ABSTRACT
The neuronal collapsin response mediator protein 2 (CRMP2) undergoes several posttranslational modifications that codify its functions. Most recently, CRMP2 SUMOylation (addition of small ubiquitin like modifier (SUMO)) was identified as a key regulatory step within a modification program that codes for CRMP2 interaction with, and trafficking of, voltage-gated sodium channel NaV1.7. In this paper, we illustrate the utility of combining sequence alignment within protein families with structural analysis to identify, from several putative SUMOylation sites, those that are most likely to be biologically relevant. Co-opting this principle to CRMP2, we demonstrate that, of 3 sites predicted to be SUMOylated in CRMP2, only the lysine 374 site is a SUMOylation client. A reduction in NaV1.7 currents was the corollary of the loss of CRMP2 SUMOylation at this site. A 1.78-Å-resolution crystal structure of mouse CRMP2 was solved using X-ray crystallography, revealing lysine 374 as buried within the CRMP2 tetramer interface but exposed in the monomer. Since CRMP2 SUMOylation is dependent on phosphorylation, we postulate that this state forces CRMP2 toward a monomer, exposing the SUMO site and consequently, resulting in constitutive regulation of NaV1.7.
KEYWORDS: CRMP2, NaV1.7, post-translational modifications, SUMOylation, trafficking, X-ray crystallography
Introduction
Small ubiquitin-like modifier 1 (SUMO1) protein was first identified in 1996 as a covalent modifier of nuclear proteins.1 In the years that immediately followed, the definition of SUMOylation expanded to include a family of 3 ∼11 kilodalton (kDa) proteins (SUMO 1–3) able to target ε-amino groups of lysine residues for posttranslational modification.1-3 It is now known that SUMO proteins are transferred via covalent interaction through the E1-SUMO-activating, E2-SUMO-conjugating, and E3-SUMO-ligating enzyme cascade allowing for recognition and binding to SUMO-targeted lysine residues.4 Despite evidence that SUMOylation is a transient and reversible modification typically bound to only ∼1% of SUMO-targeted lysine residues,5 the consequences of protein SUMOylation are numerous. The rapid turnover of SUMOylation has led to the hypothesis that the effects of protein SUMOylation persist long after the modification has been lost. Put another way, only ∼1% of substrate might be SUMOylated but a much greater fraction of protein may have undergone recent SUMOylation and this may be sufficient to actively exert longer-term effects of the modification on the protein's function.6 The effects of protein SUMOylation include alteration of conformation, regulation of stability via competition with ubiquitin modifications, and alteration of interactome via enhancement or restriction of interaction with protein binding partners (see4 for review).
Predicting clients for protein SUMOylation originally relied on protein sequence comparison to the canonical SUMOylation consensus sequence. SUMOylation most commonly occurs at a lysine within a canonical SUMO-consensus motif defined as ψKxD/E, where ψ is a large hydrophobic amino acid (∼60% of all SUMOylation7), or an inverted canonical motif (D/ExKψ).8 As SUMOylation literature expands and more non-canonical SUMOylation motifs are identified, pattern-based predictions are becoming more accurate. However, tertiary structure undoubtedly has a strong influence on whether a lysine can be efficiently SUMOylated and although some algorithms do incorporate empirical data on local secondary structure, the 3-dimensional environment of the predicted site presents a challenge to SUMO-site prediction that has yet to be met. Nevertheless, several freely available algorithms (PTMProber,9 JASSA,10 GPS-SUMO,11 SUMOhydro,12 SUMOplot (http://www.abgent.com/sumoplot)) are useful for comparing protein sequences to positively identify SUMOylation targets. These algorithms compare hydrophobicity of amino acids that flank lysine residues and rely on machine learning techniques to improve prediction of SUMOylation based on positively identified sites of modification. Analyzing the sequence of the mouse collapsin response mediator protein 2 (CRMP2; Uniprot ID: O08553) with the above SUMO site prediction algorithms consistently predicts K20, K269, K374, and K390 as likely sites of SUMOylation. As a protein interacting partner of tubulin, actin, and vimentin, CRMP2 participates in processes that regulate cytoskeletal dynamics and the balance between neurite collapse and outgrowth.13-15 Proximity of CRMP2 to cytoskeletal proteins and the cell membrane led to the discovery of additional functions in cellular trafficking.16-21 Our laboratory has further expanded this role by demonstrating that CRMP2 mediates trafficking of N-type voltage-gated calcium (CaV2.2) channels.22,23 An interaction between calcium channels and CRMP2 is regulated by Cdk5 phosphorylation.24 Indeed, CRMP2 functions are determined by an interplay of multiple posttranslational modifications including glycosylation, oxidation, proteolysis, phosphorylation, and SUMOylation (for review see25). Most recently, we identified SUMOylation as a posttranslational modification that targets CRMP2.26,27 We determined that CRMP2 SUMOylation regulates proteostasis – trafficking and surface expression – of the voltage-gated sodium channel NaV1.7 in sensory neurons and that CRMP2 SUMOylation status is controlled by CRMP2 phosphorylation by Cdk5 and Fyn kinases.26 This arrangement of interacting posttranslational modifications determines CRMP2 interactions with endocytosis related proteins Numb and Eps15, and the NaV1.7 targeting E3 ubiquitin ligase Nedd4–2 to regulate sodium current density and properties of sensory neuron excitability.
In this paper detailing the modification and signaling program for CRMP2s control of NaV1.7 in sensory neurons,26 we analyze predicted CRMP2 SUMOylation sites and illustrate how the combination of protein sequence conservation and tertiary structural information can be used to more accurately predict residues likely to be biologically relevant clients for SUMOylation. To this end, we report the X-ray crystal structure of mouse CRMP2 to 1.78 Å resolution, revealing an additional 6 amino acids at the C-terminus, compared to previous X-ray structures of CRMP2. Analyzing the environment of the top predicted SUMOylation sites in this structure reveals features relevant to SUMOylation that could not be ascertained from either the primary sequence or secondary structure predictions.
