Abstract
Genetic skin diseases encompass a vast, complex, and ever expanding field. Recognition of the features of these diseases is important to ascertain a correct diagnosis, initiate treatment, consider genetic counseling, and refer patients to specialists when the disease may impact other areas. Because genodermatoses may present with a vast array of features, it can be bewildering to memorize them. This manuscript will explain and depict some genetic skin diseases that occur in both humans and domestic animals and offer a connection and memorization aid for physicians. In addition, we will explore how animal diseases serve as a model to uncover the mechanisms of human disease.
The genetic skin diseases we will review are pigmentary mosaicism, piebaldism, albinism, Griscelli syndrome, ectodermal dysplasias, Waardenburg syndrome, and mucinosis in both humans and domesticated animals.
Introduction
Human genetics is a vast, complicated, and rapidly expanding field. Many genetic diseases affect the integumentary system; therefore, afflicted patients present first to a dermatologist. The dermatologist’s ability to recognize the features of a genodermatosis and their capability to group salient features together to ascertain a diagnosis is unique among medical specialists. It is rare for a dermatologist to encounter undiagnosed genetic skin diseases in daily practice, thus making it difficult to memorize these genodermatoses. Any connections we can draw between the work we do in our own clinic and other areas of life will aid in the remembrance of genetic skin disease.
Many of the genetic skin diseases that doctors encounter in their patients occur in animal species as well and the expression of gene mutations in animals is at times much more apparent than in human patients.
This manuscript will explore genetic skin diseases that occur both in humans and domesticated animals. Recognition of the genetic underpinnings of pigmentary variations in animals can reinforce the memorization of features of gene mutations that are important in diagnosing human disease. Furthermore, for those outside of the medical profession, knowledge of the outcome of genetic variation in animals makes the complicated topic of human genodermatoses more accessible and relatable.
Connections between human genetic disease and phenotype of domesticated animals
In domesticated animals, genetic skin diseases and particularly those that affect pigmentation may have a striking phenotypic expression. For millennia, humans have bred animals for specific desirable traits and historically, domesticated animals were bred primarily for function. For example, terriers were bred to control the population of rabbits and rats in Europe and oxen for their strength as a plow animal. More recently, domesticated animals have been bred for appearance, sometimes to the detriment of their health.
The interest in and emphasis on the appearance of purebred dogs and the popularity of dog showing was initially noted in North America in the 1870s with the establishment of the National American Kennel Club in 1876 and the Westminster Dog show in 1877. Subsequently, the National American Kennel Club developed a studbook in 1879 to help regulate breed standards and breeders started selecting dogs on the basis of beauty points instead of working talent. This trend has been notably criticized in the German Shepherd dog breed. The German Shepherd was originally bred to protect and herd flocks of sheep because of its well-muscled hind quarter and strong hind limbs. Recently, a sloping back conformation has become en vogue and breeding for the sloped back has weakened the integrity of the breed’s hips and limited their use as a working dog.
Piebaldism
Piebaldism is a rare, autosomal dominant (AD) disorder of melanocyte migration that results from a mutation in the KIT gene and can occur in nearly every species of mammal. Unlike many of the genodermatoses we will discuss, piebaldism is notable because it is solely a pigmentary skin disorder and lacks any systemic findings. Therefore, it is not surprising that when the unique pigmentary alteration was identified in domesticated animals, humans selected the gene for propagation to create new breeds of animals.
KIT gene mutations that cause piebaldism result in axial depigmentation, which usually spares the hands, feet, and back. Humans present with poliosis at birth, of which the most characteristic feature is a white forelock in 80% to 90% of cases. A piebald or pied animal has an analogous pigmentary pattern. The term piebald is a portmanteau of the words magpie and bald and originated as a reference to the distinctive black and white plumage of a magpie (Fig. 1; Skeat, 1882). Other notable examples include a black-and-white spotted Holstein cow and the Cavalier King Charles Spaniel dog breed.
