Abstract
Purpose
Posterior fossa epidural hematomas (PFEDH) are uncommon in children but usually require timely surgical intervention due to the risk of life-threatening brainstem compression. We attempt to make the surgical procedure less invasive by treating selected pediatric patients with trephination mini-craniectomy.
Methods
We retrospectively reviewed the clinical courses, radiological findings, surgical procedures, and prognoses of the pediatric patients who were treated in our departments for traumatic PFEDH from January 2010 to January 2015.
Results
During this period, a total of 17 patients were surgically treated for PFEDH and 7 were managed with trephination mini-craniectomy for hematoma evacuation. The outcomes were good in all 7 patients as evaluated with Glasgow Outcome Score. There was no mortality in this series. The on average 30-month clinical follow-up showed that patients experienced satisfactory recoveries without complications.
Conclusion
Our results suggest that trephination mini-craniectomy is a safe surgical technique for selected PFEDH patients with moderate hematoma volume and stabilized neurological functions. However, standard craniectomy is recommend when there are rapid deteriorations in patients' neurological functions or the hematomas are large and exerted severe mass effects.
Keywords: Posterior fossa epidural hematoma, Trephination mini-craniectomy, Pediatrics
Introduction
Epidural hematoma (EDH) represents a relatively uncommon entity in neurosurgical departments, with its incidence in patients with traumatic brain injury reported to be 2.7%–4%.1, 2, 3, 4 In pediatric population, traumatic EDH is estimated to represent 2%–5% of all head injuries.5, 6, 7 Posterior fossa EDH (PFEDH) is even less common as it only accounts for 1.2%–15% of all EDH,8, 9, 10 with a slightly higher incidence in children as compared with other age groups.11 The diagnosis of EDH has become easier with the increased availability of computed tomography (CT) facilities.12 Although some patients have been successfully treated with conservative approach,5, 13 most studies support timely management of PFEDH by surgical intervention in children.5, 7, 14, 15, 16, 17 For EDH, standard craniotomy is routinely used to remove the hematomas.18 There are also several reports on the successful applications of trephination mini-craniectomy for evacuation/drainage of the hematomas in adult patients under emergency setting.19, 20, 21, 22 However, little evidence is available regarding the feasibility of using trephination mini-craniectomy for traumatic PFEDH in children.23, 24 Therefore, we attempted to evaluate its safety and efficacy by retrospectively analyzing clinical data from pediatric patients who were surgically treated for PFEDH in our department.
Materials and methods
A retrospective study was undertaken to identify all pediatric patients who were surgically treated for the diagnosis of PFEDH from January 2010 to January 2015 at Department of Neurosurgery, Second Affiliated Hospital of Wenzhou Medical University. Only patients with clear evidences of head trauma as the primary etiology were included in this study. While patients with hematomas located at the clivus, tentorium, or PFEDH accompanied with brain contusions at the cerebrum or brain stems were excluded. Patients' demographic profiles, CT findings and clinical data (e.g. causes of injury, surgical procedures, complications, hospital course and clinical outcome) were collected. Consciousness level was evaluated by Glasgow Coma Scale (GCS) at admission as well as during and after treatment. CT findings assessed included hematoma volume, presence of fourth ventricle compression, hydrocephalus and obliteration of the peri-mesencephalic cistern, presence and pattern of the skull fracture, and other associated intracranial lesions.
The decision on whether standard craniectomy or trephination mini-craniectomy was used was dependent on the CT findings as well as clinical conditions. Specifically, criteria for surgical intervention with standard craniectomy were as follows: hematoma volume>30 mL or presence of severe mass effects like cerebellar tonsillar herniation. Criteria for surgical intervention with trephination mini-craniectomy were as follows: hematoma volume between 10 mL and 30 mL, with mild mass effects (e.g. fourth ventricle compression or displacement and/or obstructive ventriculomegaly) and stable neurological conditions. Clinical outcome was evaluated by Glasgow Outcome Scale (GOS) at discharge and flow-ups. Approval to conduct this study was obtained from the Medical Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University. Both patients' parents gave their consent for the use of their children's medical and personal information for the publication of this case report and any accompanying images.
