Abstract
d-amino acids are present in some peptides from amphibian skin. These residues are derived from the corresponding l-amino acids present in the respective precursors. From skin secretions of Bombinae, we have isolated an enzyme that catalyzes the isomerization of an l-Ile in position 2 of a model peptide to d-allo-Ile. In the course of this reaction, which proceeds without the addition of a cofactor, radioactivity from tritiated water is incorporated into the second position of the product. The amino acid sequence of this isomerase could be deduced from cloned cDNA and genomic DNA. After expression of this cDNA in oocytes of Xenopus laevis, isomerase activity could be detected. Polypeptides related to the frog skin enzyme are present in several vertebrate species, including humans.
Keywords: amphibia, bombinin H, isomerase, chirality
From amphibian skin, a wide variety of biologically active peptides have been isolated (1). One of these compounds, the heptapeptide dermorphin (present in the skin of the South American tree frog Phyllomedusa sauvagei), is a potent opiate that binds with high affinity and selectivity to μ-opiate receptors (2). Quite unexpectedly, dermorphin was found to contain d-Ala at position 2, and this residue is essential for binding to the receptor. Subsequently, several additional opiate peptides containing a d-amino acid were found in the skin of other Phyllomedusinae (3). The bombinins H, antibacterial and hemolytic peptides isolated from skin secretions of the frog Bombina variegata, contain either l-Ile or d-allo-Ile (d-aIle) as the second amino acid (4). Peptides with d-amino acids in their sequences were also found in different invertebrate species, such as snails (5–7), the venom of a spider (8), and crustaceans (9). The most recent addition to this group was a homologue of C-type natriuretic peptides isolated from the venom of the male platypus (10).
In principle, the biosynthesis of these d-amino acids in animal peptides has been clarified. As shown for dermorphin (11) and subsequently for several cases of vertebrate and invertebrate peptides, the secreted products are derived from larger precursors encoded by mRNAs. At the positions where a d-amino acid is present in the end product, a codon for the corresponding l-isomer has been found (reviewed in refs. 12 and 13). This finding implies that, at some stage in the processing of the precursors, a conversion of certain l-amino acids to the d-form takes place. Indeed, in many cases, the l and d forms of the mature peptides coexist in the respective secretory glands and secretions, which can be interpreted as incomplete isomerization. An enzyme that catalyzes such an unusual reaction has been characterized from the venom of a funnel web spider (8). In this case, the chirality of a Ser close to the C terminus of a peptide termed ω-agatoxin IV is inverted. This “isomerase” is related to Ser proteases and functions without any added cofactors (14, 15).
As mentioned above, skin secretions of Bombina species contain a group of hemolytic peptides that contain either l-Ile or d-aIle as the second amino acid (4). We hypothesized that an enzyme catalyzing the posttranslational l-d-isomerization should also be present in these secretions. In this article, we describe the isolation and partial characterization of this enzyme.
Materials and Methods
Enzyme Purification. Skin secretions from Bombinae (those from the closely related species B. variegata and Bombina bombina were mixed, whereas those from Bombina orientalis were studied separately) were collected and processed as described (16, 17). The glycoprotein fraction eluted from ConA-Sepharose with methylmannoside was passed over a Sephacryl S-300 column (20 mM Hepes buffer, pH 7.6, containing 1 mM EDTA). Individual fractions were tested for isomerase activity by using an enzyme immunoassay (see below). Active fractions were concentrated, rechromatographed under the same conditions, and subsequently passed over SP-Sepharose. The flow-through was adjusted to pH 8.6 and fractionated over a Q-Sepharose column. The enzyme could be eluted with a gradient of 1–2.5 mM NaCl. Active fractions were concentrated with Centricon filters retaining only proteins with a mass of 30–100 kDa. These fractions were again chromatographed over SP- and Q-Sepharose, which yielded considerable narrower peaks of activity. Individual fractions were then once more carried through the same protocol. Last, an apparently homogeneous protein with an apparent molecular mass of 52 kDa (as judged by SDS/PAGE) was obtained.
