 5.8 | 33.3 ± 15.0 | 7.3 | 128 | 0.2940 | |
| K3Glu | 27.1 ± 8.6 | 33.6 ± 11.8 | 6.5 | 124 | 0.3028 | |
| K3Gal | 11.5 ± 6.5 | 17.7 ± 8.7 | 6.2 | 153 | 0.1970 | |
| Iso3Hlu | 126 ± 88 | 132 ± 94 | 5.8 | 105 | 0.9144 | |
| Iso | 11.4 ± 10.3 | 14.2 ± 10.7 | 2.9 | 125 | 0.6478 | |
| Q3Gal | 9.93 ± 3.12 | 12.6 ± 3.8 | 2.6 | 126 | 0.2226 | |
| Q3R | 9.54 ± 2.19 | 12.0 ± 2.7 | 2.5 | 126 | 0.1127 | |
| Q | 3.05 ± 2.19 | 5.30 ± 2.00 | 2.3 | 174 | 0.0925 | |
| K | 4.16 ± 2.14 | 5.75 ± 2.25 | 1.6 | 138 | 0.2374 | |
| pHBA | 5.34 ± 0.903 | 6.79 ± 2.50 | 1.4a | 127 | 0.2121 | |
| E | 3.91 ± 0.49 | 4.60 ± 0.72 | 0.7 | 118 | 0.0811 | |
| diOHK | 60.2 ± 14.9 | 57.1 ± 16.9 | −3.1 | 95 | 0.7450 | |
| EC | 80.0 ± 30.1 | 65.8 ± 21.4 | −14.2 | 82 | 0.3666 | |
| CA | 163 ± 80 | 129 ± 53 | −34.2 | 79 | 0.4045 | |
| Sum | 1539 ± 122 | 1663 ± 241 | 123.6a | 108 | 0.2885 | |
| Almond skin alone | K3Glu | 27.1 ± 8.6 | 42.4 ± 49.1 | 15.3 | 156 | 0.4704 |
| PCA | 26.0 ± 5.8 | 38.0 ± 16.1 | 12.0 | 146 | 0.1161 | |
| N | 93.2 ± 12.0 | 102 ± 14 | 9.3 | 110 | 0.2523 | |
| pHBA | 5.34 ± 0.90 | 10.6 ± 3.1 | 5.3a | 199 | 0.0027 | |
| K3Gal | 11.4 ± 6.5 | 12.7 ± 7.4 | 1.2 | 110 | 0.7763 | |
| Q | 3.05 ± 2.19 | 4.09 ± 2.45 | 1.0 | 134 | 0.4534 | |
| Iso | 11.4 ± 10.3 | 12.3 ± 11.0 | 0.9 | 108 | 0.8868 | |
| K | 4.16 ± 2.14 | 4.84 ± 2.96 | 0.7 | 116 | 0.6570 | |
| E | 3.91 ± 0.49 | 4.43 ± 0.45 | 0.5 | 113 | 0.0855 | |
| diOHK | 60.2 ± 14.9 | 60.7 ± 21.2 | 0.5 | 101 | 0.9658 | |
| Q3R | 9.54 ± 2.19 | 9.10 ± 3.51 | −0.4 | 95 | 0.7985 | |
| Q3Gal | 9.93 ± 3.12 | 8.27 ± 3.30 | −1.7 | 83 | 0.3919 | |
| N7Glu | 55.0 ± 19.1 | 45.8 ± 19.1 | −9.2 | 83 | 0.4249 | |
| EC | 80.0 ± 30.1 | 65.1 ± 25.2 | −14.9 | 81 | 0.3738 | |
| K3R | 208 ± 117 | 179 ± 97 | −29.2 | 86 | 0.6486 | |
| Iso3Glu | 126 ± 88 | 96.1 ± 75.6 | −30.1 | 76 | 0.5399 | |
| CA | 163 ± 80 | 105 ± 53 | −57.9 | 65 | 0.1702 | |
| Iso3R | 641 ± 118 | 575 ± 118 | −65.5 | 90 | 0.3589 | |
| Sum | 1539 ± 122 | 1377 ± 103 | −162.3a | 89 | 0.0322 |
Data is mean ± SD of n = 6 almonds (3 almond varieties, PPO pasteurised and roasted). Abbreviations as in Table 2.
Significant difference between treatments, P ≤ 0.05, tested by a 2-tailed paired t-test.
The finding that whole almonds with skins are required for increases in FP content of skins undergoing an accelerated ageing treatment suggests that the mechanism is unlikely due to physical changes to the skin such as moisture accumulation or hydration of cellulose or lignin. This is because the powdered almond skins had increased surface area for moisture and oxygen exposure but had decreased FP content. Within the seeds, increases in moisture during the accelerated ageing could activate lipoxygenase, leading to formation of lipid peroxidation products which might then interact with the skin (Buranasompob, Tang, Powers, Reyes, Clark & Swanson, 2007). Further studies are warranted to elucidate the mechanisms underlying the increase of FP in almond skins during storage.