Results and discussion
Predicted CRMP2 SUMOylation motifs are phylogenetically conserved
An analysis of the CRMP2 primary amino acid sequence with several SUMOylation algorithms identified at least 5 sequences that conformed to the SUMO-consensus motif ψKxD/E (ψ, a hydrophobic amino acid, such as A, I, L, M, P, F, V or W; x, any amino acid residue) (Fig. 1A). Notably, these sequences were conserved between most of the other 4 members of the CRMP family (Fig. 1B). In addition to comparison of lysine residue conservation between CRMP family proteins, we also performed protein BLAST analysis of predicted CRMP2 SUMOylation sequences. We used 14 amino acid sequences surrounding the predicted lysine target of SUMOylation and identified 47 animal species that have perfect sequence conservation of either lysine 20, 374, or 390. Phylogenetic relationships of animals with CRMP2 sequence homology are represented in Fig. 1C. Lysine residue 374 was conserved in 100% of these species compared with 68% and 64% for lysine residues 390 and 20, respectively. For putative targets of SUMOylation, higher rates of sequence conservation between related proteins and between species may be predictive of endogenous SUMOylation.
Figure 1.

CRMP2 putative SUMOylation motifs and conservation of the lysine 374 SUMO site. (A) CRMP2 sites that conform to SUMOylation consensus motifs (ranked by Abgent SUMOplot™ Analysis Program). The SUMOplot score is given to every lysine residue within the analyzed protein sequence and assigned a value from −1 to +1 based on sequence similarity to positively identify SUMOylation motifs. Lysine residues with positive values are returned as ‘high probability targets of SUMOylation’ with higher scores indicating greater similarity to positively identified SUMOylation motifs and higher predicted likelihood of endogenous SUMOylation. The Mus musculus (mouse) CRMP2 sequence (GenBankTM accession number NP_034085.2) was used for this prediction. The canonical SUMO motif (ψKxD/E, where ψ is a large hydrophobic amino acid) is shown underneath the predicted SUMO sites (underlined). Only the top 3 sites have the leading hydrophobic residue and only K374 has the terminal acidic residue (blue box). (B) Conservation of putative SUMO-targeted lysines within family of CRMP proteins (green check marks denote conservation of residues between all CRMPs, black X denotes non-conserved residues). (C) This phylogenetic tree was created using ‘Interactive Tree of Life’ (iTOL2.0) software54 and lists the genus and species of a sample of organisms with perfect identity to Mus musculus CRMP2. Separation into different branches represents a divergent evolutionary relationship. Surrounding the tree is a sampling of species listed by their common names, matched by color of text. Due to the high number of birds identified to have homology within the CRMP2 sequence, only a selection of birds is represented.
Primacy of the K374 SUMOylation site in CRMP2 in control of NaV1.7 function
Of these sites, K374 was particularly interesting due to (i) 100% conservation between CRMPs 1–5, (ii) conformity to the canonical SUMO-consensus motif ψKxD/E, and (iii) the presence of downstream aspartic and glutamic acid residues that typify this motif as the more stringent negative charge dependent SUMO motif (NDSM).4 We previously reported that loss of CRMP2 SUMOylation, via expression of a CRMP2-K374A mutant construct, decreased NaV1.7 trafficking (by up to ∼85%) and current density (by up to ∼70%).26,27 Consistent with this reduction, we also demonstrated that expression of this SUMO-null CRMP2 mutant lead to a more than 80% reduction in biochemically detectable CRMP2 SUMOylation levels, suggesting this site is the predominant target of SUMOylation within CRMP2.26 However, as the SUMOylation algorithms predicted not only K374 but also K20 and K390 as high-probability sites for SUMOylation in CRMP2 (Fig. 1A), we also made mutants harboring lysine to alanine (K→A) at these sites (K20A and K390A) to test the possibility that these sites may also contribute to CRMP2s SUMOylation status and, consequently, to diminution of NaV1.7 function.
Catecholamine A Differentiated (CAD) cells were used in these studies as they endogenously express CRMP2 protein24 and mRNAs for voltage-gated sodium channel (VGSC) subtypes NaV1.1, NaV1.3, NaV1.7, and NaV1.9. NaV1.7 accounts for about ∼95% of the total VGSC gene transcripts in CAD cells.24 The current-voltage (IV) protocol (Fig. 2A, top) was used to survey properties of current-voltage relationship like peak current and activation, and the fast inactivation protocol (Fig. 2A, bottom) was used to survey fast inactivation. Representative families of traces for each of these protocols are illustrated in Fig. 2B. Highlighted in blue in voltage-clamp protocols (Fig. 2A), representative traces (Fig. 2B), summary data of current density (Fig. 2C), fast inactivation and activation (Fig. 2D) are the voltage pulses and resulting CAD cell current responses that produce descriptive values of peak current and half-maximal voltages of fast inactivation and activation. Notably, NaV1.7 is the dominant sodium channel in CAD cells, representing about 80% of functional sodium channels determined by NaV1.7-selective block with 125nM Huwentoxin-IV (Fig. 2E, F).
Figure 2.

Properties of CAD cell VGSC currents. (A) Protocols used to generate current-voltage (IV) relationship and activation (top) and fast inactivation (bottom) and (B) families of current traces generated by these protocols. (C) Summary of I-V relationship. Activation of currents begins near −40 mV, current peaks between 0 mV to +10 mV (+10 mV in this experiment), and reversal potential for sodium is near +70 mV. (D) Typical fast inactivation (left curve, squares) and activation (right curve, circles) observed in CAD cells. Blue data points represent half-maximal values of activation and inactivation curves fitted using Boltzmann equation: y = 1/(1 + exp(V1/2 − V)/k), in which V1/2, V, and k represent midpoint voltage of kinetics, test potential, and slope factor, respectively. Voltage pulses and current responses that are equivalent to V1/2 and peak current values are highlighted in blue in (A) and B. (E) Representative peak current traces and summary data (F) before and 5 minutes after application of 125 nM Huwentoxin-IV. Some error bars are hidden by symbols. Asterisk denotes statistical significance compared with pre-drug (Student's t-test). Data are means with SEM n = 5–9 cells per condition for each property shown.
The sodium currents of CAD cells (predominantly NaV1.7) were significantly reduced when CRMP2-K374A was expressed but were not altered by expression of CRMP2-K20A, or CRMP2-K390A (Fig. 3A). None of the mutations had any effect on activation or fast-inactivation of NaV1.7 (Fig. 3B). Total levels of CRMP2 were not affected by any of the lysine mutations (Fig. 3C, D). Mutating K374, but not K20 or K390, to alanine resulted in a dramatic loss of CRMP2 SUMOylation (Fig. 3E, F), in agreement with a reduction in NaV1.7 currents.