After general anesthesia, patient was placed in prone position with all pressure points padded. A 5 cm straight paramedian suboccipital skin incision, followed by a trephination, was made in the center of the hematoma projection on the skull according to preoperative CT images. Then the trephination hole was either used directly or enlarged with rongeur to create a mini-craniectomy of about 2 cm–3 cm in diameter. A silicon tube was then inserted into the hematoma to aspirate the blood clot as thoroughly as possible and was left in the epidural space for 1 day postoperatively to provide complete drainage of any potential residual hematoma under negative pressure. Wounds were closed in layers with stitches on the scalp. Two trephination holes would be needed if the hematoma was large. The operation took about 30 min–50 min. Blood loss due to the operation was estimated to be around 50 mL. All patients routinely underwent a cranial CT scan within 6 h postoperatively to assess the completeness of hematoma evacuation.
Results
A total of 17 patients who diagnosed with PFEDH were surgically treated during the study period, and 7 were treated with trephination mini-craniectomy (Table 1, Table 2). There were three males and four females and with an average age of 5.3 years (2 years–10 years). GCS was 13 on average (12–15) at admission and preoperative hematoma volume was 19 cm3 on average (12 cm3- 30 cm3). The time interval between injury and surgery was 24 h on average (5 h- 72 h). Six patients had one trephination and one had two trephinations. All hematomas were successfully evacuated according to postoperative CT. In all 7 patients, GCS scores were improved to 15 after surgery and symptoms such as headache, nausea and vomiting, disappeared immediately after operation. Postoperative pain due to the incision was very mild and was managed with nonsteroidal anti-inflammatory drugs when necessary. Patients were discharged 4 days on average after operation (3 days–5 days) for rehabilitation. GOS scores were 5 on discharge and clinical follow-ups, and no surgical related mortality, complications or recurrence of PFEDHs were observed during the on average 30 months' follow-up.
Table 1.
Summary of clinical characteristics of pediatric patients diagnosed with traumatic PFEDH.
| No | Sex | Age (yr) | Cause of injury | Symptoms | GCSa | GCSb | Time (h) c | Time (d) d | GOS | Follow-up (mon) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 6 | Fall | Headache, vomiting | 15 | 15 | 20 | 3 | 5 | 20 |
| 2 | F | 10 | Fall | Headache, nausea, vomiting | 15 | 15 | 24 | 4 | 5 | 27 |
| 3 | F | 3 | Motor vehicle accident | Headache, dizziness, nausea | 12 | 15 | 6 | 5 | 5 | 48 |
| 4 | M | 7 | Fall | Headache, vomiting | 12 | 15 | 24 | 3 | 5 | 22 |
| 5 | F | 5 | Fall | Crying, vomiting | 12 | 15 | 72 | 4 | 5 | 28 |
| 6 | F | 2 | Fall | Crying, vomiting | 12 | 15 | 18 | 5 | 5 | 24 |
| 7 | M | 4 | Fall | Headache, nausea, vomiting | 15 | 15 | 5 | 3 | 5 | 36 |
GCS = Glasgow Coma Scale; GOS = Glasgow Outcome Scale;
on admission;
after operation;
between injury and operation;
total hospital stay
Table 2.
Characteristics of PFEDH and related surgical managements
| No | Hematoma |
Location of bone fracture | Trephination | Complications | ||
|---|---|---|---|---|---|---|
| Mass effect | Location | Volume (cm3) | ||||
| 1 | Ambient cistern and brain stem compression | Left occipital | 12 | Left occipital | 1 | None |
| 2 | Ambient cistern and brain stem compression | Left occipital, across the transverse sinus | 15 | None | 1 | None |
| 3 | None | Left and right occipital | 21 | Occipital | 1 | None |
| 4 | Ambient cistern and brain stem compression | Right occipital | 30 | Right occipital | 1 | None |
| 5 | Ambient cistern and brain stem compression | Left occipital | 22 | Left occipital | 1 | None |
| 6 | Ambient cistern and brain stem compression | Left occipital, across the transverse sinus | 20 | Left occipital | 2 | None |
| 7 | None | Left occipital | 14 | None | 1 | None |
Case 1
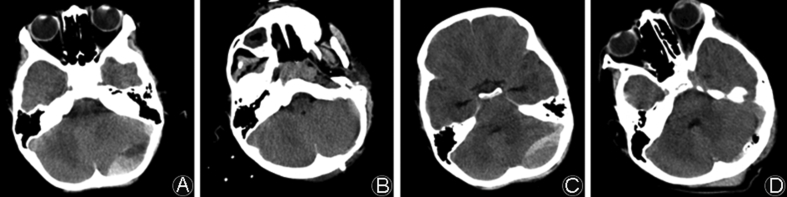
A 6-year-old male patient complained about headache, nausea and vomiting for 20 h after a fall from a height was referred to our hospital. On admission, he was alert and conscious (GCS = 15) and physical examination revealed his pupillary size and reaction were normal. Neurological exam revealed that no abnormalities and cranial CT scan showed a left occipital bone fracture combined with a left occipital PFEDH, 46.9 mm × 17.6 mm × 30 mm on the longest axis, about 12 mL in volume (Fig. 1A). The ambient cistern and brain stem were compressed slightly but without middle line shift. With a paramedian suboccipital 5 cm straight incision, a fracture in the left occipital bone was identified and a trephination mini-craniectomy (2 cm × 3 cm) was made below the transverse sinus, over the center of hematoma projection on skull. The patient's symptoms like headache and vomiting were relieved immediately after surgery and the epidural draining tube was removed 1 day after surgery. Postoperative CT scans showed the hematoma being successfully evacuated (Fig. 1B). Three days after surgery, this patient was discharged with a GOS of 5.