Assays for Enzymatic Activity. The peptide Ile-d-aIle-Gly-Pro-Val-Leu-Gly-Cys-amide, which contains the N-terminal heptapeptide sequence of bombinin H, was coupled via the SH group to keyhole limpet hemocyanin. An antibody against this construct was generated in rabbits. For the competitive immunoassay, this peptide was coupled to acetylcholinesterase, and the amount of this enzyme bound to the antibody was measured by using acetylthiocholine as substrate and the Ellmann reagent to measure the amount of free thiocholine (see refs. 18 and 19 for details).
For partly purified enzyme preparations, an assay with a fluorescent peptide was used. The peptide Ile-Ile-Gly-Pro-Val-Leu-Gly-Cys-amide was coupled via the SH-group to the fluorophor Bodipy FL IA [(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-S-indacene-3-propionyl)-N′-iodoacetylethylenediamine; Molecular Probes]. Similar derivatives were prepared for dermorphin, Ala- and Met-deltorphins, and several other peptides, each containing a Cys inserted after the last amino acid. Fluorescent peptides were separated by reverse-phase HPLC, and their mass was determined by MALDI MS. Enzyme samples were incubated with 2 nmol of substrate in 100 mM phosphate buffer/5 mM EDTA, and the pH was adjusted to 6.5. Aliquots of the reaction mixture were injected onto a 218TP C-18 column (Vydac, Hesperia, CA) with a gradient of acetonitrile (solvent A, 0.1% TFA; solvent B, 80% acetonitrile in solvent A). Fluorescence emission was recorded at 514 nm (excitation at 480 nm).
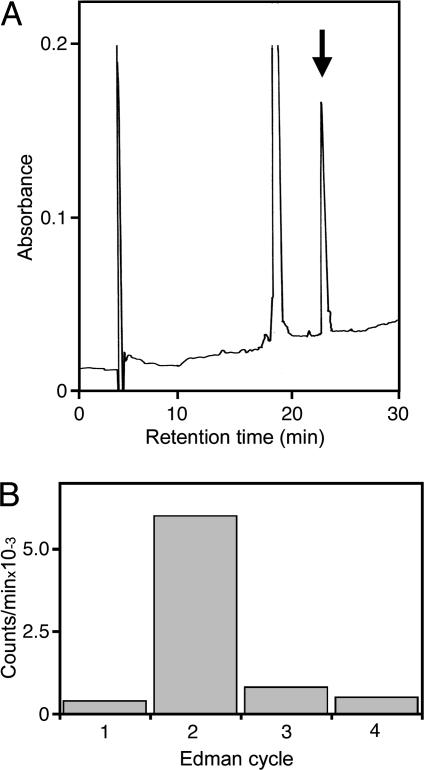
In some experiments that required larger amounts of enzyme, the peptide Ile-Ile-Gly-Pro-Val-Leu-Gly-Ser-amide was used as substrate. The product could be separated from this substrate by reverse-phase HPLC (Fig. 1A). Chiral Edman degradation to identify the d-aIle in the product was performed by following the procedure given in ref. 20. The mixtures of substrate and product were incubated with Leu aminopeptidase (sequencing grade; Sigma) by following the recommended conditions.
Fig. 1.
Characterization of the reaction product. (A) Separation of substrate and product by reverse-phase HPLC on an Aquapore RP300 column (see ref. 17 for details). Substrate (15 μg in 50 μl of buffer) and ≈1 μg of crude enzyme were incubated for 8 h at 37°C. The arrow indicates the position in the gradient where synthetic Ile-d-aIle-Gly-Pro-Val-Leu-Gly-Ser.amide is eluted from the column (at ≈25.9% acetonitrile). Product yield was ≈8%. (B) Substrate (15 μg) and enzyme were incubated in 50 μl of tritiated water (specific activity, 5 Ci/ml (1 Ci = 37 GBq); American Radiolabeled Chemicals, St. Louis) for 8 h at 37°C: Subsequently, the sample was dried, redissolved several times in water, and dried again. Manual Edman degradation was performed by using standard conditions. After each step, radioactivity was extracted into n-butyl acetate and measured in a scintillation counter. Radioactivity (counts per min × 10–3) is plotted against the step of Edman degradation. In the control incubated in the absence of enzyme, <300 counts per min could be extracted after each Edman cycle.
