ABSTRACT
Ion channels play essential roles toward determining how neurons respond to sensory input to mediate perception and behavior. Small conductance calcium-activated potassium (SK) channels are found ubiquitously throughout the brain and have been extensively characterized both molecularly and physiologically in terms of structure and function. It is clear that SK channels are key determinants of neural excitability as they mediate important neuronal response properties such as spike frequency adaptation. However, the functional roles of the different known SK channel subtypes are not well understood. Here we review recent evidence from the electrosensory system of weakly electric fish suggesting that the function of different SK channel subtypes is to optimize the processing of independent but behaviorally relevant stimulus attributes. Indeed, natural sensory stimuli frequently consist of a fast time-varying waveform (i.e., the carrier) whose amplitude (i.e., the envelope) varies slowly and independently. We first review evidence showing how somatic SK2 channels mediate tuning and responses to carrier waveforms. We then review evidence showing how dendritic SK1 channels instead determine tuning and optimize responses to envelope waveforms based on their statistics as found in the organism's natural environment in an independent fashion. The high degree of functional homology between SK channels in electric fish and their mammalian orthologs, as well as the many important parallels between the electrosensory system and the mammalian visual, auditory, and vestibular systems, suggest that these functional roles are conserved across systems and species.
KEYWORDS: envelope, information theory, optimal coding, SK channels, weakly electric fish
Introduction
Understanding the set of transformations by which sensory input gives rise to perception and behavior (i.e., the neural code) remains an important problem in neuroscience. Growing evidence shows that the responses of sensory neurons to stimulation are in part due to the presence or absence of specific ion channels on their membrane surface. One such family of ion channels are small conductance calcium-activated potassium (SK) channels. Indeed, SK channels are key determinants of cellular excitability and thus of responses to sensory input.1 Such channels are found ubiquitously throughout the CNS and mediate a variety of functions in health and disease.2-7 Interestingly, there are 3 SK subtypes (SK1, SK2, and SK3) that are differentially distributed throughout the CNS.8 While much is known about the structure, pharmacology, trafficking, and modulation of SK channels,2,7,9,10 much less is known about the functional roles of the different subtypes or the importance of somatic vs. dendritic SK channel expression.
Here we review recent evidence from the electrosensory system of weakly electric fish showing distinct functional roles for SK1 and SK2 channel subtypes that are expressed in dendrites and somata, respectively, in optimizing coding of independent behaviorally relevant stimulus attributes in parallel within the same neural population. The paper is organized as follows: we first review key background material on SK channels, natural stimulus statistics, optimized coding of natural stimuli, and weakly electric fish. We then review recent evidence showing how SK1 and SK2 channels each regulate excitability and tuning to different but behaviorally-relevant electrosensory stimulus attributes in an independent fashion. We conclude by highlighting important parallels between the electrosensory and other systems toward emphasizing the general applicability of the reviewed results as well as pointing out interesting future avenues of research.
Background
SK channels and cellular excitability: Role in spike-frequency adaptation and tuning
Here we briefly mention some key properties of SK channels on cellular excitability as these have already been extensively reviewed in the past.2,7,9,10 SK channels (Fig. 1A) share common structural similarities with other members in the potassium channel family in that they have 6 transmembrane domains as well as a pore region found in traditional voltage-gated potassium channels between S5 and S6.11 Due to the lack of a functioning voltage sensor,11,12 SK channels are not voltage-gated but are instead activated by increases in the intracellular calcium concentration. SK channels are hence named for their high calcium sensitivity as well as their small conductance of approximately 10–14 pS.13,14 The binding of small concentrations of calcium onto calmodulin leads to an important conformational change of the SK channel, causing activation of an outward potassium current.10,15,16
Figure 1.
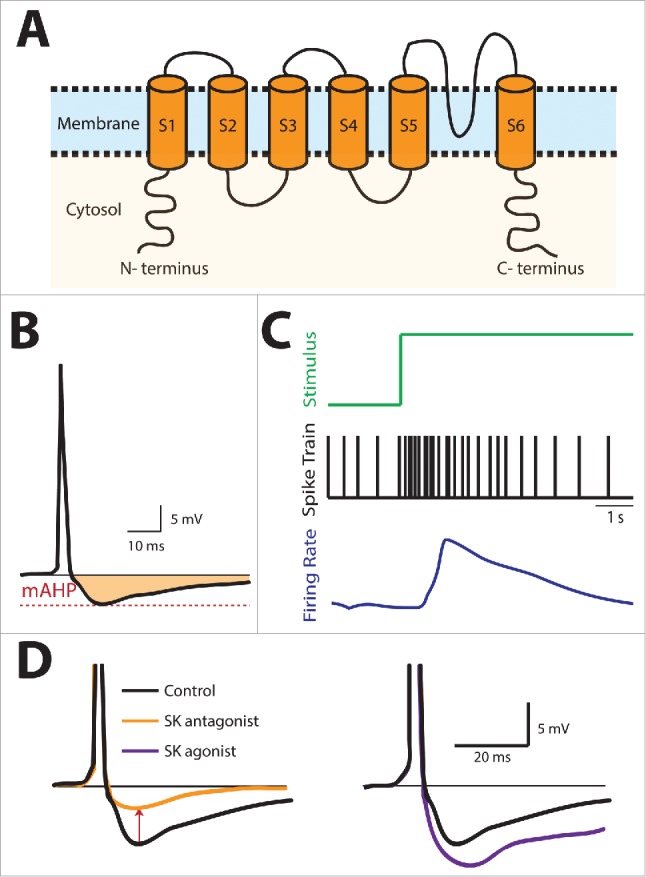
Summary of SK channel structure and effect on neuronal excitability and spike frequency adaptation. (A) Schematic of an SK Channel showing its basic structure consisting of 6 transmembrane domains. (B) SK channels are activated by increases in the intracellular calcium concentration following action potential firing and give rise to an after-hyperpolarization (AHP) (shaded region). (C) Stimulus (top, green), spiking activity (middle, black), and filtered firing rate (bottom, blue). In response to a step increase in the stimulus, there is an initial increase followed by a gradual decrease in spiking activity and firing rate during the step that is known as spike frequency adaptation (SFA). (D) SK channel antagonist application reduces the AHP while SK channel agonist application instead increases the AHP.
SK channels regulate cellular excitability by controlling the afterhyperpolarization (AHP) following action potential firing (Fig. 1B). The AHP influences how fast the neuron recovers before it can fire the next action potential, and in turn affects how often a neuron can fire given a particular stimulus. For example, a step stimulus can cause rapid firing following its onset, and as the influence of calcium from various sources become activated over time, the rate of firing decreases over time (Fig. 1C), this is known as spike frequency adaptation. In addition, it is well known that SK channels can be manipulated pharmacologically by either blocking their activation with an antagonist such as the bee venom apamin or UCL-1684, or enhancing their activation properties with an agonist such as 1-EBIO.17,18 These pharmacological manipulations of SK channels with antagonists and agonists give rise to strong reduction and enhancement in the AHP, respectively (Fig. 1D). These changes in AHP in turn affect both the degree and timecourse of spike-frequency adaptation. There is general agreement that spike frequency adaptation introduces a high-pass filter to the neuronal transfer function (i.e., the relationship between neural response and stimulus as a function of frequency), thereby attenuating or completely eliminating the transmission of low frequencies.17,19-22 It is important to note here that natural stimuli (described below) are much more complex than the simple step increases that are often used to elicit spike frequency adaptation.
Natural stimulus statistics
Growing evidence shows that the brain's coding strategies are adapted to the statistics of stimuli as found in the organism's natural environment,23-30 therefore making knowledge of these statistics paramount toward understanding the neural code. Natural stimuli have complex spatiotemporal characteristics in general. However, in many cases, their temporal variation consists of a fast waveform (i.e., the carrier or first order) whose amplitude (i.e., the envelope or second order) varies independently.31-41 Both first and second order carry behaviorally relevant information and, in general, must be processed by the brain. For example, in the auditory system, first- and second-order attributes would correspond to acoustic pressure and amplitude, respectively, and psychophysical experiments have shown that the latter are essential for proper speech perception.42 The statistics of natural sensory input have been studied extensively (visual:,43,44; auditory:36,45; electrosensory:46,47; vestibular:48,49), both in terms of their probability of occurrence in the natural environment and in terms of their frequency content. Interestingly, it was found that natural stimuli can display scale invariance (i.e., are self-similar when looked at different temporal and spatial scales) (see24 for review). Such scale invariance is manifested when looking at their frequency (either spatial or temporal) content by the fact that spectral power decays as a power law as a function of increasing frequency. This implies that natural stimuli contain more power at low than at high frequencies.
Optimized neural coding of natural stimuli
Artificial stimuli such as sine waves and contrast gratings, or auditory noise within a given range of frequencies have been frequently used to characterize neural tuning due to their relative simplicity. However, as mentioned above, growing evidence shows that neural coding strategies take advantage of the statistical structure of natural stimuli to optimize coding23-29,50 and have been reviewed extensively.24,29 In particular, it has been proposed that sensory neurons should follow the “response equalization hypothesis,” whereby neurons should have roughly the same activity in response to natural scenes irrespective of frequency (i.e., is “white”). Such coding mechanisms enhance signal-to-noise ratio and reduce redundancy in the stimulus to maximize information transmission.51,52 Since natural stimuli display spectral power that decays with increasing frequency, it is necessary for the neural sensitivity to increase to “oppose” this decay, thereby leading to a neural response whose power is independent of frequency (i.e., is “white”). In the spatial domain, the center-surround properties of receptive fields in the retina have been proposed to perform whitening of natural stimuli.53,54 Whitening in the temporal domain (i.e., “temporal whitening”) has also been observed experimentally,25-27,55 and there is much interest in understanding the underlying mechanisms.
Importantly, the statistics of natural stimuli change over time (e.g., the distribution of luminance and contrast values at a given location vary enormously during the day). It is thus necessary for coding strategies to adapt to these changes. It has been proposed that sensory adaptation (i.e., changes in a sensory neuron's response properties following a change in a stimulus attribute) optimizes coding of natural stimuli with changing statistics.56-58 Sensory adaptation has been observed in response to changes in relatively “simple” properties of the stimulus such as its mean or variance, but also to more complex attributes such as changes in frequency content as recently reviewed.57 It is important to realize that adaptation, while beneficial for optimized coding of natural stimuli with changing statistics, also introduces ambiguity in the neural code.56,59 This is because the same neural response can be elicited by different stimuli. How the nervous system resolves the problems caused by ambiguous neural representations of stimuli due to adaptation is still an ongoing area of research.
Weakly electric fish
Gymnotiform wave-type weakly electric fish have become an attractive model system for studying sensory processing and perception of natural stimuli due to well-characterized neural circuits (see60-64 for review). These fish sense amplitude modulations (AMs) of their self-generated quasi-sinusoidal electric organ discharge (EOD) through peripheral electroreceptors found on their skin. Note that it is important to realize that the relevant stimulus here is the AM. Electroreceptors send afferents that trifurcate to contact sensory pyramidal neurons within 3 parallel maps of the body surface [the lateral segment (LS), centrolateral segment (CLS), and centromedial segment (CMS)] in the electrosensory lateral line lobe (ELL). Pyramidal cells are the sole output neurons of the ELL and project to higher brain areas, thereby mediating perception and behavioral responses (Fig. 2A, see60 for review). The pyramidal cells within each segment can be further divided into ON- and OFF-type, which can be found superficial, intermediate, and deep layers of the ELL. ELL pyramidal cells are very heterogeneous in both their morphologies and function and their responses to sensory input have been reviewed extensively elsewhere.60-62,65-67 Cells whose somata are located most superficially tend to have very elongated apical dendritic trees whereas cells whose somata are located most deeply within the pyramidal cell layer tend to have short apical dendritic trees,68,69 (Fig. 2A). In between these “superficial” and “deep” cells are “intermediate” cells. Interestingly, anatomic studies have shown that ELL pyramidal cells are organized into columns, each consisting of 6 cells (3 ON-type and 3 OFF-type superficial, intermediate, and deep cells) (Fig. 2A). It is important to note that deep pyramidal cells also display the weakest expression of receptors involved in synaptic plasticity as well as release of calcium from intracellular stores70-72 which correlates well with the physiologically observed lack of plasticity in their responses to sensory input.69
Figure 2.