Fig. 1.
Preoperative (A) and postoperative (B) CT scans showed that a left occipital epidural hematoma in a 6-year-old boy was evacuated by one trephination mini-craniectomy surgery. Preoperative (C) and postoperative (D) CT scans showed that a left occipital epidural hematoma in a 10-year-old girl was evacuated by one trephination mini-craniectomy surgery.
Case 2
A 10-year-old female patient complained about headache, nausea and vomiting for 1 day after a fall from a height was referred to our hospital. On admission, she was alert and conscious (GCS = 15) and physical examination revealed normal pupillary size and reaction, with no abnormal neurological sign. CT scans revealed a left occipital PFEDH that extended across the transverse sinus. The hematoma measured about 50 mm × 20 mm × 30 mm on the longest axis, about 15 mL in volume (Fig. 1C). The ambient cistern and brain stem were slightly compressed but without middle line shift. A paramedian straight incision was made from 1 cm above the transverse sinus to the level of mandible (5 cm in total). A trephination mini-craniectomy (2 cm × 2 cm) was made below the transverse sinus. The patient's symptoms were relieved immediately after surgery and postoperative CT showed the hematoma being largely removed (Fig. 1D). Four days after surgery, this patient was discharged with a GOS of 5.
Discussion
Traumatic PFEDH is an uncommon entity in pediatric patients with head trauma and, despite improved identification by widely available CT facilities, there are few reports in the past decades on surgical treatments of PFEDH in children.9, 10, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Our hospital is a large pediatric trauma center with approximately 600 pediatric cases of head trauma treated annually. Among them, 9.3% are EDH and traumatic PFEDHs account for 1.5%. EDH usually requires emergent hematoma evacuation to prevent potential morbidity or even death by increased intracranial pressures. PFEDH could be of particular danger due to direct brain stem compression.15 Occipital bone fractures are quite common (80%) in pediatric PFEDHs5, 13 and PFEDHs are more likely to have a venous origin (venous sinus, venous vessels or diploic veins in bone) by bone fractures than arterial origin.40 Hydrocephalus or ventricular dilation is uncommon in pediatric PFEDHs and its presence might indicate the existence of severe mass effect in the posterior fossa and thus usually associate with poor prognosis.5, 13 We presented here the clinical data on the successful application of trephination mini-craniectomy in 7 pediatric patients. The surgical procedures were fast and simple and satisfactory clinical outcomes were achieved.