Cloning Experiments and Sequence Analysis. Total RNA from skin of B. variegata was prepared as described (16). cDNA was synthesized with either random or sequence-specific primers with SuperScript II (GIBCO/BRL) according to the manufacturer's instructions. PCR (Pwo polymerase, Boehringer) was performed with the following degenerate primers deduced from sequences of the cyanogen bromide-generated fragments: AAYCAYGAYCCNAARGARACNCC (forward) and ATYTCNGTRTCYTTNGCNGG (reverse). The amplified fragment was used to screen a genomic library from B. orientalis (21) constructed in Lambda FIX II (Stratagene)
Enzyme Expression in Xenopus Oocytes. cDNA coding for amino acids 4–413 of the mature Bombina isomerase was fused at the 5′ end to a cDNA coding for the signal peptide of human plasminogen activator. The construct was then cloned into the translation plasmid T7TS (22), which contains the 5′ and 3′ untranslated regions of the β-globin mRNA of Xenopus laevis as well as a multiple cloning site with stop codons in all reading frames in between. From the linearized vector, RNA was transcribed in vitro (mMessage Machine T7, Ambion, Austin, TX) and injected into Xenopus oocytes. Injected and control oocytes were incubated for 3 days at 20–25°C by using the conditions as described in ref. 23. Supernatants were collected, adjusted to 5 mM EDTA, and concentrated on a 3kD Centricon filter to a volume of ≈1 μl per two oocytes. Isomerase activity was determined with the enzyme immunoassay (30 pmol of peptide; incubation time, 8 h).
Results
Isolation and Partial Characterization of the Isomerase. From skin secretions of B. variegata and B. bombina, we purified a glycoprotein with a molecular mass of ≈52 kDa moving as a single band on an SDS/PAGE (data not shown). In three different assays, it could be shown that this protein catalyzes the conversion of l-Ile to d-aIle at position 2 of a model peptide with the N-terminal sequence of bombinin H (4). Such an experiment with the substrate Ile-Ile-Gly-Pro-Val-Leu-Gly-Ser-amide is shown in Fig. 1 A. The product could be separated from the substrate by reverse-phase HPLC and it coeluted with the synthetic peptide containing the d-amino acid. The product was found to be resistant to the action of Leu aminopeptidase, as was demonstrated earlier for other peptides with a d-amino acid at position 2 (C.M. and G.K., unpublished data). Moreover, by using chiral Edman degradation (20), the presence of d-aIle could be corroborated (data not shown). By analogy with peptidylprolyl-cis/transisomerases, this enzyme could be termed peptidylaminoacyl-l/d-isomerase, hereafter referred to as the isomerase, for brevity.
Next, we incubated this substrate and the enzyme in tritiated water. This incubation resulted in the incorporation of radioactivity into the product, selectively into position 2 as revealed by manual Edman degradation (Fig. 1B). Similar results were obtained by using the synthetic peptide containing d-aIle at position 2 (data not shown). This finding implies that the change of chirality proceeds in both directions via a deprotonation/protonation reaction at the α-carbon of the second amino acid. Such a mechanism has been demonstrated for the isomerase from spider venom (14).
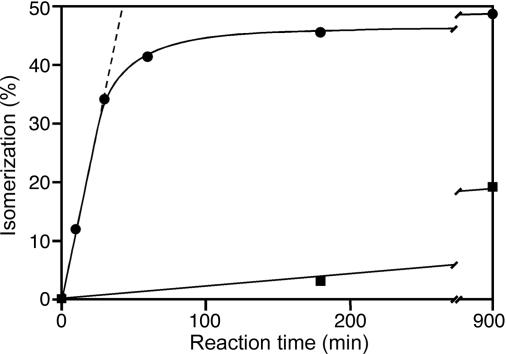
By using the peptide Ile-Ile-Gly-Pro-Val-Leu-Gly-Cys-amide (coupled to a fluorophor via the SH-group) as substrate (see Materials and Methods), a time-course experiment showed that at equilibrium, the concentration of the product, a diastereomer of the substrate, was slightly less than 50% (Fig. 2). The reaction catalyzed by the frog skin isomerase was sensitive to diethyl pyrocarbonate (Fig. 2), but it was not inhibited by iodoacetamide (data not shown). This result indicates that a His residue is part of the active site, whereas free Cys residues can be excluded. Moreover, treatment with 2.5 mM hydroxylamine had no effect (data not shown), which argues against the presence of pyridoxal phosphate, a cofactor of many amino acid racemases. The enzyme was also not inhibited by phenylmethylsulfonylfluoride, which blocks the isomerase from spider venom (13).