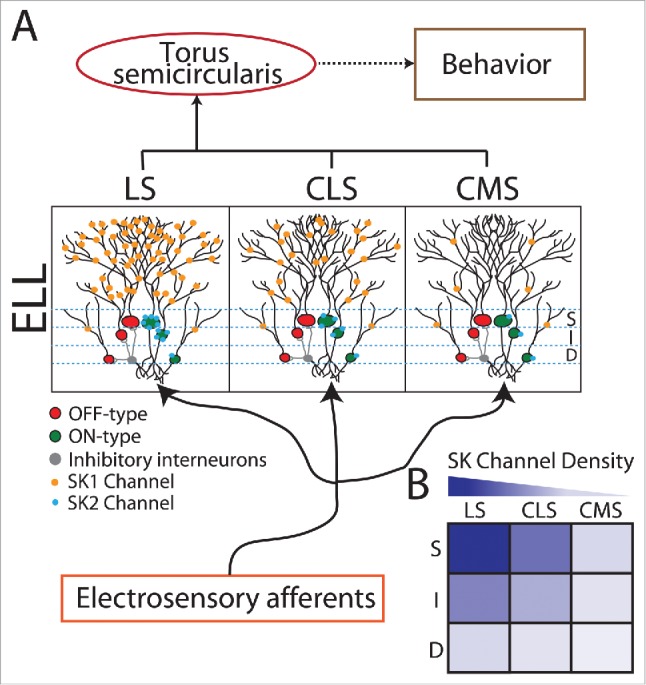
SK Channels are expressed differentially across cell types and parallel maps of the sensory epithelium within the hindbrain of weakly electric fish. (A) Schematic showing basic anatomy and circuitry of the peripheral and central electrosensory system. Peripheral electrosensory afferents trifurcate and make synaptic contact onto pyramidal cells within 3 parallel maps of the body surface: the centro-medial segment (CMS), the centro-lateral segment (CLS), and the lateral segment (LS). Within each segment, pyramidal cells are organized in a columnar organization with each column consisting of 6 cells. Three are ON-type (somata shown in green) and are excited by increases in afferent activity while the other 3 are OFF-type (somata shown in red) as they are instead inhibited by increases in afferent activity through local interneurons (gray). ON and OFF-type cells can furthermore be sub-divided into Superficial (S), Intermediate (I), and Deep (S) based on the location of their somata within the pyramidal cell layer. Only ON-type pyramidal cells express SK2 channels (cyan circles) on their somata whereas both ON and OFF-type pyramidal cells express SK1 channels (orange circles) on their dendrites. With the exception that SK2 channels are only expressed by ON-type pyramidal cells, the variation in levels of expression across segments and cell types are similar for SK1 and SK2 channels. In general, pyramidal cells within LS display the strongest while pyramidal cells within CMS instead display the weakest expression levels. Moreover, within each map, S cells display the strongest while D cells instead display the weakest expression levels. (B) Summary of SK channel expression across the maps and across S, I, and D cells.
For the purposes of this review, we will only consider the natural stimuli experienced during social interactions with conspecifics. When 2 fish come within close proximity of one another (i.e., less than 1 m), interference between the EODs give rise to a sinusoidal AM (i.e., a beat) whose amplitude depends on the distance and orientation between both fish. While the frequency of the beat is given by the difference between the EOD frequencies, the amplitude of the beat varies independently as both fish move with respect to one another.73 It is important to note that beat frequency and amplitude each carry behaviorally relevant but independent information that are encoded in the animal's brain as evidenced from appropriate behavioral responses.47,73-78 We note that weakly electric fish also emit communication calls consisting of brief changes in their EOD called chirps whose coding and perception is an ongoing area of research.19,65,79-85 that are not considered here. We will henceforth refer to the beat waveform itself as a first order attribute and to the beat amplitude (i.e., the envelope) as a second order attribute. The statistics of natural first and second order electrosensory stimulus attributes have been characterized extensively46,47,86 In particular, natural first and second order electrosensory stimulus attributes display spectral power that decays as a power law as a function of increasing temporal frequency,46,47 which is characteristic of scale invariance as mentioned above and is similar to what is seen in other systems.24
Expression patterns of SK channel subtypes in weakly electric fish and effects of neuromodulators
The SK1, SK2, and SK3 subtypes have been cloned in the weakly electric fish species Apteronotus leptorhynchus and share ∼85% sequence identity with their mammalian orthologs.18,87 In particular, only the SK1 and SK2 channel subtype were expressed in ELL (see,63,64 for review). Both in situ hybridization and immunohistochemistry have revealed differential expression patterns for SK1 and SK2 channels across ELL pyramidal cells depending on segment and cell type87 (Fig. 2A, B). In particular, SK1 and SK2 channel display similar graded expression profiles across the ELL segments. Indeed, expression is greatest in LS, intermediate in CLS, and weakest in CMS. There is moreover a graded expression patterns within each column, with expression greatest in superficial cells, intermediate in intermediate cells, and weakest in deep cells (Fig. 2A, B).
There are however differences between the expression patterns of SK1 and SK2 channels when looking at both cell type and location. Indeed, it was found that SK2 channels are only expressed in ON-type cells, whereas SK1 channels are instead expressed in both ON- and OFF-type cells (Fig. 2A). Moreover, immunohistochemistry performed using sub-type specific antibodies has revealed that SK2 channels are exclusively located near the somata of ON-type ELL pyramidal cells based on the lack of expression in dendrites as well as lack of co-localization with known dendritic markers (e.g., Map2) (Fig. 2A). In contrast, SK1 channels are expressed throughout the dendritic trees of both ON- and OFF-type cells as evidenced from co-localization with known dendritic markers87 (Fig. 2A). The implications of graded SK channel expression across segments and cell-type on response properties to different behaviorally relevant attributes of natural electrosensory stimuli are the focus of this review.
SK1 and SK2 channels in ELL can furthermore be altered by the neuromodulator serotonin (5-HT). Indeed, expression of 5-HT fibers is strongest in LS, intermediate in CLS, and weak if not absent in CMS88 which correlates well with SK channel expression. Previous studies have shown that application of 5-HT inhibits both SK1 and SK2 channels, thereby increasing cellular excitability and responses to sensory input.88,89 (see90 for review).
SK2 channels determine tuning and responses to first order electrosensory stimulus attributes
In this section, we review evidence showing that differences in SK2 channel expression across segments and cell-type strongly determines both adaptation properties and frequency tuning in the ELL pyramidal cell population. As mentioned above, SK2 channel expression is different across the ELL segments and cell-type. Figure 3A summarizes the distribution of SK2 channels. For ON-type cells (Fig. 3A, left), SK2 channel expression is graded across the segments with LS cells showing the greatest expression levels and CMS cells showing the weakest18,87 In contrast, for OFF-type cells (Fig. 3A, right), SK2 channel expression is weak and similar across segments.
Figure 3.
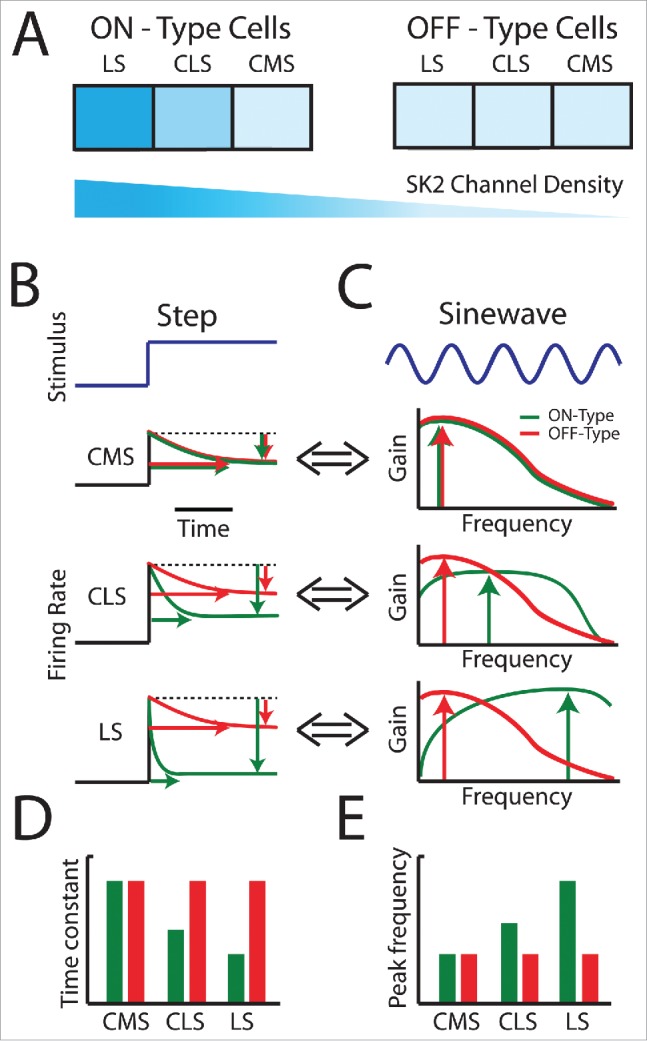
Responses to step and sinusoidal changes in stimulus mean (i.e., 1st order) for ON- and OFF-type ELL pyramidal cells across the segments. (A) Summary of SK2 channel expression across the 3 ELL segments and between ON-type (left) and OFF-type (right) neurons. Note the graded expression across segments for ON-type cells and the lack of expression in OFF-type cells. (B) Representative firing rate responses of pyramidal cells within each map for ON-type cells (green) and OFF-type cells (red) to step increases (blue). ON and OFF-type CMS cells (top) display similar adaptation properties both in terms of level (green and red vertical arrows, respectively) with the longest time constants (horizontal green and red arrows, respectively). Note that red and green arrows were offset with respect to one another for clarity. ON-type CLS cells (middle, green) display stronger adaptation with shorter time constants as compared with their CMS counterparts. In contrast, OFF-type CLS cells (middle, red) display adaptation properties that were similar to those of their CMS counterparts. ON-type LS cells (bottom, green) display the strongest adaptation with the shortest time constants while OFF-type LS cells (bottom, red) instead display adaptation properties that are similar to those of their CMS and CLS counterparts. (C) Representative tuning curves obtained by plotting gain (i.e., sensitivity) as a function of the sinusoidal stimulus' frequency for CMS (top), CLS (middle), and LS (bottom) pyramidal cells. OFF-type pyramidal cells displayed similar tuning curves across the segments (compare red curves across panels) with a maximum at low frequencies (red vertical arrows). In contrast, tuning curves for ON-type pyramidal cells are different across the segments (compare green curves across panels). Tuning becomes progressively more bandpass from CMS to LS, as reflected by a rightward shift of the frequency at which tuning is maximal (green vertical arrows). (D) Mean adaptation time constants seen across the maps for ON-type (green) and OFF-type (red) cells. Note that adaptation time constant for decreases from CMS to LS for ON-type cells only. (E) Mean peak frequency seen across the maps for ON-type (green) and OFF-type (red) cells. Note that peak frequency increases from CMS to LS only for ON-type and not for OFF-type cells.