Craniectomy has been proposed as the standard surgical management for symptomatic EDH patients,41 while several reports suggested trephination (burr hole) mini-craniectomy could be applied in EDH patients whose clinical conditions were relatively mild.42, 43, 44 However, there is no consensus on the surgical indications for PFEDH in pediatric patients.45 Previous studies have suggested that surgery should be considered when hematoma volume >10 mL, thickness >15 mm, midline shift >5 mm, obliteration of perimesencephalic cisterns, displacement of the fourth ventricle or the presence of hydrocephalus.7, 16, 17, 46 Lately, Prasad et al.13 suggested a PFEDH volume >20 mL and/or fourth-ventricle mass effect with/without ventriculomegaly should be surgically treated, while Senceret et al.5 recommended a PFEDH with thickness <5 mm could be followed up conservatively. The criteria for surgical intervention of PFEDH in our series is when the hematoma volume >10 mL or presence of fourth ventricle compression/displacement and/or obstructive ventriculomegaly. A total of 17 patients were surgically treated for PFEDH during the study period in our institution and, among them, 10 were treated with standard craniectomy and thus were excluded from subsequent analysis. Factors like the hematoma volume, severity of mass effects and patient's neurological conditions were taken into consideration when deciding whether standard craniectomy or trephination mini-craniectomy was used. For example, for patients with gradually enlarging hematomas and neurological deterioration, standard craniectomy is more suitable than trephination mini-craniectomy. On the other hand, trephination mini-craniectomy is indicated when patients showed relatively stable neurological status and the hematoma exerted mild to moderate mass effect. According to our results, trephination mini-craniectomy could be applied as a less invasive alternative to standard craniotomy in patients with stable vital signs and hematoma volumes. Those patients did not have severe mass effects like cerebellar tonsillar herniation or brain contusions at the cerebrum and brain stems. Negative pressure aspiration of the hematoma was used both during and after operation and this might be helpful for the attachment of dura matter to the inner table of the skull. In addition, through the mini-craniectomy, dural sutures were placed when possible to prevent accumulation of residual hematoma. However, when CT findings indicated that the bleeding source was from foramen spinosum, venous sinus, artery or PFEDH was accompanied with cerebellum hemorrhage, standard craniectomy would be more suitable as patients' neurological conditions are more likely to deteriorate rapidly. In addition, when patients had signs of rebleeding after trephination mini-craniectomy, reoperation with standard craniotomy is recommended for better control of the bleeding.
One advantage of trephination mini-craniectomy is its relatively minimal invasiveness as compared with standard craniectomy. For example, blood loss and time duration of the operation were decreased compared with previous report.13 The requirement for bone removal was also minimized, ideally, to the size of a burr hole, given that the hematoma can be effectively and safely evacuated. However, in order to allow for placing dural tack-up sutures after hematoma evacuation, this initial burr hole was usually extended by rongeur to a bone window of about 2 cm–3 cm in diameter. Another potential benefit of trephination mini-craniectomy is that it can be performed with hand drill and rongeur, which are widely available in regional hospitals. In comparison, standard craniectomy requires craniotome and perforator that are driven by high-speed electric system or pneumatic system, both of which are only available in large tertiary hospital. Compared with the literature,5, 13 the time intervals between injury and surgery for our cases were relatively long (24 h). The main reason was because of the delays in the transport of the patients to our hospital from regional hospitals. Therefore, adoption of this trephination mini-craniectomy technique in regional hospitals might contribute to more timely management of PFEDH locally. Nevertheless, the on average 4-day hospital stay is shorter than reported in the literature (4.5 days–6.5 days).5, 13 One possible explanation is that different discharging criteria might be used in our department. For example, patients in our study would start rehabilitation and have their scalp sutures removed after discharge. Therefore, future study with larger sample size might be needed to compare the efficacy of trephination mini-craniectomy versus standard craniectomy for the management of pediatric PFEDH.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Asanin B. Traumatic epidural hematomas in posterior cranial fossa. Acta Clin Croat. 2009;48:27–30. [PubMed] [Google Scholar]