Fig. 2.
Time course of the conversion of the peptide Ile-Ile-Gly-Pro-Val-Leu-Gly-Cys-amide, labeled with a fluorophor (see Materials and Methods), to the product containing d-aIle as the second residue. Reaction conditions were 100 mM phosphate buffer (pH 6.5) and 5 mM EDTA (•). In a parallel sample, the enzyme was preincubated in the presence of 1 mM diethyl pyrocarbonate for 16 h on ice (▪). Aliquots removed at different times were analyzed by reverse-phase HPLC.
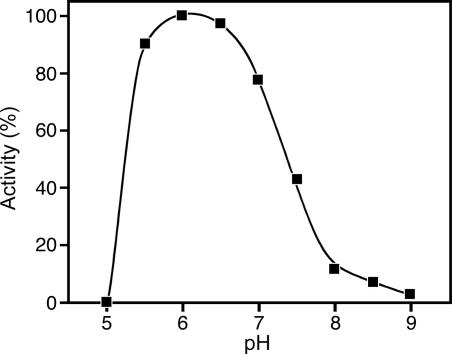
In this assay for isomerase activity, the pH optimum was found to be pH ≈6.5, with a steep decline of activity at pH <5.5. At pH 7.3–7.5 used for the enzyme immunoassays, the activity was ≈40% of the maximum (Fig. 3).
Fig. 3.
Isomerase activity at different pH values. The same substrate as described in the legend to Fig. 2 was used. Relative activity is plotted against the pH.
As a first step toward the elucidation of the substrate specificity of this enzyme, we tested fluorescent derivatives of the l-forms of dermorphins and deltorphins (2, 3). All of these peptides have the N-terminal sequence Tyr-Ala(or Met)-Phe. We found no evidence for the formation of products with a d-amino acid at position 2. However, these peptides were quite rapidly degraded, apparently by traces of proteases present in the enzyme preparations, so that a slow isomerization reaction would not have been detected. We tested two additional peptides: Leu-Leu-His-Asp-His-Pro-Cys-amide (corresponding to the N-terminal sequence of the platypus homologue of C-type natriuretic peptide; ref. 10) as well as a related peptide, Leu-Leu-Asn-Glu-His-Pro-Cys-amide (a fragment of a frog homologue belonging to the same family; ref. 24). Interestingly, the isomerase catalyzed the formation of a product with d-Leu in position 2 with the second, but not the first, of these peptides. This result indicates that a bulky side chain and/or a positive charge of the third amino acid of a peptide are not accepted by the isomerase. We also tested whether the enzyme could act on an unprocessed N terminus. For this experiment, we used the peptide acetyl-Glu-Lys-Glu-Lys-Arg-Ile-Ile-Gly-Pro-Val-Leu-Cys-amide, in which the N-terminal sequence of bombinin H is preceded by the last five residues of the pro-part of the bombinin/bombinin H precursor (4). As expected, even after prolonged incubation, no trace of the product with a d-aIle could be detected by reverse-phase HPLC.
Amino Acid Sequence of the Isomerase. From skin secretions of B. variegata collected from ≈500 “milkings” of frogs, enough purified isomerase could be obtained to determine the N-terminal sequence of the protein and of some of its cyanogen bromide fragments by automated Edman degradation (see Fig. 4). Oligonucleotides deduced from these sequences were used for PCR experiments with total mRNA isolated from Bombina skin (16). This amplification yielded a cDNA fragment representing about the first half of the isomerase. With Northern blot analyses, it could be demonstrated that the isomerase was encoded by a large mRNA containing >8,000 nucleotides (data not shown). All attempts to amplify the rest of the isomerase with 5′- and 3′-RACE failed. In a different approach, we screened a genomic library prepared from the related species B. orientalis (21). We could isolate two clones that contained the sequence of an exon with an ORF for 409 aa, starting with residue 4 of the mature isomerase and part of an intron. The first 3 aa of the enzyme and a preceding pro-sequence or spacer sequence are encoded by a separate small exon. These two genomic fragments were assembled to yield a B. orientalis cDNA, which can be translated into a polypeptide comprising 412 aa (see Fig. 4). This cDNA codes for a protein with a calculated mass of 45.0 kDa. A single N-glycosylation site is present close to the amino end. The cDNA and genomic fragments containing the genetic information for this protein do not code for a signal sequence and its ORF does not terminate with a stop codon.