Fig. 1.
Piebaldism.
(A) A magpie that exhibits midline depigmentation (Available from: https://upload.wikimedia.org/wikipedia/commons/5/50/01-Magpie.jpg User: diginatur [own work], CC BY-SA 3.0).
(B) Cavalier King Charles Spaniel dog that demonstrates axial depigmentation (Justin Finch, MD).
(C) Human with piebaldism who lives in El Cayo, British Honduras (Available from: https://commons.wikimedia.org/wiki/File:ALBINISM;_Human_Piebald_living_in_El_Cayo_Wellcome_L0032353.jpg Credit: Wellcome Library, London. Originally published in Karl Pearson and C. H. Usher. London: 1913. Plate BB).
Pigmentary mosaicism and lyonization
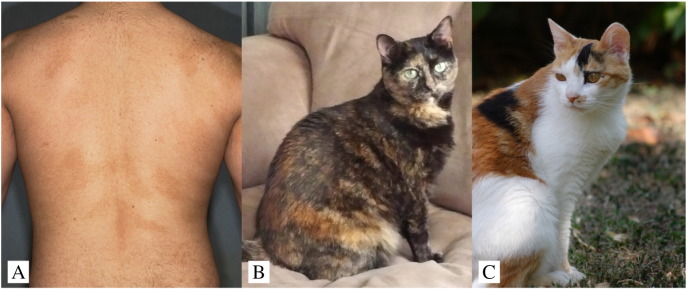
Pigmentary mosaicism is the occurrence of multiple colors or tonal variations of the skin or hair as a result of a mosaic gene mutation (Fig. 2). The exact genetic underpinnings of pigmentary mosaicism in humans remain elusive. Pigmentary mosaicism is a phenotypic endpoint that likely results from a vast array of genes. Although it is likely highly genetically heterogeneous, there are many cases of phylloid hypomelanosis among the known genes that are due to mosaic trisomy 13 (Faletra et al., 2012, González-Enseñat et al., 2009).
Fig. 2.
Variations in pigmentary mosaicism.
(A) Pigmentary mosaicism in a human (Justin Finch, MD).
(B) Pigmentary mosaicism in a tortoise shell cat (Stephanie Abrams, DVM).
(C) Pigmentary mosaicism in a calico cat (photo courtesy of Tatiana Chessa; under CC-BY SA 3.0 license).
In cats, hair color is a manifestation of different types of melanin much like in humans, with areas of pheomelanin-producing orange or red hair and eumelanin-producing black hair. Tortoiseshell cats have coat coloration that comprises of brindled swirls and stripes of red and black colored fur (Fig. 2). The orange coat color in cats is coded on the X chromosome and the tortoiseshell phenotype is produced by lyonization or X-inactivation. Therefore, tortoiseshell cats are female (or in rare cases, XXY male cats). When the tortoiseshell phenotype is combined with piebaldism, the result is a calico cat, which is also female. As the amount of piebalding increases in a calico cat, the areas of color become more distinct from one another and produce a phylloid tricolor pattern of white, orange, and brown.
Albinism
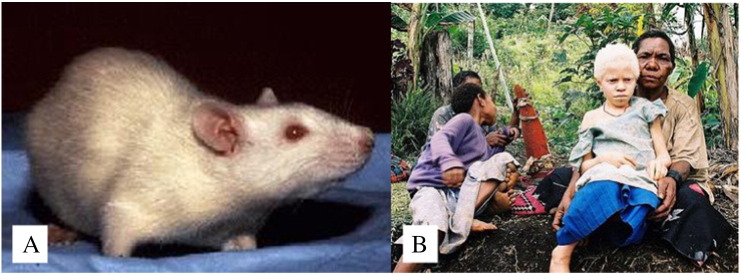
Oculocutaneous albinism is a group of pigmentary dilution disorders that affect the pigment-containing structures of the skin and eyes. Oculocutaneous albinism type 1 exhibits the greatest degree of pigmentary dilution and is characterized by very pale skin, white hair, and pink- or light-colored irises (Fig. 3). Types 2, 3, and 4 have lesser degrees of pigmentary dilution. Causative genes and their protein products include tyrosinase (TYR), OCA2 gene (a P protein), tyrosinase-related protein (TYRP), and the membrane-associated transporter protein, which is also known as solute carrier family 45 member 2 (Finch and Payette, 2016).