- 2.Karasu A., Sabanci P.A., Izgi N. Traumatic epidural hematomas of the posterior cranial fossa. Surg Neurol. 2008;69:247–251. doi: 10.1016/j.surneu.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Bor-Seng-Shu E., Aguiar P.H., de Almeida Leme R.J. Epidural hematomas of the posterior cranial fossa. Neurosurg Focus. 2004;16 doi: 10.3171/foc.2004.16.2.10. ECP1. [DOI] [PubMed] [Google Scholar]
- 4.Lui T.N., Lee S.T., Chang C.N. Epidural hematomas in the posterior cranial fossa. J Trauma. 1993;34:211–215. doi: 10.1097/00005373-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Sencer A., Aras Y., Akcakaya M.O. Posterior fossa epidural hematomas in children: clinical experience with 40 cases. J Neurosurg Pediatr. 2012;9:139–143. doi: 10.3171/2011.11.PEDS11177. [DOI] [PubMed] [Google Scholar]
- 6.Bozbuga M., Izgi N., Polat G. Posterior fossa epidural hematomas: observations on a series of 73 cases. Neurosurg Rev. 1999;22:34–40. doi: 10.1007/s101430050006. [DOI] [PubMed] [Google Scholar]
- 7.Ciurea A.V., Nuteanu L., Simionescu N. Posterior fossa extradural hematomas in children: report of nine cases. Childs Nerv Syst. 1993;9:224–228. doi: 10.1007/BF00303574. [DOI] [PubMed] [Google Scholar]
- 8.Khwaja H.A., Hormbrey P.J. Posterior cranial fossa venous extradural haematoma: an uncommon form of intracranial injury. Emerg Med J. 2001;18:496–497. doi: 10.1136/emj.18.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervoni L., Rocchi G., Salvati M. Extradural haematoma of posterior cranial fossa. J Neurosurg Sci. 1993;37:47–51. [PubMed] [Google Scholar]
- 10.Weinman D.F., Subramaniam N. Extradural haematoma in the posterior cranial fossa. Ceylon Med J. 1964;9:177–183. [PubMed] [Google Scholar]
- 11.Mohanty A., Kolluri V.R., Subbakrishna D.K. Prognosis of extradural haematomas in children. Pediatr Neurosurg. 1995;23:57–63. doi: 10.1159/000120936. [DOI] [PubMed] [Google Scholar]
- 12.Zhong W., Sima X., Huang S. Traumatic extradural hematoma in childhood. Childs Nerv Syst. 2013;29:635–641. doi: 10.1007/s00381-012-1971-x. [DOI] [PubMed] [Google Scholar]
- 13.Prasad G.L., Gupta D.K., Sharma B.S. Traumatic pediatric posterior fossa erxtradural hematomas: a tertiary-care trauma center experience from India. Pediatr Neurosurg. 2015;50:250–256. doi: 10.1159/000438488. [DOI] [PubMed] [Google Scholar]
- 14.Ammirati M., Tomita T. Posterior fossa epidural hematoma during childhood. Neurosurgery. 1984;14:541–544. doi: 10.1227/00006123-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Berker M., Cataltepe O., Ozcan O.E. Traumatic epidural haematoma of the posterior fossa in childhood: 16 new cases and a review of the literature. Br J Neurosurg. 2003;17:226–229. doi: 10.1080/0268869031000153071. [DOI] [PubMed] [Google Scholar]
- 16.Costa Clara J.M., Claramunt E., Ley L. Traumatic extradural hematomas of the posterior fossa in children. Childs Nerv Syst. 1996;12:145–148. doi: 10.1007/BF00266818. [DOI] [PubMed] [Google Scholar]
- 17.Ersahin Y., Mutluer S. Posterior fossa extradural hematomas in children. PediatrNeurosurg. 1993;19:31–33. doi: 10.1159/000120697. [DOI] [PubMed] [Google Scholar]
- 18.Choux M., Grisoli F., Peragut J.C. Extradural hematomas in children. 104 cases. Childs Brain. 1975;1:337–347. doi: 10.1159/000119585. [DOI] [PubMed] [Google Scholar]
- 19.Liu J.T., Tyan Y.S., Lee Y.K. Emergency management of epidural haematoma through burr hole evacuation and drainage. A preliminary report. Acta Neurochir (Wien) 2006;148:313–317. doi: 10.1007/s00701-005-0723-z. [DOI] [PubMed] [Google Scholar]
- 20.Brohi S.R. Exploratory burr hole and subsequent craniectomy for acute bilateral posterior fossa epidural haematoma. J Coll Physicians Surg Pak. 2005;15:665–666. [PubMed] [Google Scholar]
- 21.Springer M.F., Baker F.J. Cranial burr hole decompression in the emergency department. Am J Emerg Med. 1988;6:640–646. doi: 10.1016/0735-6757(88)90110-6. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld J.V. The emergency burr hole: indication and technique. P. N. G Med J. 1982;25:189–193. [PubMed] [Google Scholar]