Figure 3.

SUMOylation of CRMP2 at lysine 374 controls Nav1.7 currents and SUMOylation status of CRMP2 in CAD cells. (A) Box plot of peak current density (in pA/pF) from CAD cells transfected with the indicated CRMP2 plasmids. Data are normalized to the wildtype CRMP2 condition. Boxes represent quartiles, each data point represents 1 cell. Error bars are behind data symbols in some cases. The peak currents were: 1.26 ± 0.12 nA for CRMP2; 1.38 ± 0.21 nA for K20-CRMP2; 0.54 ± 0.13 nA for K374A-CRMP2; and 0.93 ± 0.05 nA for K390-CRMP2. The cell capacitances (in pF) were not different between the groups: 22.1 ± 1.9 for CRMP2; 24.9 ± 2.8 for K20-CRMP2; 20.2 ± 2.7 for K374A-CRMP2; and 19.5 ± 3.1 for K390-CRMP2. (B) Representative Boltzmann fits for activation and steady-state inactivation for CAD cells transfected with the indicated constructs are shown. The calculated values for V1/2 and k of activation and steady-state inactivation for all conditions tested and the associated statistics are presented in Table 1. Sodium current fast inactivation (left curves) and activation (right curves) were unaffected by expression of CRMP2 mutants. 5 cells per condition, data are presented mean ± SEM. Asterisk indicates statistically significant differences between CRMP2-K374A and all other conditions tested (n = 5; p < 0.05, one-way ANOVA with Tukey's post-hoc test). (C) Representative Western blot and summary (D) of CRMP2 from lysates of CAD cells transfected with indicated plasmids. (n = 3). Representative immunoblot (E) and summary (F) to detect SUMOylated CRMP2 from CAD cells transfected with the indicated plasmids (n = 3). Asterisks indicate statistically significant differences compared with wildtype CRMP2 expressing cells (p < 0.05, one-way ANOVA with Tukey's post-hoc test).
We paired this sodium current and biochemical analyses of predicted CRMP2 SUMOylation targets with a post-hoc analysis of protein sequence homology within CRMP proteins (CRMPs 1–5). The analysis compared only the presence of a lysine at the predicted residue and revealed 60% conservation of lysine at position 390, 80% conservation of lysine at position 20, and a 100% conservation of lysine at position 374 (Fig. 1B). This result allows for a hypothesis that biologic relevance of protein SUMOylation is correlated with more stringent conservation of the motif within related proteins. Within our study, the prediction of CRMP2 SUMOylation sites was aided by protein sequence analysis. When applicable, this criterion could be an additional resource for prediction of SUMOylation sites within other proteins.
Accessibility and local environments of predicted SUMOylation sites in the CRMP2 structure
Additional insight about the availability of predicted SUMOylation motifs can be inferred from 3-dimensional structures, when available. Many predicted SUMOylation motifs are not accessible to the SUMOylation machinery proteins due to positioning within internal or highly structured regions of the protein.4,28 This is especially relevant when the SUMOylation motif occurs at an interface within an oligomeric assembly. CRMP2 has been shown to exhibit multiple oligomeric states in a metal dependent manner29 as well as form heterotetramers with other CRMPs.30,31 In addition, the structure of human CRMP2 is known.32-34 We report here the X-ray crystal structure of mouse CRMP2 determined to 1.78 Å resolution (Table 2).
Table 1.
Comparative current densities and Boltzmann–fits of voltage–dependence of channel activation and fast-inactivation for the respective transfection conditions in CAD cells.
| Activation |
Fast Inactivation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Peak Current | n | V1/2 (mV) | k (mV/e-fold) | n | V1/2 (mV) | k | n | ||
| CRMP2 | 100.0 ± 9.7 | 5 | 3.0 ± 1.3 | 10.8 ± 1.0 | 5 | −64.4 ± 1.9 | 5.6 ± 0.7 | 5 | ||
| CRMP2-K374A | 42.9 ± 10.0* | 5 | 3.4 ± 1.4 | 10.5 ± 0.9 | 4 | −63.7 ± 3.1 | 5.7 ± 0.9 | 3 | ||
| CRMP2-K20A | 109.3 ± 16.5 | 5 | 2.2 ± 1.9 | 9.8 ± 0.9 | 3 | −63.5 ± 0.6 | 5.6 ± 0.4 | 3 | ||
| CRMP2-K390A | 93.4 ± 5.0 | 5 | −0.1 ± 1.3 | 9.3 ± 0.9 | 3 | −62.2 ± 2.0 | 6.7 ± 1.3 | 3 | ||
Note. Values of peak current density (picoamp/picofarad) were normalized to the CRMP2 condition. Values for V1/2, the voltage of half–maximal activation and fast inactivation (in millivolts, mV), and slope k, were derived from Boltzmann distribution fits to the individual recordings and averaged. The mean and standard error of the mean ( ± SEM) is reported. n values indicates the number of cells per condition. All transfection conditions contained dsRed for identification of construct expression. Asterisks represent statistically significant differences as compared with control (i.e., CRMP2 alone condition) within tested groups (p < 0.05, ANOVA with Tukey's post–hoc test or Student's t–test).
Table 2.
Crystallographic data collection and refinement statistics.
| Data Collection | |
|---|---|
| Wavelength (Å) | 1.13 |
| Resolution range (Å) | 39.22 – 1.78 |
| Space group | C 1 2 1 |
| Unit cell | 133.4, 107.2, 81.7, 90, 120.7, 90 |
| Total reflections | 552,156 |
| Unique reflections | 94,721 |
| Multiplicity | 5.8 (5.8) |
| Completeness (%) | 100 (99.8) |
| Mean I/sigma(I) | 23.1 (3.4) |
| Wilson B-factor | 15.3 |
| R-merge |
0.059 (0.54) |
| Refinement | |
| R-work (%) | 13.5 |
| R-free (%) | 17.3 |
| RMSD bonds (Å) | 0.018 |
| RMSD angles (°) | 1.82 |
| Ramachandran favored (%) | 97.2 |
| Clashscore | 2.4 |
The structure contains a dimer in the asymmetric unit with the primary biologically relevant tetramer being formed by crystal symmetry. This is not unusual for CRMPs, which crystallize in a variety of space groups.29,32-35 Our structure is highly similar to published CRMP2 structures32-34 with RMSDs of less than 0.30 Å among the 4 structures (Fig. 4A).