Fig. 3.
Albinism.
(A) Pet rat with albinism (Available from: http://dir.niehs.nih.gov/dirlep/Webpages/clinpath.html, public domain).
(B) An albino girl from Papua New Guinea (Available from: https://commons.wikimedia.org/wiki/File:Albinistic_girl_papua_ new_guinea.jpg, public domain).
Among domesticated animals, albinism in rats is noteworthy because the rat was the first mammalian species that was domesticated for scientific research. Work with albino rats dates back to the first half of the nineteenth century (Kuramoto et al., 2012). The albino rat was used as a model to confirm that Gregor Mendel’s famous laws on Mendelian Inheritance held true in the animal kingdom and it continues to be a popular exotic house pet today.
Temperature-sensitive albinism
Temperature-sensitive albinism is a variant of oculocutaneous albinism type 1B and is caused by an autosomal recessive (AR) mutation in the TYR gene. The temperature-sensitive variant TYR gene encodes a thermolabile tyrosinase enzyme that is inactivated at temperatures that exceed 35°C. The mutation results in decreased tyrosinase levels but with enough residual tyrosinase activity to produce some pheomelanin. As a result, cutaneous structures in warm sites of the body (eg, axilla, groin) are depigmented but normal coloration persists in cool acral sites (eg, arms, legs). Intermediate areas may have red hair (Wang et al., 2005).
These variations may be difficult to detect in humans but are striking in fur-bearing animals. The most notable example is the Siamese cat (Fig. 4).
Fig. 4.

Siamese cat (Stephanie Abrams, DVM).
Griscelli syndrome
Griscelli syndrome type 1, often classified as one of the silver hair syndromes (Finch and Payette, 2016), is a pigmentary dilution disorder that is caused by an AR mutation in Myosin Va (MYO5A). Myosin Va binds melanophilin to the actin cytoskeleton. Myosin Va is abundant in neurons of the central nervous system, cephalic ganglia, and spinal ganglia (Mercer et al., 1991) and controls oligodendrocyte morphogenesis and myelination (Sloane and Vartanian, 2007). Therefore, mutations result in profound neurologic defects and disordered melanosome trafficking. Affected humans have silvery-colored skin and hair, quadraparesis, mental retardation, and seizures.
Mutated MYO5A is present in approximately 10% of Arabian horses and homozygous transmission of the gene results in a lethal disorder known as Lavender Foal Syndrome, also known as Coat Color Dilution Lethal (Brooks et al., 2010). Affected foals are born with a coat color that is described as silver, pewter, lavender, or pink (Webb and Cullen, 2010). Afflicted foals are typically euthanized because of a constellation of severe neurologic abnormalities including opisthotonos and an inability to stand (Fig. 5).
Fig. 5.
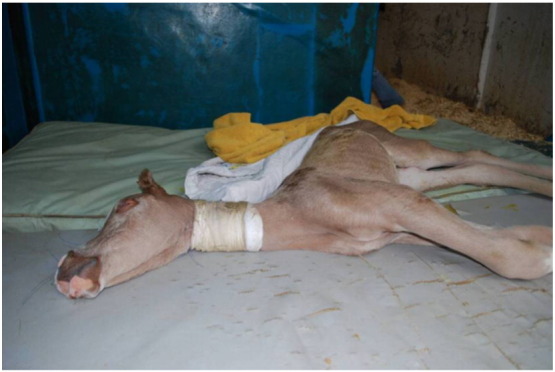
Foal with Lavender Foal Syndrome demonstrating pigmentary dilution and opisthotonos, one cardinal neurological sign of the disorder (PLoS Genet 2010;6, CC-BY 3.0).