- 23.Nath P.C., Mishra S.S., Das S. Supratentorial extradural hematoma in children: an institutional clinical experience of 65 cases. J Pediatr Neurosci. 2015;10:114–118. doi: 10.4103/1817-1745.159192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teichert J.H., Rosales P.R., Jr., Lopes P.B. Extradural hematoma in children: case series of 33 patients. Pediatr Neurosurg. 2012;48:216–220. doi: 10.1159/000345849. [DOI] [PubMed] [Google Scholar]
- 25.Abeysuriya S.C. Extradural haematoma of the posterior fossa. Report of three cases with review of the literature. Ceylon Med J. 1970;15:104–109. [PubMed] [Google Scholar]
- 26.Balik V., Lehto H., Hoza D. Posterior fossa extradural haematomas. Cent Eur Neurosurg. 2010;71:167–172. doi: 10.1055/s-0030-1249046. [DOI] [PubMed] [Google Scholar]
- 27.Brambilla G., Rainoldi F., Gipponi D. Extradural haematoma of the posterior fossa: a report of eight cases and a review of the literature. Acta Neurochir (Wien) 1986;80:24–29. doi: 10.1007/BF01809553. [DOI] [PubMed] [Google Scholar]
- 28.Devadiga K.V., Geerannavar S.S., Pai B.R. Posterior fossa extradural haematoma (case report) Neurol India. 1975;23:210–212. [PubMed] [Google Scholar]
- 29.Gelabert M., Prieto A., Allut A.G. Acute bilateral extradural haematoma of the posterior cranial fossa. Br J Neurosurg. 1997;11:573–575. doi: 10.1080/02688699745763. [DOI] [PubMed] [Google Scholar]
- 30.Joubert M.J. Extradural haematoma of the posterior fossa. S Afr Med J. 1961;35:194. [PubMed] [Google Scholar]
- 31.Lewis G.M., Brice J. Traumatic posterior fossa extradural haematoma. Proc R Soc Med. 1967;60:246. doi: 10.1177/003591576706000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan R.K., Sharma B.S., Khosla V.K. Posterior fossa extradural haematoma–experience of nineteen cases. Ann Acad Med Singap. 1993;22:410–413. [PubMed] [Google Scholar]
- 33.Mc Culloch G.A. Posterior fossa extradural haematoma. Med J Aust. 1976;2:303–305. doi: 10.5694/j.1326-5377.1976.tb130189.x. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer U.J. Extradural haematoma of the posterior fossa. Twelve years experiences with CT-scan. Acta Neurochir (Wien) 1987;87:105–111. doi: 10.1007/BF01476060. [DOI] [PubMed] [Google Scholar]
- 35.Peter J.C., Domingo Z. Subacute traumatic extradural haematomas of the posterior fossa: a clinicopathological entity of the 5- to 10-year-old child. Childs Nerv Syst. 1990;6:135–138. doi: 10.1007/BF00308489. [DOI] [PubMed] [Google Scholar]
- 36.Prusty G.K., Mohanty A. Posterior fossa extradural haematoma. J Indian Med Assoc. 1995;93:255–258. [PubMed] [Google Scholar]
- 37.Roka Y.B., Kumar P., Bista P. Traumatic posterior fossa extradural haematoma. JNMA J Nepal Med Assoc. 2008;47:174–178. [PubMed] [Google Scholar]
- 38.Subba Rao A.N., Vidyasagar C., Reddy G.N. Posterior fossa extradural haematoma. Neurol India. 1978;26:198–200. [PubMed] [Google Scholar]
- 39.Wang E.C., Lim A.Y., Yeo T.T. Traumatic posterior fossa extradural haematomas (PFEDH) Singap Med J. 1998;39:107–111. [PubMed] [Google Scholar]
- 40.Koc R.K., Pasaoglu A., Menku A. Extradural hematoma of the posterior cranial fossa. Neurosurg Rev. 1998;21:52–57. doi: 10.1007/BF01111486. [DOI] [PubMed] [Google Scholar]
- 41.Malik N.K., Makhdoomi R., Indira B. Posterior fossa extradural hematoma: our experience and review of the literature. Surg Neurol. 2007;68:155–158. doi: 10.1016/j.surneu.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 42.Liu W., Ma L., Wen L. Drilling skull plus injection of urokinase in the treatment of epidural haematoma: a preliminary study. Brain Inj. 2008;22:199–204. doi: 10.1080/02699050801895407. [DOI] [PubMed] [Google Scholar]
- 43.Habibi Z., Meybodi A.T., Haji Mirsadeghi S.M. Burr-hole drainage for the treatment of acute epidural hematoma in coagulopathic patients: a report of eight cases. J Neurotrauma. 2012;29:2103–2107. doi: 10.1089/neu.2010.1742. [DOI] [PubMed] [Google Scholar]
- 44.Nelson J.A. Local skull trephination before transfer is associated with favorable outcomes in cerebral herniation from epidural hematoma. Acad Emerg Med. 2011;18:78–85. doi: 10.1111/j.1553-2712.2010.00949.x. [DOI] [PubMed] [Google Scholar]
- 45.Bhau K.S., Bhau S.S., Dhar S. Traumatic extradural hematoma–role of non-surgical management and reasons for conversion. Indian J Surg. 2010;72:124–129. doi: 10.1007/s12262-010-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubey A., Pillai S.V., Kolluri S.V. Does volume of extradural hematoma influence management strategy and outcome? Neurol India. 2004;52:443–445. [PubMed] [Google Scholar]