Transient Receptor Potential (TRP) channels comprise a large family that in mammals includes 28 members. All TRP channels have common structural properties such as 6 transmembrane domains and cytoplasmic N- and C-termini. Several TRP channels respond to cool, warm and hot temperatures. TRPA1 (TRP ankyrin 1), a unique member of the TRPA subfamily, is a homotetrameric cation-permeable channel that has an unusually large (16–17) number of ankyrin repeats in the N-terminal region. Initially, TRPA1 was cloned from cultured human fibroblasts and named ANKTM1. TRPA1 from various species are reported to be activated by either cold or heat stimulus, and the diverse thermosensitive properties of these channels have been reviewed and analyzed.1,2 Ca2+ is an important ion for both activation and modulation of TRPA1 channel activity, and a key regulator for potentiation and inhibition of TRPA1 channel activity upon chemical stimulation.3 However, the role of extracellular Ca2+ in temperature-evoked gating is not clear.
Our earlier work showed that green anole lizard TRPA1 (gaTRPA1) requires extracellular Ca2+ for heat-evoked activation.4 We recently expanded upon this finding by demonstrating the involvement of extracellular Ca2+ in heat-evoked activation of TRPA1 from several other species.5 Even in the presence of high concentrations of intracellular Ca2+, the gaTRPA1 channel did not respond to heat stimulation in the absence of extracellular Ca2+. Extracellular Ca2+, but not other extracellular divalent cations (Ba2+ and Mg2+), was found to be necessary for heat-evoked activation of gaTRPA1. The heat-evoked gaTRPA1 activation was dependent on the extracellular Ca2+ concentration, whereas the temperature thresholds for heat-evoked gaTRPA1 activation remained unchanged. To understand how extracellular Ca2+ is involved in heat-evoked activation of gaTRPA1, we also examined heat-evoked activation of TRPA1 from chicken (chTRPA1) and rat snake (rsTRPA1).5 Both chTRPA1 and rsTRPA1 showed small, but significant heat-evoked activation in the absence of extracellular Ca2+, suggesting that extracellular Ca2+ is not essential for heat-induced activation of TRPA1 from these species. The findings that extracellular Ca2+ is important for gaTRPA1 heat-induced activation and that chTRPA1 and rsTRPA1 are activated by heat even in the absence of extracellular Ca2+ led us to hypothesize that negatively charged amino acids located on the extracellular side of the gaTRPA1 channel could be important for heat-induced gaTRPA1 activation. In particular, gaTRPA1 Glu894 could be implicated in heat activation because chTRPA1 and rsTRPA1 have Gln at the corresponding position, residue 897 and 895, respectively. Mutating gaTRPA1 Glu894 to Gln led to small, but significant channel opening by heat in the absence of extracellular Ca2+, which is similar to the phenomenon observed for wild type chTRPA1 and rsTRPA1. In addition, chTRPA1 and rsTRPA1 carrying Gln to Glu mutations at positions 897 and 895 showed significantly smaller inward currents compared with wild type in the absence, but not in the presence of extracellular Ca2+, further supporting the concept that more negative surface charges are required for heat-induced TRPA1 activation. Given that mutated gaTRPA1 and wild type chTRPA1 and rsTRPA1 channels showed significantly smaller inward currents in response to heat stimulation in the absence of extracellular Ca2+ than in the presence of extracellular Ca2+, we asked whether other negatively charged amino acids might be conserved in all 3 species. Further sequence alignment revealed that Asp and Glu residues located before and within pore helix 2 were conserved in all 3 species (Fig. 1). chTRPA1 and rsTRPA1 carrying a double mutation and a gaTRPA1 triple mutant in which Asp and Glu were mutated to non-charged amino acids showed large channel activity in the absence of extracellular Ca2+ that was similar to that seen in the presence of extracellular Ca2+, suggesting that neutralization of extracellular negative charges promotes channel activation following heat stimulation. Based on these data, we propose a new model for heat-evoked activation of TRPA1 (Fig. 1) wherein negatively charged amino acids (Glu and Asp) at the outer surface of the TRPA1 channel bind Ca2+ ions that in turn promotes channel opening in response to heat stimulation.
Figure 1.
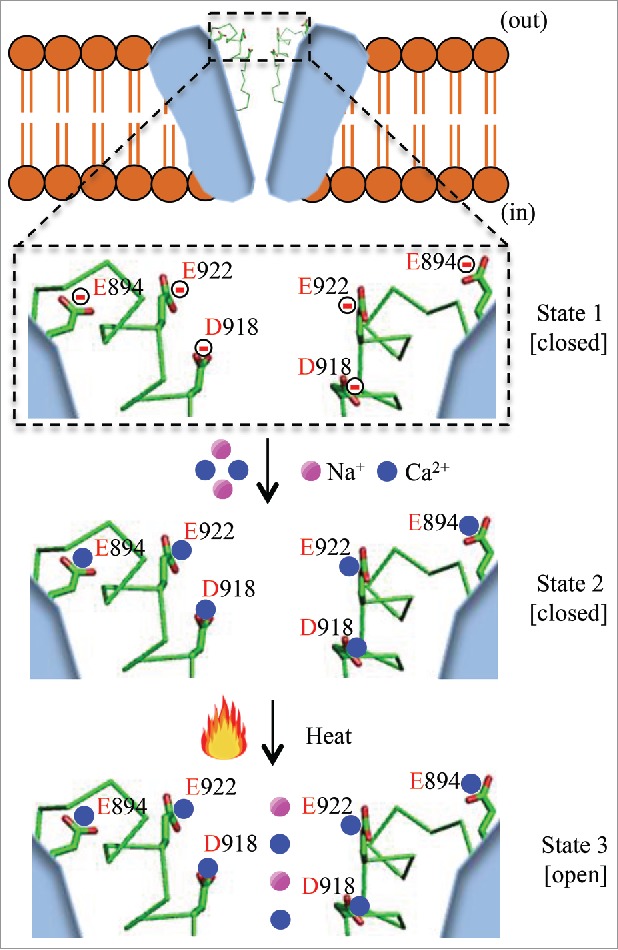
A schematic model for heat-evoked activation of gaTRPA1 in the presence of extracellular Ca2+. Ca2+ ions bind and neutralize negatively charged amino acids on the outer surface of the gaTRPA1 channel. Upon heat stimulation, the TRPA1 channel opens to permit cation influx.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Laursen WJ, Anderson EO, Hoffstaetter LJ, Bagriantsev SN, Gracheva EO. Species-specific temperature sensitivity of TRPA1. Temperature (Austin) 2015; 2(2):214-26; PMID:27227025; https://doi.org/ 10.1080/23328940.2014.1000702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saito S, Tominaga M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium 2015; 57:214-21; PMID:25533790; https://doi.org/ 10.1016/j.ceca.2014.12.001 [DOI] [PubMed] [Google Scholar]
- [3].Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 2008; 283:32691-703; PMID:18775987; https://doi.org/ 10.1074/jbc.M803568200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kurganov E, Zhou Y, Saito S, Tominaga M. Heat and AITC activate green anole TRPA1 in a membrane-delimited manner. Pflügers Arch 2014; 466:1873-84; PMID:24385018; https://doi.org/ 10.1007/s00424-013-1420-z [DOI] [PubMed] [Google Scholar]
- [5].Kurganov E, Saito S, Saito CT, Tominaga M. Requirement of extracellular Ca2+ binding to specific amino acids for heat-evoked activation of TRPA1. J Physiol 2017; PMID:28194754; https://doi.org/ 10.1113/JP274083 [DOI] [PMC free article] [PubMed] [Google Scholar]