Fig. 4.
Amino acid sequence deduced from cloned genomic DNA from B. orientalis (21). Amino acid sequences obtained from the isomerase purified from skin secretions of B. variegata are underlined. The single N-glycosylation site is indicated (+).
Starting with the sequence of the B. orientalis isomerase, we screened a cDNA library from skin of B. variegata by using PCR and suitable primers. Five different cDNAs, termed va to ve, could be amplified and were sequenced fully or in part. All of these cDNAs code for proteins that are >90% identical to the B. orientalis isomerase (see Fig. 7, which is published in the supporting information on the PNAS web site). These homologous proteins are linked by spacer segments in the following order: —va—(33)—vb— and —vc—(10)—vd—(48)—ve— (numbers in parentheses indicate the length of the spacers). The spacer sequences are relatively rich in Pro and charged amino acids. Individual isomerase domains are apparently excised from this polyprotein by unknown proteases.
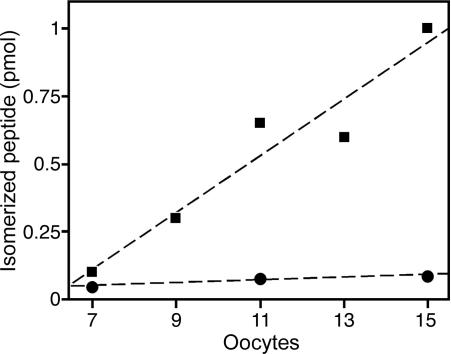
Expression of the Isomerase cDNA in Xenopus Oocytes. For the expression of the cloned isomerase cDNA, we used Xenopus oocytes. Because the C-terminus of the enzyme present in skin secretions is not known, we decided to construct a cDNA encoding amino acids 4–413 of the mature Bombina isomerase, followed by a stop codon. At the 5′ end, this fragment was fused to a cDNA coding for the signal peptide of human plasminogen activator. The construct was then cloned into the translation plasmid T7TS (22), between the 5′ and 3′ untranslated regions of the β-globin mRNA of X. laevis. From this vector, RNA was transcribed in vitro and injected into oocytes. Media from injected and control oocytes were collected, concentrated, and assayed for isomerase activity by using the enzyme immunoassay. In these experiments, formation of the product containing d-aIle could be demonstrated (Fig. 5).
Fig. 5.
Expression of isomerase cRNA in Xenopus oocytes. Product yield in pmol is plotted against the number of injected oocytes from which supernatants were collected (▪). As controls, supernatants from mock-injected oocytes were analyzed in parallel (•).
Homologues of the Frog Isomerase in Other Species. A search in the data banks for polypeptides related to the Bombina isomerase originally yielded only one major hit, namely, the N-terminal H-domain of the human IgG-Fc binding protein (25). This domain comprises ≈450 aa and has no known function. Subsequently, ESTs coding for fragments of related proteins from X. laevis and chicken were deposited in the data banks. Last, in the genome of the fugu fish, three genes potentially coding for homologous proteins are present (FuguGenscan_26525, FuguGenscan_28866, and FuguGenscan_28655). A comparison of the amino acid sequences of the frog isomerase and some of its homologues from other vertebrates are shown in Fig. 6.
Fig. 6.
Comparison of amino acid sequences of the Bombina skin isomerase with other vertebrate sequences deduced from cDNA or genomic DNA. Man, domain H of the human IgG-Fc binding protein (25); Bird, chicken EST BM488067; Fish, FuguGenscan_28655. Asterisks indicate conserved His and Cys residues.