Ectodermal dysplasia
Ectodermal dysplasias are a group of disorders in which there is abnormal development of the skin, nails, hair, teeth, or other adnexal structures such as sweat glands. Dozens of causative genes have been identified in humans. It is beyond the scope of this article to cover all the associated genes; however, it is worth discussing some specific phenotypes.
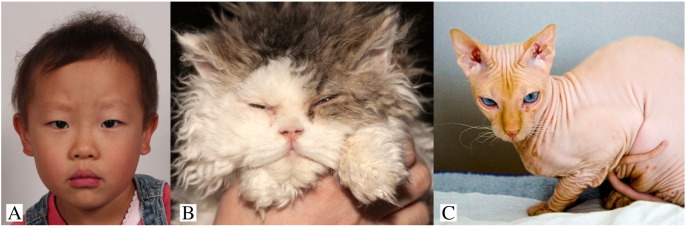
Wooly hair is an abnormal variant of hair growth in which the hair shaft is tightly coiled and imparts a wooly appearance. It is frequently associated with hypotrichosis and considered a hair growth deficiency. Keratins are a major structural component of the hair follicle and type II keratins 71, 72, 73, and 74 have been shown to localize to the inner root sheath of the hair follicle (Langbein et al., 2003). Keratin 74 mutations have been shown to underlie some pedigrees of AD wooly hair and hypotrichosis (Shimomura et al., 2010, Wasif et al., 2011), and more recently a keratin 71 mutation has been shown to cause the same phenotype (Fig. 6; Fujimoto et al., 2012).
Fig. 6.
Phenotypic variation of Keratin 71 mutations in humans and cats.
(A) Autosomal dominant wooly hair and hypotrichosis that is caused by KRT 71. Note the sparse eyebrow and wooly scalp hair (photo courtesy of Simomura Yukata, MD).
(B) Pure breed Sphinx cat. This phenotype is also caused by a KRT71 mutation. (photo: by User: Artourp [Own work], CC BY-SA 3.0; Available from: https://upload.wikimedia.org/wikipedia/commons/9/95/Shabbat.jpg).
(C) Selkirk Rex. The wooly hair is caused by a KRT71 mutation. (photo: Nickolas Titkov [own work], CC BY-SA 3.0; Available from: https://www.flickr.com/photos/titkov/5297138877/).
Interestingly, in cats the KRT71 gene is responsible for two distinct breeds: wooly-haired and hypotrichotic. The wooly-haired Selkirk Rex (Gandolfi et al., 2013) carries a KRT71 mutation (Fig. 6). Likewise, the very distinctive phenotype of the profoundly hypotrichotic hairless Sphinx cat is the result of a KRT71 mutation that was bred into the feline to create this notable breed (Fig. 6; Gandolfi et al., 2010). These cats, like affected humans, do not appear to have any systemic deficits.
Interest in hairless pets is not limited to cats. Among dogs, the Chinese Crested and Xoloitzcuintli dogs (commonly known as the Mexican hairless dog) are also bred for their hairlessness. Mutations in the FOXI3 gene that encode a forkhead box (FOX) protein are responsible for ectodermal dysplasia in both the Chinese Crested and Xoloitzcuintli dog breeds (Drögemüller et al., 2008, Wiener et al., 2013). Forkhead box proteins are a family of transcription factors that are important in regulating the expression of genes that are involved in cell growth, proliferation, and differentiation.
Today, we have not implicated forkhead box proteins in ectodermal dysplasias in humans but this discovery in dogs may prompt an investigation for new paths of human disease. Among human genodermatoses, FOX gene mutations that are worthy of a mention include FOXP3, which is responsible for IPEX Syndrome, and FOXC2, which is responsible for a range of disorders that exhibit lymphedema as a prominent feature including Meige Lymphedema, Lymphedema-Distichiasis, and Lymphedema-Yellow Nail Syndrome. But again, ectodermal dysplasia is not a feature of these disorders.