The responses of ELL pyramidal cells to first order electrosensory stimulus attributes have been extensively investigated91-94 (see60 for review). Interestingly, ELL pyramidal cell adaptation and tuning are strikingly similar whether in response to sensory input in vivo or to current injection in vitro,91,95 indicating that frequency tuning is primarily due to intrinsic properties rather than network mechanisms. In general, adaptation in to step increases correlate well with frequency tuning measured using either sinusoidal or noisy stimuli.91,93,94,96,97 The adaptation properties and frequency tuning of ON and OFF-type cells across the different segments are summarized in Fig. 3B and C, respectively. In general, there is a strong relationship between known levels of SK2 channel expression in ELL pyramidal cells and their response properties. Indeed, CMS ON- and OFF-type cells both display the weakest spike frequency adaptation with the longest time constant to steps (Fig. 3B, top panel). LS ON-type cells instead display strong spike frequency adaptation with a short time constant (Fig. 3B, bottom panel) with CLS cells in between (Fig. 3B, middle panel). Interestingly, both LS and CLS OFF-type cells display adaptation properties that are similar to those displayed by their CMS counterparts (Fig. 3B). Thus, while adaptation time constant decreases for ON-type cells from CMS to LS, this is not the case for OFF-type cells (Fig. 3D, compare green and red bars).
The differential adaptation properties seen across segments and cell-type for ELL pyramidal cells are reflected in their tuning curves obtained in response to sinusoidal stimuli with varying frequencies. Indeed, OFF-type cells display similar tuning curves that are maximal at low frequencies irrespective of segment (Fig. 3C, compare red curves across panels). In contrast, ON-type cells in LS are tuned to the highest frequencies, followed by CLS ON-type cells, while CMS ON-type cells are tuned to the lowest frequencies (Fig. 3C, compare green curves across panels). Interestingly, CMS ON- and OFF-type display similar tuning curves that are maximal at low frequencies (Fig. 3C, compare green and red curves in top panel). Thus, while peak frequency increases for ON-type cells from CMS to LS, this is not the case for OFF-type cells (Fig. 3E, compare green and red bars).
Overall, these results show that there is a strong relationship between response properties as quantified from both adaptation and frequency tuning and SK2 channel expression in ELL pyramidal cells. The fact that OFF-type cells displayed similar response properties across segments despite differential levels of SK1 channel expression argues against the hypothesis that it is SK1 channels that determine response properties to 1st order attributes of electrosensory stimuli. Rather, these results support the hypothesis that it is actually SK2 channels that determine such properties.
Causal evidence strongly supporting the fact that SK2 channels determine response properties comes from pharmacological manipulations. Indeed, for LS ON-type pyramidal cells, application of SK channel antagonists will reduce their adaptation level and increase their adaptation time constants (Fig. 4A).17,18 Application of SK channel antagonists will also increase tuning to low frequencies, thereby lowering the frequency at which tuning is maximal (Fig. 4B). The effects of SK channel antagonists on adaptation and tuning of OFF-type LS cells has not been tested although it is assumed that these will have no effect in vitro. This is based on the fact application of SK channel antagonists or agonists has no effect on OFF-type LS and CLS cell excitability in the first place.18 Interestingly, application of SK channel antagonists made the adaptation and frequency tuning of LS and CLS ON-type cells more similar to those displayed by their CMS counterparts (compare orange curves of Figs. 4A, B to curves from the top panels of Fig. 3B, C). Further evidence for the role of SK channels on determining frequency tuning comes from evidence that deep ON-type pyramidal cells display broadband tuning irrespective of segment,91,93,96,97 which correlates well with their weak SK channel expression.
Figure 4.
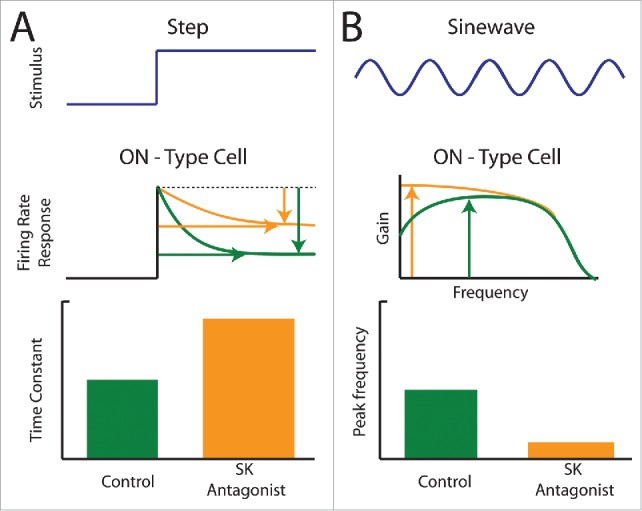
Application of SK channel antagonists alters adaptation and frequency tuning of ELL pyramidal cells to changes in stimulus mean (i.e., 1st order). (A) Firing rate responses (middle) of an ON-type pyramidal cell to a 1st order step stimulus (blue, top) under control (green) conditions and after application of an SK antagonist (orange). The antagonist increased the time constant and decreased the level of adaptation. (B) Frequency tuning (middle) of an ON-type pyramidal cell to a 1st order sinusoidal stimulus (blue, top) under control (green) conditions and after SK channel antagonist application (orange). SK channel antagonist application increased tuning to low frequencies, thereby lowering the frequency at which frequency tuning is maximum (compare position of green and orange arrows).
In summary, there is a strong correlation between SK2 channel expression and response properties to 1st order feature of electrosensory stimuli in ELL pyramidal cells across segments and cell-types. Indeed, tuning for ON-type cells becomes progressively more high-pass from CMS to LS, correlating well with increased SK2 channel expression. Further, OFF-type cells in all segments, which display weak SK2 channel expression, tend to display tuning properties similar to those of CMS ON-type cells, which also display weak SK2 channel expression. Finally, application of SK channel antagonists in LS ON-type cells makes their response properties more similar to those of their CMS counterparts. These results strongly suggest that it is SK2 rather than SK1 channels that primarily, if not exclusively, determine response properties and frequency tuning to first order electrosensory stimulus attributes.
We end this section by highlighting the potential advantages of graded SK2 channel expression across segments and cell types. It is thought that the high-pass tuning properties of peripheral receptors.97,98 will offset the decaying spectral power of natural first-order stimulus attributes and lead to a temporally whitened representation that is sent to ELL pyramidal cells46 Thus, ON-type pyramidal cells within each segment will best respond to different ranges of frequencies that are required for eliciting different behaviors.99-102 The functional roles of OFF-type pyramidal neurons in this context is not well understood. Importantly, deep pyramidal cells within each map are thought to provide a faithful representation of sensory input that is unaltered by adaptation of other filtering to higher brain centers. As further discussed below, this unaltered representation is not only necessary to enable feature selectivity by superficial and intermediate pyramidal cells but is also likely necessary to resolve the aforementioned ambiguity problem caused by adaptation.
SK1 channels determine adaptation and tuning to second-order electrosensory stimulus attributes
We now review the role played by SK channels in the encoding of second-order electrosensory stimulus attributes. In this context, it is useful to consider the expression of SK1 channels across the ELL segments and across cell types. Similar to what is seen for SK2 channels, SK1 channel expression is graded across the segments with LS cells showing the most expression and CMS cells showing the least (Fig. 5A). SK1 channel expression is furthermore graded within a column with superficial cells displaying the most and deep cells the least expression.18,87 However, unlike SK2 channel expression (Fig. 3A), there is no difference in SK1 channel expression in ON- vs. OFF-type cells within a given segment (Fig. 5A, compare left and right panels).
Figure 5.
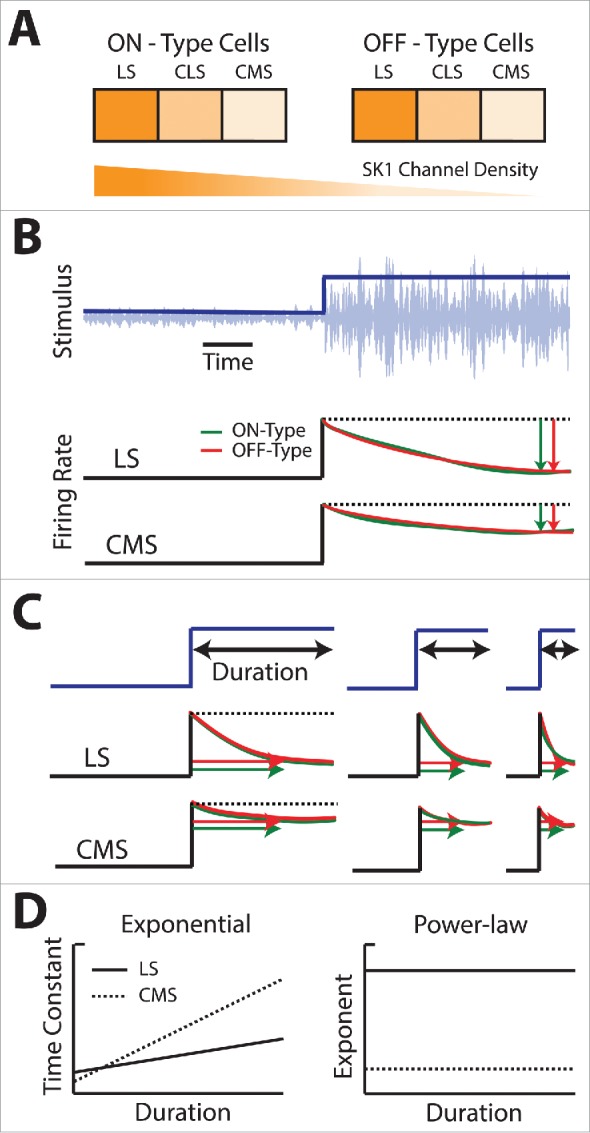
Summary of the effects of SK1 channels on responses to step and sinusoidal changes in stimulus variance (i.e., 2nd order) for ELL pyramidal cells. (A) Summary of SK1 channel expression across the 3 ELL segments and between ON-type (left) and OFF-type (right) neurons. (B) Firing rate responses of LS (middle) and CMS (bottom) neurons in response to a step increase in stimulus variance (blue, top) for an ON-type (green) and OFF-type (red) cell. Notice the lesser adaptation displayed by the CMS neuron as compared with the LS as quantified by a lesser decrease in firing rate from the maximum value (vertical red and green arrows). (C) Firing rate responses of LS (middle) and CMS (bottom) to step increases in variance with different durations (blue, top) for an ON-type (green) and OFF-type (red) cell. In each case, the stimulus waveform shown was repeated in a periodic fashion and the neuronal responses averaged. For both LS and CMS neurons, the time constant of adaptation apparently decreases with decreasing stimulus duration (horizontal arrows). Note the similarity in responses for both ON- and OFF-type cells. (D) Graphs showing adaptation constant τ (left) and power-law exponent α (right) as a function of duration for pooled LS (black) and CMS (gray) populations of neurons. Note that a single power-law exponent can fit for all durations of LS or CMS neural responses.