Figure 4.
Structural analysis of predicted and validated CRMP2 SUMOylation motifs in the crystal structure of mCRMP2 at 1.78 Å. (A) Structural alignment of asymmetric unit contents of 3 published CRMP232-34 structures against our structure. Ribbon representation overlaid on smoothed surface with key residues shown as spheres. Letters in parentheses indicate subunits; the tetramer is formed by interaction between the A:B dimer and the C:D dimer. (B) Subunit A of our structure, rotated approximately 90° from panel A to show the interface between subunits A and B. Ribbon colored by B-factor overlaid on smoothed surface. Low B-factors (dark blue) consistent with regions of contact with subunit B and high B-factors (orange) indicative of flexibility. (C) Accessible surface area (1.4 Å probe) for each amino acid in the top SUMOylation motifs averaged over all subunits in 4 structures, calculated for asymmetric unit contents (with interfaces intact, top) and as isolated monomers (with interfaces exposed, bottom). (D) Close up view of each 4 residue SUMOylation motif (carbon atoms white) with the surrounding protein shown in green surface representation. Top row shows dimer, bottom row shows monomer, total accessible surface area of SUMOylation motif in lower right corner of each panel. Solvent accessible motif residues are labeled.
The 3 top-predicted SUMOylation sites are all present and well-ordered. Site K20 is located in the solvent accessible, flexible N-terminus and is within 10 Å of the Fyn kinase phosphorylation site Y32, which may have an impact on whether K20 gets SUMOylated in vivo. Sites K374 and K390 are located in the interface between subunits and could be accessible in the monomeric state (Fig. 4B). Although no monomeric structure is available, we can predict the change in solvent accessibility by calculating the solvent accessible surface area for the oligomer as well as each subunit in isolation (Fig. 4C). K20 is highly accessible to solvent in either case and K374 is partially buried in the oligomer but more exposed in the isolated subunit. In contrast, K390 is largely solvent inaccessible in the tetramer, though slightly more accessible in the monomer. More importantly, comparing the accessibility of the 4 residue SUMOylation motif reveals that 2 key residues of the K20 motif (I19 and G22) and 3 residues of the K390 motif (AG389, MV391, and F392) are, in fact, not solvent accessible in either the tetramer or monomer. On the other hand, the 3 residues of the K374 motif (G373, M3375, and D376) are highly accessible in the monomeric state. Examining the local structural environment of each site corroborates these values (Fig. 4D). Although K20 is present at the surface, it sits in a shallow pocket with only 2 residues of the motif accessible to solvent. Furthermore, the surrounding residues likely present a barrier to interaction with the SUMOylation machinery; at the very least some conformational changes would be required for efficient SUMOylation of this residue. The K390 motif sits in a deep pocket in both the tetramer and monomer with only the lysine accessible to solvent, making this site unlikely to be accessible to the SUMOylation machinery without drastic conformational changes. In contrast, the K394 site, though buried in the tetrameric interface, is nearly 4-fold more exposed in the monomeric state and furthermore is presented on the surface without any local barriers to protein-protein interactions required for SUMOylation (Fig. 4D, middle panel).
Thus, from our analysis as well as that of a recent publication,34 SUMOylation of K374 will require exposure of the interface. This could be triggered by interaction with the SUMOylation machinery, but since the K374 motif is partially obscured in the interface this would require an additional recognition site outside of the interface. However, as mentioned above, CRMP2 forms heterotetramers with other CRMPs.30,31 This implies a certain level of equilibrium between the tetrameric and monomeric states as we have observed with analytical ultracentrifugation.36 Additionally, with this new structure, we confirm for the first time that CRMP2 forms a domain-swap handshake across the AC and BD interfaces as previously seen in CRMP4,37 CRMP5,29 and for one subunit in CRMP138 as well as other DHPs (for example, see39) (Fig. 5A). We observe clear electron density for the backbone and several side chains out to conserved residue arginine 496 (Fig. 5B), confirming that the conserved residues R496 and E221 form a salt bridge as previously observed for other CRMPs.29,37,38 The CRMP2 sequence extends another 76 residues beyond this point and contains multiple phosphorylation sites (see review25) but has been predicted to be disordered.34 We speculate that the role of this conserved salt bridge is to anchor and position the C-terminus and it's multiple regulation sites at the surface of the protein to facilitate recognition and modification. It is likely that there are other points of contact as well, but structural evidence has been elusive. Further, in our recent paper,26 we have shown that SUMOylation at site 374 is dependent on phosphorylation at S522 but not vice-versa. Thus we suggest that phosphorylation at site S522 weakens the handshake, driving the equilibrium toward the monomeric state and exposing the K374 SUMOylation motif, ultimately culminating in constitutive regulation of Nav1.7.
Figure 5.