Waardenburg syndrome
Waardenburg syndrome is a group of heterogeneous disorders that are caused by mutations in at least half a dozen genes (Table 1). In humans, characteristic phenotypic features include poliosis that resembles piebaldism, synophrys (ie, medial eyebrow hyperplasia), and sensorineural deafness. Waardenburg syndrome commonly results in ophthalmic findings and most notably dystopia canthorum (ie, increased intercanthal distance and a broad nasal root with normal interpupillary distance) and heterochromia iridis. Affected patients have normal vision. Mutations in the endothelin-B receptor (ENDRB), endothelin-3, or SRY box 10 underlie type 4 Waardenburg Syndrome in which affected patients also exhibit Hirschsprung’s disease.
Table 1.
Human disease and corresponding animal breed that result from the same gene mutation
| Human Disease | Gene | Protein Product | Animal Breed |
|---|---|---|---|
| Piebaldism | KIT | ckit | Cavalier King Charles Spaniel dog Holstein cow Many others |
| Phylloid Pigmentary Mosaicism | Humans: unknown, some due to mosaic trisomy 13 | Unknown | Calico cat |
| Oculocutaneous Albinism, Type 1 | TYR | Tyrosinase | White rat Many others |
| Temperature-Sensitive Albinism | TYR | Tyrosinase | Siamese cat |
| Griscelli Syndrome | MYO5A | Myosin Va | Some Arabian horses (Lavender Foal Syndrome) |
| Autosomal Dominant Wooly Hair and Hypotrichosis | KRT 71 | Keratin 71 | Hairless Sphinx cat (KRT71) Selkirk Rex cat |
| Waardenburg Syndrome type 1 | PAX3 | Paired Box Gene 3 | Blaze-colored ferret (exact gene unknown) Blue-eyed white-coat cats (exact gene unknown) |
| SLUG | Zinc finger transcription factor | ||
| Waardenburg Syndrome type 2 | MITF | Microphthalmia-associated transcription factor | |
| Waardenburg syndrome type 3 | PAX3 | Paired Box Gene 3 | |
| Waardenburg Syndrome type 4 | SOX-10 | SRY box 10 | |
| ENDRB | Endothelin-B receptor | Frame overo horse | |
| EDN3 | Endothelin-3 |
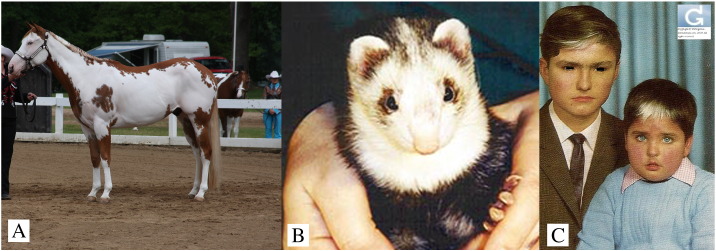
Waardenburg syndrome has several corollaries in the animal kingdom. For example, among horses, the unique pigmentation of the American Paint Horse breed Frame Overo results from an AD mutation in ENDRB (Fig. 7A; Magdesian et al., 2009). Like affected humans, these horses are deaf. Foals homozygous for this mutation suffer from a fatal condition known as Lethal White Overo, with lethality that results from severe Hirschsprung’s disease. Among ferrets, the blaze coloration is a ferret with a white stripe down its forehead (Fig. 7B). One hundred percent of ferrets with a white blaze are deaf (Piazza et al., 2014) and between 20% to 40% of white odd-eyed (heterochromic) cats are deaf (Cvejic et al., 2009). The association between deafness and dappled coat color also extends to llamas, alpacas, and dogs (Gauly et al., 2005, Strain et al., 1992).
Fig. 7.
Pigmentary alteration in Waardenburg syndrome.
(A) Deaf Frame Overo horse displayed at a show (photo: by Sandysphotos2009; licensed under CC BY 2.0).