4. Conclusions
Processing and storage of almonds changed the polyphenol and antioxidant content of skins. Almond skin TP and FRAP values decreased ~30% by roasting, but FP remained unchanged. Pasteurisation did not significantly affect FP, TP, or FRAP of almond skins. Almond stored at 4 and 23 °C in darkness had nearly double FP, TP, and FRAP values after 15 mo. Further study is needed to understand the physical and/or biochemical mechanisms of these changes.
Supplementary Material
Acknowledgments
This work was supported by U.S. Department of Agriculture (USDA)/Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707 and a grant from the Almond Board of California. Dr. Bolling was supported by award K12GM074869 from the National Institute of Medical Sciences. The authors are grateful for the excellent technical assistance of Gregory Dolnikowski, Jennifer O’Leary, Desire Kelley, and Sisca Bolling.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.foodchem.2010.05.058.
Footnotes
Supported by the Almond Board of California, US Department of Agriculture (USDA)/Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, and K12GM074869 from the National Institute of General Medical Sciences. The contents of this publication do not necessarily reflect the official views or policies of the NIGMS, NIH, or USDA nor does mention of trade names, commercial products or organizations imply endorsement by the US government.
References
- Açar OC, Gökmen V, Pellegrini N, Fogliano V. Direct evaluation of the total antioxidant capacity of raw and roasted pulses, nuts, and seeds. European Food Research and Technology. 2009;229:961–969. [Google Scholar]
- Alasalvar C, Shahidi F, editors. Tree nuts: Composition, phytochemicals and health effects. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Almond Board of California. Almond Almanac. Modesto, CA: 2009. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bolling BW, Dolnikowski G, Blumberg JB, Chen CY. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chemistry. 2010;122:819–825. doi: 10.1016/j.foodchem.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling BW, Dolnikowski G, Blumberg JB, Chen CY. Quantification of almond skin polyphenols by liquid chromatography–mass spectrometry. Journal of Food Science. 2009;74:C326–C332. doi: 10.1111/j.1750-3841.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buranasompob A, Tang J, Powers JR, Reyes J, Clark S, Swanson BG. Lipoxygenase activity in walnuts and almonds. LWT-Food Science and Technology. 2007;40:893–899. [Google Scholar]
- Chen CY, Blumberg JB. Phytochemical composition of nuts. Asia Pacific Journal of Clinical Nutrition. 2008;17:329–332. [PubMed] [Google Scholar]
- Chen CY, Milbury PE, Chung SK, Blumberg JB. Effect of almond skin polyphenolics and quercetin on human LDL and apolipoprotein B-100 oxidation and conformation. Journal of Nutritional Biochemistry. 2007;18:785–794. doi: 10.1016/j.jnutbio.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Chen CY, Milbury PE, Lapsley KG, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. Journal of Nutrition. 2005;135:1366–1373. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- Chukwumah Y, Walker L, Bogler B, Verghese M. Changes in the phytochemical composition and profile of raw, boiled, and roasted peanuts. Journal of Agricultural and Food Chemistry. 2007;55:9266–9273. doi: 10.1021/jf071877l. [DOI] [PubMed] [Google Scholar]
- Davis JP, Dean LL, Price KM, Sanders TH. Roast effects on the hydrophilic and lipophilic antioxidant capacities of peanut flours, blanched peanut seed and peanut skins. Food Chemistry. 2010;119:539–547. [Google Scholar]
- Estruch R, Martinez-Gonzalez MA, Corella D. Effects of a Mediterranean-style diet on cardiovascular risk factors. Annals of Internal Medicine. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Fraser GE, Sabate J, Beeson WL, Strahan M. A possible protective effect of nut consumption on risk of coronary heart disease. Archives of Internal Medicine. 1992;152:1416–1424. [PubMed] [Google Scholar]
- Friedman M, Levin CS, Lee SU, Kozukue N. Stability of green tea catechins in commercial tea leaves during storage for 6 months. Journal of Food Science. 2009;74:H47–H51. doi: 10.1111/j.1750-3841.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- Garrido I, Monagas M, Gomez-Cordoves C, Bartolomé B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. Journal of Food Science. 2008;73:C106–C115. doi: 10.1111/j.1750-3841.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Gentile C, Tesoriere L, Butera D, Fazzari M, Monastero M, Allegra M, et al. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. Journal of Agricultural and Food Chemistry. 2007;55:643–648. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. Journal of Nutrition. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of US Fruits, vegetables, and nuts. Journal of Agricultural and Food Chemistry. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- Jaramillo S, Rodríguez R, Jiménez A, Guillén R, Fernández-Bolaños J, Heredia A. Effects of storage conditions on the accumulation of ferulic acid derivatives in white asparagus cell walls. Journal of the Science of Food and Agriculture. 2007;87:286–296. [Google Scholar]
- Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, et al. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: A randomized controlled crossover trial. Metabolism. 2008;57:882–887. doi: 10.1016/j.metabol.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Li N, Jia X, Chen CY, Blumberg JB, Song Y, Zhang W, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. Journal of Nutrition. 2007;137:2717–2722. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]
- Mandalari G, Tomaino A, Arcoraci T, Martorana M, Turco VL, Cacciola F, et al. Characterization of polyphenols, Lipids and dietary fibre from almond skins (Amygdalus communis L.) Journal of Food Composition and Analysis. 2010;23:166–174. [Google Scholar]
- Mexis SF, Badeka AV, Chouliara E, Riganakos KA, Kontominas MG. Effect of γ-irradiation on the physicochemical and sensory properties of raw unpeeled almond kernels (Prunus dulcis) Innovative Food Science and Emerging Technology. 2009;10:87–92. doi: 10.1002/jsfa.4225. [DOI] [PubMed] [Google Scholar]
- Mexis SF, Kontominas MG. Effect of oxygen absorber, nitrogen flushing, packaging material oxygen transmission rate and storage conditions on quality retention of raw whole unpeeled almond kernels (Prunus dulcis) LWT – Food Science and Technology. 2010;43:1–11. [Google Scholar]
- Milbury PE, Chen CY, Dolnikowski GG, Blumberg JB. Determination of flavonoids and phenolics and their distribution in almonds. Journal of Agricultural and Food Chemistry. 2006;54:5027–5033. doi: 10.1021/jf0603937. [DOI] [PubMed] [Google Scholar]
- Monagas M, Garrido I, Lebrón-Aguilar RM, Gómez-Cordovés MC, Rybarczyk A, Amarowicz R, et al. Comparative flavan-3-ol profile and antioxidant capacity of roasted peanut, hazelnut, and almond skins. Journal of Agricultural and Food Chemistry. 2009;57:10590–10599. doi: 10.1021/jf901391a. [DOI] [PubMed] [Google Scholar]
- Prodanov M, Garrido I, Vacas V, Lebron-Aguilar R, Duenas M, Gomez-Cordoves C, et al. Ultrafiltration as alternative purification procedure for the characterization of low and high molecular-mass phenolics from almond skins. Analytica Chimica Acta. 2008;609:241–251. doi: 10.1016/j.aca.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Sang S, Lapsley KG, Jeong WS, Lachance PA, Ho CT, Rosen RT. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch) Journal of Agricultural and Food Chemistry. 2002;50:4709–4712. doi: 10.1021/jf011533+. [DOI] [PubMed] [Google Scholar]
- Severini C, Gomes T, De Pilli T, Romani S, Massini R. Autoxidation of packed almonds as affected by Maillard reaction volatile compounds derived from roasting. Journal of Agricultural and Food Chemistry. 2000;48:4635–4640. doi: 10.1021/jf0000575. [DOI] [PubMed] [Google Scholar]
- Severini C, Pilli TD, Baiano A, Gomes T. Autoxidation of packed roasted almonds as affected by two packaging films. Journal of Food Processing and Preservation. 2003;27:321–335. [Google Scholar]
- Sies H. Total antioxidant capacity: Appraisal of a concept. Journal of Nutrition. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Sobolev VS, Horn BW, Potter TL, Deyrup ST, Gloer JB. Production of stilbenoids and phenolic acids by the peanut plant at early stages of growth. Journal of Agricultural and Food Chemistry. 2006;54:3505–3511. doi: 10.1021/jf0602673. [DOI] [PubMed] [Google Scholar]
- Sreeramulu N. Auxins, inhibitors and phenolics in bambarranut seeds (Voandzeia subterranean Thouars) in relation to loss of viability during storage. Annals of Botany. 1983;51:209–216. [Google Scholar]
- Talcott ST, Passeretti S, Duncan CE, Gorbet DW. Polyphenolic content and sensory properties of normal and high oleic acid peanuts. Food Chemistry. 2005;90:379–388. [Google Scholar]
- Teets AS, Minardi CS, Sundararaman M, Hughey CA, Were LM. Extraction, identification, and quantification of flavonoids and phenolic acids in electron beam-irradiated almond skin powder. Journal of Food Science. 2009;74:C298–C305. doi: 10.1111/j.1750-3841.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- Urpi-Sarda M, Garrido I, Monagas M, Gomez-Cordoves C, Medina-Remon A, Andres-Lacueva C, et al. Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. Journal of Agricultural and Food Chemistry. 2009;57:10134–10142. doi: 10.1021/jf901450z. [DOI] [PubMed] [Google Scholar]
- USDA. [Accessed Jan 20, 2010];Database for the flavonoid content of foods. 2007 www.nal.usda.gov/fnic/foodcomp/Data/Flav/Flav02-1.pdf.
- Zacheo G, Cappello MS, Gallo A, Santino A, Cappello AR. Changes associated with post-harvest ageing in almond seeds. LWT-Food Science and Technology. 2000;33:415–423. [Google Scholar]
- Zacheo G, Cappello AR, Perrone LM, Gnoni GV. Analysis of factors influencing lipid oxidation of almond seeds during accelerated ageing. LWT-Food Science and Technology. 1998;31:6–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.