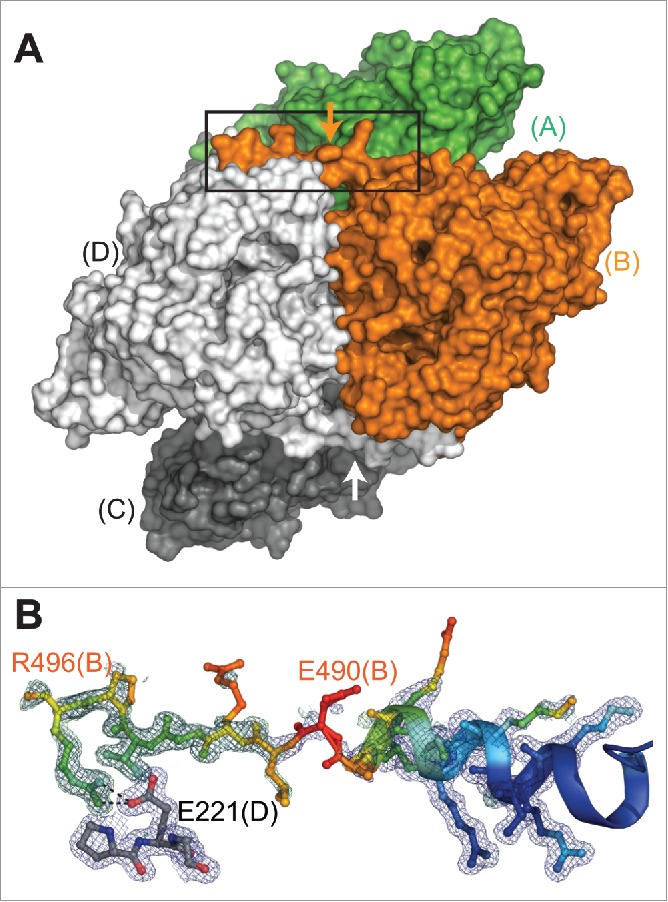
CRMP2 handshake in the crystal structure of mCRMP2 at 1.78 (A). Surface representation of tetramer (viewed from asterisk position in panel A of Fig. 4), illustrating domain-swap “handshake” formed across the tetramer interface by the C-terminal extension (subunits C and D generated by crystal symmetry). Arrow marks the position of residue 490, the limit of currently published CRMP2 structures. (B) Close-up view from panel D (box) showing simulated annealing omit map for C-terminal extension (subunit B, colored by B-factor). Dashed lines between R496 (B subunit) and E221 (D subunit) show distances consistent with salt-bridge formation. 2Fo-Fc map contoured at 1.0σ (blue), Fo-Fc map contoured at 3.0σ (green). Molecular figures generated with PyMol 1.8 (Schrödinger, LLC.)
A crosstalk among posttranslational modifications – the case of CRMP2
Protein structure analysis may aid in prediction of the interplay between SUMOylation and phosphorylation. Our recent publication indicated that CRMP2 phosphorylation by Fyn kinase at tyrosine 32 prevented CRMP2 SUMOylation of lysine 374 and that CRMP2 phosphorylation by cyclin depend kinase 5 (Cdk5) at serine 522 enabled CRMP2 SUMOylation of lysine 374.26 Previous work has indicated that phosphorylation and SUMOylation can interact within the same protein to alter prevalence of the other modification. Examples of this include focal adhesion kinase where SUMOylation promotes autophosphorylation,40 heat-shock factors 1 and 4b, myocyte enhancer factor 2, GluK2 kainate receptor, or estrogen receptor β that require phosphorylation for secondary SUMOylation,41-45 DNA binding protein SATB1 where phosphorylation prevents SUMOylation,46 and microtubule stabilizing Tau protein where either phosphorylation or SUMOylation promotes the other modification.47 In the case of CRMP2, Fyn phosphorylation reduces SUMOylation, consequently reducing CRMP2s interaction with NaV1.7.26 It is possible that this relationship results from Fyn phosphorylation of tyrosine 32 blocking access to lysine 374 by SUMOylation machinery and proximity within the CRMP2 crystal structure supports this hypothesis. Other relationships between phosphorylation and SUMOylation have been described as proximity-dependent but only in terms of primary protein structure, i.e. within about 10 amino acids.42
Conclusions
In summary, our data show that multiple levels of CRMP2 sequence analysis aided our prediction of CRMP2 SUMOylation and enhanced our understanding of CRMP2 structure-function relationships. This description of our analysis puts forward evidence that protein sequence conservation and positive identification of protein SUMOylation sites can be correlative. However, additional studies of SUMOylated proteins and sequence conservation are needed to determine if these properties have predictive value.
Methods
Plasmids, antibodies and other materials
Biochemical analysis of protein content by Western blot analysis used the following antibodies: βIII-Tubulin (Promega Cat# G7121 RRID:AB_430874), CRMP2 (Sigma-Aldrich Cat# C2993 RRID:AB_1078573).
Mutations to plasmid dsRed2-N1-CRMP224 used in this study were introduced by QuickChange II XL mutagenesis kit (Cat# 200521, Agilent, Santa Clara, CA) in the pdsRed2-CRMP2 plasmid. Plasmids were purified from DH5α E. coli using the NucleoBond® Xtra Maxi kit (Cat# 740414, Macherey-Nagel, Germany). The coding sequence of mouse CRMP2 (residues 1–500) was amplified by PCR using primers 5′-ACCATCACAGCCATATGTCTTATCAGGGGAAGAAAAATAT-3′ (forward) and 5′-GAAGTTCCCAGTCGACTTAGTCATACAGGCCACGAGG-3′ (reverse) (Eurofins Genomics (Louisville, KY, USA)), and subcloned into the pET15_NESG vector (purchased from DNAsu, Phoenix, AZ, USA) between NdeI and SalI restriction sites, resulting in a construct with an N-terminal His tag. Amplified sequences and introduced mutations were verified by DNA sequencing.
Protein expression and purification
Recombinant mouse CRMP2 (residues 1–500) without the C-terminal tail was expressed and purified as described previously.24,36 The pure CRMP2 protein was concentrated to 30 mg/mL, flash frozen in liquid nitrogen and stored at −80°C.
Crystallization
CRMP2 1–500 was crystallized using the hanging drop method at room temperature, by mixing 2 μL of the well solution (0.1 M Bis-Tris pH 6, 25% PEG3,350) and 2 μL of protein (16 mg/mL diluted in 50mM HEPES pH7.5, 300 mM Na Cl, 0.5mM TCEP, 10% glycerol, 200mM CaCl2) and equilibrating against 0.5 mL of the well solution. Crystals were soaked in a cryoprotectant solution (100 mM HEPES, pH 7.4, 45% PEG 3,350) before data collection.
Data collection and processing
X-ray data were collected at Stanford Synchrotron Radiation Lightsource (SSRL) beamline BL7–1. Data were processed using X-ray Detector Software48 to 1.78 Å and the structure was solved by molecular replacement using the published human CRMP2 structure (2GSE.pdb32). The asymmetric unit contains a dimer. The appropriate amino acid substitutions were made and the structure was refined using Refmac49 in the CCP450 suite with automatic weighting with manual rebuilding in Coot.51 The final model contains residues 15 – 496 (subunit A) and residues 15 – 49 and 54 – 496 (subunit B). Density for residues 50–53 was poorly resolved in subunit B, likely due to disorder caused by crystal packing. Simulated annealing omit maps were generated with PHENIX.52 Coordinates and structure factors for CRMP2 are deposited in the Protein Data Bank (accession code 5UQC).