Adaptation to second-order electrosensory stimulus attributes is strongly correlated with SK1 channel expression
We first consider how graded expression of SK1 channels across the segments contributes to shaping adaptation to second-order stimulus attributes. Figure 5B shows a step increase in a noisy stimulus's variance. It is important to note the difference between the stimuli shown in Fig. 3B and Fig. 5B: while the step change shown in Fig. 3B was in the stimulus mean (i.e., first-order), the step change shown in Fig. 5B is instead in the stimulus variance (i.e., second-order). A recent study has characterized adaptation to step changes in variance in both CMS and LS pyramidal neurons in vivo103: it was found that ELL pyramidal neurons responded to a change in variance (step envelope) of the stimulus (Fig. 5B, top) with a rapid increase in firing rate at the stimulus onset that subsequently decayed over time. In addition, the decay was greater in LS neurons than in CMS neurons, implying that the former display stronger adaptation than the latter (Fig. 5B, compare middle and bottom). Interestingly, ON and OFF-type pyramidal cells within a given segment always displayed similar adaptation profiles (Figs. 5B, compare red and green curves).30,103
The experimenters then varied the duration of the step and compared the adaptation profiles (Fig. 5C). Such comparisons are useful in determining whether the timecourse of adaptation is scale-invariant or not.59,104 Indeed, while it is often assumed that the timecourse of adaptation is exponential (i.e., has a timescale), several studies have reported that the timecourse of adaptation follows a power law (i.e., has no timescale).25,26,35,37,56,59,105,106 To distinguish between an exponential timecourse and a power law, it is necessary to investigate adaptation over multiple timescales, which can be achieved by varying the step duration. One can then fit both exponential and power law functions to the adaptation and then plot both the best-fit exponential time constant (Fig. 5D, left) and power law exponent (Fig. 5D, right) as a function of step duration. If the timecourse of the adaptation has a characteristic timescale, then this timescale should be independent of stimulus duration. One would then expect that the best-fit exponential time constant would be independent of stimulus duration. If, on the other hand, the timecourse of adaptation is scale-invariant, then we would expect that the timecourse of adaptation be well-fit by a power law with a given exponent that is independent of stimulus duration. It was found that adaptation to second-order electrosensory stimulus attributes by ELL pyramidal cells is scale-invariant as it was well-fit by a power law whose exponent is independent of stimulus duration (Fig. 5D).
Thus, it was found that ELL pyramidal cells display adaptation to second-order stimulus attributes.103 This is in contrast to peripheral receptor afferents that do not display such adaptation.76 It was hypothesized that the strong adaptation displayed by LS pyramidal cells gives rise to ambiguity and that the weaker adaptation displayed by CMS pyramidal cells helps the brain resolve ambiguity caused by adaptation by providing an unaltered representation of sensory input in parallel.103 It is very unlikely that adaptation to second-order attributes is due to SK2 channels. This is because, as mentioned above, both ON and OFF-type cells display similar adaptation properties yet only the former express SK2 channels. As further argued below, it is most likely that the strong adaptation properties of LS pyramidal cells to second-order attributes are due to larger levels of SK1 channel expression although this has not been tested directly to date.
Tuning to second order electrosensory stimulus attributes is strongly related to SK1 channel expression
In this section, we review the relationship between SK1 channel expression and tuning to second-order electrosensory stimulus attributes. Theoretical studies predict that stronger adaptation should give rise to reduced tuning at low frequencies, thereby increasing peak frequency.107 Thus, the answer to the question “does stronger adaptation give rise to enhanced tuning at high frequencies for second-order attributes?” is yes in general as described below.
To explore responses to second order attributes, both artificial sinusoidal (Fig. 6A) and naturalistic (Fig. 6B) stimuli were used. In the latter case, previous studies have shown that natural second-order electrosensory stimulus attributes are scale invariant (i.e., spectral power decays as 1/fα, where f is the temporal frequency and α = −0.8 is the power-law exponent).46,47 The tuning curves of both ON- and OFF- type ELL pyramidal cells to second-order attributes obtained by varying the frequency of the sinusoidal stimulus are summarized in Fig. 6C while their responses to natural stimuli are summarized in Fig. 6D. While there were important differences seen across the segments, ON- and OFF-type pyramidal cells within a given segment displayed similar tuning curves (Fig. 6C, compare green and red curves). It is seen that sensitivity is independent of frequency for CMS cells (Fig. 6C, top), which correlates well with their weak adaptation properties. In contrast, sensitivity strongly increased as a function of increasing temporal frequency for LS cells (Fig. 6C, bottom). For CLS cells, sensitivity also increased as a function of increasing temporal frequency but at a weaker rate (Fig. 6C, middle). It is important to note that the summaries plotted in Fig. 6C are plotted in log-log coordinates. Thus, the linear increase in gain as a function of increasing temporal frequency implies that gain increases as a power law but with different exponents. The fact that tuning to second order stimulus attributes is similar between ON- and OFF-type cells within a given segment strongly argues against the hypothesis that SK2 channels play a role in mediating such tuning. This is because SK2 channels are expressed in ON- but not OFF-type cells. Rather, the differences in tuning seen across the segments strongly correlate with SK1 expression levels.
Figure 6.
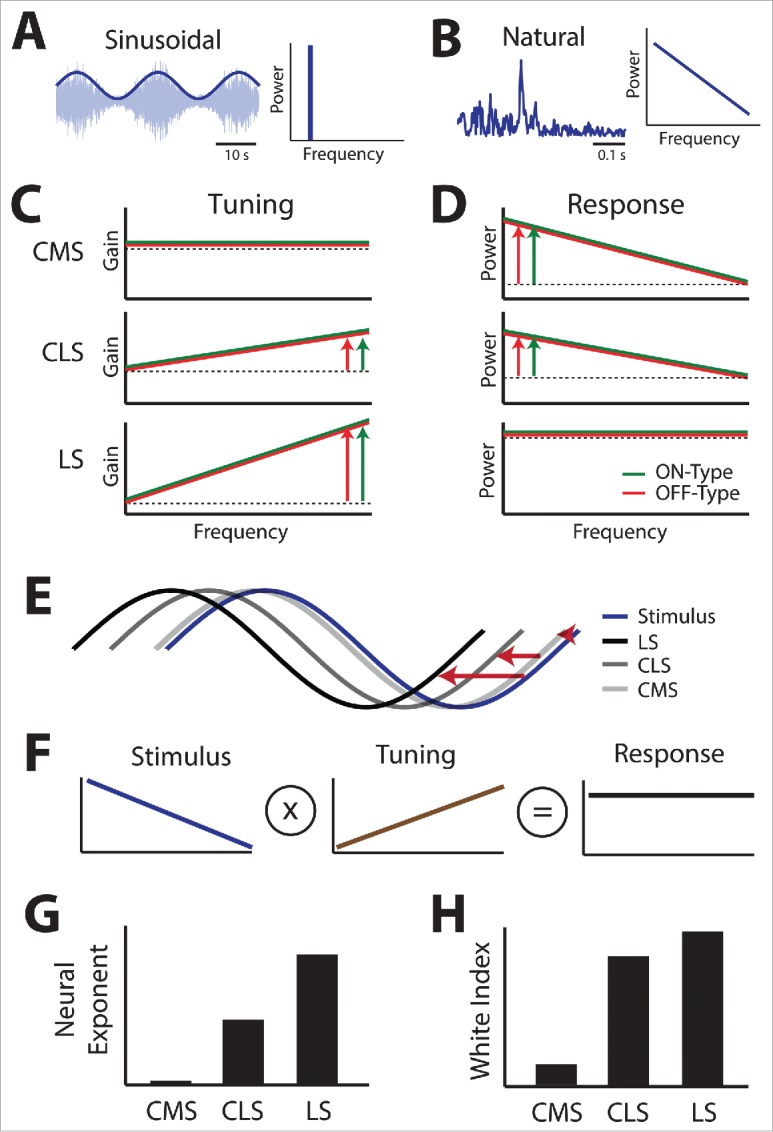
Summary of the differential tuning to sinusoidal 2nd order stimuli seen across maps and their consequences on coding of natural 2nd order stimuli in relationship to SK1 channel expression. (A) Left: example noisy waveform (light blue) whose amplitude (dark blue) is modulated sinusoidally. Right: all spectral power is found at the sinusoidal frequency. (B) Left: example amplitude waveform obtained under natural conditions. Right: spectral power of the natural stimulus decays as a power law as a function of frequency. (C) Tuning curves to 2nd order for CMS (top), CLS (middle), and CMS (bottom) obtained using sinusoidal stimulation for ON-type (green) and OFF-type (red) cells. In each case, a constant tuning (dashed black) is shown for comparison. The vertical arrows indicate how strongly gain increases as a function of increasing frequency. (D) Neural response power spectra for CMS (top), CLS (middle), and LS (bottom) under natural 2nd order stimulation for ON-type (green) and OFF-type (red) cells. In each case, a horizontal line (dashed black) is shown for comparison. The vertical arrows indicate how strongly response power decays as a function of increasing frequency. Note the similarity in responses for both ON- and OFF-type cells. E) Stimulus (blue) and neural responses of CMS (light gray), CLS (darker gray), and LS (black) neurons. The horizontal red arrows quantify the phase advance between the neural responses and stimulus. F) Schematic showing that, to optimize coding, the tuning curve (middle) must oppose stimulus statistics (left) to give rise to a neural response that is independent of frequency (right). G) Summary of the different neural tuning exponents seen across the different maps. H) Summary of the different white index quantifying response constancy as a function of frequency across the different maps.
The observed tuning curves to second-order electrosensory stimulus attributes have important implications for 2 reasons: A) they imply that ELL pyramidal cells perform fractional differentiation of second-order stimulus attributes. Fractional differentiation is a mathematical operation that is thought to be advantageous for neural coding as it results in a phase lead of the response (Fig. 6E) that is independent of frequency.26,55 B) they imply that the increase in gain can effectively compensate for the observed decrease in spectral power of natural second-order stimulus attributes (Fig. 6B, right), such that the resulting neural response power is independent of frequency (Fig. 6F). As mentioned above, this is known as temporal whitening. A recent study has shown that LS pyramidal cells effectively perform temporal whitening of natural envelope stimuli.30,77 (Fig. 6D, bottom). This is because the power law exponent at which gain increases (i.e., the “neural” exponent) is precisely matched to the power law exponent of natural second-order attributes30 (Fig. 6G). Further studies have shown that this is, however, not the case for CLS and CMS neurons23 Indeed, CMS neurons, with their flat tuning curves, essentially perform little of any filtering of second-order attributes (Fig. 6D, top) as their neural exponent was near zero (Fig. 6G). While CLS pyramidal cells do perform some fractional differentiation of envelopes, their neural exponent (Fig. 6G) is not large enough to effectively compensate for the spectral power decay of natural second-order attributes, such that the resulting neural response still decays as a function of increasing temporal frequency (Fig. 6D, middle). Thus, CMS cells perform little if any temporal whitening of natural stimuli whereas LS cells perform the most (Fig. 6H). These results suggest that SK channel expression is directly linked to the level of fractional differentiation as further discussed below.
Further evidence that SK1 channel expression regulates tuning to second-order electrosensory stimulus attributes comes from comparing the tuning curves of deep, intermediate, and superficial pyramidal cells. This is because, as mentioned above (Fig. 2), SK1 channel expression is weakest in deep cells, intermediate in intermediate cells, and strongest in superficial cells.18,87 First, similar response profiles were always observed for ON and OFF-type deep, intermediate, and superficial cells.23 Second, superficial cells within LS and CLS display the largest neural exponents (i.e., performed the most fractional differentiation) whereas deep cells instead display neural exponents that are close to zero (i.e., performed little if any fractional differentiation). Interestingly, in CMS, all cells displayed neural exponents close to zero (i.e., performed little if any fractional differentiation),23 similar to peripheral afferents.76 Thus, the tuning curves of superficial, intermediate, and deep cells across segments also strongly correlate with SK1 channel expression.
In general, it is thought that the lack of fractional differentiation of second-order stimulus attributes by deep and CMS cells, which is most likely due to lack of SK1 channel expression, provides an unaltered and thus faithful representation of these to higher brain centers that is necessary to unambiguously decode the optimized representation sent by superficial LS cells. This hypothesis has however not been rigorously tested to date. While it is known that midbrain electrosensory neurons that receive synaptic input from ELL pyramidal cells can respond selectively to second-order electrosensory stimulus attributes,73,108 their tuning properties or their responses to natural stimulation have not been investigated to date.
Thus, both adaptation and tuning to second-order stimulus attributes display a strong correlation with SK1 but not SK2 channel expression. This is primarily because, within each segment, both ON- and OFF-type ELL pyramidal cells display similar adaptation and tuning properties. In the following, we review both theoretical and experimental results showing a causal link between SK channels and response properties to second-order stimulus attributes.