(B) Deaf blaze-color ferret. (image courtesy of John Porter and Erika Matulich, PhD, PCM).
(C) Waardenburg syndrome in two siblings (image courtesy of Chirsophe Hsu, MD).
Mucinosis
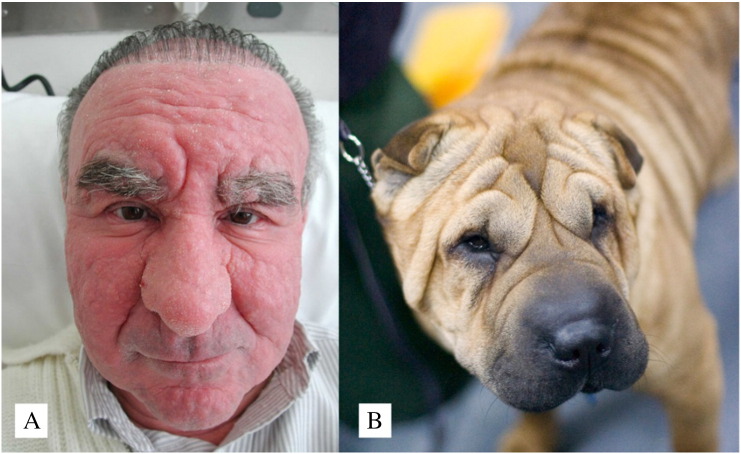
Mucinoses are a diverse group of skin disorders that involve the deposition of mucopolysaccharides in the skin and most notably the jelly-like protein hyaluronic acid. Mucinoses in humans can range in severity from minor nuisances to life-threatening systemic diseases that affect internal organs. Among the more severe forms of mucinosis, scleromyxedema and follicular mucinosis are notable for their propensity to extensively infiltrate the dermis and cause deep furrows in the skin, which impart a leonine appearance to the facial features of afflicted patients (Fig. 8A).
Fig. 8.
(A) Leonine facies due to follicular mucinosis (photo: Peter Heald, MD).
(B) Shar-Pei dog with facial features that result from hereditary mucinosis
(Available from: https://www.flickr.com/photos/48685334@N00/388814720; User: whartonds; licensed under CC BY-SA 2.0).
In domesticated animals, the Shar-Pei dog is a breed that is prized for its distinctive wrinkly skin (Fig. 8). Humans have bred a hereditary cutaneous mucinosis into this species to produce the characteristic wrinkled phenotype (Zanna et al., 2008, Zanna et al., 2009). Some Shar-Pei dogs suffer from such severe mucinosis that the mucin forms papules and nodules on the surface of the skin that rupture, ooze, and become pyodermatous or infected.
It has long been known that Shar-Pei dogs are predisposed to aggressive mast cell cancers. In humans, mast cells are found also in increased numbers in skin that is affected by mucinosis (Kobayasi et al., 1976). Mast cells in other diseases are associated with increased angiogenesis but mast cells in mucinosis appear to not be associated with neovascularization (Martins et al., 2010). Much remains to be learned about mucinoses and the Shar-Pei dog breed may serve as a model for ongoing studies of this disease.
Conclusions
Genetic skin diseases can present with a vast array of features. Because of humans’ pursuit of desirable visible traits in domesticated animals, we have bred some of the same mutations that cause disease in humans into our pets. The phenotype of these genodermatoses is at times more apparent in animals than in humans and familiarization with the features can aid in the recollection of the important aspects of genodermatoses in humans. The genetics of skin disorders in animals also carries relevance to humans because animal diseases serve as a model for uncovering the mechanism of human disease. Without murine models of disease, the field of genetics probably would not exist at all. By recognizing the similarities between animal and human disease, dermatologists can better serve their patients.
Footnotes
Conflicts of interest: None.