Discussion
From skin secretions of Bombinae, we have isolated an enzyme that catalyzes the conversion of the second amino acid of a model peptide from the l- to the d-configuration. The change in chirality probably proceeds by means of a deprotonation/protonation mechanism at the α-carbon of the second residue. Such a reaction mechanism has been demonstrated for the isomerase from spider venom (14) as well as for some bacterial racemases (26, 27). In these cases, different amino acids (e.g., Cys, Lys, or His) can function as proton donors and acceptors. In the comparison of related genes from other species (Fig. 6), two His residues are present in the most highly conserved segment in all four species. Because the isomerization reaction is inhibited by diethyl pyrocarbonate, it seems to be likely that one or both of these residues is part of the active site. The pH dependence (Fig. 3) also suggests that isomerase activity is blocked if one or more His residues carries a positive charge.
For the tests of isomerase activity, we used a peptide with the N-terminal sequence Ile-Ile-Gly... of bombinin H. The only other substrate for the enzyme was a peptide derived from the sequence of C-type natriuretic peptide from the frog Rana catesbiana (24) starting with the sequence Leu-Leu-Asn.... In this case, the second Leu was converted to d-Leu. With a few other peptides that we tested, l-d-isomerization could not be detected (see Results). Many more experiments will be necessary to determine the substrate specificity of the isomerase.
Enzymes catalyzing the posttranslational maturation and modification of biologically active peptides are usually highly conserved in evolution. For example, the enzyme from Conus snails that catalyzes the vitamin K-dependent formation of the γ-carboxy glutamic acids present in some venom peptides is highly homologous to the corresponding carboxylase from bovine liver (28, 29). Thus, it seems to be surprising that structurally unrelated enzymes catalyze the l-d-isomerization of amino acids in peptide linkage in spider venom (15) and frogs. Evidence indicates that, in both cases, the catalytic mechanism is basically the same (namely, removal of a proton from the α-carbon and concomitant addition from the opposite side in an acid-base type of catalysis). However, one must bear in mind that the amino acids that are subject to this isomerization reaction reside at very different positions in the respective substrates. In case of the spider enzyme, it is the third residue from the carboxyl end, both in the ω-agatoxins IVB and IVC as well as in the C-terminal fragments that were tested as substrates (8, 14). Conversely, results presented here indicate that the frog isomerase acts exclusively on the second amino acid from the N terminus. Evidence also indicates that the active-site residues involved in proton abstraction and addition are different. The enzyme from spider venom has a catalytic triad characteristic for Ser proteases, and it is indeed inhibited by phenylmethylsulfonylfluoride (13), an inhibitor of this type of enzymes. The activity of the frog skin enzyme is blocked by diethyl pyrocarbonate, which reacts with His residues, whereas phenylmethylsulfonylfluoride has no effect. These results indicate that two different classes of enzymes catalyzing such a reaction exist. According to this hypothesis, one type would act on the variety of peptides containing a d-residue at position 2, which have been isolated from vertebrate and invertebrate species (5, 6, 10, 12). The other class would target residue 3 from the carboxyl end, such as in the ω-agatoxins IV. Another potential substrate for an enzyme of this group would be a recently characterized peptide from the venom of a Conus snail (30), which contains a d-Phe at position 3 from the C terminus. Still other isomerases may catalyze the formation of a d-amino acid at other positions in the respective peptide chains (7, 9). Both the spider and the frog isomerase act on small peptides corresponding to the C- or N-terminal sequences, respectively, of the mature products. These findings imply that in vivo as well, formation of the d-amino acid is a late reaction in the processing of the precursors. Indeed, a peptide containing the last five amino acids of the pro-region linked to the N-terminal hexapeptide of bombinin H is not a substrate. However, in case of the more complex precursors of the amphibian opioid peptides, evidence for a different mechanism has been presented (31, 32). From skin of P. sauvagei, a precursor protein has been isolated that, upon digestion with trypsin and carboxypeptidase, yielded the processing intermediates of dermorphin and deltorphin (also termed dermenkephalin) predicted from the cDNA sequence (11). By using specific antibodies, it could be shown that d-Ala was already present in these fragments (31). This result suggests that in the skin of these frogs, l-d-isomerization already takes place at an early stage of processing, before the action of prohormone convertases.