Understanding how changes in SK channel expression alters adaptation and frequency tuning to second-order attributes
To understand how SK channels give rise to adaptation in the temporal domain and fractional differentiation in the frequency domain, it is beneficial to use mathematical models. Indeed, multiple mathematical formalisms have been proposed to model scale invariant adaptation.26,105,106,109,110 One of the most commonly used formalism to model neurons is called “leaky integrate-and-fire”111 and has been reviewed extensively.112,113 While the leaky integrate-and-fire model by itself does not produce scale invariant adaptation, addition of an activity dependent kernel that effectively models the activation properties of SK channels following an action potential can give rise to such adaptation (Fig. 7A). The interested reader should consult the following for details.23,26,30,105,106,109 Importantly, the model assumes that the strength of the adaptation kernel is equivalent to the total maximum conductance of a population of SK channels (henceforth referred to as the total conductance). Thus, changes in the strength of the adaptation kernel could mimic either changes in the maximum conductance of individual channels or changes in channel number (i.e., changes in channel density).
Figure 7.
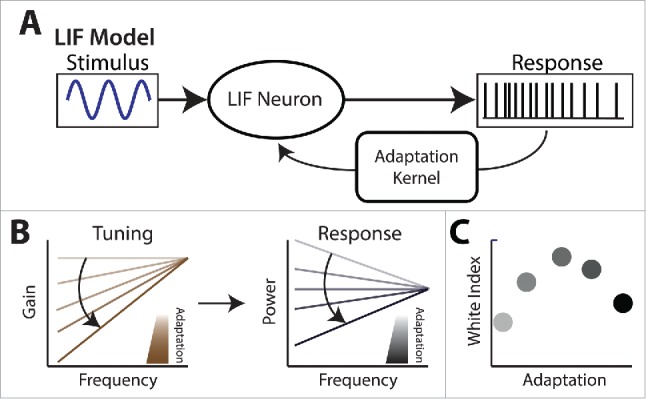
Understanding how SK channels affect both spike frequency adaptation in tuning to second-order stimulus attributes. (A) Schematic showing a simple mathematical model based on the leaky integrate-and-fire formalism. Adaptation is implemented by feeding back a current whose time course decays after each action potential (i.e., the adaptation kernel). (B) Left: varying the adaptation strength in the model leads to tuning curves that are more and more high-pass. Right: when considering natural stimuli for which spectral power decays with increasing frequency, increasing adaptation strength initially leads to a progressive whitening of the response (i.e., the response power spectrum becomes more and more independent of frequency). There is then a given value of adaptation strength for which the response is white. Further increasing adaptation strength leads to a response whose spectral power will increase more and more steeply with increasing frequency (i.e., will not be white). (C) The white index displays a maximum when increasing adaptation strength.
In general, it is found that increasing the strength of the adaptation kernel in the model led to tuning curves that increased more steeply as a function of increasing temporal frequency (i.e., increased the neural exponent) (Fig. 7B, left). This has important consequences when considering the neural response to a natural stimulus whose spectral power decays with a given power law exponent α. If the neural exponent is too weak, then the resulting neural response will display spectral power that decays as a function of frequency. If, instead, the neural exponent is too strong, then the resulting neural response will display spectral power that increases as a function of frequency. This implies that there is a neural exponent for which the neural response to a natural stimulus will display spectral power that is independent of frequency (i.e., is white), which will maximize information transmission (Fig. 7B). Thus, the white index must display a maximum as a function of adaptation strength (Fig. 7C).
The modeling exercise presented therefore has important implications for the role of SK channels toward determining tuning and response properties to second-order stimulus attributes. First, increasing the total SK channel conductance (e.g., by increasing the single channel maximum conductance or by increasing channel density) will lead to greater adaptation and a steeper tuning function. A recent study has shown that the different tuning profiles of ELL pyramidal cells across segments and cell types could be reproduced by simply varying the strength of the adaptation kernel in the model in accordance with known levels of SK channel expression.23 Importantly, the model also predicts that the total SK channel conductance strongly affects the tuning properties and responses of ELL pyramidal cells to second-order stimulus attributes.
Role of SK1 channels in determining responses to second-order stimulus attributes
So far, we have reviewed experimental evidence showing a strong correlation between SK1 channel expression and response properties to second-order stimulus attributes. We have also reviewed modeling results showing that changes in SK channel conductance can affect adaptation and frequency tuning. In this section, we review causal evidence showing experimental evidence that pharmacological manipulation of SK channels using agonists and antagonists give rise to changes in tuning properties that are consistent with modeling predictions.
Application of SK channel antagonists changes the tuning properties of LS ELL pyramidal cells as the tuning curve becomes more independent of frequency, thereby reducing fractional differentiation and the neural exponent (Fig. 8A).30 In contrast, the tuning curve after application of SK channel agonists increased more steeply with frequency, thereby increasing fractional differentiation and the neural exponent (Fig. 8A). These changes in tuning are in excellent agreement with modeling predictions and consistent with effective reduction and increase of SK-mediated currents by antagonists and agonists, respectively (compare Fig. 8A with Fig. 7B, left panel). Further, these changes in tuning give rise to important changes in responses to natural stimuli. Indeed, SK channel antagonist application caused neural response power to decrease with increasing frequency while SK channel agonist application instead led to neural response power to increase with increasing frequency (Fig. 8B). These changes in neural responses to natural stimuli are also in excellent agreement with modeling predictions (compare Fig. 8B with Fig. 7B, right panel).
Figure 8.
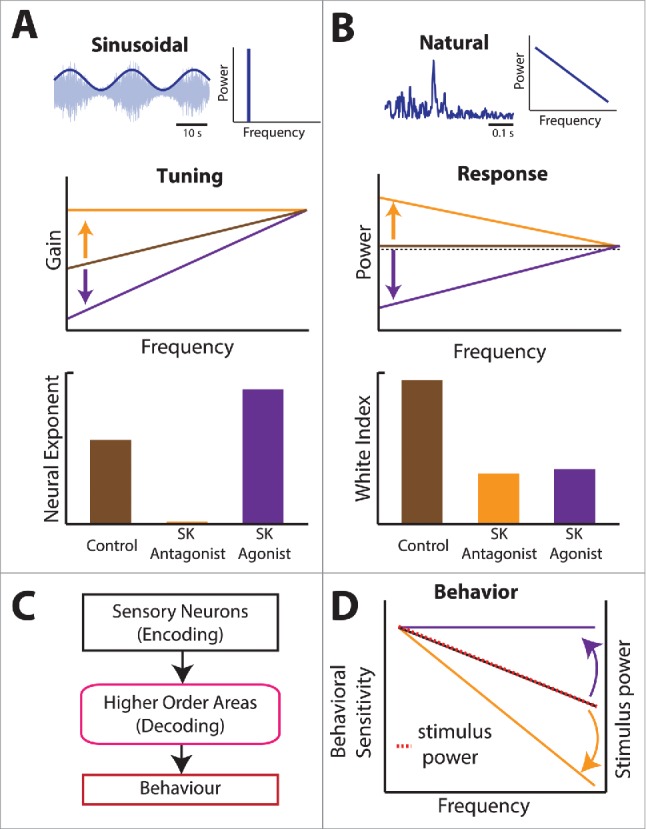
SK1 channels are key determinant of frequency tuning and optimized coding of natural 2nd order stimuli. (A) Effects of SK channel antagonists (orange) and agonists (purple) on frequency tuning to sinusoidal 2nd order stimulus (top). SK antagonists make tuning less high-pass, while agonists make tuning more high-pass (middle), thereby leading to a lower and higher neural exponent respectively (bottom). (B) Effects of SK channel antagonists (orange) and agonists (purple) on the neural response to natural 2nd order stimulus. SK channel antagonists lead to a neural response whose spectral power decreases with increasing frequency, while agonists lead to a neural response whose spectral power increases with increasing frequency (middle). In both cases, the neural response power is not independent of frequency as reflected by a lower white index (bottom). (C) Summary of information flow in the brain. Information about the stimulus is encoded by sensory neurons and are decoded by higher brain areas, thereby leading to perception and behavior. (D) Application of SK channel antagonists and agonists in ELL pyramidal cells has strong influence on the organism's behavioral responses as quantified by sensitivity. Under control conditions, there is a match between stimulus power (dashed red) and behavioral sensitivity (solid black). Application of SK channel agonists makes behavioral sensitivity more independent of frequency (purple). In contrast, application of SK channel antagonists makes behavioral sensitivity decrease more steeply with increasing frequency (orange). In both cases, there is a mismatch between behavioral sensitivity and stimulus power.
Importantly, the effects of SK channel agonist and antagonist application on tuning and responses to natural stimuli were not different when comparing ON-type and OFF-type LS ELL pyramidal cells.30 This strongly support the hypothesis that it is SK1 channels that largely if not exclusively determine tuning and responses to second-order electrosensory stimulus attributes.
The changes in tuning and responses brought about by application of SK channel agonists and antagonists in ELL also had important consequences on perception and behavior (Fig. 8C). Indeed, previous studies have shown that weakly electric fish display robust and easily elicited behavioral responses to second-order stimulus attributes.47,75 One such behavior is that the animal's EOD frequency will track second-order electrosensory stimulus attributes in a one-to-one fashion. Interestingly, it was found that behavioral sensitivity matched the spectral power content of natural second-order electrosensory stimulus attributes for temporal frequencies ranging over 3 order of magnitude.47 What was found was that application of SK channel agonists and antagonists that were confined to ELL gave rise to important changes in the animal's perception of second order electrosensory stimulus attributes as evidenced from its behavioral responses to these (Fig. 8D). Indeed, the behavioral sensitivity curve decreased less steeply after application of SK channel agonists and decreased more steeply after application of SK channel agonists, thereby creating a mismatch with natural stimulus statistics in both cases. These results are important as they show that there is a one-to-one relationship between the tuning properties of sensory neurons in ELL and the animal's behavioral responses. These results are, to our knowledge, the first to show that SK1 channels determine neural responses to second-order electrosensory stimulus attributes.
Importantly, these results imply that there must be a match between the total SK1 channel conductance and natural stimulus statistics to optimize coding. Any change in total conductance (e.g., due to changes in either single channel maximum conductance or changes in channel density) from this “optimal” value will lead to sub-optimal coding and, consequently, less information transmission. In the following section, we consider some possible implications of how changes in the total SK1 channel conductance could mediate adaptive optimized coding of natural stimuli with changing statistics.
Possible role of SK1 channels in adaptive optimized coding of natural second-order electrosensory stimulus attributes
As mentioned above, the statistics of natural sensory stimuli change over time. As such, to remain optimized to statistics, coding strategies must adapt to these changes.56,57 While there is experimental evidence that neurons can adapt their response properties to optimally encode a new stimulus following a change in statistics,58 the underlying mechanisms are not well understood to date. Here we speculate as to how regulation in the total SK1 channel conductance in LS ELL pyramidal cells could mediate adaptive optimized coding of second-order natural electrosensory stimulus attributes with changing statistics by regulating both the tuning function and adaptation (Fig. 9A).
Figure 9.