Culturing and transfection of catecholamine A differentiated (CAD) cells
Mouse neuron derived CAD (ECACC Cat# 08100805, RRID:CVCL_0199) cells were grown in standard cell culture conditions (DMEM/F12 media supplemented with 10% fetal bovine serum (Hyclone)), 37°C in 5% CO2, as described previously.24,53
For patch clamp experiments, CAD cells were transfected with 1 µg/µl of polyethyleneimine (PEI, Sigma) and 2 µg of various CRMP2 constructs. Under these conditions efficiencies of ∼50% were observed. For biochemistry experiments, CAD cells were transfected with Lipofectamine 2000 (Invitrogen) with ∼80–95% efficiency. All experiments were performed 48 h after transfection.
Patch clamp electrophysiology
Whole cell voltage clamp recordings were performed at room temperature using an EPC 10 Amplifier-HEKA as described previously.27 The internal solution for voltage clamp cell recordings contained (in mM): 110 CsCl, 5 MgSO4, 10 EGTA, 4 ATP Na2-ATP, and 25 HEPES (pH 7.3, 290–300 mOsm/L) and external solution contained (in mM): 100 NaCl, 10 tetraethylammonium chloride, 1 CaCl2, 1 CdCl2, 1 MgCl2, 10 D-glucose, 4 4-aminopyridine, 0.1 NiCl2, 10 HEPES (pH 7.3, 310–315 mosM/L).
Voltage clamp protocols
CAD cells were subjected to current-density (I-V) and fast-inactivation voltage protocols. In the I-V protocol, cells were held at a −80 mV holding potential before depolarization by 20 ms voltage steps from −70 mV to +60 mV in 5 mV increments. This allowed for collection of current density data to analyze activation of sodium channels as a function of current versus voltage and also peak current density which was typically observed near ∼0 to −10 mV and normalized to cell capacitance (pF). Values of current in nA and cell size in pF are available in figure legends. In the fast-inactivation protocol, cells were held at a −80 mV holding potential before hyperpolarizing and depolarizing pulses for 500 ms between −120 mV to −10 mV in 5 mV increments. This step conditioned various percentages of channels into fast-inactivated states so that a 0 mV test pulse for 20 ms could reveal relative fast inactivation normalized to maximum current. The current density of CAD cells undergoes a slight drift between passages, but averages around 60 pA/pF peak currents. Due to drift, controls were repeated within each experiment to normalize data. Importantly, all relationships between conditions were conserved regardless of drift.
Immunoprecipitation (IP) of endogenously SUMOylated proteins from cell lysates
CAD cells were lysed into the immunoprecipitation buffer as described previously.23 Buffer composition was 20 mM Tris-HCl pH = 7.4, 50 mM NaCl, 2 mM MgCl2, 10 mM N-Ethylmaleimide (NEM), 1% (vol/vol) NP-40, 0.5% (mass/vol) sodium deoxycholate, 0.1% (mass/vol) sodium dodecyl sulfate (SDS) with Protease inhibitors (Cat# B14002, Biotool, Houston, TX), phosphatase inhibitors (Cat# B15002, Biotool, Houston, TX) and BitNuclease (Cat# B16002, Biotool, Houston, TX). Total protein concentration was determined by BCA protein assay (Cat# PI23225, Thermo Fisher Scientific, Waltham, MA). For immunoprecipitation of endogenously SUMOylated proteins, SDS was added to the lysates at 0.5% (mass/vol) final concentration, before boiling for 5 min at 95°C. Then, 500 µg of total proteins were incubated with 5 μg of SUMO1 antibody overnight at 4°C under gentle agitation. Protein G magnetic beads (Cat# 10009D, Thermo Fisher Scientific, Waltham, MA), pre-equilibrated with the immunoprecipitation buffer, were then added to the lysates and incubated for 1 h at 4°C to capture immuno-complexes. Beads were washed 4 times with immunoprecipitation buffer to remove non-specific binding of proteins, before re-suspension in Laemmli buffer and boiling at 95°C for 5 min before immunoblotting.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of the Khanna laboratory for technical assistance regarding this project: Isabel N. Angeles for assistance with plasmid cloning. We thank Andrzej Weichsel, X-ray Diffraction Facility, University of Arizona College of Science, for assistance with X-ray data collection and processing.
Funding
This work was supported by a Career Development Award from the Arizona Health Science Center to M.K., a National Scientist Development grant SDG5280023 from the American Heart Association and a Neurofibromatosis New Investigator Award NF1000099 from the Department of Defense Congressionally Directed Military Medical Research and Development Program to R.K. A.M. was partially supported by a Young Investigator Award from the Children's Tumor Foundation.