References
- Brooks S.A., Gabreski N., Miller D., Brisbin A., Brown H.E., Streeter C. Whole genome SNP association in the horse: identification of a deletion in myosin Va responsible for lavender foal syndrome. PLoS Genet. 2010;6:e1000909. doi: 10.1371/journal.pgen.1000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvejic D., Steinberg T.A., Kent M.S., Fischer A. Unilateral and bilateral congenital sensorineural deafness in client-owned pure-breed white cats. J Vet Intern Med. 2009;23:392–395. doi: 10.1111/j.1939-1676.2008.0262.x. [DOI] [PubMed] [Google Scholar]
- Drögemüller C., Karlsson E.K., Hytönen M.K., Perloski M., Dolf G., Sainio K. A mutation in hairless dogs implicates FOXI3 in ectodermal development. Science. 2008;321:1462. doi: 10.1126/science.1162525. [DOI] [PubMed] [Google Scholar]
- Faletra F., Berti I., Tommasini A., Pecile V., Cleva L., Alberini E. Phylloid pattern of hypomelanosis closely related to chromosomal abnormalities in the 13q detected by SNP array analysis. Dermatology. 2012;225:294–297. doi: 10.1159/000342884. [DOI] [PubMed] [Google Scholar]
- Finch J.J., Payette M. Genoderms Made Ludicrously Easy. Journal of Drugs in Dermatology. 2017 New York, NY, 2017 (in press) [Google Scholar]
- Fujimoto A., Farooq M., Fujikawa H., Inoue A., Ohyama M., Ehama R. A missense mutation within the helix initiation motif of the keratin K71 gene underlies autosomal dominant woolly hair/hypotrichosis. J Invest Dermatol. 2012;132:2342–2349. doi: 10.1038/jid.2012.154. [DOI] [PubMed] [Google Scholar]
- Gandolfi B., Alhaddad H., Joslin S.E., Khan R., Filler S., Brem G. A splice variant in KRT71 is associated with curly coat phenotype of Selkirk Rex cats. Sci Rep. 2013;3:2000. doi: 10.1038/srep02000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi B., Outerbridge C.A., Beresford L.G., Myers J.A., Pimentel M., Alhaddad H. The naked truth: Sphynx and Devon Rex cat breed mutations in KRT71. Mamm Genome. 2010;21:509–515. doi: 10.1007/s00335-010-9290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauly M., Vaughan J., Hogreve S.K., Erhardt G. Brainstem auditory evoked potential assessment of auditory function and congenital deafness in llamas (Lama glama) and alpacas (L pacos) J Vet Intern Med. 2005;19:756–760. doi: 10.1892/0891-6640(2005)19[756:bapaoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- González-Enseñat M.A., Vicente A., Poo P., Catalá V., Mar Pérez-Iribarne M., Fuster C. Phylloid hypomelanosis and mosaic partial trisomy 13: two cases that provide further evidence of a distinct clinicogenetic entity. Arch Dermatol. 2009;145:576–578. doi: 10.1001/archdermatol.2009.37. [DOI] [PubMed] [Google Scholar]
- Kobayasi T., Danielsen L., Asboe-Hansen G. Ultrastructure of localized myxedema. Acta Derm Venereol. 1976;56:173–185. [PubMed] [Google Scholar]
- Kuramoto T., Nakanishi S., Ochiai M., Nakagama H., Voigt B., Serikawa T. Origins of albino and hooded rats: implications from molecular genetic analysis across modern laboratory rat strains. PLoS One. 2012;7:e43059. doi: 10.1371/journal.pone.0043059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L., Rogers M.A., Praetzel S., Winter H., Schweizer J. K6irs1, K6irs2, K6irs3, and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol. 2003;120:512–522. doi: 10.1046/j.1523-1747.2003.12087.x. [DOI] [PubMed] [Google Scholar]