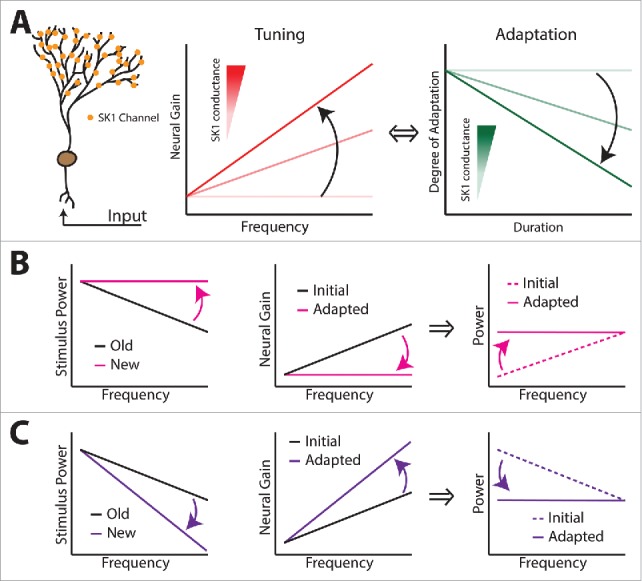
Is SK channel regulation a key determinant of adaptive optimized coding of natural stimuli? (A) Summary showing that SK1 channels present on the apical dendritic trees of ELL pyramidal cells (left) regulate frequency tuning (middle) and adaptation (right) to 2nd order stimuli. Increasing the total SK1 channel conductance will make the neural tuning curve more high-pass (middle) by increasing the degree of adaptation (right). (B) Following a change in stimulus statistics (left), neural tuning should also progressively adapt (middle, compare black and magenta curves) such as to optimize coding of the new stimulus (right, compare dashed and solid curves). (C) Same as B but for another change in stimulus statistics.
Let us assume the total SK1 channel conductance is such that the pyramidal neurons' tuning curves are set such as to temporally whiten a natural stimulus with given statistics as found experimentally (Fig. 9B, C, black curves). We will consider changes in natural stimulus statistics such that the power spectrum now decays with a different power law exponent. Specifically, we will consider 2 cases: 1) the power law exponent is lower than before (Fig. 9B, left) and; 2) the power law exponent is higher than before (Fig. 9C, left). It is then clear that the existing neural tuning curve is not set such as to optimize coding of the new stimulus in both cases (Fig. 9B, C, dashed lines). However, appropriate changes in SK1 channel total conductance will lead to changes in tuning that will optimize coding of the new stimulus in both cases. In case 1), a decrease in SK1 channel total conductance will lead to a tuning curve that increases less steeply (Fig. 9B, magenta, middle), thereby optimizing coding of the new stimulus through temporal whitening (Fig. 9B, magenta, right). In case 2), an increase in SK1 channel total conductance will lead to a tuning curve that increases more steeply (Fig. 9C, purple, middle), thereby optimizing coding of the new stimulus through temporal whitening (Fig. 9C, purple, right). Such changes in SK1 channel total conductance could be achieved by changes in channel trafficking or, possibly, by activation of serotonergic input onto ELL pyramidal cells which, as discussed above, will inhibit SK channels.88,89 It is important to note that further experiments are needed to test these predictions.
Future directions and unsolved problems
In this paper, we have reviewed the role of SK2 and SK1 channels in the encoding of first- and second-order stimulus attributes in the electrosensory system of weakly electric fish. Both SK1 and SK2 channel subtypes showed graded expression across segments and cell types, which determines how cells will respond to different stimulus attributes. On top of proposing a novel functional role for SK2 channels that would complement other documented functions (see2 for review), the results in the electrosensory system are the first, to our knowledge, to propose a functional role for SK1 channels. Indeed, progress on the role of SK1 channels has been hampered by the fact that, in rodents, these cannot be expressed without heteromerization with the SK2 channel subunits, which contrasts with results obtained in humans.114,115 The lack of SK2 channel expression in OFF-type cells, together with pharmacology showing clear effects of SK channel agonists and antagonists on these cells when applied near dendrites in vivo, strongly suggest that SK1 channels form homomultimeric channels in weakly electric fish, as observed for humans.2 The large homology (85%) between SK channels in weakly electric fish and their orthologs in humans makes it very likely that the functional roles of SK1 and SK2 channel subtypes found in the electrosensory system will be relevant for understanding the human brain. In the following, we highlight some interesting future directions for research aimed at understanding how SK channels regulate information transmission by sensory neurons.
As mentioned above, while SK2 channels are found on the somata of ON-type pyramidal cells, SK1 channels are instead found on the dendrites of ON- and OFF-type pyramidal cells.18,87 A future area of study should investigate how SK1 channels affect dendritic integration and processing of second-order electrosensory stimulus attributes, thereby leading to optimized and, possibly, adaptive processing of these. This area of research will be particularly interesting as almost nothing is known about the location of SK channels on a neuron's cellular membrane affects their function.
An important area of research should focus on the mechanisms by which SK channels can give rise to scale invariant adaptation, which is necessary for optimized coding of scale invariant natural stimuli. It is likely that such mechanisms will include the fact that SK channels can activate on multiple timescales. Indeed, SK channels can be rapidly activated by sources such as voltage-gated calcium channels, which follow the time course of the upstroke of action potentials and the available free Ca2+ within the vicinity.116,117 Slower changes in SK channel activation occurs via transmitter-gated NMDA receptors and nicotinic acetylcholine receptors associated with the time course of activation of transmitter release and receptor activation.118,119 Finally, calcium could also be released from intracellular stores at longer timescales via metabotropic-activated IP3 receptors as well as ryanodine receptors.120-122 It is likely that these multiple timescales of activation mediate the observed scale invariant adaptation, although further studies are needed to test this hypothesis. It is also likely that other ion channels are responsible for this power-law adaptation as many previous studies have investigated extensively on the different ionic currents and their various effects on the AHP and spike-frequency adaptation.123-125 For example, in the electrosensory system, M-type potassium channels also give rise to an AHP and to spike frequency adaptation.17 Further studies are needed to investigate these interesting questions in detail.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Canada Research Chairs and the Canadian Institutes of Health Research (MJC).
References
- [1].Faber ES. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+channels regulates synaptic excitability in the medial prefrontal cortex. J Physiol-London 2010; 588:1281-92; PMID:20194128; https://doi.org/ 10.1113/jphysiol.2009.185645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Ann Rev Physiol 2012; 74:245-69; PMID:21942705; https://doi.org/ 10.1146/annurev-physiol-020911-153336 [DOI] [PubMed] [Google Scholar]
- [3].Deschaux O, Bizot JC, Goyffon M. Apamin improves learning in an object recognition task in rats. Neurosci Lett 1997; 222:159-62; PMID:9148239; https://doi.org/ 10.1016/S0304-3940(97)13367-5 [DOI] [PubMed] [Google Scholar]
- [4].Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 2002; 22:10163-71; PMID:12451117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Inan SY, Aksu F, Baysal F. The effects of some K+ channel blockers on scopolamine- or electroconvulsive shock-induced amnesia in mice. Eur J Pharmacol 2000; 407:159-64; PMID:11050303; https://doi.org/ 10.1016/S0014-2999(00)00736-6 [DOI] [PubMed] [Google Scholar]
- [6].Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron 2010; 65:682-94; PMID:20223203; https://doi.org/ 10.1016/j.neuron.2010.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stocker M. CA2+-Activated K+ Channels: Molecular Determinants and Function of the SK Family. Nat Rev Neurosci 2004; 5:758-70; PMID:15378036; https://doi.org/ 10.1038/nrn1516 [DOI] [PubMed] [Google Scholar]
- [8].Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 2000; 15:476-93; PMID:10833304; https://doi.org/ 10.1006/mcne.2000.0842 [DOI] [PubMed] [Google Scholar]
- [9].Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 2003; 9:181-94; PMID:15065814; https://doi.org/ 10.1177/1073858403009003011 [DOI] [PubMed] [Google Scholar]
- [10].Adelman JP. SK channels and calmodulin. Channels 2016; 10:1-6; PMID:25942650; https://doi.org/ 10.1080/19336950.2015.1029688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996; 273:1709-14; PMID:8781233; https://doi.org/ 10.1126/science.273.5282.1709 [DOI] [PubMed] [Google Scholar]
- [12].Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature 1986; 323:718-20; PMID:2430185; https://doi.org/ 10.1038/323718a0 [DOI] [PubMed] [Google Scholar]
- [13].Hirschberg B, Maylie J, Adelman JP, Marrion NV. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J Gen Physiol 1998; 111:565-81; PMID:9524139; https://doi.org/ 10.1085/jgp.111.4.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Christophersen P, Wulff H. Pharmacological gating modulation of small- and intermediate-conductance Ca(2+)-activated K(+) channels (KCa2.x and KCa3.1). Channels 2015; 9:336-43; PMID:26217968; https://doi.org/ 10.1080/19336950.2015.1071748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, et al.. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 1998; 395:503-7; PMID:9774106; https://doi.org/ 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- [16].Griffith T, Tsaneva-Atanasova K, Mellor JR. Control of Ca2+ influx and calmodulin activation by SK-channels in dendritic spines. PLoS Comput Biol 2016; 12:e1004949; PMID:27232631; https://doi.org/ 10.1371/journal.pcbi.1004949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deemyad T, Kroeger J, Chacron MJ. Sub- and suprathreshold adaptation currents have opposite effects on frequency tuning. J Physiol-London 2012; 590:4839-58; PMID:22733663; https://doi.org/ 10.1113/jphysiol.2012.234401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ellis LD, Mehaffey WH, Harvey-Girard E, Turner RW, Maler L, Dunn RJ. SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons. J Neurosci 2007; 27:9491-502; PMID:17728462; https://doi.org/ 10.1523/JNEUROSCI.1106-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benda J, Longtin A, Maler L. Spike-frequency adaptation separates transient communication signals from background oscillations. J Neurosci 2005; 25:2312-21; PMID:15745957; https://doi.org/ 10.1523/JNEUROSCI.4795-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].French AS, Hoger U, Sekizawa S, Torkkeli PH. Frequency response functions and information capacities of paired spider mechanoreceptor neurons. Biol Cybernet 2001; 85:293-300; PMID:11592626; https://doi.org/ 10.1007/s004220100260 [DOI] [PubMed] [Google Scholar]
- [21].Glantz RM, Schroeter JP. Analysis and simulation of gain control and precision in crayfish visual interneurons. J Neurophysiol 2004; 92:2747-61; PMID:15240762; https://doi.org/ 10.1152/jn.00448.2004 [DOI] [PubMed] [Google Scholar]
- [22].Benda J, Hennig RM. Spike-frequency adaptation generates intensity invariance in a primary auditory interneuron. J Comput Neurosci 2008; 24:113-36; PMID:17534706; https://doi.org/ 10.1007/s10827-007-0044-8 [DOI] [PubMed] [Google Scholar]
- [23].Huang CG, Chacron MJ. Optimized parallel coding of second-order stimulus features by heterogeneous neural populations. J Neurosci 2016; 36:9859-72; PMID:27656024; https://doi.org/ 10.1523/JNEUROSCI.1433-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Simoncelli EP, Olshausen BA. Natural image statistics and neural representation. Ann Rev Neurosci 2001; 24:1193-216; PMID:11520932; https://doi.org/ 10.1146/annurev.neuro.24.1.1193 [DOI] [PubMed] [Google Scholar]