References
- [1].Shen Z, Pardington-Purtymun PE, Comeaux JC, Moyzis RK, Chen DJ. UBL1, a human ubiquitin-like protein associating with human RAD51/RAD52 proteins. Genomics 1996; 36:271-9; PMID:8812453; https://doi.org/ 10.1006/geno.1996.0462 [DOI] [PubMed] [Google Scholar]
- [2].Kamitani T, Kito K, Nguyen HP, Fukuda-Kamitani T, Yeh ET. Characterization of a second member of the sentrin family of ubiquitin-like proteins. J Biol Chem 1998; 273:11349-53; PMID:9556629; https://doi.org/ 10.1074/jbc.273.18.11349 [DOI] [PubMed] [Google Scholar]
- [3].Kamitani T, Nguyen HP, Kito K, Fukuda-Kamitani T, Yeh ET. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J Biol Chem 1998; 273:3117-20; PMID:9452416; https://doi.org/ 10.1074/jbc.273.6.3117 [DOI] [PubMed] [Google Scholar]
- [4].Henley JM, Craig TJ, Wilkinson KA. Neuronal SUMOylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiol Rev 2014; 94:1249-85; PMID:25287864; https://doi.org/ 10.1152/physrev.00008.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnson ES. Protein modification by SUMO. Annual Rev Biochem 2004; 73:355-82; PMID:15189146; https://doi.org/ 10.1146/annurev.biochem.73.011303.074118 [DOI] [PubMed] [Google Scholar]
- [6].Hardeland U, Steinacher R, Jiricny J, Schar P. Modification of the human thymine-DNA glycosylase by ubiquitin-like proteins facilitates enzymatic turnover. EMBO J 2002; 21:1456-64; PMID:11889051; https://doi.org/ 10.1093/emboj/21.6.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ulrich HD. The SUMO system: an overview. Methods Mol Biol 2009; 497:3-16; PMID:19107407 [DOI] [PubMed] [Google Scholar]
- [8].Xu J, He Y, Qiang B, Yuan J, Peng X, Pan XM. A novel method for high accuracy sumoylation site prediction from protein sequences. BMC Bioinformatics 2008; 9:8; PMID:18179724; https://doi.org/ 10.1186/1471-2105-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen X, Shi SP, Xu HD, Suo SB, Qiu JD. A homology-based pipeline for global prediction of post-translational modification sites. Sci Rep 2016; 6:25801; PMID:27174170; https://doi.org/ 10.1038/srep25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beauclair G, Bridier-Nahmias A, Zagury JF, Saib A, Zamborlini A. JASSA: a comprehensive tool for prediction of SUMOylation sites and SIMs. Bioinformatics 2015; 31:3483-91; PMID:26142185; https://doi.org/ 10.1093/bioinformatics/btv403 [DOI] [PubMed] [Google Scholar]
- [11].Zhao Q, Xie Y, Zheng Y, Jiang S, Liu W, Mu W, Liu Z, Zhao Y, Xue Y, Ren J. GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res 2014; 42:W325-30; PMID:24880689; https://doi.org/ 10.1093/nar/gku383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen YZ, Chen Z, Gong YA, Ying G. SUMOhydro: a novel method for the prediction of sumoylation sites based on hydrophobic properties. PLoS One 2012; 7:e39195; PMID:22720073; https://doi.org/ 10.1371/journal.pone.0039195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vincent P, Collette Y, Marignier R, Vuaillat C, Rogemond V, Davoust N, Malcus C, Cavagna S, Gessain A, Machuca-Gayet I, et al.. A role for the neuronal protein collapsin response mediator protein 2 in T lymphocyte polarization and migration. J Immunol 2005; 175:7650-60; PMID:16301675; https://doi.org/ 10.4049/jimmunol.175.11.7650 [DOI] [PubMed] [Google Scholar]
- [14].Vuaillat C, Varrin-Doyer M, Bernard A, Sagardoy I, Cavagna S, Chounlamountri I, Lafon M, Giraudon P. High CRMP2 expression in peripheral T lymphocytes is associated with recruitment to the brain during virus-induced neuroinflammation. J Neuroimmunol 2008; 193:38-51; PMID:18006081; https://doi.org/ 10.1016/j.jneuroim.2007.09.033 [DOI] [PubMed] [Google Scholar]
- [15].Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, et al.. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 2002; 4:583-91 [DOI] [PubMed] [Google Scholar]
- [16].Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol 2003; 5:819-26 [DOI] [PubMed] [Google Scholar]
- [17].Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Ménager C, Kawabata S, Fujii K, et al.. Anterograde transport of TrkB in Axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell 2009; 16:675-86; PMID:19460344; https://doi.org/ 10.1016/j.devcel.2009.03.005 [DOI] [PubMed] [Google Scholar]
- [18].Rahajeng J, Giridharan SS, Naslavsky N, Caplan S. Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J Biol Chem 2010; 285(42):31918-22; PMID:20801876; https://doi.org/ 10.1074/jbc.C110.166066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kimura T, Watanabe H, Iwamatsu A, Kaibuchi K. Tubulin and CRMP-2 complex is transported via Kinesin-1. J Neurochem 2005; 93:1371-82; PMID:15935053; https://doi.org/ 10.1111/j.1471-4159.2005.03063.x [DOI] [PubMed] [Google Scholar]
- [20].Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovascular Res 2007; 4:143-51; PMID:17504212; https://doi.org/ 10.2174/156720207780637216 [DOI] [PubMed] [Google Scholar]
- [21].Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K. CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol Cell Biol 2005; 25:9920-35; PMID:16260607; https://doi.org/ 10.1128/MCB.25.22.9920-9935.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem 2009; 284:31375-90; PMID:19755421; https://doi.org/ 10.1074/jbc.M109.009951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci 2009; 122:4351-62; PMID:19903690; https://doi.org/ 10.1242/jcs.053280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brittain JM, Wang Y, Eruvwetere O, Khanna R. Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS Letters 2012; 586:3813-8; PMID:23022559;https://doi.org/ 10.1016/j.febslet.2012.09.022 [DOI] [PubMed] [Google Scholar]
- [25].Khanna R, Wilson SM, Brittain JM, Weimer J, Sultana R, Butterfield A, Hensley K. Opening Pandora's jar: a primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurol 2012; 7:749-71; PMID:23308041; https://doi.org/ 10.2217/fnl.12.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dustrude ET, Moutal A, Yang X, Wang Y, Khanna M, Khanna R. Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proc Natl Acad Sci U S A 2016; 113:E8443-E52; PMID:27940916; https://doi.org/ 10.1073/pnas.1610531113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dustrude ET, Wilson SM, Ju W, Xiao Y, Khanna R. CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. J Biol Chem 2013; 288:24316-31; PMID:23836888; https://doi.org/ 10.1074/jbc.M113.474924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pichler A, Knipscheer P, Oberhofer E, van Dijk WJ, Korner R, Olsen JV, Jentsch S, Melchior F, Sixma TK. SUMO modification of the ubiquitin-conjugating enzyme E2-25K. Nat Struct Mol Biol 2005; 12:264-9; https://doi.org/ 10.1038/nsmb903 [DOI] [PubMed] [Google Scholar]