- Magdesian K.G., Williams D.C., Aleman M., Lecouteur R.A., Madigan J.E. Evaluation of deafness in American Paint Horses by phenotype, brainstem auditory-evoked responses, and endothelin receptor B genotype. J Am Vet Med Assoc. 2009;235:1204–1211. doi: 10.2460/javma.235.10.1204. [DOI] [PubMed] [Google Scholar]
- Martins C., Nascimento A.P., Monte-Alto-Costa A., Alves Mde F., Carneiro S.C., Porto L.C. Quantification of mast cells and blood vessels in the skin of patients with cutaneous mucinosis. Am J Dermatopathol. 2010;32:453–458. doi: 10.1097/DAD.0b013e3181b1c593. [DOI] [PubMed] [Google Scholar]
- Mercer J.A., Seperack P.K., Strobel M.C., Copeland N.G., Jenkins N.A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Piazza S., Abitbol M., Gnirs K., Huynh M., Cauzinille L. Prevalence of deafness and association with coat variations in client-owned ferrets. J Am Vet Med Assoc. 2014;244:1047–1052. doi: 10.2460/javma.244.9.1047. [DOI] [PubMed] [Google Scholar]
- Skeat Walter W. 1st ed. Clarendon Press; Hertfordshire: 1882. The Concise Dictionary of English Etymology. [Google Scholar]
- Shimomura Y., Wajid M., Petukhova L., Kurban M., Christiano A.M. Autosomal-dominant woolly hair resulting from disruption of keratin 74 (KRT74), a potential determinant of human hair texture. Am J Hum Genet. 2010;86:632–638. doi: 10.1016/j.ajhg.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane J.A., Vartanian T.K. Myosin Va controls oligodendrocyte morphogenesis and myelination. J Neurosci. 2007;27:11366–11375. doi: 10.1523/JNEUROSCI.2326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain G.M., Kearney M.T., Gignac I.J., Levesque D.C., Nelson H.J., Tedford B.L. Brainstem auditory-evoked potential assessment of congenital deafness in Dalmatians: Associations with phenotypic markers. J Vet Intern Med. 1992;6:175–182. doi: 10.1111/j.1939-1676.1992.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Wang T., Waters C.T., Jakins T., Yates J.R., Trump D., Bradshaw K. Temperature sensitive oculocutaneous albinism associated with missense changes in the tyrosinase gene. Br J Ophthalmol. 2005;89:1383–1384. doi: 10.1136/bjo.2005.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasif N., Naqvi S.K., Basit S., Ali N., Ansar M., Ahmad W. Novel mutations in the keratin-74 (KRT74) gene underlie autosomal dominant woolly hair/hypotrichosis in Pakistani families. Hum Genet. 2011;129:419–424. doi: 10.1007/s00439-010-0938-9. [DOI] [PubMed] [Google Scholar]
- Webb A.A., Cullen C.L. Coat color and coat color pattern-related neurologic and neuro-ophthalmic diseases. Can Vet J. 2010;51:653–657. [PMC free article] [PubMed] [Google Scholar]
- Wiener D.J., Gurtner C., Panakova L., Mausberg T.B., Müller E.J., Drögemüller C. Clinical and histological characterization of hair coat and glandular tissue of Chinese crested dogs. Vet Dermatol. 2013;24:274-e62. doi: 10.1111/vde.12008. [DOI] [PubMed] [Google Scholar]
- Zanna G., Docampo M.J., Fondevila D., Bardagí M., Bassols A., Ferrer L. Hereditary cutaneous mucinosis in shar pei dogs is associated with increased hyaluronan synthase-2 mRNA transcription by cultured dermal fibroblasts. Vet Dermatol. 2009;20:377–382. doi: 10.1111/j.1365-3164.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- Zanna G., Fondevila D., Bardagí M., Docampo M.J., Bassols A., Ferrer L. Cutaneous mucinosis in shar-pei dogs is due to hyaluronic acid deposition and is associated with high levels of hyaluronic acid in serum. Vet Dermatol. 2008;19:314–318. doi: 10.1111/j.1365-3164.2008.00703.x. [DOI] [PubMed] [Google Scholar]