- [25].Rodriguez FA, Chen C, Read HL, Escabi MA. Neural modulation tuning characteristics scale to efficiently encode natural sound statistics. J Neurosci 2010; 30:15969-80; PMID:21106835; https://doi.org/ 10.1523/JNEUROSCI.0966-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pozzorini C, Naud R, Mensi S, Gerstner W. Temporal whitening by power-law adaptation in neocortical neurons. Nat Neurosci 2013; 16:942-8; PMID:23749146; https://doi.org/ 10.1038/nn.3431 [DOI] [PubMed] [Google Scholar]
- [27].Dan Y, Atick JJ, Reid RC. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. J Neurosci 1996; 16:3351-62; PMID:8627371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laughlin S. A simple coding procedure enhances a neuron's information capacity. Z Naturforsch C 1981; 36:910-2; PMID:7303823 [PubMed] [Google Scholar]
- [29].Theunissen FE, Elie JE. Neural processing of natural sounds. Nat Rev Neurosci 2014; 15:355-66; PMID:24840800; https://doi.org/ 10.1038/nrn3731 [DOI] [PubMed] [Google Scholar]
- [30].Huang CG, Zhang ZD, Chacron MJ. Temporal decorrelation by SK channels enables efficient neural coding and perception of natural stimuli. Nat Commun 2016; 7:11353; PMID:27088670; https://doi.org/ 10.1038/ncomms11353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heil P. Coding of temporal onset envelope in the auditory system. Speech Commun 2003; 41:123-34; https://doi.org/ 10.1016/S0167-6393(02)00099-7 [DOI] [Google Scholar]
- [32].Zeng FG, Nie K, Stickney GS, Kong YY, Vongphoe M, Bhargave A, Wei C, Cao K. Speech recognition with amplitude and frequency modulations. PNAS 2005; 102:2293-8; PMID:15677723; https://doi.org/ 10.1073/pnas.0406460102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Derrington A, Cox M. Temporal resolution of dichoptic and second-order motion mechanisms. Vision Res 1998; 38:3531-9; PMID:9893787; https://doi.org/ 10.1016/S0042-6989(98)00050-9 [DOI] [PubMed] [Google Scholar]
- [34].Baker CL., Jr Central neural mechanisms for detecting second-order motion. Curr Opin Neurobiol 1999; 9:461-6; PMID:10448168; https://doi.org/ 10.1016/S0959-4388(99)80069-5 [DOI] [PubMed] [Google Scholar]
- [35].Lundstrom BN, Fairhall AL, Maravall M. Multiple timescale encoding of slowly varying whisker stimulus envelope in cortical and thalamic neurons in vivo. J Neurosci 2010; 30:5071-7; PMID:20371827; https://doi.org/ 10.1523/JNEUROSCI.2193-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Attias H, Schreiner CE. Low-order temporal statistics of natural sounds. Advances in Neural Information Proc Systems 1997; 9:27-33 [Google Scholar]
- [37].Lewicki MS. Efficient coding of natural sounds. Nat Neurosci 2002; 5:356-63; PMID:11896400; https://doi.org/ 10.1038/nn831 [DOI] [PubMed] [Google Scholar]
- [38].Joris PX. Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive. J Neurophysiol 1996; 76:2137-56 [DOI] [PubMed] [Google Scholar]
- [39].Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev 2004; 84:541-77; PMID:15044682; https://doi.org/ 10.1152/physrev.00029.2003 [DOI] [PubMed] [Google Scholar]
- [40].Mante V, Frazor RA, Bonin V, Geisler WS, Carandini M. Independence of luminance and contrast in natural scenes and in the early visual system. Nat Neurosci 2005; 8:1690-7; PMID:16286933; https://doi.org/ 10.1038/nn1556 [DOI] [PubMed] [Google Scholar]
- [41].de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. PNAS 2009; 106:16446-50; PMID:19805318; https://doi.org/ 10.1073/pnas.0904143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shannon RV, Zeng FG, Wygonski J. Speech recognition with altered spectral distribution of envelope cues. J Acoust Soc Am 1998; 104:2467-76; PMID:10491708; https://doi.org/ 10.1121/1.423774 [DOI] [PubMed] [Google Scholar]
- [43].Dong DW, Atick JJ. Statistics of natural time-varying images. Network 1995; 6:345-58; https://doi.org/ 10.1088/0954-898X_6_3_003 [DOI] [Google Scholar]
- [44].Tolhurst DJ, Tadmor Y, Chao T. Amplitude spectra of natural images. Ophthalmic Physiol Opt 1992; 12:229-32; PMID:1408179; https://doi.org/ 10.1111/j.1475-1313.1992.tb00296.x [DOI] [PubMed] [Google Scholar]
- [45].Torralba A, Oliva A. Statistics of natural image categories. Network 2003; 14:391-412; PMID:12938764; https://doi.org/ 10.1088/0954-898X_14_3_302 [DOI] [PubMed] [Google Scholar]
- [46].Fotowat H, Harrison RR, Krahe R. Statistics of the electrosensory input in the freely swimming weakly electric fish apteronotus leptorhynchus. J Neurosci 2013; 33:13758-72; PMID:23966697; https://doi.org/ 10.1523/JNEUROSCI.0998-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Metzen MG, Chacron MJ. Weakly electric fish display behavioral responses to envelopes naturally occurring during movement: implications for neural processing. J Exp Biol 2014; 217:1381-91; PMID:24363423; https://doi.org/ 10.1242/jeb.098574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Carriot J, Jamali M, Chacron MJ, Cullen KE. Statistics of the vestibular input experienced during natural self-motion: implications for neural processing. J Neurosci 2014; 34:8347-57; PMID:24920638; https://doi.org/ 10.1523/JNEUROSCI.0692-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schneider AD, Jamali M, Carriot J, Chacron MJ, Cullen KE. The increased sensitivity of irregular peripheral canal and otolith vestibular afferents optimizes their encoding of natural stimuli. J Neurosci 2015; 35:5522-36; PMID:25855169; https://doi.org/ 10.1523/JNEUROSCI.3841-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Avila-Akerberg O, Chacron MJ. Nonrenewal spike train statistics: causes and functional consequences on neural coding. Exp Brain Res 2011; 210:353-71; PMID:21267548; https://doi.org/ 10.1007/s00221-011-2553-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Field DJ. Relations between the statistics of natural images and the response properties of cortical-cells. J Opt Soc Am A 1987; 4:2379-94; PMID:3430225; https://doi.org/ 10.1364/JOSAA.4.002379 [DOI] [PubMed] [Google Scholar]
- [52].Rieke F, Warland D, de Ruyter van Steveninck RR, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA: MIT, 1996 [Google Scholar]
- [53].Atick JJ, Redlich A. Towards a Theory of Early Visual Processing. Neural Computation 1990; 2:308-20; https://doi.org/ 10.1162/neco.1990.2.3.308 [DOI] [Google Scholar]
- [54].Atick JJ. Could information theory provide an ecological theory of sensory processing? Network 2011; 22:4-44; PMID:22149669 [DOI] [PubMed] [Google Scholar]
- [55].Lundstrom BN, Higgs MH, Spain WJ, Fairhall AL. Fractional differentiation by neocortical pyramidal neurons. Nat Neurosci 2008; 11:1335-42; PMID:18931665; https://doi.org/ 10.1038/nn.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol 2007; 17:423-9; PMID:17714934; https://doi.org/ 10.1016/j.conb.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sharpee TO, Calhoun AJ, Chalasani SH. Information theory of adaptation in neurons, behavior, and mood. Curr Opin Neurobiol 2014; 25:47-53; PMID:24709600; https://doi.org/ 10.1016/j.conb.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sharpee TO, Sugihara H, Kurgansky AV, Rebrik SP, Stryker MP, Miller KD. Adaptive filtering enhances information transmission in visual cortex. Nature 2006; 439:936-42; PMID:16495990; https://doi.org/ 10.1038/nature04519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fairhall AL, Lewen GD, Bialek W, de Ruyter van Steveninck RR. Efficiency and ambiguity in an adaptive neural code. Nature 2001; 412:787-92; PMID:11518957; https://doi.org/ 10.1038/35090500 [DOI] [PubMed] [Google Scholar]
- [60].Krahe R, Maler L. Neural maps in the electrosensory system of weakly electric fish. Curr Opin Neurobiol 2014; 24:13-21; PMID:24492073; https://doi.org/ 10.1016/j.conb.2013.08.013 [DOI] [PubMed] [Google Scholar]
- [61].Clarke SE, Longtin A, Maler L. Contrast coding in the electrosensory system: parallels with visual computation. Nat Rev Neurosci 2015; 16:733-44; PMID:26558527; https://doi.org/ 10.1038/nrn4037 [DOI] [PubMed] [Google Scholar]
- [62].Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Curr Opin Neurobiol 2011; 21:752-60; PMID:21683574; https://doi.org/ 10.1016/j.conb.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Maler L. Receptive field organization across multiple electrosensory maps. II. Computational analysis of the effects of receptive field size on prey localization. J Comp Neurol 2009; 516:394-422 [DOI] [PubMed] [Google Scholar]
- [64].Maler L. Receptive field organization across multiple electrosensory maps. I. Columnar organization and estimation of receptive field size. J Comp Neurol 2009; 516:376-93; PMID:19655387; https://doi.org/ 10.1002/cne.22124 [DOI] [PubMed] [Google Scholar]
- [65].Marsat G, Longtin A, Maler L. Cellular and circuit properties supporting different sensory coding strategies in electric fish and other systems. Curr Opin Neurobiol 2012; 22:686-92; PMID:22326255; https://doi.org/ 10.1016/j.conb.2012.01.009 [DOI] [PubMed] [Google Scholar]
- [66].Bell C, Maler L. Central neuroanatomy of electrosensory systems in fish In: Bullock TH, Hopkins CD, Popper AN, Fay RR, eds. Electroreception. New York: Springer, 2005:68-111 [Google Scholar]
- [67].Metzen MG, Krahe R, Chacron MJ. Burst Firing in the Electrosensory System of Gymnotiform Weakly Electric Fish: Mechanisms and Functional Roles. Front Comput Neurosci 2016; 10:81; PMID:27531978; https://doi.org/ 10.3389/fncom.2016.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bastian J, Nguyenkim J. Dendritic modulation of burst-like firing in sensory neurons. J Neurophysiol 2001; 85:10-22; PMID:11152701 [DOI] [PubMed] [Google Scholar]
- [69].Bastian J, Chacron MJ, Maler L. Plastic and non-plastic cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron 2004; 41:767-79; PMID:15003176; https://doi.org/ 10.1016/S0896-6273(04)00071-6 [DOI] [PubMed] [Google Scholar]
- [70].Berman NJ, Hincke MT, Maler L. Inositol 1,4,5-trisphosphate receptor localization in the brain of a weakly electric fish (Apteronotus leptorhynchus) with emphasis on the electrosensory system. J Comp Neurol 1995; 361:512-24; PMID:8550896; https://doi.org/ 10.1002/cne.903610313 [DOI] [PubMed] [Google Scholar]
- [71].Zupanc GKH, Airey JA, Maler L, Sutko JL, Ellisman MH. Immunohistochemical localization of ryanodine binding protein in the central nervous system of gymnotiform fish. J Comp Neurol 1992; 325:135-51; PMID:1460110; https://doi.org/ 10.1002/cne.903250202 [DOI] [PubMed] [Google Scholar]
- [72].Harvey-Girard E, Dunn RJ. Excitatory amino acid receptors of the electrosensory system: the NR1/NR2B N-methyl-D-aspartate receptor. J Neurophysiol 2003; 89:822-32; PMID:12574460; https://doi.org/ 10.1152/jn.00629.2002 [DOI] [PubMed] [Google Scholar]
- [73].Stamper SA, Fortune ES, Chacron MJ. Perception and coding of envelopes in weakly electric fishes. J Exp Biol 2013; 216:2393-402; PMID:23761464; https://doi.org/ 10.1242/jeb.082321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bastian J, Schniederjan S, Nguyenkim J. Arginine vasotocin modulates a sexually dimorphic communication behavior in the weakly electric fish Apteronotus leptorhynchus. J Exp Biol 2001; 204:1909-23; PMID:11441033 [DOI] [PubMed] [Google Scholar]
- [75].Stamper SA, Madhav MS, Cowan NJ, Fortune ES. Beyond the Jamming Avoidance Response: weakly electric fish respond to the envelope of social electrosensory signals. J Exp Biol 2012; 215:4196-207; PMID:23136154; https://doi.org/ 10.1242/jeb.076513 [DOI] [PubMed] [Google Scholar]