Based on the sequence comparison of homologues from different species (see Fig. 6), we consider it to be likely that enzymes related to the frog isomerase are widely distributed among vertebrate species. This finding raises the intriguing possibility that peptides containing a d-amino acid are also present in these species. These may be unknown peptides that have not been isolated. In addition, the presence of d-isomers may have been overlooked in hormones, antibacterial or modulatory peptides of the immune system, or neuropeptides that have earlier been characterized. Indeed, d-amino acids cannot be detected with the usual sequencing techniques and by MS. In case of dermorphin, the first animal peptide that was found to contain a d-amino acid, this residue was discovered only because the synthetic all-l-isomer was biologically inactive (2). In other cases, both naturally occurring isomers are active, albeit with subtle differences. For example, such differences have been demonstrated for the two forms of ω-agatoxin IV from spider venom (8), the hypoglycemic hormone of lobsters (9), as well as the bombinins H (33). Similar cases may exist, but have not been noted, in peptides isolated from different vertebrate species.
Supplementary Material
Acknowledgments
We thank Dr. Rossella Miele (Università di Roma La Sapienza) for the genomic library from B. orientalis, Anita Weber (Austrian Academy of Sciences, Salzburg) for expert technical assistance, and Dr. Gabriele Seethaler (Austrian Academy of Sciences, Salzburg) for advice in the early part of this project. This work was supported by Austrian Fonds zur Förderung der Wissenschaftlichen Forschung Grant P13279, a grant from the State of Salzburg, the Italian Ministero dell'Istruzione dell'Università e della Ricerca (Università di Roma La Sapienza), and the Istituto Pasteur Fondazione Cenci Bolognetti (to D.B.).
Abbreviation: d-aIle, d-allo-Ile.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY916557, AY916558, and AY916559).
References
- 1.Lazarus, L. H. & Attila, M. (1993) Prog. Neurobiol. 41, 473–507. [DOI] [PubMed] [Google Scholar]
- 2.Montecucchi, P. C., de Castiglione, R., Piani, S., Gozzini, L. & Erspamer, V. (1981) Int. J. Pept. Protein Res. 17, 275–283. [DOI] [PubMed] [Google Scholar]
- 3.Erspamer, V., Melchiorri, P., Falconieri Erspamer, G., Negri, L., Corsi, R., Severini, C., Barra, D., Simmaco, M. & Kreil, G. (1989) Proc. Natl. Acad. Sci. USA 86, 5188–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mignogna, G., Simmaco, M., Kreil, G. & Barra, D. (1993) EMBO J. 12, 4829–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamatani, Y., Minakata, H., Iwashita, T., Nomoto, K., In, Y., Doi, M. & Ishita, T. (1990) FEBS Lett. 276, 95–97. [DOI] [PubMed] [Google Scholar]
- 6.Ohta, N., Kubota, I., Takao, T., Shimonishi, A., Yasuda-Kamatani, Y., Minakata, H., Homoto, K., Mueoka Y. & Kobayashi, M. (1991) Biochem. Biophys. Res. Commun. 178, 486–493. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez, E. C., Olivera, B. M., Gray, W. R. & Cruz, L. J. (1996) J. Biol. Chem. 271, 28002–28005. [DOI] [PubMed] [Google Scholar]
- 8.Heck, S. D., Siok, C. J., Krapcho, K. J., Kelbaugh, P. R., Thadeio, P. F., Welch, W. F., Williams, R. D., Ganong, A. H., Kelly, M. E., Lanazetti, A. J., et al. (1994) Science 266, 1065–1068. [DOI] [PubMed] [Google Scholar]
- 9.Soyez, D., Van Herp, F., Rossier, J., Le Caer, J. P., Tensen, C. P. & Lafont, R. (1994) J. Biol. Chem. 269, 18295–18298. [PubMed] [Google Scholar]
- 10.Torres, A. M., Menz, I., Alewood, P. F., Bansal, P., Lahnstein, J., Gallagher, C. H. & Kuchel, P. W. (2002) FEBS Lett. 524, 172–176. [DOI] [PubMed] [Google Scholar]
- 11.Richter, K., Egger, R. & Kreil G. (1987) Science 238, 200–202. [DOI] [PubMed] [Google Scholar]
- 12.Kreil, G. (1997) Annu. Rev. Biochem. 66, 337–345. [DOI] [PubMed] [Google Scholar]
- 13.Volkmann, R. A. & Heck, S. D. (1998) in d-Amino Acids in Sequences of Secreted Peptides of Multicellular Organisms, ed. Jollès, P. (Birkhäuser, Basel), pp. 87–105.