- [29].Ponnusamy R, Lohkamp B. Insights into the oligomerization of CRMPs: crystal structure of human collapsin response mediator protein 5. J Neurochem 2013; 125:855-68; PMID:23373749; https://doi.org/ 10.1111/jnc.12188 [DOI] [PubMed] [Google Scholar]
- [30].Wang LH, Strittmatter SM. Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. J Neurochem 1997; 69:2261-9; PMID:9375656; https://doi.org/ 10.1046/j.1471-4159.1997.69062261.x [DOI] [PubMed] [Google Scholar]
- [31].Fukada M, Watakabe I, Yuasa-Kawada J, Kawachi H, Kuroiwa A, Matsuda Y, Noda M. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem 2000; 275:37957-65; PMID:10956643; https://doi.org/ 10.1074/jbc.M003277200 [DOI] [PubMed] [Google Scholar]
- [32].Stenmark P, Ogg D, Flodin S, Flores A, Kotenyova T, Nyman T, Nordlund P, Kursula P. The structure of human collapsin response mediator protein 2, a regulator of axonal growth. J Neurochem 2007; 101:906-17; PMID:17250651; https://doi.org/ 10.1111/j.1471-4159.2006.04401.x [DOI] [PubMed] [Google Scholar]
- [33].Majava V, Loytynoja N, Chen WQ, Lubec G, Kursula P. Crystal and solution structure, stability and post-translational modifications of collapsin response mediator protein 2. FEBS J 2008; 275:4583-96; PMID:18699782; https://doi.org/ 10.1111/j.1742-4658.2008.06601.x [DOI] [PubMed] [Google Scholar]
- [34].Myllykoski M, Baumann A, Hensley K, Kursula P. Collapsin response mediator protein 2: high-resolution crystal structure sheds light on small-molecule binding, post-translational modifications, and conformational flexibility. Amino Acids 2017; https://doi.org/10.1007/s00726-016-2376-z; PMID:2804420614685275 [DOI] [PubMed] [Google Scholar]
- [35].Deo RC, Schmidt EF, Elhabazi A, Togashi H, Burley SK, Strittmatter SM. Structural bases for CRMP function in plexin-dependent semaphorin3A signaling. EMBO J 2004; 23:9-22; PMID:14685275; https://doi.org/ 10.1038/sj.emboj.7600021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moutal A, Francois-Moutal L, Perez-Miller S, Cottier K, Chew LA, Yeon SK, Dai J, Park KD, Khanna M, Khanna R. (S)-Lacosamide Binding to Collapsin Response Mediator Protein 2 (CRMP2) regulates CaV2.2 activity by subverting its phosphorylation by Cdk5. Mol Neurobiol 2016; 53:1959-76; PMID:25846820; https://doi.org/ 10.1007/s12035-015-9141-2 [DOI] [PubMed] [Google Scholar]
- [37].Ponnusamy R, Lebedev AA, Pahlow S, Lohkamp B. Crystal structure of human CRMP-4: correction of intensities for lattice-translocation disorder. Acta Crystallogr D Biol Crystallogr 2014; 70:1680-94; PMID:24914979; https://doi.org/ 10.1107/S1399004714006634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu SH, Huang SF, Hsu YL, Pan SH, Chen YJ, Lin YH. Structure of human collapsin response mediator protein 1: a possible role of its C-terminal tail. Acta Crystallogr F Struct Biol Commun 2015; 71:938-45; PMID:26249678; https://doi.org/ 10.1107/S2053230X15009243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martinez-Rodriguez S, Martinez-Gomez AI, Clemente-Jimenez JM, Rodriguez-Vico F, Garcia-Ruiz JM, Las Heras-Vazquez FJ, Gavira JA. Structure of dihydropyrimidinase from Sinorhizobium meliloti CECT4114: new features in an amidohydrolase family member. J Struct Biol 2010; 169:200-8; PMID:19895890; https://doi.org/ 10.1016/j.jsb.2009.10.013 [DOI] [PubMed] [Google Scholar]
- [40].Kadare G, Toutant M, Formstecher E, Corvol JC, Carnaud M, Boutterin MC, Girault JA. PIAS1-mediated sumoylation of focal adhesion kinase activates its autophosphorylation. J Biol Chem 2003; 278:47434-40; PMID:14500712; https://doi.org/ 10.1074/jbc.M308562200 [DOI] [PubMed] [Google Scholar]
- [41].Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol 2003; 23:2953-68; PMID:12665592; https://doi.org/ 10.1128/MCB.23.8.2953-2968.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A 2006; 103:45-50; PMID:16371476; https://doi.org/ 10.1073/pnas.0503698102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gregoire S, Tremblay AM, Xiao L, Yang Q, Ma K, Nie J, Mao Z, Wu Z, Giguère V, Yang XJ. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J Biol Chem 2006; 281:4423-33; PMID:16356933; https://doi.org/ 10.1074/jbc.M509471200 [DOI] [PubMed] [Google Scholar]
- [44].Konopacki FA, Jaafari N, Rocca DL, Wilkinson KA, Chamberlain S, Rubin P, Kantamneni S, Mellor JR, Henley JM. Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proc Natl Acad Sci USA 2011; 108:19772-7;PMID:22089239;https://doi.org/ 10.1073/pnas.1111575108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Picard N, Caron V, Bilodeau S, Sanchez M, Mascle X, Aubry M, Tremblay A. Identification of estrogen receptor beta as a SUMO-1 target reveals a novel phosphorylated sumoylation motif and regulation by glycogen synthase kinase 3beta. Mol Cell Biol 2012; 32:2709-21; PMID:22586270; https://doi.org/ 10.1128/MCB.06624-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tan JA, Song J, Chen Y, Durrin LK. Phosphorylation-dependent interaction of SATB1 and PIAS1 directs SUMO-regulated caspase cleavage of SATB1. Mol Cell Biol 2010; 30:2823-36; PMID:20351170; https://doi.org/ 10.1128/MCB.01603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Luo HB, Xia YY, Shu XJ, Liu ZC, Feng Y, Liu XH, Yu G, Yin G, Xiong YS, Zeng K, et al.. SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc Natl Acad Sci U S A 2014; 111:16586-91; PMID:25378699; https://doi.org/ 10.1073/pnas.1417548111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr 2010; 66:125-32; PMID:20124692; https://doi.org/ 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 1997; 53:240-55; PMID:15299926; https://doi.org/ 10.1107/S0907444996012255 [DOI] [PubMed] [Google Scholar]
- [50].Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al.. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 2011; 67:235-42; PMID:21460441; https://doi.org/ 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 2010; 66:486-501; PMID:20383002; https://doi.org/ 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al.. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010; 66:213-21; PMID:20124702; https://doi.org/ 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brittain JM, Wilson SM, Wang Y, Khanna R. Regulation of CREB signaling through L-Type Ca 2+ channels by Nipsnap-2. Channels 2012; 6:94-102 [DOI] [PubMed] [Google Scholar]
- [54].Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 2011; 39:W475-8; PMID:21470960; https://doi.org/ 10.1093/nar/gkr201 [DOI] [PMC free article] [PubMed] [Google Scholar]