- [76].Metzen MG, Chacron MJ. Neural heterogeneities determine response characteristics to second-, but not first-order stimulus features. J Neurosci 2015; 35:3124-38; PMID:25698748; https://doi.org/ 10.1523/JNEUROSCI.3946-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Martinez D, Metzen MG, Chacron MJ. Electrosensory processing in Apteronotus albifrons: implications for general and specific neural coding strategies across wave-type weakly electric fish species. J Neurophysiol 2016; 116:2909-21; PMID:27683890; https://doi.org/ 10.1152/jn.00594.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Metzen MG, Jamali M, Carriot J, Avila-Akerberg O, Cullen KE, Chacron MJ. Coding of envelopes by correlated but not single-neuron activity requires neural variability. PNAS 2015; 112:4791-6; PMID:25825717; https://doi.org/ 10.1073/pnas.1418224112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Marsat G, Maler L. Neural heterogeneity and efficient population codes for communication signals. J Neurophysiol 2010; 104:2543-55; PMID:20631220; https://doi.org/ 10.1152/jn.00256.2010 [DOI] [PubMed] [Google Scholar]
- [80].Marsat G, Proville RD, Maler L. Transient signals trigger synchronous bursts in an identified population of neurons. J Neurophysiol 2009; 102:714-23; PMID:19474165; https://doi.org/ 10.1152/jn.91366.2008 [DOI] [PubMed] [Google Scholar]
- [81].Benda J, Longtin A, Maler L. A synchronization-desynchronization code for natural communication signals. Neuron 2006; 52:347-58; PMID:17046696; https://doi.org/ 10.1016/j.neuron.2006.08.008 [DOI] [PubMed] [Google Scholar]
- [82].Vonderschen K, Chacron MJ. Sparse and dense coding of natural stimuli by distinct midbrain neuron subpopulations in weakly electric fish. J Neurophysiol 2011; 106:3102-18; PMID:21940609; https://doi.org/ 10.1152/jn.00588.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Metzen MG, Hofmann V, Chacron MJ. Neural correlations enable invariant coding and perception of natural stimuli in weakly electric fish. Elife 2016; 5:e12993; PMID:27128376; https://doi.org/ 10.7554/eLife.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Walz H, Grewe J, Benda J. Static frequency tuning accounts for changes in neural synchrony evoked by transient communication signals. J Neurophysiol 2014; 112:752-65; PMID:24848476; https://doi.org/ 10.1152/jn.00576.2013 [DOI] [PubMed] [Google Scholar]
- [85].Walz H, Hupe GJ, Benda J, Lewis JE. The neuroethology of electrocommunication: how signal background influences sensory encoding and behaviour in Apteronotus leptorhynchus. J Physiology Paris 2013; 107:13-25; PMID:22981958 [DOI] [PubMed] [Google Scholar]
- [86].Yu N, Hupe G, Garfinkle C, Lewis JE, Longtin A. Coding conspecific identity and motion in the electric sense. PLoS Comput Biol 2012; 8:e1002564; PMID:22807662; https://doi.org/ 10.1371/journal.pcbi.1002564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ellis LD, Maler L, Dunn RJ. Differential distribution of SK channel subtypes in the brain of the weakly electric fish Apteronotus leptorhynchus. The J Comparat Neurol 2008; 507:1964-78; PMID:18273887; https://doi.org/ 10.1002/cne.21597 [DOI] [PubMed] [Google Scholar]
- [88].Deemyad T, Maler L, Chacron MJ. Inhibition of SK and M channel-mediated currents by 5-HT enables parallel processing by bursts and isolated spikes. J Neurophysiol 2011; 105:1276-94; PMID:21209357; https://doi.org/ 10.1152/jn.00792.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Deemyad T, Metzen MG, Pan Y, Chacron MJ. Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics. Proc Natl Acad Sci U S A 2013; 110:19609-14; PMID:24218585; https://doi.org/ 10.1073/pnas.1314008110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Marquez BT, Krahe R, Chacron MJ. Neuromodulation of early electrosensory processing in gymnotiform weakly electric fish. J Exp Biol 2013; 216:2442-50; PMID:23761469; https://doi.org/ 10.1242/jeb.082370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Krahe R, Bastian J, Chacron MJ. Temporal processing across multiple topographic maps in the electrosensory system. J Neurophysiol 2008; 100:852-67; PMID:18509073; https://doi.org/ 10.1152/jn.90300.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chacron MJ, Longtin A, Maler L. Delayed excitatory and inhibitory feedback shape neural information transmission. Physical Review E 2005; 72:051917; https://doi.org/ 10.1103/PhysRevE.72.051917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chacron MJ. Nonlinear information processing in a model sensory system. J Neurophysiol 2006; 95:2933-46; PMID:16495358; https://doi.org/ 10.1152/jn.01296.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Avila-Akerberg O, Krahe R, Chacron MJ. Neural heterogeneities and stimulus properties affect burst coding in vivo. Neuroscience 2010; 168:300-13; PMID:20298764; https://doi.org/ 10.1016/j.neuroscience.2010.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mehaffey WH, Maler L, Turner RW. Intrinsic frequency tuning in ELL pyramidal cells varies across electrosensory maps. J Neurophysiol 2008; 99:2641-55; PMID:18367702; https://doi.org/ 10.1152/jn.00028.2008 [DOI] [PubMed] [Google Scholar]
- [96].Chacron MJ, Maler L, Bastian J. Feedback and Feedforward Control of Frequency Tuning to Naturalistic Stimuli. J Neurosci 2005; 25:5521-32; PMID:15944380; https://doi.org/ 10.1523/JNEUROSCI.0445-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chacron MJ, Maler L, Bastian J. Electroreceptor Neuron Dynamics Shape Information Transmission. Nat Neurosci 2005; 8:673-8; PMID:15806098; https://doi.org/ 10.1038/nn1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bastian J, Electrolocation I. How the electroreceptors of Apteronotus albifrons code for moving objects and other electrical stimuli. J Comparat Physiol A 1981; 144:465-79; https://doi.org/ 10.1007/BF01326832 [DOI] [Google Scholar]
- [99].Heiligenberg W, Metzner W, Wong CJH, Keller CH. Motor control of the jamming avoidance response of Apteronotus leptorhynchus: evolutionary changes of a behavior and its neuronal substrates. J Comp Physiol A 1996; 179:653-74; https://doi.org/ 10.1007/BF00216130 [DOI] [PubMed] [Google Scholar]
- [100].Engler G, Zupanc GK. Differential production of chirping behavior evoked by electrical stimulation of the weakly electric fish, Apteronotus leptorhynchus. J Comp Physiol A 2001; 187:747-56; PMID:11778836; https://doi.org/ 10.1007/s00359-001-0248-8 [DOI] [PubMed] [Google Scholar]
- [101].Hagedorn M, Heiligenberg W. Court and Spark - Electric Signals in the Courtship and Mating of Gymnotoid Fish. Anim Behav 1985; 33:254-65; https://doi.org/ 10.1016/S0003-3472(85)80139-1 [DOI] [Google Scholar]
- [102].Metzner W, Juranek J. A sensory brain map for each behavior? PNAS 1997; 94:14798-803; PMID:9405693; https://doi.org/ 10.1073/pnas.94.26.14798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhang ZD, Chacron MJ. Adaptation to second order stimulus features by electrosensory neurons causes ambiguity. Scient Rep 2016; 6:28716; PMID:27349635; https://doi.org/ 10.1038/srep28716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Brenner N, Bialek W, de Ruyter van Steveninck R. Adaptive rescaling maximizes information transmission. Neuron 2000; 26:695-702; PMID:10896164; https://doi.org/ 10.1016/S0896-6273(00)81205-2 [DOI] [PubMed] [Google Scholar]
- [105].Drew PJ, Abbott LF. Models and properties of power-law adaptation in neural systems. J Neurophysiol 2006; 96:826-33; PMID:16641386; https://doi.org/ 10.1152/jn.00134.2006 [DOI] [PubMed] [Google Scholar]
- [106].Clarke SE, Naud R, Longtin A, Maler L. Speed-invariant encoding of looming object distance requires power law spike rate adaptation. PNAS 2013; 110:13624-9; PMID:23898185; https://doi.org/ 10.1073/pnas.1306428110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Benda J, Herz AV. A universal model for spike-frequency adaptation. Neural Computation 2003; 15:2523-64; PMID:14577853; https://doi.org/ 10.1162/089976603322385063 [DOI] [PubMed] [Google Scholar]
- [108].McGillivray P, Vonderschen K, Fortune ES, Chacron MJ. Parallel coding of first- and second-order stimulus attributes by midbrain electrosensory neurons. J Neurosci 2012; 32:5510-24; PMID:22514313; https://doi.org/ 10.1523/JNEUROSCI.0478-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Teka W, Marinov TM, Santamaria F. Neuronal spike timing adaptation described with a fractional leaky integrate-and-fire model. PLoS Comput Biol 2014; 10:e1003526; PMID:24675903; https://doi.org/ 10.1371/journal.pcbi.1003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Teka W, Stockton D, Santamaria F. Power-Law Dynamics of Membrane Conductances Increase Spiking Diversity in a Hodgkin-Huxley Model. PLoS Comput Biol 2016; 12:e1004776; PMID:26937967; https://doi.org/ 10.1371/journal.pcbi.1004776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lapicque L. Recherches quantitatives sur l'excitation électrique des nerfs traitée comme une polarisation. J Physiol Pathol Genet 1907; 9:620-35 [Google Scholar]
- [112].Burkitt AN. A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biol Cybernet 2006; 95:1-19 [DOI] [PubMed] [Google Scholar]
- [113].Burkitt AN. A review of the integrate-and-fire neuron model: II. Inhomogeneous synaptic input and network properties. Biol Cybernet 2006; 95:97-112 [DOI] [PubMed] [Google Scholar]
- [114].Benton DC, Monaghan AS, Hosseini R, Bahia PK, Haylett DG, Moss GW. Small conductance Ca2+-activated K+ channels formed by the expression of rat SK1 and SK2 genes in HEK 293 cells. J Physiol 2003; 553:13-9; PMID:14555714; https://doi.org/ 10.1113/jphysiol.2003.054551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].D'Hoedt D, Hirzel K, Pedarzani P, Stocker M. Domain analysis of the calcium-activated potassium channel SK1 from rat brain. Functional expression and toxin sensitivity. J Biol Chem 2004; 279:12088-92 [DOI] [PubMed] [Google Scholar]
- [116].Sah P. Role of calcium influx and buffering in the kinetics of Ca(2+)-activated K+ current in rat vagal motoneurons. J Neurophysiol 1992; 68:2237-47; PMID:1491269 [DOI] [PubMed] [Google Scholar]
- [117].Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 2008; 59:873-81; PMID:18817728; https://doi.org/ 10.1016/j.neuron.2008.09.001 [DOI] [PubMed] [Google Scholar]
- [118].Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 2005; 8:635-41; PMID:15852010; https://doi.org/ 10.1038/nn1450 [DOI] [PubMed] [Google Scholar]
- [119].Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 2005; 8:642-9; PMID:15852011; https://doi.org/ 10.1038/nn1449 [DOI] [PubMed] [Google Scholar]
- [120].Arima J, Matsumoto N, Kishimoto K, Akaike N. Spontaneous miniature outward currents in mechanically dissociated rat Meynert neurons. J Physiol 2001; 534:99-107; PMID:11432995; https://doi.org/ 10.1111/j.1469-7793.2001.00099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 2005; 25:10308-20; PMID:16267239; https://doi.org/ 10.1523/JNEUROSCI.2697-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci 2008; 28:3209-20; PMID:18354024; https://doi.org/ 10.1523/JNEUROSCI.4310-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kuznetsova MS, Higgs MH, Spain WJ. Adaptation of firing rate and spike-timing precision in the avian cochlear nucleus. J Neurosci 2008; 28:11906-15; PMID:19005056; https://doi.org/ 10.1523/JNEUROSCI.3827-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol 1988; 59:424-49; PMID:3351569 [DOI] [PubMed] [Google Scholar]
- [125].Schwindt PC, Spain WJ, Crill WE. Calcium-dependent potassium currents in neurons from cat sensorimotor cortex. J Neurophysiol 1992; 67:216-26; PMID:1313080 [DOI] [PubMed] [Google Scholar]