- 14.Heck, S. D., Faraci, W. S., Kelbaugh, P. R., Saccomono, N. A., Thadeio, P. F. & Volkmann. R. A. (1996) Proc. Natl. Acad. Sci. USA 93, 4036–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shikata, Y., Watanabe, T., Teramoto, T., Inoue, A., Kawakami, Y., Nishizawa, Y., Katayama, K. & Kuwada, M. (1995) J. Biol. Chem. 270, 16719–16723. [DOI] [PubMed] [Google Scholar]
- 16.Simmaco, M., Barra, D., Chiarini, F., Noviello, L., Melchiorri, P., Kreil, G. & Richter, K. (1991) Eur. J. Biochem. 199, 217–222. [DOI] [PubMed] [Google Scholar]
- 17.Mignogna, G., Pascarella, S., Wechselberger, C., Hinterleitner, C., Mollay, C., Amiconi, C., Barra, D. & Kreil, G. (1996) Protein Sci. 5, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradelles, P., Grassi, J. & Maclouf, J. (1985) Anal. Chem. 57, 1170–1173. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin, L. L., Wei, Y. F., Stockmann, P. T., Leahy, K. M., Needleman, P., Grassi, J. & Pradelles, P. (1987) Biochem. Biophys. Res. Comm. 144, 469–476. [DOI] [PubMed] [Google Scholar]
- 20.Scaloni, A., Simmaco, M. & Bossa, F. (1991) Anal. Biochem. 197, 305–310. [DOI] [PubMed] [Google Scholar]
- 21.Miele, R., Ponti, D., Boman, H.G., Barra, D. & Simmaco, M. (1998) FEBS Lett. 431, 23–28. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, P. A. & Melton. D. A. (1984) Nucleic Acids Res. 12, 7057–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay, B. K. (1991) Methods Cell Biol. 36, 663–669. [PubMed] [Google Scholar]
- 24.Kojima, M., Ohyama, Y., Miyamoto, K., Minamino, N., Kangawa, K. & Matsuo, H. (1994) J. Biol. Chem. 269, 13136–13140. [PubMed] [Google Scholar]
- 25.Harada, N., Iijima, S., Kobayashi, K., Yoshida, T., Brown, W. H., Hibi, T., Oskima, A. & Morikawa, M. (1997) J. Biol. Chem. 272, 15232–15241. [DOI] [PubMed] [Google Scholar]
- 26.Tanner, M. E., Gallo, K. A. & Knowles, J. R. (1993) Biochemistry 32, 3998–4006. [DOI] [PubMed] [Google Scholar]
- 27.Babbitt, P. C., Mraschko, G. T., Hasson, M. S., Huisman, G. W., Kolt, R., Ringe, D., Petsko, G. A., Kenyon, G. L. & Gerlt, J. A. (1995) Science 267, 1159–1181. [DOI] [PubMed] [Google Scholar]
- 28.Bandyopadhyay, P. K., Garren, J. E., Shetty, R. P., Keate, T., Walker, C. S. & Olivera, B. M. (2002) Proc. Natl. Acad. Sci. USA 99, 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czerwiec, E., Begley, G. S., Bronstein, M., Stenflo, J., Taylor, K., Furie, B. C. & Furie, B. (2002) Eur. J. Biochem. 269, 6162–6172. [DOI] [PubMed] [Google Scholar]
- 30.Buczek, O., Yoshikami, D., Bulaj, G., Jimenez, E. C. & Olivera, B. M. (2004) J. Biol. Chem. 280, 4247–4253. [DOI] [PubMed] [Google Scholar]
- 31.Mor, A., Delfour, A. & Nicolas, P. (1991) J. Biol. Chem. 266, 6264–6270. [PubMed] [Google Scholar]
- 32.Mor, A., Amiche, M. & Nicolas, P. (1992) Trends Biochem. Sci. 17, 481–485. [DOI] [PubMed] [Google Scholar]
- 33.Mangoni, M. L., Grovale, N., Giorgi, A., Mignogna, G., Simmaco, M. & Barra, D. (2000) Peptides 21, 1673–1679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.